Abstract
Three experiments investigated the size and sources of age-related changes in visual imitation. In Experiment 1, young and older adults viewed sequences of quasi-random movements and then reproduced from memory what they had seen. As expected, older adults made more errors in imitation than their younger counterparts. However, older adults seemed to supplement their memory by exploiting an abstracted representation (gist) of a sequence. Experiments 2 and 3 apportioned the observed age-related changes in imitation performance among several possible causes. Experiment 2 showed that changes in precision of visual perception and motor control together accounted for only a small fraction of age-related changes in imitation quality; Experiment 3 showed that the bulk of the age-related changes arose from the older participants’ reduced ability to accommodate for increases in memory load, likely caused by diminished ability to encode or retain detailed information about movement sequences. Guided by these results, strategies are proposed for enhancing older adults’ imitation learning.
Keywords: aging, imitation, gist, motor skill, memory
What do tying shoelaces, hitting a golf ball, and square dancing have in common? Each can be learned by imitation, which is a major way that people of all ages acquire and master important skills. Imitation has been widely studied in infants (Elsner, 2007) and young children (Lepage & Théoret, 2007), but almost no attention has been given to the imitation ability of older adults (but see Celnik et al., 2006; Leonard & Tremblay, 2007). This lack of attention is noteworthy because imitation learning is especially important for older adults. For example, imitation learning makes it possible to maximize the physical and cognitive benefits of participation in exercise, dance, and sports activities (Lautenschlager & Almeida, 2006; Studenski et al., 2006). Additionally, imitation is key for mastering everyday tasks that are essential to the maintenance of older adults’ healthy independent lifestyle (Czaja et al., 2006).
We anticipate that imitation ability changes with age. After all, successful imitation of seen actions requires the cooperation of several processes that change with age. In particular, age-related changes have been demonstrated for visual perception of motion (Ball & Sekuler, 1986; Bennett, Sekuler, & Sekuler, 2007; Betts, Sekuler, & Bennett, 2007; Billino, Bremmer, & Gegenfurtner, 2008; Sekuler & Ball, 1986; Snowden & Kavanagh, 2006), motor control and motor imagery (Christou & Carlton, 2001; Smiley-Oyen, Lowry, & Kerr, 2007), and working memory (Craik & Salthouse, 1992; Gomez-Perez & Ostrosky-Solis, 2006; Peelle & Wingfield, 2005; Salthouse & Coon, 1993; Small, 2001; Verhaeghen, Marcoen, & Goossens, 1993), each of which is essential for successful imitation. We devised tasks that made it possible to characterize each component process’s contribution to imitation’s overall quality.
Most of our experiments ask participants to view and then reproduce from memory a sequence of quasi-random, linked movements (Sekuler, Siddiqui, Goyal, & Rajan, 2003). Each sequence is enacted by a disk that moves across a computer screen while leaving no visible trail. After observing an entire movement sequence, the participant guides a handheld stylus across the surface of a graphics tablet to reproduce from memory the movements that had just been seen. For our purposes, this task confers important advantages over others that have been used to study imitation. First, the task is well suited to disentangling imitation’s various components. Second, the task lends itself to an objective, quantitative assessment of imitation accuracy (Ambrosoni, Della Sala, Motto, Oddo, & Spinnler, 2006; Buxbaum, Kyle, & Menon, 2005; Leonard & Tremblay, 2007; Rizzolatti & Craighero, 2004), and it uses stimuli of sufficient complexity that performance need not be reduced to a binary, pass–fail scale (e.g., Celnik et al., 2006; Iacoboni et al., 1999). Finally, by accommodating stimuli with distinct statistical properties, our task makes it possible to characterize some of the supplementary information that participants of different ages exploit. Such supplementary information, which aids successful retrieval of items from memory, can include semantic or other relationships (Baddeley, 1964; Miller, 1956; Okada & Burrows, 1973; Reynolds & Goldstein, 1974). Although the sequences of quasi-random movements used in our imitation task do not involve semantic relationships, participants might be able to abstract the gist of the stimulus’s spatiotemporal structure and then use that gist to supplement imperfect recall of movement sequence details. This possibility is of interest because a number of studies, using pictures or words as stimuli, have shown that older adults rely more heavily than young adults on an abstracted version, or gist, of what they have seen rather than the details of each experience (Castel, Farb, & Craik, 2007; Koutstaal, 2006; Koutstaal, Schacter, Galluccio, & Stofer, 1999; Tun, Wingfield, Rosen, & Blanchard, 1998). By examining imitations of stimuli with distinct spatiotemporal structures, we sought to identify the form of gist that participants exploited.
Additionally, by varying our basic task, we attempted to isolate imitation’s key components, identifying the sources of age-related change in imitation quality. To preview what follows, Experiment 1 employs converging measures to capture the overall age-related decrease in imitation performance. Additionally, by comparing performance with different, specially designed movement sequences, Experiment 1 attempts to reveal how an age-dependent use of supplementary information contributes to overall age-related differences in imitation. Experiment 2 quantifies the contributions that age-related changes in perception and motor control might make to the age-related difference in imitation accuracy. By manipulating the retention interval and the memory load imposed by the imitation task, Experiment 3 evaluates several ways by which age-related changes in memory might impact imitation performance in young and older adults. Together all three experiments provide insights into the processes that support imitative performance and illuminate the ways in which those processes change with age.
General Method
The three experiments reported here shared the same basic task and data analyses, with variations introduced as required by each experiment’s distinct purpose. The following sections summarize the task’s general properties and the general methods used to analyze performance.
Experimental Task
Participants observed a small disk that made one or more linear movements. Then, after some delay, participants reproduced the movements that they had seen. The sequence of events on a typical trial is illustrated in Figure 1. At the start of a trial, a stationary yellow disk (0.54° visual angle) appeared for 1 s (Figure 1A) before moving along a pseudo-random path comprising a series of connected straight segments (Figure 1B). Traveling at a constant rate of 4° visual angle/s, the disk took 350 ms to move through the 1.40° visual angle length of each segment. In addition, the disk paused for 250 ms between successive segments. After a sequence’s movements were completed, the yellow disk disappeared. Then, after 3.75 s (Figure 1C), a blue disk appeared (Figure 1D), signaling the participant to move a handheld stylus over the surface of a graphics tablet (31 × 24 cm), reproducing from memory the movements that had been seen (Figure 1E). During the reproduction, the movement of the blue disk was yoked to the movement of the stylus’s tip on the graphics tablet. Participants were instructed to “make the blue disk do what the yellow disk had done.”
Figure 1.
