Abstract
Current treatment for advanced, metastatic melanoma is not very effective, and new modalities are needed. ADI-PEG20 is a drug that specifically targets ASS-negative malignant melanomas while sparing the ASS-expressing normal cells. Although laboratory research and clinical trials showed promising results, there are some ASS-negative cell lines and patients that can develop resistance to this drug. In this report, we combined ADI-PEG20 with another antitumor drug TRAIL to increase the killing of malignant melanoma cells. This combination can greatly inhibit cell growth (to over 80%) and also enhanced cell death (to over 60%) in four melanoma cell lines tested compared with control. We found that ADI-PEG20 could increase the cell surface receptors DR4/5 for TRAIL and that caspase activity correlated with the increased cell death. These two drugs could also increase the level of Noxa while decrease that of survivin. We propose that these two drugs can complement each other by activating the intrinsic and extrinsic apoptosis pathways, thus enhance the killing of melanoma cells.
Keywords: ADI-PEG20, TRAIL, ASS, melanoma, arginine-deprivation, apoptosis
Introduction
Melanoma is usually refractory to conventional chemotherapy and immunotherapy [1]. New strategies are needed to improve the treatment outcome and survival of these patients.
We and others have previously shown that many melanoma cell lines or primary culture do not express an enzyme called argininosuccinate synthetase (ASS) [2; 3], and hence cannot utilize citrulline and aspartate to synthesize arginine. The loss of expression of ASS takes place at the transcriptional level [4; 5]. As a result, for those ASS-negative melanoma cells, arginine becomes an essential amino acid for growth and survival. Arginine deprivation can eventually lead to cell death for these ASS-negative tumors.
Arginine deiminase (an enzyme originally from mycoplasmas) which degrades arginine into citrulline and ammonium has been shown to have in vitro antitumor activity for melanoma. In order to improve its half life and decrease its antigenicity, a pegylated form of ADI (ADI-PEG20) has been developed by Polaris Inc. [3] and has been shown to have clinical antitumor activity in ASS-negative melanoma [6]. The toxicity is minimal due to the fact that normal cells express ASS and can synthesize arginine from citrulline. This makes ADI-PEG20 a promising new drug for malignant melanoma. However, not all ASS-negative patients respond to this treatment while stable disease was achieved in some patients [6]. Furthermore, re-expression of ASS can occur at the time of relapse in certain patients. Corresponding with our clinical findings, we also have found that the sensitivity to ADI-PEG20 varies in ASS-negative melanoma cell lines [7]. Certain melanoma cell lines such as A2058 and SK-Mel-2 which do not express ASS can be induced to express ASS after exposure to ADI-PEG20, while in other cell lines like A375 and Mel-1220, the induction does not occur. In order to kill ASS-negative melanoma cells more effectively using this strategy, one has to increase cell death by combining with another drug.
At the present time, the mechanism of how ADI-PEG20 induces cell death is not clear. Nevertheless, it has been shown that various kinds of cellular stress such as reactive oxygen species and proteasome inhibitors, as well as nutritional stress can activate apoptosis through the mitochondrial pathway [8]. Thus, it is likely that arginine deprivation from ADI-PEG20 treatment can cause apoptosis via the mitochondrial pathway (intrinsic pathway). However, it is known that melanoma is a Type II cells which need the participation of both the intrinsic and extrinsic pathway to effectively carry out apoptosis [9]. This could explain why ADI-PEG20 alone did not result in wide spread apoptotic cell death. We have chosen TRAIL to combine with ADI-PEG20 because the former compound is capable of inducing apoptosis via extrinsic pathway. Importantly, TRAIL selectively kills tumor cells while sparing normal ones [10]. This property of TRAIL makes it also a targeted therapy for tumor tissues just as ADI-PEG20. Currently, recombinant human TRAIL and agonistic antibody against TRAIL receptor have been developed for cancer treatment [11].
