Abstract
Childhood asthma is not distributed evenly throughout the population, and children who grow up in crowded urban neighborhoods have higher rates of asthma and experience greater morbidity due to asthma. There are several environmental and lifestyle factors associated with urban living that are suspected to promote the development of asthma, particularly in the first few years of life. Collectively, this information suggests the hypothesis that exposure in early life to adverse environmental and lifestyle factors associated with disadvantaged urban environments modifies immune development to increase the risk for allergic diseases and asthma. The Urban Environment and Childhood Asthma birth cohort study was initiated in 2004 to test this hypothesis. The study population was recruited prenatally, and consisted of 560 families from four urban areas who were at high risk for allergies and/or asthma on the basis of parental histories, along with an additional 49 families without atopic parents. Immune development, respiratory illnesses, and exposure to stress, indoor pollutants, microbial products, and allergens were measured prospectively, and the major study outcomes are recurrent wheeze at three years of age and asthma at age seven. This review summarizes the study design, methods, and early findings of the URECA study.
Introduction
Most asthma begins in childhood, and a number of environmental and lifestyle factors are thought to contribute to the onset of asthma. These factors include outdoor pollutants, including ozone and diesel exhaust, and indoor pollutants such as tobacco smoke and NO2. A number of recent studies have focused on the hygiene hypothesis, and the latest iteration of this theory proposes that exposure to farm animals, pets, and nonpasteurized milk or fermented beverages may promote healthy development of the immune system to reduce rates of allergic diseases and asthma.1 On the other hand, lack of exposure could lead to a Th2-bias in immune responses, allergic sensitization, and asthma. Lifestyle and nutrition may also be important for lung and immune development, and several studies suggest that nutrients (e.g. omega-3 fatty acids, vitamin D) and consumption of fruits and vegetables protect against asthma, while obesity and lack of exercise could have the opposite effect.2-4 Virus-induced wheezing episodes in the first 2-3 years of life are a strong risk factor for asthma, particularly in children with other atopic features such as allergic sensitization or atopic dermatitis.5 Whether these episodes contribute to asthma causation or instead reveal an underlying predisposition to asthma has not yet been established. Finally, other factors that have been linked to the development of wheezing and asthma include genetics, ethnicity, month of birth, and exposure to high levels of stress.6-8
It is expected that diseases with a strong environmental component would not be evenly distributed throughout the population, and this is the case for asthma. The ISAAC study has documented over 30-fold differences in the local prevalence of childhood wheezing and asthma on a global scale,9 and although there is less information about the distribution of asthma within the US population, the same appears to be true.10 Asthma in the US appears to more frequent in ethnic minorities and in children growing up in poor urban neighborhoods, and is least common in rural areas in combination with farm animal exposure.11 Notably, asthma morbidity follows a similar pattern.
These findings raise questions about the reasons for increased asthma in densely populated urban areas. What are the specific factors or combinations of factors that lead to asthma in American inner cities? Considering the list of asthma risk factors, the urban environment has a number of features that could have adverse effects on children's respiratory health, especially during the first few years of life when the lung and immune system are rapidly developing. It is possible that asthma risk is high because of exposure to adverse conditions, such as pollutants, cockroach or mouse allergens, stress, or the development of obesity. Conversely, it is also possible that children who grow up in an urban environment lack exposures or experiences that are necessary for healthy lung and immune development. Potential examples include reduced exercise, less availability of nutrients such as vitamin D, or low exposure to beneficial microbes that promote normal immune development. Although the urban environment is not usually considered hygienic, it may in fact be deficient in dirt (soil)!
To begin to identify environmental and lifestyle factors related to asthma causation in economically disadvantaged neighborhoods in large US cities, the Inner City Asthma Consortium initiated the Urban Environment and Childhood Asthma (URECA) study in 2004.12 The study hypothesis, design, and early findings from this observational birth cohort study are described in the following sections.