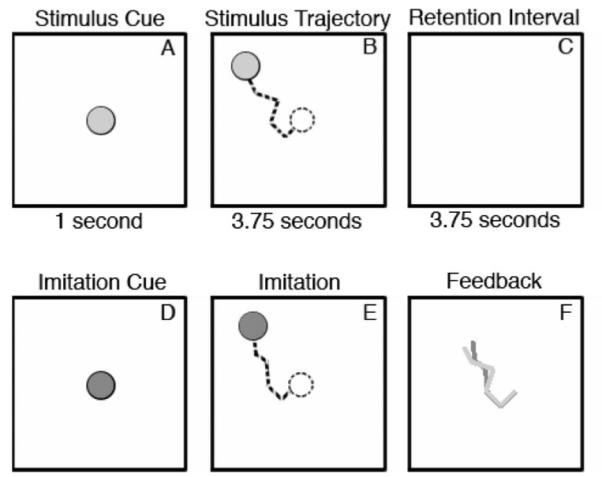
Sequence of events on an imitation trial. At the trial’s start a yellow disk appeared at the center of the display (Figure 1A). After 1 s the disk began to move in a series of connected linear segments without leaving any visible trail (Figure 1B). After a retention interval of 3.75 s (Figure 1C), a blue disk appeared (Figure 1D) signaling the participant to begin imitating the remembered path taken previously, by the yellow disk (Figure 1E). When the participant finished, feedback was provided by displaying the path of the participant’s imitation superimposed on the path that had been taken by the stimulus (Figure 1F). Figure adapted from “Geometric Structure and Chunking in Reproduction of Motion Sequences,” by Y. Agam and R. Sekuler, 2008, Journal of Vision, 8, p. 3. Copyright 2008 by the Association for Research in Vision and Ophthalmology.
After a participant’s reproduction had been completed, feedback was provided in the form of a superimposed display of a static image of the path taken by disk in the original movements and a static image of the participant’s reproduction (Figure 1F). The next trial began when the participant tapped the stylus on the graphics tablet. The computer display was viewed binocularly from a distance of 65 cm; each participant positioned the graphics tablet so that he or she would be comfortable while controlling the stylus.
Note that neither the blue nor the yellow disk left a visible trail as it moved across the display. As a result, any representation of the complete path would have to be generated in the participant’s mind’s eye and then maintained in memory until reproduction was called for (Geisler, Albrecht, Crane, & Stern, 2001; Jancke, 2000).
Stimuli
When an experiment required stimuli comprising multiple movements, each trial’s unique sequence of movements was generated with an algorithm similar to that described in Agam, Bullock, and Sekuler (2005). The direction of the first movement segment for each trial was chosen randomly from integer values spanning 1°–360°. The direction of each successive segment can be described as a change relative to the direction of its immediate predecessor, with each change being chosen randomly from a uniform distribution of 30°–150°.
Note that whatever its precise direction, each movement segment after the first can be described broadly, as representing a clockwise or a counterclockwise turn relative to the previous movement segment. Further, from the third segment on, each clockwise or counterclockwise turn can be described as either consistent (+) or inconsistent (−) with the previous turn. Thus, in a stimulus with n segments, there are n−1 turns and n−2 potential loci of consistency. Figure 2 illustrates a movement sequence containing both consistent and inconsistent turns. In this figure, the first two segments define a clockwise turn; the third segment also turns clockwise, which makes it a consistent turn (+). However, the fourth segment reverses direction, turning counterclockwise and therefore qualifying as an inconsistent turn (−). The number of possible types of stimuli that could result from permuting consistent and inconsistent turns varies with n: the number of possible types = 2n−2. As Figure 3 shows, in a five-segment stimulus (the common variety in our experiments) there are eight possible types (23), each described by a triplet of +’s and −’s (one at each of the three potential loci of consistency). In each block of trials, exemplars of all possible types of stimuli occurred with equal frequency in an order that was rerandomized for each block of 2n−2 trials. Stimuli comprising different patterns of consistent and inconsistent turns afford distinct forms of stimulus gist, as explained below. By comparing imitation performance across different types of stimuli, we hoped to be able to characterize the forms of gist that participants exploited in making their reproductions of what they had seen.
Figure 2.
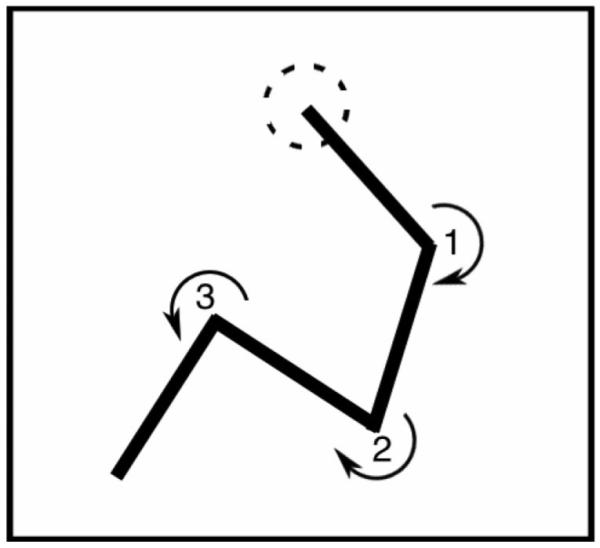
A stimulus that includes consistent and inconsistent turns. The dashed circle indicates the stimulus’s starting point. The first turn (1), from Segment 1 to Segment 2, is in a clockwise direction; the second turn (2) is also in a clockwise direction, which is consistent (+) with the immediately preceding turn. However, the third turn (3) is counterclockwise, which is inconsistent (−) with the previous, clockwise turn (2).
Figure 3.
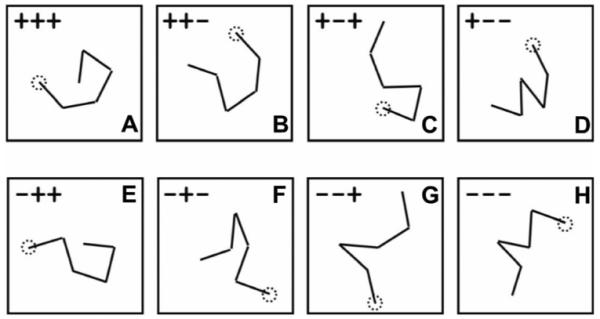
Examples of the eight types of stimuli that are possible with five segments. There are three potential loci of consistency at which a turn can be compared with the previous turn: at the start of the third, fourth, and fifth movement segments. The turn at each one of these potential loci of consistency can be either consistent (indicated by +) or inconsistent (indicated by −). The dotted circle in each figure indicates the start point of the sequence. Figure adapted from “Geometric Structure and Chunking in Reproduction of Motion Sequences,” by Y. Agam and R. Sekuler, 2008, Journal of Vision, 8, p. 3. Copyright 2008 by the Association for Research in Vision and Ophthalmology.
Evaluating Imitation Quality
During each reproduction, a computer recorded the stylus’s position whenever the stylus moved by one or more pixels; the horizontal and vertical coordinates of the stylus’s new position were written to computer disk along with a time stamp. These data were processed offline with an algorithm that broke each reproduction into segments based on spatial (changes in the direction of the reproduction) and temporal (changes in the timing of the pen movement) criteria (see Agam et al., 2005, for details). The algorithm also estimated the orientation of each segment, fitting a line to the segment’s beginning and end points and calculating the slope of the fitted slope.
A trial was deemed valid if the number of segments recovered by the algorithm from the reproduction matched the number of segments that had been in the stimulus; invalid trials were excluded from further analysis. To minimize the number of invalid trials and maximize the precision with which segment direction could be determined, participants were instructed to try to produce the same number of segments that had been in the stimulus. The percentage of trials that were invalid for young and older adults were not reliably different from one another: Experiment 1: 7.5% (SD = 11.2%) and 12.8% (SD = 12.2%), for young and older participants, respectively (p > .05); Experiment 2: no trials invalid for either group; Experiment 3: 12.6% (SD = 12.5%) and 12.5% (SD = 7.6%), respectively (p > .05).