TRAIL-induced apoptosis starts with the binding of TRAIL to its cognate receptors DR4/5. This binding will lead to the formation of death-inducing signaling complex (DISC) and the subsequent activation of caspase-8. Caspase-8 is then able to activate downstream effector caspases such as caspase-3 and 6, ultimately resulting in apoptosis in Type I cells. This pathway is termed the extrinsic pathway for apoptosis. However, TRAIL alone treatment is not effective enough to kill melanoma, thus a series of different combinations either with conventional chemotherapeutic drugs or signal transduction inhibitors have been tested to increase the therapeutic efficacy of TRAIL [12] . Nevertheless, some of these drugs tested also affect normal cells. Since arginine deprivation does not affect normal cell, and can prime melanoma cells toward the intrinsic pathway, it may be a good combination with TRAIL to kill melanoma cells. The apoptotic signal initiated by ADI-PEG20 can be further strengthened through the cleavage of a cytosolic protein Bid by caspase-8 activation through the extrinsic pathway. The truncated Bid (tBid) will translocate to mitochondria outer membrane and help to initiate the intrinsic pathway. The net effect is the release of cytochrome c, SMAC/Diablo, and AIF from the mitochondria and the activation of caspase-9. In turn, caspase-9 can activate downstream effector caspases, and importantly, activation of caspase-9 can also leads to the activation of more caspase-8, thus amplifying the effect of the extrinsic pathway. Furthermore, SMAC/Diablo released from mitochondria will neutralize caspase inhibitors such as XIAP and survivin, whereas AIF can directly enter nuclei to induce caspase-independent cell death. Thus, the combination of TRAIL and ADI-PEG20 should enhance the apoptosis of ASS-negative melanoma cells. In this communication, we have tested this hypothesis.
Materials and Methods
Reagents
All cell culture reagents and Versene were from Invitrogen except that the fetal bovine serum is from Atlanta Biologicals. ADI-PEG20 was provided by Polaris, Inc., and the recombinant human TRAIL was from National Cancer Institute. The ApoStat FITC-VD-FMK caspase probe, caspase-3 inhibitor Z-DEVD and biotinylated-IgG, -DR4, -DR5 antibodies and PE-SA conjugates were obtained from R&D System. Antibodies against Bid, XIAP, Bcl-2, Bcl-xL, Mcl-1, and survivin were purchased from Cell Signaling. Anti-β-actin was from Sigma. Anti-Noxa and anti-α-tubulin were from Calbiochem. Secondary antibodies for western blot were from Promega.
Cell lines and culture
Melanoma cell lines A2058, A375 and SK-Mel-2 were purchased from American Type Culture Collection. Mel-1220 was established in our own laboratory from patient biopsy (with informed consent). All these cell lines were maintained in MEM supplemented with 10% fetal bovine serum and penicillin/streptomycin.
MTS assay
The CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit from Promega was used to determine the number of live cells following a modified protocol according to Huang et al. [13].
Flow cytometry for cell death analysis
Treated cells were collected with trypsin/EDTA treatment for attached cells and combined with floating ones in the medium. Collected cells were washed in PBS and incubated with the FITC-VD-FMK ApoStat caspase probe and PI (Sigma) for 30 min at 37 °C. The cells were then washed with PBS and resuspended in PBS. The prepared cells were analyzed in a Coulter EPIC XL-MCL bench top flow cytometer within 1 hour.
Flow cytometry for death receptor analysis
Cells were collected with Versene treatment and washed with PBS. Then the cells were incubated with biotinylated-IgG or biotinylated specific death receptor antibodies for 30 min at room temperature. After washing with PBS, the cells were incubated with PE-SA for another 30 min at room temperature in the dark. After washing in PBS, cells were resuspended with 1% paraformaldehyde in PBS and kept in the dark at 4 °C. Analysis was done in a Beckton-Dickinson bench top flow cytometer.
Western blot
Cells from culture dish were washed with PBS and scraped into cold PBS containing protease inhibitors. After the removal of PBS, cell pellet was resuspended in 1× lysis buffer (Cell Signaling) and sonicated to prepare the cell lysate. Lysate was then mixed with SDS-PAGE sample buffer and boiled at 100 °C for 10 min. The prepared sample was used for SDS-PAGE. Resolved proteins were electro-transferred onto a PVDF membrane and probed with specific antibodies.
Knocking down of Bid in A2058 cells
ON-TARGETplus SMARTpool of siRNA for human Bid were purchased from Dharmacon, and the transfection of siRNA into cells were done following the manufacturer recommended protocol. At 24 hr post transfection, the transfected cells were seeded into 96-well plates. TRAIL, ADI-PEG20 or combination was added for assaying the cytotoxicity as stated in MTS assay.
