Abstract
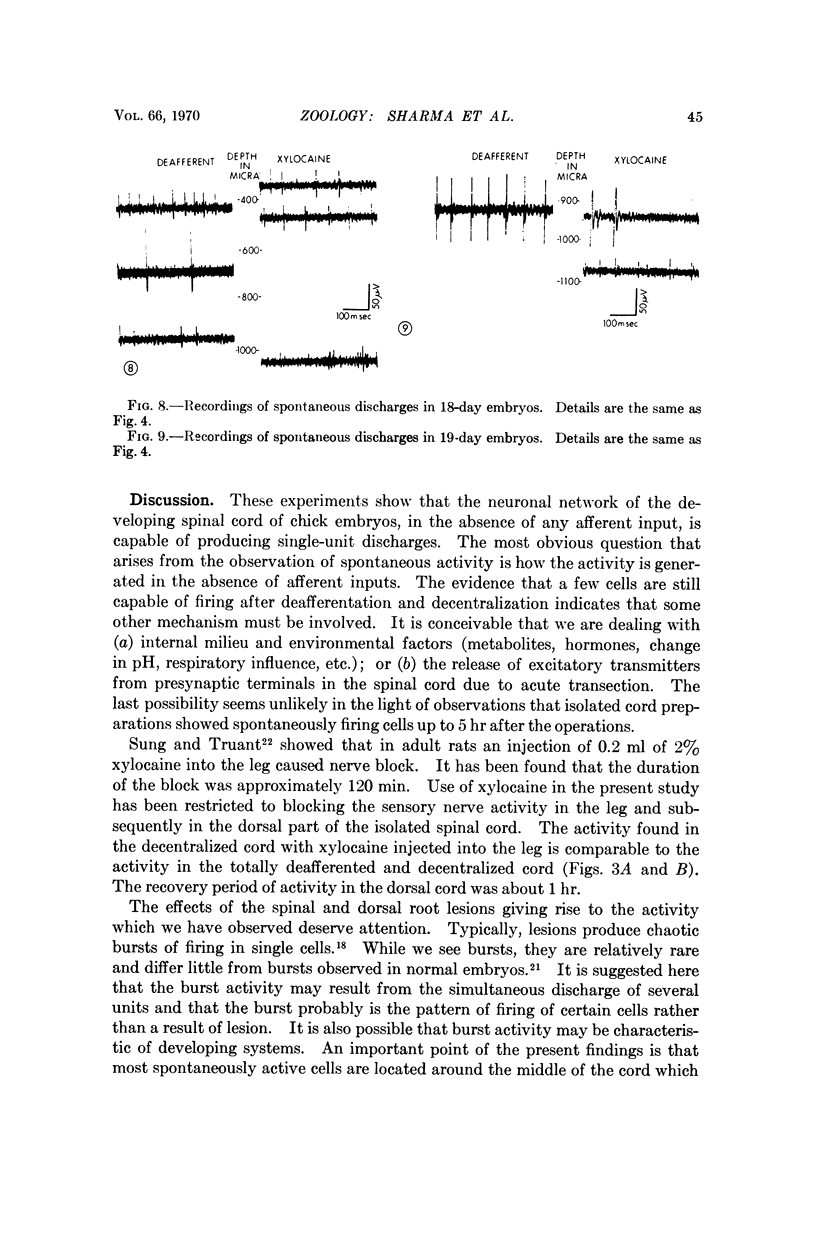
Unit electrical activity was recorded from single neurons in the isolated lumbo-sacral spinal cord of 14- to 19-day chick embryos, in situ. Spinal cord transection was combined with transection of all lumbo-sacral dorsal roots. The spontaneous discharge of cells is confined, for the most part, to the lower two thirds of the cord. A quasilinear reduction in the number of spontaneously active units was found during the developmental period studied. Comparable results were obtained in decentralized cord with sensory inputs blocked by xylocaine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Bronk D. W., Phillips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932 Feb 8;74(2):115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEACHAM W. S., PERL E. R. BACKGROUND AND REFLEX DISCHARGE OF SYMPATHETIC PREGANGLIONIC NEURONES IN THE SPINAL CAT. J Physiol. 1964 Aug;172:400–416. doi: 10.1113/jphysiol.1964.sp007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., GRAFSTEIN B., OLSZEWSKI J. Identification of neurones giving burst response in isolated cerebral cortex. J Neurophysiol. 1957 Mar;20(2):200–210. doi: 10.1152/jn.1957.20.2.200. [DOI] [PubMed] [Google Scholar]
- CRAIN S. M. Resting and action potentials of cultured chick embryo spinal ganglion cells. J Comp Neurol. 1956 Apr;104(2):285–329. doi: 10.1002/cne.901040207. [DOI] [PubMed] [Google Scholar]
- Corner M. A., Bot A. P. Electrial activity in the isolated forebrain of the chick embryo. Brain Res. 1969 Feb;12(2):473–476. doi: 10.1016/0006-8993(69)90017-1. [DOI] [PubMed] [Google Scholar]
- GRAFSTEIN B., SASTRY P. B. Some preliminary electrophysiological studies on chronic neuronally isolated cerebral cortex. Electroencephalogr Clin Neurophysiol. 1957 Nov;9(4):723–725. doi: 10.1016/0013-4694(57)90096-2. [DOI] [PubMed] [Google Scholar]
- HENRY C. E., SCOVILLE W. B. Suppression-burst activity from isolated cerebral cortex in man. Electroencephalogr Clin Neurophysiol. 1952 Feb;4(1):1–22. doi: 10.1016/0013-4694(52)90027-8. [DOI] [PubMed] [Google Scholar]