Study hypothesis and outcomes
The study hypothesis was based on the concept that postnatal lung and immune system development occur most rapidly in utero and during the first few years of life, and during this period of time, may be especially susceptible to environmental influences.13 Furthermore, specific patterns of immune development in early life, such as enhanced Th2-like cytokines and reduced interferon responses, affect the risk of developing atopic diseases, wheezing illnesses, and asthma.14,15 The URECA study has a two-stage hypothesis (Figure 1). First, the unique environmental exposures in the inner city interact with genetic factors during the prenatal and postnatal periods to adversely influence the development of innate and adaptive immunity, which in turn increases the risk for allergic sensitization and atopic diseases. Second, immune dysregulation in infancy increases the risk of developing lower respiratory infections caused by viruses and perhaps atypical bacteria. Those infections cause airway inflammation and structural changes during a particularly vulnerable period of lung development, leading to an increased risk of asthma by age 7 years.
Figure 1.
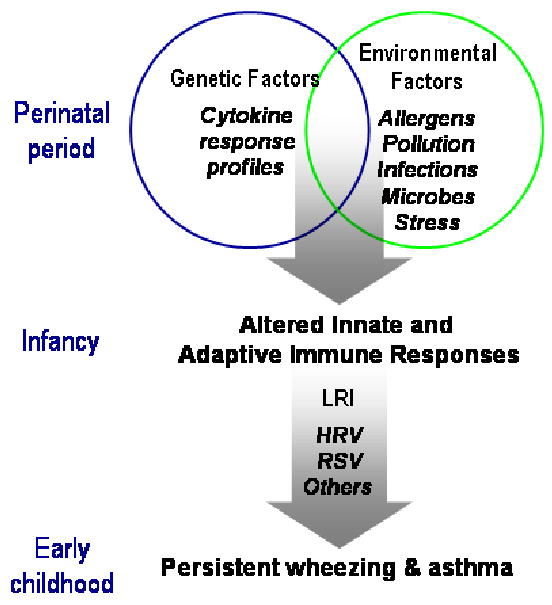
Factors influencing the onset of asthma in urban settings (from reference 12). Abbreviations: LRI, lower respiratory infection; HRV, human rhinoviruses; RSV, respiratory syncytial virus.
The primary objective of URECA is to identify in inner-city children the immunologic risk factors for the development of recurrent wheeze by age 3 years and asthma by age 7 years. As an indicator of immune development, blood mononuclear cells from each infant are stimulated ex vivo yearly beginning with cells from cord blood to measure cytokine responses. Patterns of cytokine responses over time will be compared between children with and without recurrent wheeze at age 3 years and with and without asthma at age 7 years. The two main secondary objectives are to identify environmental exposures that modify the developmental pattern of cytokine responses, and to identify the immunologic correlates of the development of atopic features such as total IgE, allergic sensitization, and atopic dermatitis.
Study population
To obtain sufficient power to test the main hypothesis, 500 subjects were needed, and 560 were recruited, from four study sites: Baltimore, Boston, New York, and St. Louis. The entry criteria specified that the families had to live in neighborhoods where at least 20% of the population had income below the poverty line, and one of the parents had allergic diseases or asthma. The participants were recruited prenatally, and to be study eligible had to be born at 34 weeks gestation or later and without significant respiratory problems in the neonatal nursery. The goal was to recruit a high risk population with as few restrictions as possible, while excluding children who had other respiratory conditions (e.g. hyaline membrane disease, bronchopulmonary dysplasia) that would confound the diagnosis of asthma.
Some studies have shown that babies of atopic vs. nonatopic families have distinct patterns of blood mononuclear cell cytokine responses at the time of birth.16 With this in mind, a second group of babies born to non-atopic parents were enrolled during the latter stages of the study. The goal was to enroll 50-60 families and 49 were enrolled; the sample size was based on power calculations to enable detection of a 50% reduction in interferon responses in the atopic families, as had been suggested by previous studies.