Results
ADI-PEG20 and TRAIL combination effectively increases cell death
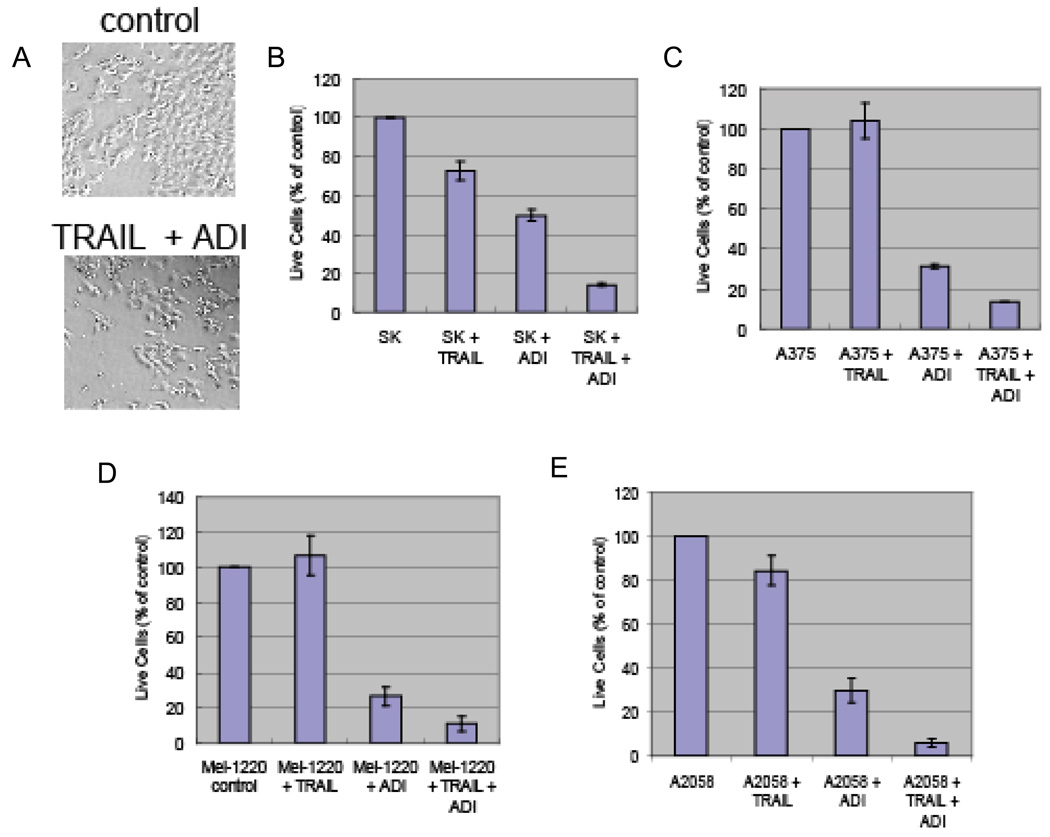
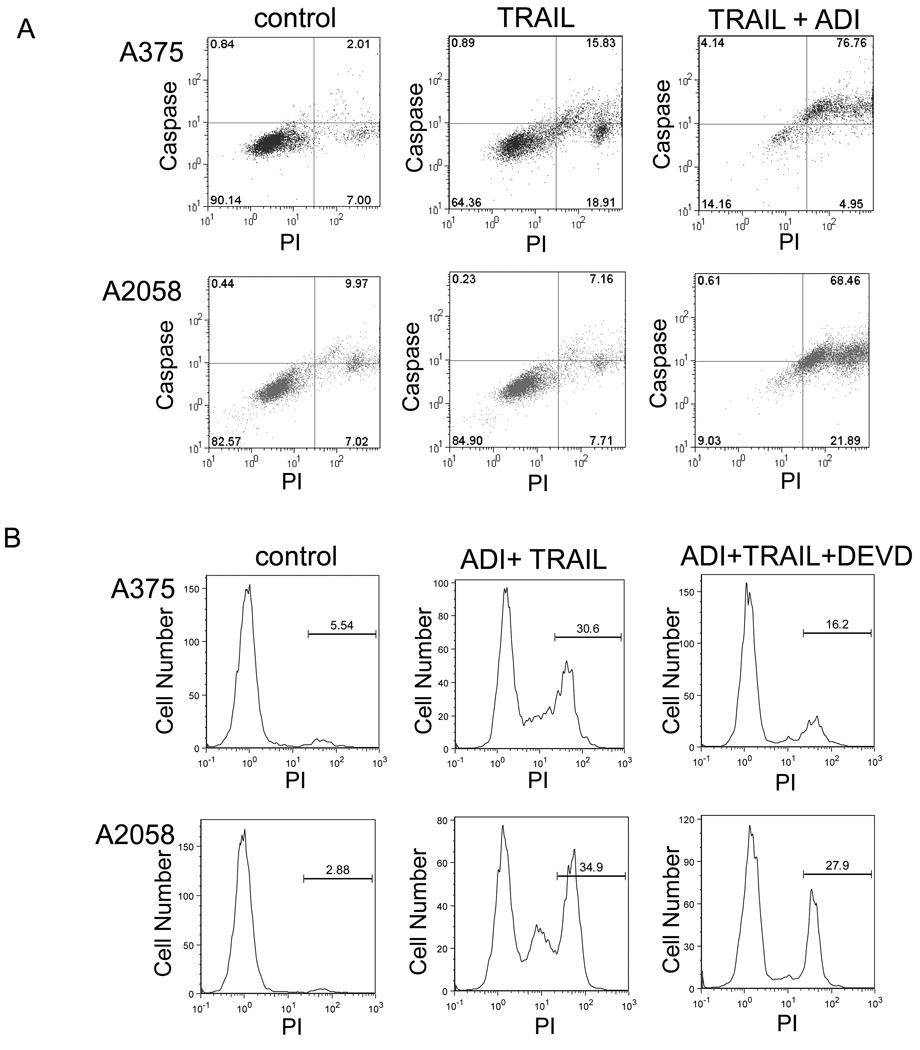
We first determined the effect of TRAIL and ADI-PEG20 on the survival of melanoma cell lines in cell culture. When these cell lines were treated with ADI-PEG20 (0.05 ug/ml) plus recombinant TRAIL (100 ng/ml), cell death was apparent after 72 hr as shown in Figure 1A for A375. The dead cell also showed pyknotic morphology which resembles apoptosis. To measure the cytotoxicity effect of these treatments, we used the MTS assay to determine the remaining live cells after the treatment. Cells were treated with ADI-PEG20 at 0.1 ug/ml with or without TRAIL at 100 ng/ml. We treated SK-Mel-2 and A375 for 48 hr while A2058 and Mel-1220 for 72 hr. This is based on the fact that the former two cell lines are more sensitive to ADI-PEG20 treatment. At this dosage of ADI-PEG20, arginine was completely depleted in the medium. The results for SK-Mel-2, A375, Mel-1220, and A2058 were shown in Figure 1B to E, respectively. TRAIL alone showed some inhibition effect (15% to 25%) in SK-Mel-2 and A2058 tested while there was not apparent effect for A375 and Mel-1220. The ADI-PEG20 alone results in 50% to 75% of cell growth inhibition for these melanoma cells. However, the combination of ADI-PEG20 and TRAIL could further increase the growth inhibition to over 80% in all the cell lines tested. Since the morphology of dead cells after the treatment resembled that of apoptotic cells, we used flow cytometry to investigate whether caspases played any role in the cell death caused by ADI-PEG20 and TRAIL combination. In this experiment, PI was