Mean Orientation Error
Our basic measure of imitation accuracy was mean orientation error. This measure is the mean absolute difference between the orientation of a segment in the reproduction and the orientation of the corresponding segment in the stimulus (see Agam et al., 2005, for details of this computation). Note that this measure is unsigned, making its value independent of whether the reproduced segment’s orientation was clockwise or counterclockwise relative to the stimulus’s segment.
Experiment 1
Experiment 1 evaluated possible differences between the performance of young and older adults in imitating sequences of five quasi-random movements. In addition to exploring the overall accuracy of each imitation, we explored supplementary measures designed to reveal theoretically significant age-related differences in imitation performance.
Method
Participants
Thirteen young adults (7 women, 6 men; M = 21.5 years of age, SD = 4.2) and 13 older adults (7 women, 6 men; M = 74.3 years of age, SD = 3.9) participated in this experiment. Young adults were recruited through posters and a website advertisement at Brandeis University and were either undergraduate or graduate students at the university. Older adults were recruited from an adult education program at Brandeis University and from participant directories maintained by laboratories on campus.
Informed consent was obtained from all participants, and the Institutional Review Board of Brandeis University approved all procedures. Each participant was compensated for time spent in the experiment, and older adults were also reimbursed for travel expenses. Each participant had a best-corrected Snellen acuity of 20/30 or better. For inclusion in the study, older adults had to achieve a Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) score that equaled or exceeded age norms. One 80-year-old participant produced an MMSE score well below the age norm and was therefore excluded from the study. Older adults who were retained for the study achieved a mean MMSE score of 28.9 (SD = 0.8) out of a possible score of 30.
Stimuli
In Experiment 1 each stimulus comprised a sequence of five quasi-randomly directed movement segments that were to be reproduced in the same order in which they had been seen, as explained in the General Method section. Exemplars of the eight patterns of consistent and inconsistent turns (see Figure 3) were presented in block-randomized order, ensuring that all types would be seen equally often.
Design and procedure
Young adults participated in two 50-min sessions of 168 trials each. One young adult was available for just a single session and was therefore excluded from the study. Older adults served in two sessions of 144 trials each. Frequent rest breaks extended the older adults’ sessions to between 90 and 120 min. The difference in the number of trials for young and older participants proved to be inconsequential: When the young participants’ results were reanalyzed with data limited to the same number of trials as for the older adults, there were no changes in the results.
Each participant was allowed a number of practice trials before each session. Participants performed at least four practice trials but were free to continue until they felt comfortable with the task. The older adults typically took additional practice. In addition, the first eight trials in each session were treated as practice for all participants and were excluded from the analysis. Even so, we found a small but significant difference in performance between Sessions 1 and 2, F(1, 22) = 6.498, p < .05, . However, this practice effect did not differ between age groups, F(1, 22) = 0.003, p < .05,
Results and Discussion
Each imitation was segmented and scored by the process described in the General Method section.
Mean orientation error
As explained earlier, mean orientation error is the mean absolute difference between the orientation of a segment in the participant’s reproduction and the orientation of the corresponding segment in the stimulus sequence. When interpreting values of mean orientation error, it should be kept in mind that higher values (greater errors) represent poorer performance. The values of mean error for each age group are represented by the height of the arrows in Figure 4. As that figure shows, older adults’ mean error (44.63°, SEM = 1.69°) was significantly higher than young adults’ mean error (29.04°, SEM = 2.99°), F(1, 22) = 20.49, p < .001, .
Figure 4.
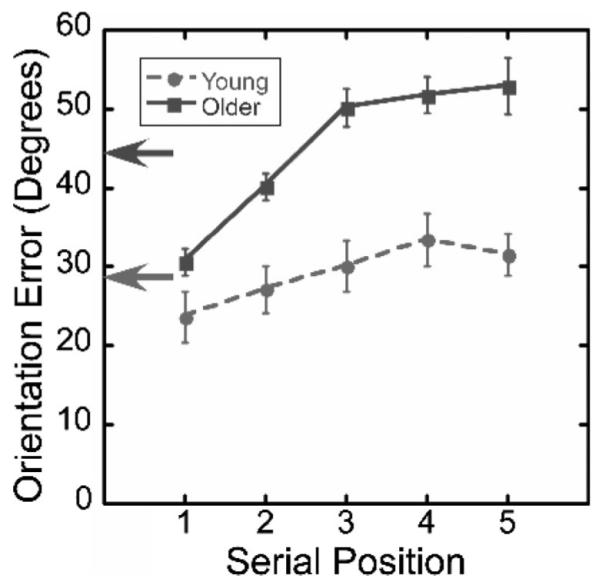
Mean orientation error in Experiment 1. The arrows indicate the overall mean orientation error for each age group. The x-axis represents the serial order of the movement segment. Error bars are standard errors of the mean.
The curves in Figure 4 show each group’s orientation error as a function of a segment’s serial position within an imitation. For both groups, performance was more accurate early in an imitation and then degraded as successive segments must be recalled and produced. Additionally, with successive segments, older adults’ error tended to increase faster than that of the young adults, F(4, 88) = 7.14, p < .001, . To understand the origin of the divergence between the groups, we considered the possibility that older adults’ relatively greater errors might have been caused by the propagation, segment by segment, of the error associated with the initial segment. If this were true, older adults would have correctly reproduced the orientation of later segments but only in relation to the orientation of the initial segment. To assess this prediction, we rigidly rotated each reproduction so that the first segment’s orientation error was reduced to zero. If older adults’ increased error with successive serial positions came solely from the propagation of the original segment’s error, the rigid rotation should decrease the errors on the last four segments for older adults only. However, the error for both groups increased significantly, F(1, 22) = 153.6, p < .001, , suggesting that neither group’s increase in mean error with serial position was linked to the error made in imitating the initial segment of a stimulus. Instead, the increase in error arose from some other source, such as failures of perception and/or memory. These possibilities are explored below, in Experiments 2 and 3.
Order-related errors
On other serial recall tasks, older adults make significantly more order-related errors than young adults (e.g., Maylor, Vousden, & Brown, 1999). To learn whether this age difference held for imitation, we focused on transposition errors, which were the easiest form of order error to identify in our task. Transposition errors signal that although item information has been preserved, order information has been degraded or lost (Histed & Miller, 2006; Rhodes, Bullock, Verwey, Averbeck, & Page, 2004). We identified an imitation as containing a transposition if interchanging two of the imitation’s segments succeeded in reducing the errors computed for both transposed segments. Figure 5A illustrates a trial containing a transposition, in this case between the third and fourth segments; the stimulus (thick line) and its reproduction (thin line) are superimposed. In Figure 5B the reproduction’s third and fourth segments have been swapped, producing a reproduction that was a better fit to the stimulus. Our analysis excluded all trials on which any single segment participated in more than one transposition; logically, a segment could only have been transposed with one other segment. Research with serial recall of verbal stimuli has shown that older participants exhibit a heightened tendency to transpose nearest-neighbor items in a series (Maylor et al., 1999; Schmitter-Edgecombe & Simpson, 2001). We therefore focused on nearest-neighbor transpositions in participants’ imitations, that is, cases in which the transposed segments were adjacent to one another in serial order. We hypothesized that transposition errors (particularly nearest-neighbor transpositions) would be more numerous in older adults, which would indicate some loss of order information.
Figure 5.