Both study groups are predominantly ethnic minority. Of the mothers in the allergic cohort, 71% are black and 19% are Hispanic (predominantly of Dominican and Puerto Rican heritage), and the nonatopic cohort has similar demographics. The ethnicity of this population, together with high rates of exposure to stress, tobacco smoke, cockroaches, and poor housing conditions and low socioeconomic status represent many of the potential risk factors for allergies and asthma in the US.
Study procedures
Overview
The URECA protocol was approved by human subjects committees at each of the study centers. Following informed consent, a series of questionnaires were administered to the mother at the prenatal visit (Table I). After birth, the child's mother responds to quarterly telephone questionnaires to assess the child's respiratory and allergy symptoms, medications, tobacco smoke exposure, and diet. Stress-related questionnaires were administered to the mother at the prenatal visit and are re-administered at selected annual clinic visits. Each year, the child visits the study site for a physical examination, eczema assessment, and a blood sample. A sample of the mother's blood was collected at the child's 12-month visit. The child undergoes allergy skin testing at selected visits, and annual pulmonary function testing begins at age 3 years. Each year, URECA staff visit the child's home to collect settled dust samples for analysis of allergens and selected microbial products. Airborne nicotine and nitrogen dioxide (NO2) are measured in the homes at month 3, and at age 4 and 6 years. A home environment questionnaire is administered at month 3, and annually thereafter. The goal of the periodic environmental sampling is to provide data for estimating longitudinal exposures for the individual study participants.
Table 1.
Urban Environment and Childhood Asthma (URECA) Study Overview.
| PN | D | 3 | Ongoing Visits | Clinic Visits (number represents child's age in months) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QC | HV | 12 | 24 | 33 | 36 | 48 | 60 | 72 | 81 | 84 | ||||
| Questionnaires | x | x | x | x | x | x | x | x | x | x | x | x | ||
| Respiratory Illness Score Card | As often as illness is reported | |||||||||||||
| Study Procedures | ||||||||||||||
| Cord blood sample | x | |||||||||||||
| Maternal blood sample | x | |||||||||||||
| Child blood sample | x | x | x | x | x | x | x | |||||||
| Eczema evaluation | x | x | x | x | x | x | x | x | ||||||
| Physical examination | x | x | x | x | x | x | x | |||||||
| Nasal lavage sample* | x | x | x | x | ||||||||||
| Allergen skin testing | x | x | x | |||||||||||
| Lung function testing | x | x | x | x | ||||||||||
| Bronchodilator reversibility | x | |||||||||||||
| Methacholine challenge | x | |||||||||||||
| Lung volume (plethysmography) | x | |||||||||||||
| Exhaled nitric oxide | x | |||||||||||||
| Bioelectrical impedance analysis | x | x | x | x | x | x | x | |||||||
| Home Environment Assessment | ||||||||||||||
| Dust sample collection | x | x | ||||||||||||
| Airborne nicotine and NO2 | x | x† | ||||||||||||
Notes: PN=prenatal visit; D=delivery; 3= home visit at age 3 months; QC=quarterly calls (occurring at 6, 9, 15, 18, 21, 27, 30, 39, 42, 45, 51, 54, 57, 63, 66, 69, 75, 78, and 81 months);
HV=home visits occur once between each yearly clinic visit, except between 5 and 6 years of age.
Also collected during respiratory illnesses
Collected at 3 months, and at age 4 and 6 years.
In addition to these scheduled activities, mothers are instructed to contact URECA staff whenever their children experience respiratory symptoms, such as rhinorrhea, cough, or wheezing. Nasal lavage specimens are collected for moderate colds or worse, as assessed by a respiratory symptom score card based on the questionnaire used in the Childhood Origins of Asthma (COAST) study.17
Cytokine secretion assays
In designing this multicenter study, one of the first tasks was to design cytokine secretion assays using peripheral blood mononuclear cells as an indicator of immune development in children. A panel of stimulants was selected to elicit innate, adaptive, and antiviral responses, along with mitogenic stimuli to induce polyclonal T cell responses (Table II). Two cytokine panels were also designed to correspond with the innate and adaptive stimuli.
