Abstract
Th17 (T-helper-17) cytokine responses have been recently recognized as an important component for the protective immunity produced by vaccination. However, the mechanism by which immune adjuvants induce Th17 immunity has not been defined. We have developed a novel mucosal nanoemulsion (NE) adjuvant that produces a robust humoral and Th1 cellular immunity. Herein, we demonstrate that immunization with NE adjuvant induces a Th17 response to diverse antigens in both outbred and inbred mice. CD86 deficiency had a limited effect on the induction of IL-17, however, double CD80/CD86, CD40, and IL-6 (interleukin 6) mutant mice failed to produce Th17 immunity in response to NE adjuvant. Mice deficient in TLR2 and TLR4 (Toll-like receptors 2 and 4) had a diminished IL-17 response. Our data indicate that nasal mucosal immunization with NE adjuvant produces Th1 and Th17 immunity; that this process requires IL-6, CD40, and at least one of the CD80/CD86 molecules; and that the induction of TH17 is enhanced by the presence of TLR2 and TLR4 receptors. This unique approach to vaccination may have a significant role in protection against mucosal and intracellular pathogens.
Keywords: mucosal adjuvant, nanoemulsion, Th17, cellular immunity
I. INTRODUCTION
Presentation of antigen to CD4+ T cells in the setting of appropriate co-stimulatory molecules facilitates the differentiation of CD4+ cells into one of three helper T-cell (Th) subtypes: Th1, Th2, and Th17. While the function of Th1 and Th2 cells is well documented, the newly recognized subset of Th17 cells produce interleukin (IL)-17A, IL-17F, and IL-22; however, its function is not entirely clear.1 Although IL-17 was initially found to play an important role in inflammation and autoimmunity, more recently it has been shown to be important in protective immune responses induced by vaccinations.2,3 While it is known that IL-17 induction requires IL-6, TGF-β (transforming growth factor-beta), and IL-23 to establish a persistent Th17 cell population (at least in mice), the role adjuvants play in the enhancing Th17 immunity has not been well studied.4–6
Nanoemulsions (NEs) are oil-in-water formulations that adjuvant viral and bacterial antigens in vaccine preparations. NEs are produced by mixing a water-immiscible soybean oil phase into an aqueous phase by high-stress mechanical extrusion, yielding a uniform population of droplets with an average diameter of approximately 350 nm. The emulsion is then simply mixed with an antigen or pathogen, and the latter is inactivated by the inherent, surface-active nature of the emulsion.7–11 When administered intranasally, NEs have been shown to induce robust humoral response with neutralizing serum antibodies to the protective antigen of anthrax (PA), whole vaccinia virus, whole and split influenza virus, HIV-gp120, and hepatitis B surface antigen (HBs), and produce a Th1-biased cellular immunity in response to these antigens, as well as the production of IL-17.7–11 This yields a unique immune response to antigen reminiscent of that observed with natural respiratory viral infections.
To investigate the mechanism of the Th17 response with NE-based vaccines, we measured antigen-specific IL-17 expression in spleen cells of mice immunized with PA, HBs, and enhanced green fluorescent protein (EGFP) in NE adjuvant. We also examined the role of IL-6, co-stimulatory molecules such as CD40, CD80, and CD86, and the innate PPR (pathogen pattern recognition) receptors TLR2 and TLR4 (Toll-like receptors 2 and 4) in the IL-17 recall response in mice immunized with this novel intranasal adjuvant.
II. MATERIALS AND METHODS
A. Adjuvant and Antigens
NE (W805EC formulation) was supplied by NanoBio Corporation (Ann Arbor, MI), and was manufactured by a high-speed emulsification of soybean oil (64%) with cetyl pyridinium chloride (1%), Tween 80 (5%), and ethanol (8%) in water, with resultant NE droplets averaging 350 nm in diameter.
Recombinant PA was obtained from List Biological Laboratories, Inc. (Campbell, CA). Recombinant, endotoxin-free EGFP was purchased from BioVision Research Products (Mountain View, CA). The recombinant adw serotype of HBs was supplied by Human Biologicals Institute (Indian Immunologics, Ltd., Hyderabad, India). The HBs protein was purified from pPIC3K plasmid-transfected Pichia pastoris cells using standard techniques (Indian Immunologicals standard operation procedures and good manufacturing practices). The endotoxin concentration was determined to be < 7.5 endotoxin units (EU)/20 μg of HBs protein; which is below the internationally accepted standard of ≤ 30 EU/20 μg of protein. Antigens were reconstituted at 5 mg/mL in either endotoxin-free sterile water (PA) or in phosphate-buffered saline (PBS; HBs and EGFP) and stored at −20°C until used.