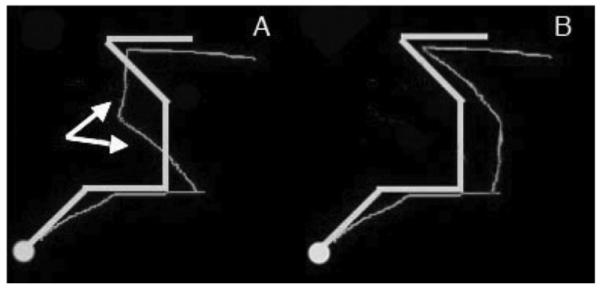
Example of a transposition in an imitation. In Figure 5A is the stimulus (thick line) and its imitation (thin line). In Figure 5B is the result of interchanging the imitation’s third and fourth segments. In each figure the small disk represents the stimulus’s (or imitation’s) starting location.
In general, older adults produced more trials with one or more transpositions than young adults (, SD = 8.3% vs. , SD = 9.4%), t(22) = −4.470, p < .001). Of the approximately 16% difference between age groups, around 13% arose from nearest-neighbor transpositions, that is, transpositions of two successive segments (older adults: , SD = 7.0%; young adults: , SD = 8.3%), t(22) = −4.327, p < .001. Though older adults made many more transposition errors than young adults, these errors alone do not explain the overall age-related difference seen in mean orientation error: When transposition errors are discounted—by reversing all transposed segments and then recomputing the mean orientation error—older adults’ overall error remains significantly higher than young adults’, F(1, 22) = 19.07, p < .001, . These results suggest that older adults were not only less successful in recalling the precise direction of a segment but also less successful in recalling the order of the segments whose directions were remembered—a finding that mirrors those in studies of memory for verbal materials (Maylor et al., 1999).
Gist information
As anticipated, participants in both age groups failed to display perfect, detailed memory for every movement in a sequence. In other serial recall tasks, when detailed memory for individual items in a series is imperfect, participants are able to exploit supplementary information, including a nondetailed, abstracted version of the stimulus series (Chapman, Anand, Sparks, & Cullum, 2006). Just as the details of a word list or prose narrative can be represented in some abstract form, so too can the spatiotemporal information in our sequences be represented in abstract form. We wanted to know whether the older adults, whose performance was consistently below that of their younger counterparts, might have exploited some abstracted gist information to supplement their imperfect memory for a sequence’s component movements. But given the makeup of our stimuli, what form might gist take? For an answer we considered the eight distinct patterns of consistent and inconsistent turns in the stimuli shown in Figure 3 and examined whether participants’ errors were systematically related to those patterns.
As can be seen in Figures 6A and 6B, the overall mean imitation error varied with the number of consistent turns contained in a stimulus sequence, F(3, 66) = 19.976, p < .001, . Generally, with one important exception, imitation tended to be more accurate for sequences whose successive turns were more consistent and less accurate for sequences whose turns were less consistent. However, the number of consistent turns had a differential effect on young and older adults, F(3, 66) = 3.135, p < .05, . As Figure 6A suggests, older adults’ imitations were most accurate when the stimulus had either the most (three) or the fewest (zero) consistent turns, a fact that was confirmed by pairwise t tests: Older adults produced smaller mean errors when stimuli had three consistent turns rather than two and when stimuli had zero consistent turns rather than one, t(11) = −6.096, p < .001, and t(11) = 3.041, p < .01, respectively (both one-tailed tests). Finally, older adults showed no difference in mean imitation error when stimuli had one or two consistent turns. In contrast, as Figure 6B suggests, for young adults, stimuli containing three consistent turns were imitated with significantly lower error than were stimuli with two, t(11) = −3.914, p < .001 (one-tailed test), which in turn had lower error than those with only a single consistent turn, t(11) = −1.856, p < .05 (one-tailed test). There was no difference between young adults’ mean error with stimuli containing one and stimuli containing zero consistent turns.
Figure 6.
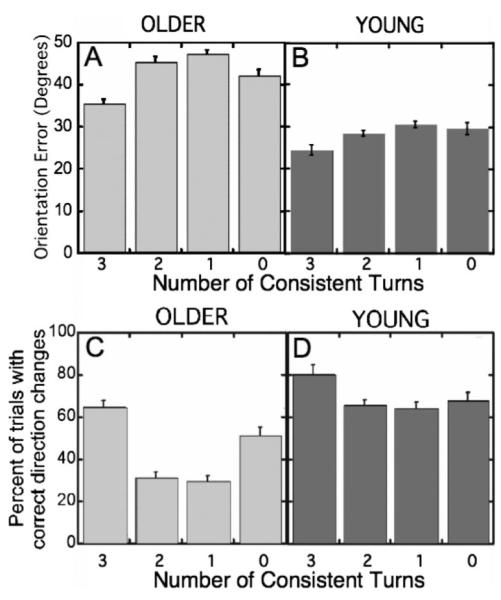
Reproduction fidelity as a function of the number of consistent turns in a stimulus for older and young adults. Figures 6A and 6B express reproduction fidelity in terms of mean orientation errors. Figures 6C and 6D express imitation fidelity in terms of the percent of trials on which an imitation’s pattern of turn consistencies matched the stimulus’s pattern of turn consistencies. Error bars represent within-subject standard error (Loftus & Masson, 1994).
The older and young adults’ results shown in Figures 6A and B were confirmed with a different but related dependent measure. As explained earlier, each stimulus model can be characterized by a pattern of +’s and −’s across the trio of potential loci of consistency. Ignoring the exact orientation of segments in a model and in the corresponding reproduction, we determined for each trial whether the pattern of turn consistency in the reproduction matched the pattern of turn consistency in the model. Note that this comparison takes account only of whether a turn was consistent or inconsistent relative to the preceding turn but ignores a turn’s angular magnitude. The results of this analysis are shown in Figures 6C and 6D, for older and young adults, respectively. As Figure 6C suggests, older adults most accurately reproduced the pattern of turn consistencies when the stimulus had either the most (three) or the fewest (zero) consistent turns. Specifically, reproduction of a stimulus’s pattern of turns was more accurate with three consistent turns than with two consistent turns and was more accurate with zero consistent turns than with a single consistent turn, t(11) = 8.971, p < .001, and t(11) = −4.059, p < .001, respectively. Note this pattern mirrors the older adults’ pattern when performance was measured in terms of mean orientation error (Figure 6A). As can be seen in Figure 6D, with this measure, young adults produced their best performance when stimuli had the greatest number of consistent turns, t(11) = 3.071, p < .01 (one-tailed test).
One striking result seen with both dependent measures was the relatively good fidelity of older adults’ imitation of stimulus models of the two most extreme types, that is, models with the most consistent turns and with no consistent turns. One explanation for this result is that the older adults were supplementing their imperfect memory of a model’s details by some broad categorization of model type. Then, during the production of an imitation, category information could be used to supplement any more detailed item information that was available to the participant. To appreciate what these categories might be, consider Figure 3. Stimuli whose successive movements were all clockwise or all counterclockwise form a convex hull (roughly an interrupted ellipse) and could be categorized as spirals or interrupted ellipses (Figure 3A). As long as some information about the direction of the initial movement was available to the participant, the application of a category label could be used to produce a set of movements roughly paralleling those of the stimulus. Likewise, stimuli whose successive directions alternated regularly between clockwise and counterclockwise turns could be categorized as zigzag or sawtooth (Figure 3H). Again, this category label, together with some information about the initial direction of movement, would allow a participant to produce a more accurate reproduction than might be possible with imperfect segment memory alone. Thus, identifying and remembering the categories of these stimuli could supplement the participant’s ability to reproduce stimuli in these two groups; thus, using categorizable gist should improve performance but only on stimuli of these two types (Figures 3A and H). Although additional experiments will be needed for confirmation, the older adults’ results lead us to hypothesize that older adults supplement their memory of a model’s details with broad categorizations of a model’s gist. A modified version of this proposal could account for young participants’ decreasing performance as stimuli decreased in number of consistent turns.