used to label the dead cell, and activated caspase was also labeled with FITC-conjugated probes. The results are presented in Figure 2A. In A375 cell line, ADI-PEG20 alone or TRAIL alone results in 25% cell death while with the two drug combination, the cell death increased to 70% when compared with control. In A2058 cells, TRAIL alone did not induce cell death, and ADI-PEG20 alone results in 10% cell death (data not shown) while with the combination the cell death increased to 64% when compared with control. Similar results were obtained with Mel-1220 and SK-Mel-2 (data not shown). Thus, this combination treatment was effective in killing all the four cell lines tested. Furthermore, the percentage of cells with positive caspase activity paralleled with cell death. This suggests that caspase activation may be one of the driving forces for cell death in this combination treatment.
Figure 1.
Figure 2.
To further confirm that caspase activation is important in cell death caused by the combination treatment, we used caspase-3 inhibitor to determine whether reversal of cell death could be achieved. Our results are shown in Fig. 2B. The dead cell fraction (PI-positive) was reduced considerably in A375 (from 30% to 16%) and from 35% to 28% in A2058 after the addition of caspase-3 inhibitor. However, these results also indicate that the cell death was not completely reversed by inhibiting caspase-3. One reason for this is the other effector caspases such as caspase-6 and caspase-7 may also be involved in the apoptosis process. Another explanation may be that caspase-independent cell death caused by the release of Endo G or AIF also accounted for part of the cell death [14; 15].