B. Animals
Pathogen-free outbred CD-1 and inbred BALB/c mice (females 6–8 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). Pathogen-free IL-6, CD86, CD80/CD86, TLR2, and TLR4 knock-out and mutant mice, and their respective wild-type background C57BL/6J or C57BL/6N control mice (JAXR Mice), were purchased from Jackson Laboratories (Bar Harbor, ME). DO11.10 transgenic BALB/c mice were a kind gift from Dr. Gary Huffnagel at the University of Michigan Medical School. During all experiments, mice were housed at the University of Michigan animal facility in accordance with the American Association for Accreditation of Laboratory Animal Care standards. All procedures involving animals were performed according to the University Committee on the Use and Care of Animals at the University of Michigan.
C. Reagents
Saline and PBS (1 × PBS and 10 × PBS, pH 7.4), RPMI-1640, and fetal bovine serum were purchased from Cellgro (Medtech, Inc., Manassas, VA). De-ionized water was prepared using a Milli-Q® Ultrapure water purification system (Millipore, Billerica, MA). Bovine serum albumin was purchased from Sigma-Aldrich (St. Louis, MO). Alkaline phosphatase-conjugated rabbit anti-mouse immunoglobulin G, (IgG) secondary antibodies were purchased from Rockland Immunochemicals, Inc. (Gilbertsville, PA).
D. Immunizations
All immunizations were conducted in mice anesthetized with isoflurane using an IMPAC6 precision vaporizer. Antigen-NE formulations were prepared 30 to 60 min prior to immunization by mixing antigen solutions with NE, using either normal saline or PBS as a diluents. The intranasal immunizations were performed with a pipette tip applied to the nares, and the animals were slowly administered 10 μL (5 μL/nare) of an NE formulation containing 20 μg of antigen mixed with 20% NE. Antigen mixed with PBS alone served as a control. Intramuscular immunizations were performed by 50-μL injection into mouse epaxial muscle with 20 μg of antigen adsorbed on aluminum hydroxide (Sigma-Aldrich), as described previously.10 Mice were immunized twice 2 to 6 weeks apart. For immune response analysis, mouse sera were obtained by saphenous vein bleeding at 2-week intervals, and splenocytes were harvested at the end of experiments, 10 to 12 weeks after primary immunization.
E. Determination of IgG Titers
Serum antibody titers were determined with ELISA using plates coated with 5 μg/mL of PA, HBs, or EGFP proteins, as described previously. 10
F. Splenocyte Stimulation and Analysis of Cytokine Expression
Freshly isolated mouse murine splenocytes were seeded at 4 × 106 cells/mL (RPMI 1640, 2% fetal bovine serum) and incubated with individual antigens (5–10 μg/mL) for 72 h. Cell-culture supernatants were harvested and analyzed for the presence of cytokines using the Milliplex mouse cytokine/chemokine immunoassay kit (Millipore) according to the manufacturer’s instructions.
G. Intracellular Cytokine Staining
Splenocytes were stimulated with antigen (PA, 10 μg/mL) for 24 h. The GolgiPlug reagent with leukocyte activation cocktail (BD Biosciences, San Jose, CA) was added for the last 4 h of incubation. For flow-cytometry analysis, cells were first stained with anti-CD4 (FITC conjugate) and anti-CD8 (PE conjugate) antibodies (ABcam), then fixed and permealized with Perm/Fix (BD Biosciences). Subsequently, cells were stained with anti-IL-17A (AlexaFluor 647 conjugate) and anti-interferon (IFN)-γ (PE-Cy5 conjugate). Samples were acquired on an LSR II (BD Biosciences) and data were analyzed with DIVA software (BD Biosciences).
H. Statistical Analysis
Results are expressed as mean ± standard deviation (SD) or standard error of the mean (SEM) as indicated. Analysis of statistical significance between multiple groups was determined by ANOVA (analysis of variance). The analyses were based on 95% confidence limits and two-tailed tests. A p value < 0.05 was considered to be statistically significant.