Experiment 2
Experiment 2 was designed to determine how imitation accuracy might be affected by each of three factors: the participants’ ability to control the stylus as it was moved across the graphics tablet, the accuracy with which participants perceived the orientation of a static line, and the accuracy with which they perceived the orientation defined by a disk moving over a single linear path. Research with young adults shows that there is some inherent error in judging the orientation of a line (Andrews, 1967a, 1967b; Whitaker, Levi, & Kennedy, 2008). Additionally, tests with random dot cinematograms and sinusoidal gratings illustrate that older adults show deficits in performance on direction identification, orientation discrimination, and motion perception (Ball & Sekuler, 1986; Bennett et al., 2007; Betts et al., 2007; Sekuler & Ball, 1986; Snowden & Kavanagh, 2006). These results raise the possibility that older adults’ increased error in Experiment 1 could have resulted from age-related differences in perception and/or motor control.
Method
Participants
Fourteen young adults (9 women, 5 men; M = 20.9 years of age, SD = 1.3; one repeat from Experiment 1) and 12 older adults (7 women, 5 men; M = 73.1 years of age, SD = 4.8; one repeat from Experiment 1; mean MMSE = 29.5, SD = 0.7) participated in this experiment, recruited and screened the same way as in Experiment 1. Both young and older adults participated in a single session of 480 trials; each session lasted approximately 50 min. Two young adults and 1 older adult participated in an early version of the experiment with fewer trials. None of these participants’ performance was found to be an outlier, so all were included in the final analysis. However, 2 of the young adults failed to follow the instructions for the experiment, and their data were excluded from the analyses.
Stimuli
Stimuli here differed in several ways from those in Experiment 1. First, only a single segment was shown on each trial, and that segment was either defined by the path of a moving disk, which left no trail (moving disk condition), as in Experiment 1, or shown in its entirety with a disk at one end to indicate the end from which to start the reproduction (static line condition). The length and timing of these segments were the same as in the General Method section. The static line stayed on the screen for the same time that the disk took to traverse a single segment, 350 ms. In addition, the interval between the stimulus and the beginning of the reproduction was reduced from 3.75 s to 250 ms, which minimized the potential impact of the retention interval. The orientations of the segments were generated randomly from 1° to 360°.
Design and procedure
The two conditions (static line and moving disk) were presented in blocks of 20 trials. The order of conditions was counterbalanced among and between groups. Participants were instructed to begin to make their reproduction of what they had seen as soon as a blue disk appeared. As before, the movement of the blue disk was yoked to a participant’s movement of the stylus across the graphics table. Also as before, feedback was provided after the completion of each reproduction.
Scoring
We examined errors from two complementary perspectives. The between-groups difference is the difference in performance between the groups on the static line condition and reflects the amount of increased error that is likely due to a decrease in motor control and/or line perception in older adults. The within-group change is the change between the two conditions (static line vs. moving disk) for each group and reflects the amount of each group’s error that is likely due to difficulties in interpolating the orientation of a line traced out by a moving disk. The difference between age groups for the within-group change reflects the amount of the older adults’ increased error over young adults that is likely due to the difference between the two groups’ perceptual ability to derive stimulus orientation from a moving disk.
Orientation errors were calculated in the same way as in Experiment 1; however, participants occasionally seemed to confuse the start point of the stimulus and drew their reproduction backward. Therefore, trials were censored prior to analysis if they would have represented an error greater than 150°. Because such trials were more likely to occur with older adults (older adults: ; young adults: ), t(22) = −3.555, p < .01, including those trials would have had a disproportionate impact on the older adults’ mean orientation error, artificially inflating the observed difference between age groups.
Results and Discussion
Figure 7 shows each age group’s mean orientation error in reproducing static and moving stimuli. In general, older adults produced larger errors than young adults, F(1, 22) = 8.841, p < .01, . Additionally, participants in each age group made larger errors in reproducing the direction of the moving disk (horizontal striped bars) than in reproducing the orientation of the static stimulus line (vertical striped bars), F(1, 22) = 5.310, p < .05, , indicating that judging the orientation traced out by the moving disk was less precise than judging the orientation of a static line. A post hoc t test showed that the between-groups difference was not significant and thus that age-related differences in motor control and line estimation were not likely to have had a large effect on imitation performance in Experiment 1. Most importantly, the within-group change was significantly greater for older adults than for younger adults, confirmed by a significant interaction between age group and presentation condition, F(1, 22) = 4.905, p < .05, . This indicates that most of the age-related error in this experiment likely arose from an age-related change in ability to extract the orientation of a line defined by the movements of a disk.
Figure 7.
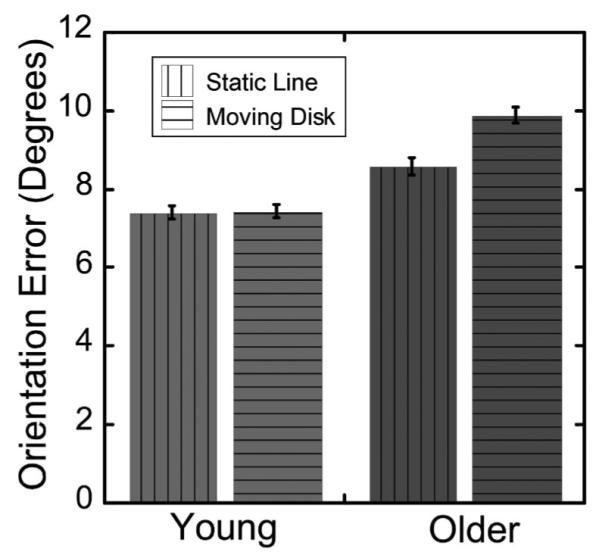
Mean orientation error for static line and moving disk condition for young and older adults in Experiment 2. Error bars are within-subject errors of the mean (Loftus & Masson, 1994).
Because the mean errors seen in Experiment 2 were so small, it seems likely that the processes studied here (motor control and perception) probably made only limited contributions to Experiment 1’s age-related differences. In addition, although motor control and line estimation cannot be separated by the methods of Experiment 2, an unpublished pilot experiment found that older adults performed close to perfectly (error of less than 2°) if both the line and the disk representing the stylus were on-screen as they were drawing the line. This error is within the range of our algorithm’s ability to estimate correctly the orientation of a drawn line. As a result, the error seen in the static line condition likely reflects the error associated with line estimation and not motor control. Together these two sources of imprecision, motor control and/or line orientation estimation and estimation of a moving stimulus’s direction, have only a minute effect on overall performance, suggesting that the bulk of the age-related difference in imitation quality in Experiment 1 reflects one or more other sources of error.
Experiment 3
Some time-dependent failure of memory might have contributed to Experiment 1’s demonstrated age-related changes in imitation. Several older adult participants in that experiment commented, during or directly after completing the experiment, that they were frustrated by the enforced retention interval, which kept them from making their imitation as soon as the stimulus ended. These older participants contended that this interval substantially impacted their performance. Consistent with this contention, Sekuler et al. (2003) found that young adults’ errors in a similar imitation task increased slightly with the length of the interval between the end of the stimulus and the start of reproduction. It may be that the retention interval in Experiment 1 allowed memory to degrade and that such degradation was more substantial for older adults.