Table II.
Stimulants used and cytokines measured in the mononuclear cell assays.12
| Innate Immune Responses | Adaptive Immune Responses | ||
|---|---|---|---|
| Stimulants | Cytokines | Stimulants | Cytokines |
| Lipopolysaccharide | IFN-α | Phytohemagglutinin | IFN-γ |
| Polyinosinic-polycytidylic acid | IFN-γ | Cockroach extract | IL-10 |
| Peptidoglycan | IL-10 | Dust mite (D. pteronyssinus) extract | IL-13 |
| CpG | IL-12p40 | Tetanus toxoid | IL-4 |
| Respiratory syncytial virus | TNF-α | CD3 + CD28 Mab* | IL-5† |
| Rhinovirus* | IL-8 | Medium alone | |
| Medium alone | |||
Stimulation not conducted on cells from the umbilical cord samples
Not measured in umbilical cord samples
Previous multicenter studies had used different protocols to isolate mononuclear cells from blood specimens; sometimes fresh cells had been used, and in other studies cells were cryopreserved so that the cells could be sent to a central laboratory for processing. Since technical details of mononuclear cell stimulation protocols and outcome measures vary widely, the URECA group conducted preliminary experiments to determine the optimal conditions for our four-center study. In these experiments, cryopreservation vs. use of fresh cells changed patterns of cytokine secretion, and did not improve reproducibility.18 As a result, processing of fresh mononuclear cells was selected for the URECA study. These preliminary studies underline the need to test the performance characteristics of immunologic assays before their adoption into a clinical protocol.
Viral diagnostics
Recurrent bouts of virus-induced wheezing in infancy signal an increased risk of developing asthma, and the viral etiology of these wheezing illnesses appears to modify the degree of risk. Results from two birth cohort studies suggest that children who wheeze with rhinoviruses (HRV) may be at especially high risk for asthma, especially if there is concurrent atopy.19,20 Viruses in urban environments could differ significantly from those reported in other populations, and this could affect the subsequent risk of asthma. Given the large sample size and repeated sampling that was expected in this study, URECA investigators worked with a biotechnology company (EraGen Biosciences, Madison, WI) to develop a high throughput multiplex PCR-based system that detects all common respiratory viruses.21 This system has been validated by comparison with standard diagnostic techniques, and like other PCR-based systems, greatly improves detection of respiratory viruses that are difficult to grow in tissue culture (e.g. HRV, bocaviruses, metapneumoviruses). Furthermore, this technique, when paired with partial sequencing of viral genomes, has been used successfully to identify additional members of the newly discovered HRV-C species, which so far have only been detected with molecular techniques.22
Preliminary Results
Maternal and prenatal influences on cytokine responses at birth
Prenatal exposures and maternal characteristics can affect immune development, and these effects are measurable in cord blood mononuclear cells.16,23,24 To test for these relationships in the URECA population, several selected maternal, perinatal, and newborn characteristics were examined as predictors of cytokine responses in the URECA newborns.25 The predictors with the most associations with innate immune responses included season of birth, ethnicity, birth weight/gestational age (which are highly correlated), and maternal asthma/use of inhaled corticosteroids. Although cytokine responses to protein antigens were low, it was nevertheless possible to evaluate associations between maternal and child characteristics and antigen-induced responses in cord blood cells.
Over half of the cytokine responses, including both innate and adaptive responses, varied by season of birth. There were up to 3-fold fluctuations in specific IFN-α and IFN-γ responses, and antigen-induced IFN-γ responses were enhanced during the winter months (Figure 2). Ethnicity, which in this study was largely a comparison between babies of black vs. Hispanic backgrounds, affected a variety of innate, but not antigen-induced, cytokine responses. Birth weight was inversely associated with IFN-γ responses to RSV (R = −0.16), but positively associated with IL-8 responses to a variety of innate stimuli (R = 0.08-0.12). Interestingly, children of mothers with asthma had generally lower RSV–induced cytokine responses. This finding suggests that babies born to mothers with asthma could have impaired antiviral responses, perhaps increasing the risk of virus-induced wheeze. Finally, cytokine responses of the 49 babies from non-atopic families were compared to an equal number of babies from allergic families, matched for season of birth and study center. Cytokine responses were generally lower in babies born to parents with allergy/asthma, and this was most pronounced for IL-12p40 and IL-8 responses.