ADI-PEG20 treatment upregulated the death receptor expression
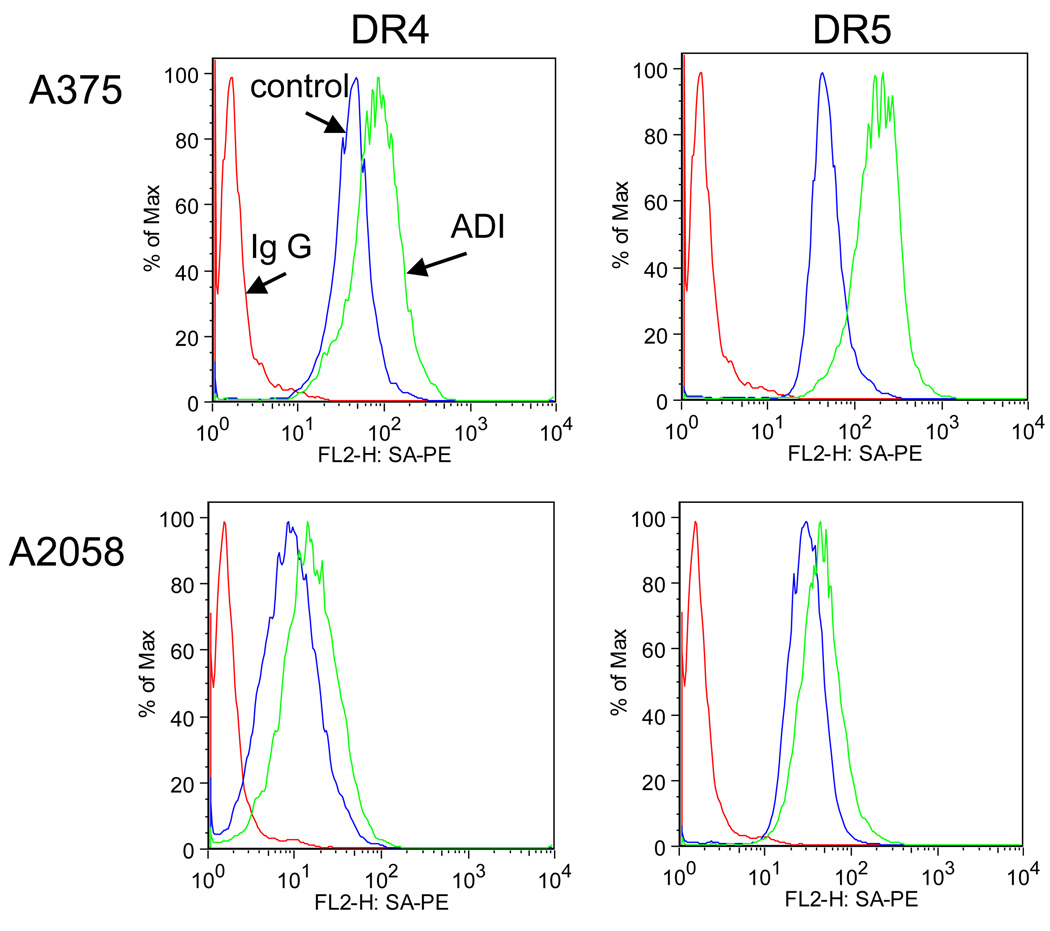
It is known that death receptor expression on the cell surface is required for the responsiveness to TRAIL treatment. The ligation of TRAIL to the death receptor DR4 or DR5 is the first step leading to apoptosis for TRAIL sensitive cells. Melanoma cells are normally regarded as TRAIL-resistant. After ADI-PEG20 treatment, we found by flow cytometry that the surface DR4/5 expression increased to varying extent (Figure 3). For A2058, both DR4 and DR5 were increased to a similar extent after ADI-PEG20 treatment, while A375 showed more increase in DR5 than DR4. However, the increase in both receptors is higher in A375 when compared with A2058. The greater extent of DR4 and DR5 increase in A375 seemed to be consistent with its higher sensitivity to the ADI-PEG20 or the combination with TRAIL. The increase in surface death receptor expression may be one of the reasons for the enhanced cytotoxicity of TRAIL and ADI-PEG20 combination treatment. Elevated levels of surface DR4/5 also have been shown after treatment with other antitumor drugs in many tumors including melanoma [16; 17]. The above results partly explain why ADI-PEG20 and TRAIL combination is more cytotoxic to melanoma.
Figure 3.