In addition to the delay in initiating reproduction, another possible source of older adults’ increased error might be the memory load associated with each stimulus’s multiple segments. Several reports have demonstrated a decrease in memory capacity with age, which causes memory-dependent performance to decrease with increases in load (Giambra, Arenberg, Kawas, Zonderman, & Costa, 1995; Perlmutter, Metzger, Nezworski, & Miller, 1981; Riege & Inman, 1981; Salthouse, 1994; Stern et al., 2005; Wingfield & Kahana, 2002; Wingfield, Stine, Lahar, & Aberdeen, 1988; but see Fastenau, Denburg, & Abeles, 1996). Despite demonstrated differences between verbal and visuospatial memory (Park et al., 2002), an age-related decrease in capacity might mean that in our imitation task, older adults would have more trouble than young adults as the number of segments in a model increased. An experiment similar to the serial imitation task of Experiment 1 examined these two possible sources of the increased error in older adults: retention interval and memory load.
Method
Participants
Twelve young adults and 13 older adults participated in this experiment, recruited and screened the same way as in Experiments 1 and 2. Both young and older adults participated in two sessions with 240 and 260 trials each. A participant was excluded from the analysis if he or she did not complete all required tasks, had an effect that was more than three standard deviations above the mean, did not meet the MMSE criterion, or failed a Snellen acuity test. A total of 3 young adults and 4 older adults were removed because of these constraints. The remaining young adults (4 women, 5 men; one that repeated from Experiments 1 and 2) were on average 20.67 years old (SD = 2.34). The older adults (5 women, 4 men; one that repeated from Experiment 2; MMSE , SD = 0.50) were on average 77.11 years old (SD = 6.75).
Stimuli
In one session, the task was the same as in Experiment 1 for five segments but with a retention interval that varied between blocks. The retention intervals were 0, 1.8, 3.75, or 7.5 s, values that bracket the one interval used in Experiment 1. This variation in retention interval comprises the retention manipulation. In the other session, the number of segments in a model varied between three and seven segments, and the retention interval was reduced to zero. This variation in number segments comprises the load manipulation.
Design and procedure
The two manipulations were tested in orders that were counterbalanced across both participants and groups. Trials were presented in blocks of 20 trials that all had the same retention interval or number of segments. These blocks were counterbalanced within a session; the first four trials in each block were discarded to minimize the effect of task switching.
In addition, to evaluate their short-term memory spans for stimulus materials different from those used in our imitation experiments (Park et al., 2002), we asked participants to take several tests of verbal memory drawn from the Wechsler Adult Intelligence Scale–Revised, including the Forward and Backward Digit Spans and the Letter–Number Sequencing task (Wechsler, 1981; see Table 1). None of the three standard tests of memory capacity showed an effect of age: Forward Digit Span, t(16) = −0.371, p > .05; Backward Digit Span, t(16) = −1.209, p > .05; and Letter–Number Sequencing, t(16) = −1.439, p > .05.
Table 1.
Mean and Standard Errors of Scores on Wechsler Adult Intelligence Scale–Revised Memory Span Tests
| Forward digit |
Backward digit |
Letter–number sequence |
||||
|---|---|---|---|---|---|---|
| Group | M | SE | M | SE | M | SE |
| Young | 11.33 | 0.6 | 7.78 | 0.9 | 11.56 | 0.8 |
| Old | 11.67 | 0.7 | 9.00 | 0.5 | 10.22 | 0.5 |
Scoring
Participants’ reproductions were analyzed and segmented as explained earlier, in the General Method section.
Results and Discussion
For the retention manipulation, older adults’ mean orientation error for the five-segment models was significantly higher than that of young adults, F(1, 16) = 9.019, p < .01, . Interestingly, although there was a marginally significant decrease in performance as retention interval increased, F(3, 48) = 2.391, p = .08, , this effect was similar for young and older adults, as can be seen in Figure 8A, F(3, 48) = 0.391, p > .05, . As that figure also shows, the older participants’ mean error with zero delay was considerably higher than the young participants’ mean error at 3.75 s. Together these two results suggest that retention interval played little or no role in the age-related differences seen in Experiment 1. Although older adults contended that a long retention interval was at the root of their poor performance, our results show that performance was fairly constant across variation in retention interval. Thus, older adults had misattributed the difficulty that they experienced in performing the imitation task.
Figure 8.
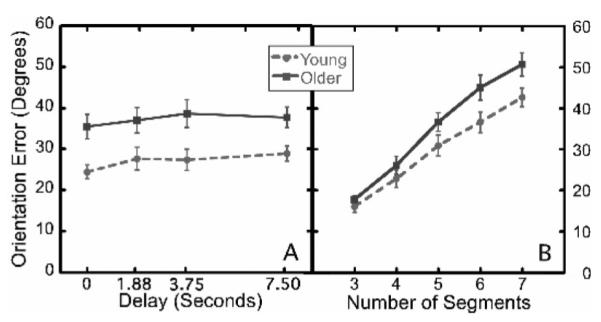
Mean orientation error in Experiment 3 for various retention intervals (Figure 8A) and as a function of the number of segments in the stimulus (Figure 8B). In each figure separate curves are shown for each age group. Error bars are standard error of the mean.
In the load manipulation, at the lowest load (three segments), the mean errors produced by young and older participants were indistinguishable from one another, t(16) = −0.928, p > .05. Further, as the number of segments increased, the older adults’ mean error grew more rapidly than did the young adults (see Figure 8B), F(4, 64) = 2.984, p < .05, . Though our tests of verbal memory did not show age-related changes, it has often been shown that age differences exist in visuospatial working memory and that they are stronger in visuospatial tasks (such as our imitation task) than in verbal tasks (Adamowicz, 1976; Dobbs & Rule, 1989; Giambra et al., 1995). In addition, the absence of age-dependence in the effect of retention interval suggests that the results of the capacity manipulation did not arise from the fact that sequences with larger number of segments took longer to display.
General Discussion
The experiments reported here explored the magnitude, characteristics, and possible sources of age-related performance differences on a serial imitation task. Experiment 1 showed that older adults’ imitation performance was poorer than that of young adults. In addition, when trials were sorted according to the number of consistent turns in their stimuli, it appeared that older adults might have been categorizing stimuli broadly, which improved performance, but only on a limited set of stimulus types.
Experiment 2 suggested that age differences in imitation were only partly attributable to age-related differences in perception, whereas Experiment 3 suggested that load (in these experiments, the number of segments) had a large effect on performance, and more so for older adults than for young.
The effect of one variable that is often implicated in age-related decreases in performance, encoding time, was not directly measured in the experiments reported here. However, in an unpublished preliminary experiment, we manipulated this variable indirectly, by increasing the length of time the stimulus disk took to traverse each movement segment. We reasoned that this increased stimulus duration would allow older participants additional encoding time. However, the manipulation had no effect on older adults’ performance. In addition, the results of Experiment 3 show that increased retention interval (which increases encoding time) did not boost performance, for either young or older adults, but instead decreased it slightly. One other source of encoding time that was not directly manipulated in these experiments is the pause between segments. However, lengthening the pause between segments could undermine participants’ ability to build a coherent representation of the spatiotemporal sequence of segments in memory. In fact, several studies have shown that increasing encoding time only added to age-related differences in performance (Craik & Rabinowitz, 1985; Rabinowitz, 1989). Together these results suggest that encoding time probably does not account for the age differences in our imitation task.