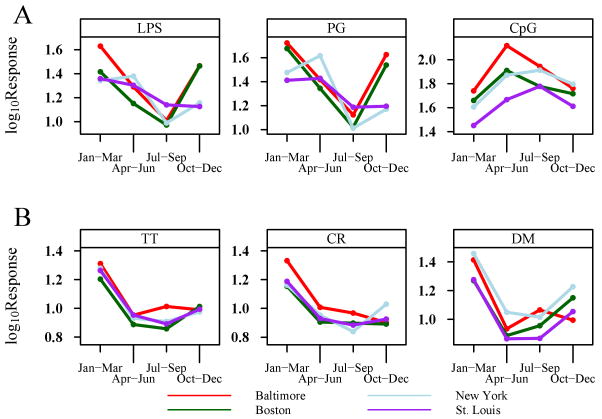
Figure 2.
Seasonal patterns of selected cytokine mean responses. A, IFN-α responses to LPS, peptidoglycan (PG), and CpG according to season (3-month intervals). B, IFN-γ responses to cockroach (CR) and dust mite (DM) extracts and tetanus toxoid (TT). Seasonal patterns from the 4 individual study sites are depicted by the colored lines. The depicted cytokine mean responses are those with the strongest seasonality patterns. All figures demonstrate a statistically significant seasonal effect, with each panel having P < .001 (from reference 25).
The results of this study provide evidence that the variability of newborn immune responses is not random, and, that development of the fetal immune system during the perinatal and postnatal periods appears to be responsive to maternal characteristics and experiences. Many of the associations between environmental predictors and cytokine responses were of relatively low magnitude; however, from an immunoepidemiologic point of view, such correlations could have biologic significance. Our findings also suggest that newborns of parents with allergy/asthma could differently to postnatal environmental experiences. This maternal influence may be partially mediated by genetics; however, seasonal variations suggest that either environmental or epigenetic mechanisms also affect the developing immune system. This cohort of infants will be analyzed to determine the long-term effect of these maternal-fetal interactions on immune development, recurrent wheezing (age 3 years), and asthma (age 7 years).
Ancillary studies
In addition to the main outcomes of the study, the prospective cohort study design provided an opportunity to address several other scientific questions related to the development of allergies and asthma. A collaboration was established with researchers in the Channing Laboratory at Harvard University to determine whether deficiencies in CD4+ T regulatory cell number or function increased the risk for asthma in the infants enrolled at the Boston URECA site. Early findings in this study show that CD4+ T cells from the newborns vs. mothers had similar expression of Foxp3, but cells from newborns had lower suppressive function.26 In addition, investigators at Washington University performed prospective analyses of selected antiviral responses to determine whether deficient antiviral signaling or interferon responses predispose to recurrent wheezing and more severe viral infections. Finally, all URECA participants undergo yearly testing for obesity, consisting of bioelectrical impedance analysis to estimate percent body fat, anthropomorphic measurements, and immunologic tests to assess potential evidence of systemic inflammation associated with overweight status.