Pro- and anti-apoptotic proteins change after treatment
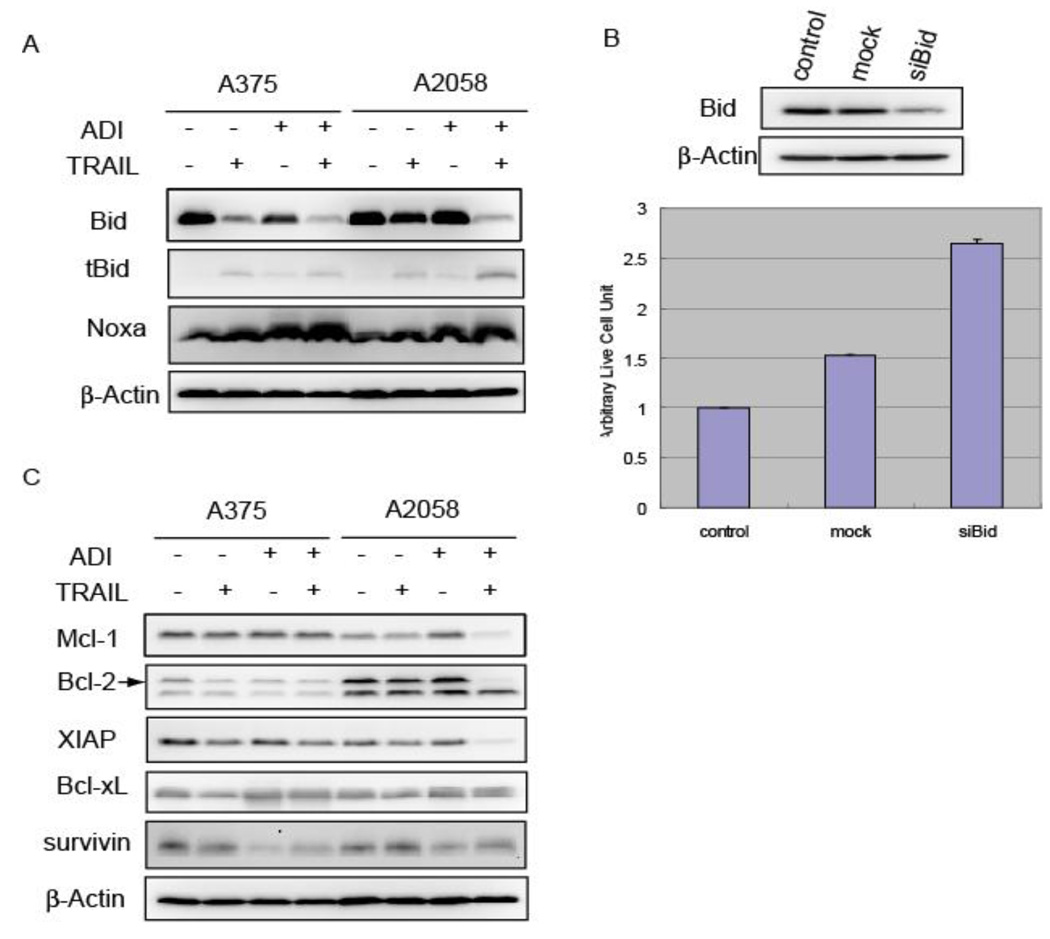
In addition to the increased expression of surface death receptors, it is possible that the ADI-PEG20 treatment can also affect the apoptotic machinery (pro/anti-apoptotic proteins) which enhances the downstream TRAIL signaling pathway. In this regard, it is logical to examine Bid, a BH3-only protein which links the extrinsic and intrinsic pathway. The link starts at the step where Bid is cleaved by activated caspase-8 or -10. The cleaved product of Bid called truncated Bid (tBid) can interact with Bcl-xL, and Bax/Bak. These interactions result in the inhibition of Bcl-xL but activation of Bax/Bak. The net effect is the activation of the mitochondrial pathway through the release of cytochrome c. As shown in Figure 4A, ADI-PEG20 alone did not affect the cleavage of Bid in A2058, and TRAIL alone treatment only resulted in minor decrease of full-length Bid. Cleavage of Bid became apparent in the combination treatment for A2058 as indicated by the appearance of tBid and the decrease of full-length Bid. For A375, which is more sensitive to both drugs as compared with A2058, the truncation of Bid could be observed in ADI-PEG20 or TRAIL alone treatment. However, there was a further decrease of full-length Bid when these two drugs were combined. This processing of Bid was also observed in cell line SK-Mel-2 which is also susceptible to the combined treatment (data now shown). To further investigate the role of Bid in the combination treatment, we used siRNA for Bid to knock down the endogenous Bid level and assayed the cytotoxic effect of ADI-PEG20 plus TRAIL in A2058 cell lines. As shown in Figure 4B, siBid led to the apparent lowered level of Bid while no tBid was observed, and in the same time, there was a significant increase of cell viability in the Bid knock-down cells upon combination treatment. This result confirmed that Bid is indeed an important player in the cell death process caused by the ADI-PEG20 and TRAIL combination. Another pro-apoptotic protein that showed apparent change upon the treatment is Noxa. This protein has been reported to play a crucial role in inducing apoptosis in several types of malignant tumor cells including melanoma [18]. In both A375 and A2058, Noxa was up-regulated in ADI-PEG20 alone, TRAIL alone or in combination. However, the extent of increase was the highest in the combination treatment (Figure 4A).
