Abstract
The diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is based on Task Force Criteria published in 1994 that included imaging abnormalities of the right ventricle as well as diagnostic pathological evaluation of the right ventricular myocardium by endomyocardial biopsy. These have recently been modified to include evaluation by cardiac magnetic resonance (CMR). In addition quantitative criteria for the percent of fibrosis and decrease in myocyte are included in the new criteria. The pitfalls of determining the presence of ARVC/D at autopsy and the difficulty of assessing the presence of this disease in family members are well illustrated in the following report. In conclusion, this report illustrates the need to subscribe to the modified criteria to avoid misdiagnosis.
Keywords: Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia, Misdiagnosis, Cardiac MRI, Pathology
Introduction
Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) is an uncommon genetic disease principally affecting the right ventricle by fatty and fibrous tissue replacement of the myocardium. 1 The clinical manifestations are primarily due to ventricular arrhythmias such as ventricular premature complexes (VPCs) and nonsustained or sustained ventricular tachycardia (VT). Occasionally, sudden cardiac death may be the first event. If this occurs, it frequently and appropriately initiates clinical evaluation of family members. The pitfalls of determining the presence of ARVC/D at autopsy and the difficulty of assessing the presence of this disease in family members are well illustrated in the following report.
Report
A 32 year asymptomatic woman (A) sought medical advice because the patient’s paternal grandmother was diagnosed as having ARVC/D at autopsy. She had had a routine electrocardiogram two years previously and was noted to have VPCs. Her 12-lead electrocardiogram was normal; the T wave was inverted in V1 but the T waves were upright in V2 – V6. A signal averaged electrocardiogram was normal. She had excellent exercise tolerance on a stress test. Several VPCs were noted during recovery but there was no sustained VT. A 24-hour Holter monitor showed 3,025 VPCs per 24 hours and 1 episode of 3 consecutive VPCs. The VPCs had a right bundle branch block pattern.
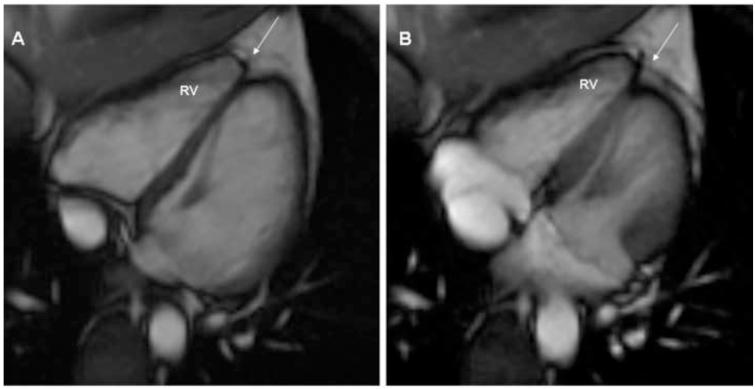
A 2 dimensional echocardiogram showed mildly redundant mitral leaflets and minimal mitral regurgitation but no mitral valve prolapse. A cardiac magnetic resonance (CMR) imaging was interpreted at the referring institution as showing “a subtle abnormality involving the right ventricular apex. There was slightly diminished contractility and delayed enhancement involving the right ventricular apex. This could represent a mild form of right ventricular dysplasia”. The above studies were sent to a tertiary referral center for evaluation. The imaging studies were interpreted by a CMR specialist, who has extensive experience in interpretation of these tests in patients with suspected ARVC/D. He could not find any abnormality suggestive of ARVC/D either by echocardiography or CMR. (Figure 1A,1B) The patient (A) was reassured and no further studies for ARVC/D were done. No treatment was advised.
Figure 1.
CMR diastolic (1A) and systolic (1B) images showing normal RV function and volume. Normally the cardiac “apex” is formed by the LV alone. Not uncommonly, the apices are separate; the so-called butterfly apex (arrow). In this situation, the normally thin, minimally contracting RV apex is not tethered by the LV apex and may be reported as akinetic, dyskinetic or even aneurysmal.