It is interesting that although older adults in Experiment 1 attributed their performance difficulties to the retention interval, that attribution was not supported by Experiment 3’s manipulation of that interval. The difference in mean error between groups was essentially preserved when the older group’s performance at zero delay was compared with the young group’s performance at a delay of 3.75 s, the delay used in Experiment 1. This is a reminder that participants’ attributions of their errors can be unreliable and that empirical tests are needed to identify errors’ true source. It may be that older adults blamed the delay for their perceived performance challenges because in other situations delay actually did limit serial recall (e.g., remembering a phone number that has been heard).
Current models of serial recall tend to be blind to the possibility that participants can exploit supplementary information to aid recall of some series of studied items. Instead, such models have traditionally focused on the storage, retrieval, and sequencing of the studied items alone. This focus has certainly characterized models of memory for sequences of motor behaviors (Rhodes et al., 2004). Our findings are a reminder that participants can and do exploit supplementary information, possibly of different forms. In our imitation task, that supplementary information seems to comprise sequence gist, which is extracted trial by trial from the spatiotemporal structure of a movement sequence. A complete model must take account of how a sequence’s gist is actually extracted, encoded, and then retrieved in a way that aids memory for a movement sequence.
As mentioned earlier, a few researchers have examined the role of aging in imitation performance. In one such study, Celnik et al. (2006) used simple finger movements to examine the effect of imitation training on motor performance. Although their study found that observing and imitating a movement improves performance in older adults, they did not examine why older adults’ performance is initially reduced or how effective training is in reducing age-related changes in imitation performance. Similarly, although Leonard and Tremblay (2007) showed that brain areas that are activated during imitation tasks are largely intact in older adults, they did not show what may underlie the age-related changes in imitation. The experiments reported here explored not only how much imitation performance changes with age but also why. Our results also suggest some ways that older adults and those who use imitation to instruct older adults might be able to improve older adults’ imitation-based skill learning. All the movement segments in our stimuli had the same duration, which deprived the stimuli of distinctive temporal structure that participants could use to organize the unfolding sequence that they saw. However, with other stimuli, one might impose and exaggerate the temporal boundaries between successive components, which could decrease the load on working memory and possibly improve subsequent recall and imitation. This basic strategy has been used to improve young adults’ recognition of observed actions (Hill & Pollick, 2000) but has not been applied to serial recall or to the performance of older adults. This would have to be done carefully, as research in our laboratory suggests that experimenter-imposed temporal boundaries can impair young adults’ performance (Rice & Sekuler, 2008). Instead, care must be taken to couple any use of this strategy with viewing time that is sufficient to accommodate the added processing time that might be needed, particularly by older adults. The introduction of appropriate temporal structure into a stimulus could help older adults generate useful organizational structures that would facilitate serial recall and imitation learning. An exploration of varied sets of stimuli and presentation rates is currently under way in our laboratory. These studies might well provide support for approaches that could help older adults exploit useful organizational strategies and reduce the load on memory, thereby improving older adults’ imitation learning.
Acknowledgments
This study was supported by National Science Foundation Center for Excellence in Science, Education and Technology Grant SBE-0354378. We thank Shivakumar Viswanathan for helpful suggestions and comments and Yigal Agam for sharing code and providing advice during this project.
Contributor Information
Jessica Maryott, Volen Center for Complex Systems, Brandeis University.
Robert Sekuler, Volen Center for Complex Systems, Brandeis University, and Department of Cognitive and Neural Systems, Boston University..
References
- Adamowicz JK. Visual short-term memory and aging. Journal of Gerontology. 1976;31:39–46. doi: 10.1093/geronj/31.1.39. [DOI] [PubMed] [Google Scholar]
- Agam Y, Bullock D, Sekuler R. Imitating unfamiliar sequences of connected linear motions. Journal of Neurophysiology. 2005;94:2832–2843. doi: 10.1152/jn.00366.2005. [DOI] [PubMed] [Google Scholar]
- Agam Y, Sekuler R. Geometric structure and chunking in reproduction of motion sequences. Journal of Vision. 2008;8:1–12. doi: 10.1167/8.1.11. [DOI] [PubMed] [Google Scholar]
- Ambrosoni E, Sala S. Della, Motto C, Oddo S, Spinnler H. Gesture imitation with lower limbs following left hemisphere stroke. Archives of Clinical Neuropsychology. 2006;21:349–358. doi: 10.1016/j.acn.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Andrews DP. Perception of contour orientation in the central fovea: I. Short lines. Vision Research. 1967a;7:975–997. doi: 10.1016/0042-6989(67)90014-4. [DOI] [PubMed] [Google Scholar]
- Andrews DP. Perception of contour orientation in the central fovea: II. Spatial integration. Vision Research. 1967b;7:999–1013. doi: 10.1016/0042-6989(67)90015-6. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Immediate memory and the “perception” of letter sequences. Quarterly Journal of Experimental Psychology. 1964;16:364–367. [Google Scholar]
- Ball K, Sekuler R. Improving visual perception in older observers. Journal of Gerontology. 1986;41:176–182. doi: 10.1093/geronj/41.2.176. [DOI] [PubMed] [Google Scholar]
- Bennett PJ, Sekuler R, Sekuler AB. The effects of aging on motion detection and direction identification. Vision Research. 2007;47:799–809. doi: 10.1016/j.visres.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Research. 2007;47:1769–1780. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Billino J, Bremmer F, Gegenfurtner KR. Differential aging of motion processing mechanisms: Evidence against general perceptual decline. Vision Research. 2008;48:1254–1261. doi: 10.1016/j.visres.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research. 2005;25:226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Castel AD, Farb NAS, Craik FIM. Memory for general and specific value information in younger and older adults: Measuring the limits of strategic control. Memory & Cognition. 2007;35:689–700. doi: 10.3758/bf03193307. [DOI] [PubMed] [Google Scholar]
- Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. Encoding a motor memory in the older adult by action observation. NeuroImage. 2006;29:677–684. doi: 10.1016/j.neuroimage.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Anand R, Sparks G, Cullum M. Gist distinctions in healthy cognitive aging versus mild Alzheimer’s disease. Brain Impairment. 2006;7:223–233. [Google Scholar]