- IGGO A., VOGT M. Preganglionic sympathetic activity in normal and in reserpine-treated cats. J Physiol. 1960 Jan;150:114–133. doi: 10.1113/jphysiol.1960.sp006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGVAR D. H. Electrical activity of isolated cortex in the unanesthetized cat with intact brain stem. Acta Physiol Scand. 1955;33(2-3):151–168. doi: 10.1111/j.1748-1716.1955.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Kellaway P., Gol A., Proler M. Electrical activity of the isolated cerebral hemisphere and isolated thalamus. Exp Neurol. 1966 Mar;14(3):281–304. doi: 10.1016/0014-4886(66)90115-4. [DOI] [PubMed] [Google Scholar]
- MARK V. H., GASTEIGER E. L. Observations on the role of afferent and descending impulses on the spontaneous potentials of the spinal cord. Electroencephalogr Clin Neurophysiol. 1953 May;5(2):251–258. doi: 10.1016/0013-4694(53)90012-1. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., BRANCH C. L. Spontaneous activity of Betz cells in cats with midbrain lesions. J Neurophysiol. 1958 Jul;21(4):368–370. doi: 10.1152/jn.1958.21.4.368. [DOI] [PubMed] [Google Scholar]
- Polosa C. Spontaneous activity of sympathetic preganglionic neurons. Can J Physiol Pharmacol. 1968 Nov;46(6):887–896. doi: 10.1139/y68-138. [DOI] [PubMed] [Google Scholar]
- Provine R. R., Sharma S. C., Sandel T. T., Hamburger V. Electrical activity in the spinal cord of the chick embryo, in situ. Proc Natl Acad Sci U S A. 1970 Mar;65(3):508–515. doi: 10.1073/pnas.65.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNG C. Y., TRUANT A. P. The physiological disposition of lidocaine and its comparison in some respects with procaine. J Pharmacol Exp Ther. 1954 Dec;112(4):432–443. [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. THE VARIABILITY OF CENTRAL NEURAL ACTIVITY IN A SENSORY SYSTEM, AND ITS IMPLICATIONS FOR THE CENTRAL REFLECTION OF SENSORY EVENTS. J Neurophysiol. 1963 Nov;26:958–977. doi: 10.1152/jn.1963.26.6.958. [DOI] [PubMed] [Google Scholar]