Figure 4.
Several anti-apoptotic proteins were investigated as well. One interesting protein is Bcl-2, an important member of the anti-apoptotic proteins for the mitochondrial pathway. In Figure 4C, the Bcl-2 level was lowered in both cell lines when they were treated with the combination of TRAIL and ADI-PEG20. Another anti-apoptotic protein of the Bcl-2 family, Bcl-xL seemed to be unaffected by all these treatments. The level of Mcl-1 and XIAP did not show much change in A375, while a decrease was observed in A2058 when treated with the combination. These disparities may result from the inherent differences of cell lines. Lastly, a member of the IAP family, survivin, decreased in ADI-PEG alone or combination treatment in all four cell lines tested (Figure 4B and not shown). In non-proliferating normal cells, survivin protein is usually not detectable. On the other hand, the expression of survivin is up-regulated in tumor cells [19]. Knocking down survivin level by siRNA or by expressing a mutant form of survivin was able to induce apoptosis in some tumor cell lines [20]. It appears that changing in the pro-apoptotic protein of Noxa and anti-apoptotic protein of survivin could be found in all the cell lines tested. These two proteins seemed to account for the increased apoptosis in melanoma cells treated with the combination.
Discussion
We have previously shown that arginine deprivation using ADI-PEG20 inhibited cell growth in ASS-negative melanoma cells while sparing ASS-expressing normal cells. Apoptosis does occur upon prolonged arginine deprivation with non detectable arginine levels which can be achieved in vitro. However, clinically it is difficult to achieve cell death by apoptosis in vivo due to the fact normal endothelial cells can supply arginine to the tumor cells. Furthermore, these ASS-negative melanoma cells can also undergo autophagy to avoid apoptosis while others can turn on ASS expression upon arginine deprivation [7]. Thus, in order to increase the efficacy of ADI-PEG20, combination treatment is needed. In this communication, we have shown that the addition of TRAIL greatly enhanced the apoptotic effect of ADI-PEG20 while TRAIL alone resulted in only 0–25% cell kills. This is not surprising since melanoma is known to be resistant to TRAIL. Thus, this new strategy can overcome the resistance to TRAIL while also increasing the antitumor effect of ADI-PEG20. We have further shown that the cell death caused by this combination was primarily through caspase-dependent apoptosis.
Our data suggests that there are multiple factors contributing to the effectiveness of this combination treatment in melanoma. First, we have found that arginine deprivation in ASS-negative melanoma cells resulted in the increase of DR4/DR5 while having no effect in ASS-expressing cells. The increase in death receptors may sensitize the cells to TRAIL. This finding also is consistent with previously published data which showed that certain cellular stress such as radiation or other chemicals can induce up regulation of surface death receptors. Of these receptors, DR4 is more important in sensitizing tumor cells to TRAIL. Since arginine deprivation can cause nutritional stress in ASS-negative cells, it is reasonable that this treatment resulted in the up regulation of DR4/5.
On the other hand, these chemical or other stresses also induce changes in the apoptosis pathways. A whole range of protein molecules can be affected and ultimately change the fate of the cancer cells. The basic theme is downregulation of anti-apoptotic proteins or upregulation of pro-apoptotic proteins. In this regard, we have found that treatment with ADI-PEG20 up-regulates Noxa, which may neutralize the anti-apoptotic protein Mcl-1, and hence release Bak to activate the mitochondria pathway. We also found the decrease of the anti-apoptotic protein survivin, which can inhibit the caspase activity. The decrease could be induced in ADI-PEG20 alone or in combination with TRAIL. However, the apoptotic signal from ADI-PEG20 treatment alone may not be sufficient to induce large scale cell death. This is supported by the findings that no cleavage of Bid (tBid) is seen. Once TRAIL is added, tBid can be visualized. To further support this notion, we have found that silencing Bid decreases the effectiveness of this combination. We conclude that the addition of TRAIL complements the apoptotic effect of ADI-PEG20 through the extrinsic pathway. On the other hand, ADI-PEG20 enhances the effect of TRAIL by up-regulating the receptors and possibly through activation of mitochondria pathway via increasing Noxa and decreasing survivin.