- Christou EA, Carlton LG. Old adults exhibit greater motor output variability than young adults only during rapid discrete isometric contractions. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2001;56:B524–B532. doi: 10.1093/gerona/56.12.b524. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Rabinowitz JC. The effects of presentation rate and encoding task on age-related memory deficits. Journal of Gerontology. 1985;40:309–315. doi: 10.1093/geronj/40.3.309. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Czaja SJ, Charness N, Fisk AD, Hertzog C, Nair SN, Rogers WA, Sharit J. Factors predicting the use of technology: Findings from the Center for Research and Education on Aging and Technology Enhancement (CREATE) Psychology and Aging. 2006;21:333–352. doi: 10.1037/0882-7974.21.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Elsner B. Infants’ imitation of goal-directed actions: The role of movements and action effects. Acta Psychologica. 2007;124:44–59. doi: 10.1016/j.actpsy.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Fastenau PS, Denburg NL, Abeles N. Age differences in retrieval: Further support for the resource-reduction hypothesis. Psychology and Aging. 1996;11:140–146. doi: 10.1037//0882-7974.11.1.140. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geisler WS, Albrecht DG, Crane AM, Stern L. Motion direction signals in the primary visual cortex of cat and monkey. Visual Neuroscience. 2001;18:501–516. doi: 10.1017/s0952523801184014. [DOI] [PubMed] [Google Scholar]
- Giambra LM, Arenberg D, Kawas C, Zonderman AB, Costa PTJ. Adult life span changes in immediate visual memory and verbal intelligence. Psychology and Aging. 1995;10:123–139. doi: 10.1037//0882-7974.10.1.123. [DOI] [PubMed] [Google Scholar]
- Gomez-Perez E, Ostrosky-Solis F. Attention and memory evaluation across the life span: Heterogeneous effects of age and education. Journal of Clinical and Experimental Neuropsychology. 2006;28:477–494. doi: 10.1080/13803390590949296. [DOI] [PubMed] [Google Scholar]
- Hill H, Pollick FE. Exaggerating temporal differences enhances recognition of individuals from point light displays. Psychological Science. 2000;11:223–228. doi: 10.1111/1467-9280.00245. [DOI] [PubMed] [Google Scholar]
- Histed MH, Miller EK. Microstimulation of frontal cortex can reorder a remembered spatial sequence. PLoS Biology. 2006;4:e134. doi: 10.1371/journal.pbio.0040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods R, Brass M, Bekkering H, Mazziotta J, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999 December 24;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jancke D. Orientation formed by a spot’s trajectory: A two-dimensional population approach in primary visual cortex. Journal of Neuroscience. 2000;20:RC86. doi: 10.1523/JNEUROSCI.20-14-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutstaal W. Flexible remembering. Psychonomic Bulletin & Review. 2006;13:84–91. doi: 10.3758/bf03193817. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL, Galluccio L, Stofer KA. Reducing gist-based false recognition in older adults: Encoding and retrieval manipulations. Psychology and Aging. 1999;14:220–237. doi: 10.1037//0882-7974.14.2.220. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Almeida OP. Physical activity and cognition in old age. Current Opinion in Psychiatry. 2006;19:190–193. doi: 10.1097/01.yco.0000214347.38787.37. [DOI] [PubMed] [Google Scholar]
- Leonard G, Tremblay F. Corticomotor facilitation associated with observation, imagery and imitation of hand actions: A comparative study in young and old adults. Experimental Brain Research. 2007;177:167–175. doi: 10.1007/s00221-006-0657-6. [DOI] [PubMed] [Google Scholar]
- Lepage J-F, Théoret H. The mirror neuron system: Grasping others’ actions from birth? Developmental Science. 2007;10:513–523. doi: 10.1111/j.1467-7687.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Maylor EA, Vousden JI, Brown GD. Adult age differences in short-term memory for serial order: Data and a model. Psychology and Aging. 1999;14:572–594. doi: 10.1037//0882-7974.14.4.572. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63:81–97. [PubMed] [Google Scholar]
- Okada R, Burrows D. Organizational factors in high-speed scanning. Journal of Experimental Psychology. 1973;101:77–81. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Peelle J, Wingfield A. Dissociations in perceptual learning revealed by adult age differences in adaptation to time-compressed speech. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:1315–1330. doi: 10.1037/0096-1523.31.6.1315. [DOI] [PubMed] [Google Scholar]
- Perlmutter M, Metzger R, Nezworski T, Miller K. Spatial and temporal memory in 20 to 60 year olds. Journal of Gerontology. 1981;36:59–65. doi: 10.1093/geronj/36.1.59. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JC. Age deficits in recall under optimal study conditions. Psychology and Aging. 1989;4:378–380. doi: 10.1037//0882-7974.4.3.378. [DOI] [PubMed] [Google Scholar]
- Reynolds J, Goldstein J. The effects of category membership on memory scanning for words. American Journal of Psychology. 1974;87:487–495. [Google Scholar]
- Rhodes B, Bullock D, Verwey W, Averbeck B, Page M. Learning and production of movement sequences: Behavioral, neurophysiological, and modeling perspectives. Human Movement Science. 2004;23:699–746. doi: 10.1016/j.humov.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Rice NJ, Sekuler R. Teaching a sequence in chunks impairs ability to form compound cues to recall: Effects on skill learning, retention and transfer. Proceedings of the 12th International Conference on Cognitive and Neural Systems; Boston University, Department of Cognitive and Neural Systems; Boston. 2008. p. 81. [Google Scholar]
- Riege WH, Inman V. Age differences in nonverbal memory tasks. Journal of Gerontology. 1981;36:51–58. doi: 10.1093/geronj/36.1.51. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging associations: Influence of speed on adult age differences in associative learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1486–1503. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Coon VE. Influence of task-specific processing speed on age differences in memory. Journal of Gerontology. 1993;48:P245–P255. doi: 10.1093/geronj/48.5.p245. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Simpson AL. Effects of age and intentionality on content memory and temporal memory for performed activities. Aging, Neuropsychology, and Cognition. 2001;8:81–97. [Google Scholar]
- Sekuler R, Ball K. Visual localization: Age and practice. Journal of the Optical Society of America, A: Optics, Image, and Science. 1986;3:864–867. doi: 10.1364/josaa.3.000864. [DOI] [PubMed] [Google Scholar]
- Sekuler R, Siddiqui A, Goyal N, Rajan R. Reproduction of seen actions: Stimulus-selective learning. Perception. 2003;32:839–854. doi: 10.1068/p5064. [DOI] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline: Current concepts and future directions. Archives of Neurology. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Smiley-Oyen AL, Lowry KA, Kerr JP. Planning and control of sequential rapid aiming in adults with Parkinson’s disease. Journal of Motor Behavior. 2007;39:103–114. doi: 10.3200/JMBR.39.2.103-114. [DOI] [PubMed] [Google Scholar]
- Snowden RJ, Kavanagh E. Motion perception in the ageing visual system: Minimum motion, motion coherence, and speed discrimination thresholds. Perception. 2006;35:9–24. doi: 10.1068/p5399. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson K, Hilton H, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S, Carlson MC, Fillit H, Greenough WT, Kramer A, Rebok GW. From bedside to bench: Does mental and physical activity promote cognitive vitality in late life? Science of Aging Knowledge Environment. 2006:pe21. doi: 10.1126/sageke.2006.10.pe21. 2006. [DOI] [PubMed] [Google Scholar]
- Tun PA, Wingfield A, Rosen MJ, Blanchard L. Response latencies for false memories: Gist-based processes in normal aging. Psychology and Aging. 1998;13:230–241. doi: 10.1037//0882-7974.13.2.230. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. Journal of Gerontology. 1993;48:P157–P171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–Revised. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- Whitaker D, Levi DM, Kennedy GJ. Integration across time determines path deviation discrimination for moving objects. PLoS ONE. 2008;3:e1930. doi: 10.1371/journal.pone.0001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield A, Kahana MJ. The dynamics of memory retrieval in older adulthood. Canadian Journal of Experimental Psychology. 2002;56:187–199. doi: 10.1037/h0087396. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Stine E, Lahar CJ, Aberdeen JS. Does the capacity of working memory change with age? Experimental Aging Research. 1988;14:103–107. doi: 10.1080/03610738808259731. [DOI] [PubMed] [Google Scholar]