Conclusions
Birth cohort studies have been a rich source of information about factors that influence the development of asthma, including viral infections, environmental factors, immune development, and wheezing phenotypes in early life. In the USA, asthma incidence and morbidity are greater in urban areas, and yet there is relatively little information about the relationship between early life exposures and asthma in this high-risk environment. The goal of the URECA birth cohort study is to identify mechanisms by which urban-specific lifestyle and environmental factors modify immune development in early life, and the subsequent risk of asthma. There are a number of potential explanations for the increased incidence of asthma and increased morbidity due to this disease in large urban areas. In theory, it is possible to improve adverse urban environmental or social conditions to prevent asthma, but due to the magnitude of the problems this is unlikely to occur without definitive information to link specific exposures or lifestyle factors to asthma onset. The design and population of the URECA study are well suited to identify relationships between urban exposures or lifestyles and childhood asthma.
Acknowledgments
Funding: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, under Contracts number NO1-AI-25496 and NO1-AI-25482, and from the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Mutius E. Allergies, infections and the hygiene hypothesis--the epidemiological evidence. Immunobiology. 2007;212(6):433–9. doi: 10.1016/j.imbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Prescott SL. Early origins of allergic disease: a review of processes and influences during early immune development. Current Opinion in Allergy & Clinical Immunology. 2003;3(2):125–32. doi: 10.1097/00130832-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol. 2005;115(5):928–34. doi: 10.1016/j.jaci.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–6. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178(11):1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7(2):95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 8.Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165(3):358–65. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 9.Weinmayr G, Weiland SK, Bjorksten B, Brunekreef B, Buchele G, Cookson WO, et al. Atopic sensitization and the international variation of asthma symptom prevalence in children. Am J Respir Crit Care Med. 2007;176(6):565–74. doi: 10.1164/rccm.200607-994OC. [DOI] [PubMed] [Google Scholar]
- 10.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 11.Adler A, Tager I, Quintero DR. Decreased prevalence of asthma among farm-reared children compared with those who are rural but not farm-reared. J Allergy Clin Immunol. 2005;115(1):67–73. doi: 10.1016/j.jaci.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O'Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38(10):1635–43. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 14.Gern JE, Brooks GD, Meyer P, Chang AM, Shen KL, Evans MD, et al. Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–8. doi: 10.1016/j.jaci.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Heaton T, Rowe J, Turner S, Aalberse RC, de Klerk N, Suriyaarachchi D, et al. An immunoepidemiological approach to asthma: identification of in-vitro T-cell response patterns associated with different wheezing phenotypes in children. Lancet. 2005;365(9454):142–9. doi: 10.1016/S0140-6736(05)17704-6. [DOI] [PubMed] [Google Scholar]
- 16.Rinas U, Horneff G, Wahn V. Interferon-gamma production by cord-blood mononuclear cells is reduced in newborns with a family history of atopic disease and is independent from cord-blood IgE levels. Pediatr Allergy Immunol. 1993;4:60–4. doi: 10.1111/j.1399-3038.1993.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 17.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans ME, Li Z, Shult P, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Shreffler WG, Visness CM, Burger M, Cruikshank WW, Lederman HM, de la MM, et al. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multicenter study. BMC Immunol. 2006;7:29. doi: 10.1186/1471-2172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119(5):1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WM, Grindle KA, Pappas TE, Marshall D, Moser M, Beaty E, et al. A high-throughput, sensitive and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. Journal of Clinical Microbiology. 2007;45(8):2626–34. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2(10):e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan Dillie KT, Tisler CJ, DaSilva DF, Pappas TE, Roberg KA, Carlson-Dakes KT, et al. The influence of processing factors and non-atopy-related maternal and neonate characteristics on yield and cytokine responses of cord blood mononuclear cells. Clin Exp Allergy. 2008;38(2):298–304. doi: 10.1111/j.1365-2222.2007.02891.x. [DOI] [PubMed] [Google Scholar]
- 24.Pfefferle PI, Buchele G, Blumer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: The PASTURE Study. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Gold DR, Bloomberg GR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol. 2009;124(5):1078–87. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ly NP, Ruiz-Perez B, McLoughlin RM, Visness CM, Wallace PK, Cruikshank WW, et al. Characterization of regulatory T cells in urban newborns. Clin Mol Allergy. 2009;7:8. doi: 10.1186/1476-7961-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]