Our results which showed that that nutritional stress, like arginine deprivation, is an effective way of priming ASS-negative tumor cells for cell death is new. Although the mechanism is not quite clear, it is most likely mediated through increased Noxa, decreased surviving, and upregualtion of death receptors. Thus, by combination with TRAIL which mediated apoptosis via death receptor/extrinsic pathway, one can significantly enhance cell death. Since both ADI-PEG20 and TRAIL are relatively more toxic to tumor cells due to the fact that normal cells possess ASS and relatively insensitive to TRAIL, this combination bears a great potential to eradicate ASS-negative melanoma or other tumors as well. This enhancement may be due to a combination of factors which govern the apoptosis-related proteins, and may vary among cell lines. However, the Bid protein which links the extrinsic and intrinsic pathways appears to be important since silencing Bid decrease the potency of this combination. The role of Noxa and survivin are not fully understood and is currently being investigated.
Acknowledgement
Supported by NIH grant R01CA109578. We wish to thank Polaris Inc. for supplying ADI-PEG20.
Abbreviations
- ADI
arginine deiminase
- ADI-PEG20
pegylated ADI
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- ASS
argininosuccinate synthetase
- DR
death receptor
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- FITC
fluorescein isothiocyanate
- PBS
phosphate buffered saline
- MEM
minimum essential medium
- PE-SA
phycoerythrocin-streptavidin
- PI
propidium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 2.Wheatley DN. Arginine deprivation and metabolomics: important aspects of intermediary metabolism in relation to the differential sensitivity of normal and tumour cells. Seminars in Cancer Biology. 2005;15:247–253. doi: 10.1016/j.semcancer.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Current Pharmaceutical Design. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–1899. doi: 10.1046/j.1432-1033.2003.03559.x. [DOI] [PubMed] [Google Scholar]
- 5.Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, Grimshaw MJ, Steele JP, Rudd RM, Balkwill FR, Fennell DA. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12:7126–7131. doi: 10.1158/1078-0432.CCR-06-1101. [DOI] [PubMed] [Google Scholar]
- 6.Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT, Ensor CM, Prestayko AW, Holtsberg FW, Bomalaski JS, Clark MA, Savaraj N, Feun LG, Logan TF. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23:7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- 7.Savaraj N, Wu C, Kuo M, You M, Wangpaichitr M, Robles C, Spector S, Feun L. The relationship of arginine deprivation, argininosuccinate synthetase and cell death in melanoma. Drug Target Insights. 2007;2:119–128. [PMC free article] [PubMed] [Google Scholar]
- 8.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310:66–67. doi: 10.1126/science.1117105. [DOI] [PubMed] [Google Scholar]
- 9.Chetoui N, Sylla K, Gagnon-Houde JV, Alcaide-Loridan C, Charron D, Al-Daccak R, Aoudjit F. Down-regulation of mcl-1 by small interfering RNA sensitizes resistant melanoma cells to fas-mediated apoptosis. Mol Cancer Res. 2008;6:42–52. doi: 10.1158/1541-7786.MCR-07-0080. [DOI] [PubMed] [Google Scholar]
- 10.Kruyt FAE. TRAIL and cancer therapy. Cancer Letters. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 13.Huang KT, Chen YH, Walker AM. Inaccuracies in MTS assays: major distorting effects of medium, serum albumin, and fatty acids. Biotechniques. 2004;37:406, 408, 410–412. doi: 10.2144/04373ST05. [DOI] [PubMed] [Google Scholar]
- 14.Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Benito M, Martinez-Lorenzo MJ, Anel A, Marzo I, Naval J. Membrane expression of DR4, DR5 and caspase-8 levels, but not Mcl-1, determine sensitivity of human myeloma cells to Apo2L/TRAIL. Experimental Cell Research. 2007;313:2378–2388. doi: 10.1016/j.yexcr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJS. Increased Death Receptor 5 Expression by Chemotherapeutic Agents in Human Gliomas Causes Synergistic Cytotoxicity with Tumor Necrosis Factor-related Apoptosis-inducing Ligand in Vitro and in Vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 17.Ivanov VN, Partridge MA, Johnson GE, Huang SXL, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Experimental Cell Research. 2008;314:1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan M, Alaoui A, Feyen O, Mirmohammadsadegh A, Essmann F, Tannapfel A, Gulbins E, Schulze-Osthoff K, Hengge UR. The BH3-only member Noxa causes apoptosis in melanoma cells by multiple pathways. Oncogene. 2008;27:4557–4568. doi: 10.1038/onc.2008.90. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci. 2008;99:1709–1714. doi: 10.1111/j.1349-7006.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]