This individual related her experience with regard to the possible misdiagnosis of ARVC/D by CMR to her cousin (B), a woman aged 27 who had been recently diagnosed with ARVC/D, who then sought reevaluation of her diagnosis. The two patients were first cousins with the same grandmother who was diagnosed with ARVC/D at autopsy. The cousin’s sister had recently died unexpectedly at age 40 and was diagnosed as having ARVC/D at autopsy. This 27 year old woman (B) reported she had noted palpitations after her sister’s death.
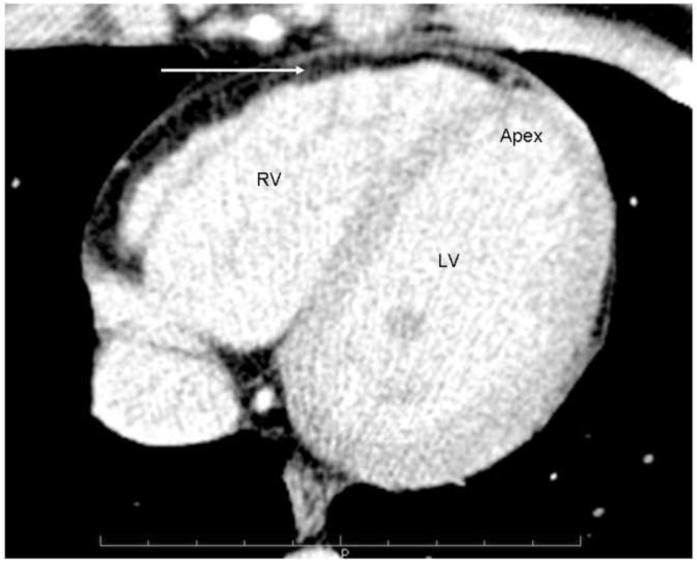
The cousin’s (B) electrocardiogram was normal. The 2-dimensional echocardiogram showed mild left ventricular enlargement; a CMR report showed “fatty infiltration of the right ventricular wall”. There were no wall motion abnormalities. The right ventricular ejection fraction was reported as 46%. A cardiac computed tomography (CT) of the chest was interpreted as showing diffuse fatty infiltration of the right ventricular wall. (Figure 2)
Figure 2.
Cardiac CT demonstrates extensive fat along the RV wall, (see arrow) but it is difficult to clearly separate epicardial from myocardial fat.
The cousin (B) was referred to an electrophysiologist who implanted an implantable cardioverter defibrillator (ICD) based on the family history of ARVC/D as well as the imaging studies that showed fatty infiltration of the right ventricular myocardium. She has not had ICD therapy over the 9 month period since her implantation. The patient expressed concern that her 4 children might be affected with this disease.
The CMR and CT scan were reviewed at our tertiary medical center and it was concluded that there was normal left ventricular and right ventricular global systolic function. There was fat around the right ventricle but no fatty infiltration in the right ventricular myocardium. (Figure 2) There were no right ventricular wall motion abnormalities. It was concluded that there were no imaging criteria of ARVC/D in this patient.
Records were obtained on the 2 family members who had had a postmortem diagnosis of ARVC/D. There was only a death certificate available of the grandmother who died in 2000 at age 68. The immediate cause of death was reported as due to heart failure and “ARVC/D”. However, she had adenocarcinoma of the liver with metastasis to the lungs and also chronic obstructive pulmonary disease. Carcinoma was the most likely cause of her death. No further details were available regarding the morphology of the right ventricle.
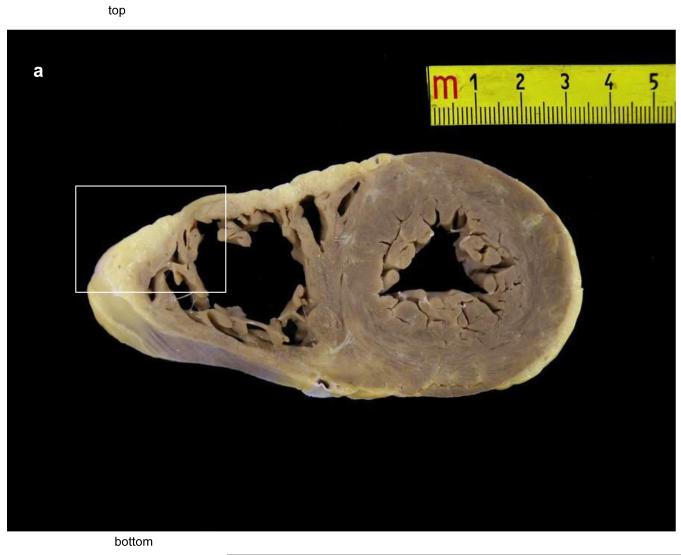
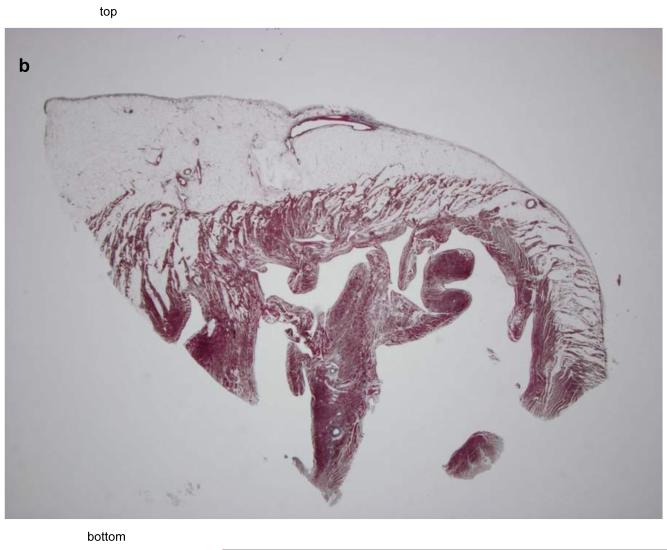
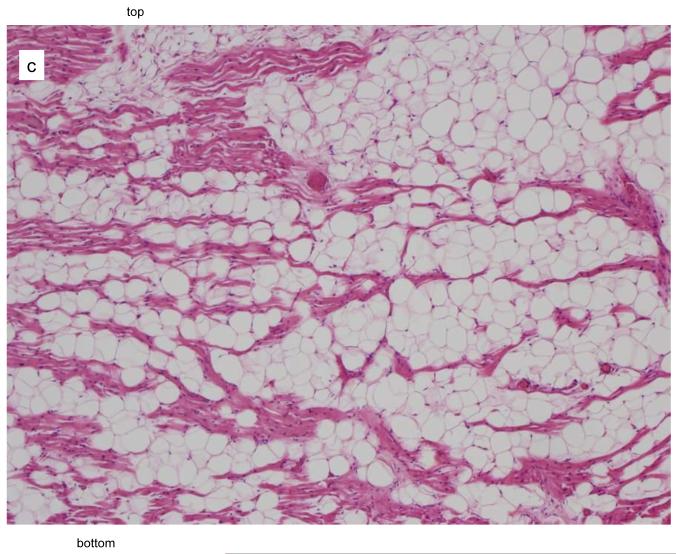
The autopsy of the 40-year-old sister who died suddenly and unexpectedly was reported to show fibrofatty infiltration of the right and left ventricular myocardium. The cause of death was stated to be ARVC/D. A complete cross sectional slice of the heart, including the right and left ventricles with ventricular septum, were provided by the medical examiner and sent to the co-director of the pathology core lab (Padua, Italy) of a NIH research study of ARVC/D. A large amount of fat was present in the right ventricle but this was within physiological limits. (Figure 3A) The panoramic sections were stained with trichrome and did not show any evidence of fibrosis. (Figure 3B) At high magnification, the hematoxylin eosin stain did not show myocyte abnormality. It was concluded that there was no pathological evidence for ARVC/D, only fatty infiltration (adipositas cordis). (Figure 3C)
Figure 3.
Pathological features in the 40 year old sister who died suddenly with a postmortem misdiagnosis of ARVC/D.
A Transverse section of the heart at the mid-ventricular level: note the presence of fatty tissue in the antero-lateral wall of the right ventricle.
B The panoramic section of the boxed area in Figure 3A shows fatty infiltration of the myocardium with dissociation of the myofibers in the absence of fibrous tissue, trichrome stain, x3 magnification.
C At higher magnification (x50), the hematoxylin eosin stain shows normal myocytes in the presence of mature adipocytes.
This case report illustrates the difficulty in the diagnosis of this disease even with 2 autopsy reports that stated that the deaths were due to ARVC/D.
Discussion
Review of this family’s experience with the pathological and clinical diagnosis of ARVC/D lead us to the conclusion that the autopsy diagnosis of ARVC/D in the 40 year old woman was incorrect as was this diagnosis in 2 family members one of whom had an ICD implanted. A critical analysis of the possible reasons for these diagnostic errors is instructive. First, the pathological diagnosis of ARVC/D consists of replacement of myocardial cells by fibrous and fatty tissue. The pathological diagnosis should minimize the extent of fatty infiltration as a diagnostic criteria and this has been recently well defined for the in vivo diagnosis. 2-3 In particular, at endomyocardial biopsy a major pathological criterion consists of < 60% of residual myocytes by morphometric analysis or < 50% if estimated, with fibrous replacement of the right ventricular free wall myocardium with or without fatty replacement of tissue. A minor criterion consists of residual myocytes of 60-75% by morphometric analysis or 50-65% if estimated, with fibrous replacement as stated above. In addition to myocardial atrophy with fibro fatty replacement, there may be degenerative changes of the myocytes. 4
In the case cited above the autopsy report stated that there was fatty infiltration and fibrosis of the right and left ventricles. They did not mention the extent of the fibrosis. Review of the pathological slides with trichrome stain showed minimal if any fibrosis.(Figure 3) The fact that there was extensive amount of fat in the right ventricle was undoubtedly the basis for the pathological misdiagnosis of ARVC/D. In normals, fat in the right ventricle but not in the left ventricle may be interspersed with myocardial fibers but without fibrosis or signs of inflammation. 5,6 The amount of subepicardial fat increases with body weight. 7 The presence of extensive fatty infiltration has not been found to be familial and is not associated with arrhythmic death during exercise. 8
Since it is of vital importance to the family members to be certain of the diagnosis of ARVC/D, it is pertinent that the pathologist assess the presence and extent of replacement-type fibrosis and fat in the free wall of the right ventricle sections stained with trichrome and of myocyte abnormalities.
Both family members had CMR studies that were interpreted at the referring center to be suggestive or consistent with ARVC/D. One study was interpreted as showing a subtle wall motion abnormality near the apex of the right ventricle. A study by Sievers et al. found that outward systolic motion interpreted as hypokinesia or bulging may be seen near the insertion of the moderator band in healthy subjects. 9 This finding is frequently misdiagnosed as indicative of ARVC/D. In the other patient, a CMR and CT scan was interpreted as showing fatty infiltration in the right ventricular wall. In the absence of right ventricular wall motion abnormalities in the same area, this finding should be interpreted with caution. 10,11 Incorrect interpretation of the CMR is a frequent cause for the misdiagnosis of ARVC/D. 12 One possible reason for misinterpretation of the CMR is that the original Task Force Criteria did not provide guidelines for the diagnosis of ARVC/D by CMR since the technology was just being introduced at that time.
Fat in the right ventricular wall by CT scan can also be misinterpreted to indicate ARVC/D. In a recent study of 165 patients who underwent electron beam computed tomography (EBCT) for coronary calcium scoring, 28 patients (17%) had macroscopic fat deposit in the right ventricular wall. 13
The problem of incorrect interpretation of the autopsy findings and imaging studies is a distinct problem for management of patients suspected with this disease, since the diagnosis carries a risk of sudden cardiac death. On the other hand, incorrect diagnosis of ARVC/D may lead to unnecessary insertion of an ICD, and initiate a costly and extensive screening of family members with the possibility of overdiagnosis as a result of the screening process. Therefore, the clinician should not hesitate to request a second opinion if there is any doubt that the imaging studies and postmortem findings have not been properly interpreted. The diagnosis of ARVC/D should not be based on any one criterion but should meet the recently published modified Task Force Criteria. 14
Acknowledgements
Supported in part by grant U01 – HL65594 from the National Heart, Lung and Blood Institute, Bethesda, MD and by the Registry of Cardio-Cerebro-Vascular Pathology, Veneto Region, Venice, Italy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009;373:1289–1300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Burke M, Fornes P, Gallagher PJ, de Gouveia R Henriques, Sheppard M, Thiene G, van der Wal A. On behalf of the association for European Cardiovascular Pathology. Guidelines for autopsy investigation of sudden cardiac death. Virchows Arch. 2008;452:11–18. doi: 10.1007/s00428-007-0505-5. [DOI] [PubMed] [Google Scholar]
- 3.Basso C, Ronco F, Marcus F, Abudureheman A, Rizzo S, Frigo AC, Bauce B, Maddalena F, Nava A, Corrado D, Grigoletto F, Thiene G. Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria. Eur Heart J. 2008;29:2760–2771. doi: 10.1093/eurheartj/ehn415. [DOI] [PubMed] [Google Scholar]
- 4.Basso C, Thiene G. Adipositas cordis, fatty infiltration of the right ventricle, and arrhythmogenic right ventricular cardiomyopathy. Just a matter of fat? Cardiovasc Pathol. 2005;14:37–41. doi: 10.1016/j.carpath.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Fontaliran F, Fontaine G, Fillette F, Aouate P, Chomette G, Grosgogeat Y. Frontieres nosologiques de la dysplasie arythmogene. Arch. Mal Coeur. 1991;84:33–38. [PubMed] [Google Scholar]
- 6.Fontaine G, Fontaliran F, Zenati O, Guzman CE, Rigoulet J, Berthier JL, Frank R. Fat in the heart. A feature unique to the human species? Observational reflections on an unsolved problem. Acta Cardiol. 1999;54:189–194. [PubMed] [Google Scholar]
- 7.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (Cor Adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol. 1995;76:414–418. doi: 10.1016/s0002-9149(99)80116-7. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium. Are they different diseases? Circ. 1998;97:1571–1580. doi: 10.1161/01.cir.97.16.1571. [DOI] [PubMed] [Google Scholar]
- 9.Sievers B, Addo M, Franken U, Trappe HJ. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magn Reson. 2004;6:601–608. doi: 10.1081/jcmr-120038528. [DOI] [PubMed] [Google Scholar]
- 10.Bluemke DA, Krupinski EA, Gear K Ovitt., Unger E, Axel L, Boxt LM, Casolo G, Ferrari VA, Funaki B, Globits S, Higgins CB, Julsrud P, Lipton M, Mawson J, Nygren A, Pennell DJ, Stillman A, White RD, Wichter T, Marcus F. MRI imaging of arrhythmogenic right ventricular cardiomyopathy: Morphologic findings and interobserver reliability. Cardiol. 2003;99:153–162. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 11.Macedo R, Prakasa K, Tichnell C, Marcus F, Calkins C, Lima JAC, Bluemke DA. Marked lipomatous infiltration of the right ventricle: MRI findings in relation to arrhythmogenic right ventricular dysplasia. AJR. 2007;188:W423–W427. doi: 10.2214/AJR.06.0161. [DOI] [PubMed] [Google Scholar]
- 12.Bomma C, Rutberg J, Tandri H, Tichnell C, Marcus F, Calkins H, Lima JAC, Bluemke DA. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2004;15:300–306. doi: 10.1046/j.1540-8167.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirsch J, Williamson EE, Glockner JF. Focal macroscopic fat deposition within the right ventricular wall in asymptomatic patients undergoing screening EBCT coronary calcium scoring examinations. Int J Cardiovasc Imaging. 2008;24:223–227. doi: 10.1007/s10554-007-9232-x. [DOI] [PubMed] [Google Scholar]
- 14.Marcus F, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado DA, Cox MGPJ, Daubert J, Fontaine G, Gear K, Hauer RNW, Nava A, Picard MH, Protonotarios N, Saffitz JE, Yoerger Sanborn DM, Steinberg JS, Tandri HM, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D); Proposed Modification of the Task Force Criteria. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.840827. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]