III. RESULTS
A. Nasal Immunization With NE Adjuvant Produces Th1 and Th17 Cellular Responses
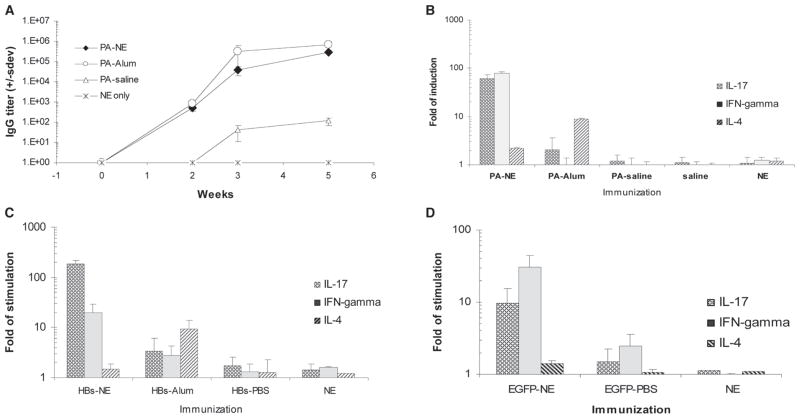
Having recently demonstrated that NE mucosal adjuvants are effective in the induction of antibody responses and Th1-biased cellular immunity,7–10 we further examined whether NE adjuvant produces Th17 responses using genetically diverse CD-1, BALB/c, and C57BL/6 mice. The serum anti-PA IgG titers in CD-1 mice immunized intranasally with a single dose of PA-NE, PA-saline, or with intramuscular injection of PA-alum were followed for at least 5 to 8 weeks (Fig. 1A). Verifying the adjuvant activity of the NE, nasal PA-NE immunization produced robust antibody response with anti-PA IgG titers (~106) comparable to those obtained after parenteral immunization using conventional alum adjuvant. Nasal immunization with antigen in PBS produced only sporadic and low IgG titers (≤102). As previously reported, similar kinetics and titers of IgG were obtained after immunization with other antigens, such as HBs and EGFP,8,10 showing the adjuvant activity of the NE for multiple antigens.
FIGURE 1.
IL-17 and Th1-type splenocyte responses after immunization with diverse antigens mixed with NE adjuvant. A, Nasal immunization with NE adjuvant produces robust IgG antibody response. CD1 mice were immunized intranasally with PA mixed with either NE (PA-NE) or saline (PA-saline) or by the intramuscular injection of PA adsorbed onto alum (PA-alum). Control mice were mock immunized with NE alone (NE only), or antigen in PBS (data not shown). Results are presented as anti-PA IgG serum endpoint titers (± SD). B–D, Antigen-specific induction of IL-17, IFN-γ, and IL-4 cytokines in splenic lymphocytes. B, Cytokine expression in splenocytes shows the CD-1 mice immunized with PA. Mice immunized with PA in alum intramuscularly or intranasally with PA in saline showed no response. There is also no background cytokine response in animals exposed to either saline or NE without antigen. Statistically significant differences with p values < 0.05 were detected for all three cytokines between mice immunized with PA-NE and any other immunization groups. For the NE and alum adjuvants, the p values were 0.0037, 0.0012, and 0.031 for IL-17, INF-γ, and IL-4, respectively. C, Cytokine expression in splenocytes from CD-1 mice immunized with HBs antigen. Statistically significant differences with p values < 0.05 were detected for all three cytokines between mice immunized with HBs-NE and other immunization groups. Note that the saline control was omitted from the figure, but again showed no response. For the NE and alum adjuvants, the p values were 0.0109, 0.0272, and 0.0417 for IL-17, INF-γ, and IL-4, respectively. D, Antigen-specific induction of cytokine expression in T cells from EGFP-NE immunized mice, showing a similar pattern of response. The animals were not intramuscularly immunized with EGFP in alum due to animal use limitations. Statistically significant differences with p values < 0.05 were detected for all three cytokines between mice immunized with EGFP-NE and any other immunization groups. For the NE and PBS group, the p values were 0.024, 0.0019, and 0.031 for IL-17, INF-γ, and IL-4, respectively. Intramuscular immunizations with antigen adsorbed onto alum were performed using PA and HBs antigens (PA-alum and HBs-alum) and demonstrated no IL-17 response. Control IN immunizations with EGFP in PBS or NE alone also demonstrated no IL-17 production. To accommodate for the differences in cytokine-induction values, the representative results from two experiments are presented in the logarithmic scale as an average-fold of cytokine induction in antigen-stimulated splenocytes over nonstimulated control cultures (± SEM).
T-cell responses were examined ex vivo in splenic lymphocytes isolated from mice intranasally immunized with PA, HBs, and EGFP antigens mixed with NE adjuvant. Analysis of antigen-specific cytokine expression demonstrated strong activation of both Th1-type cytokine IFN-γ and IL-17 in response to all tested antigenic proteins in both CD-1 and BALB/c mice (Fig. 1, B, C, and D). In contrast, intramuscular immunization with alum adjuvant resulted in the production of IL-4, but weak IFN-γ and IL-17 induction compared with intranasal NE adjuvant (Fig. 1, B and C). This was compatible with the expected Th2 response to alum. Immunization with antigens in PBS or saline did not result in antigen-specific cellular responses.8,9 These results show that NE adjuvant induces both Th1 and Th17 responses to diverse antigens.
B. NE Adjuvant Enhances Antigenspecific Th17 Cells In Vivo
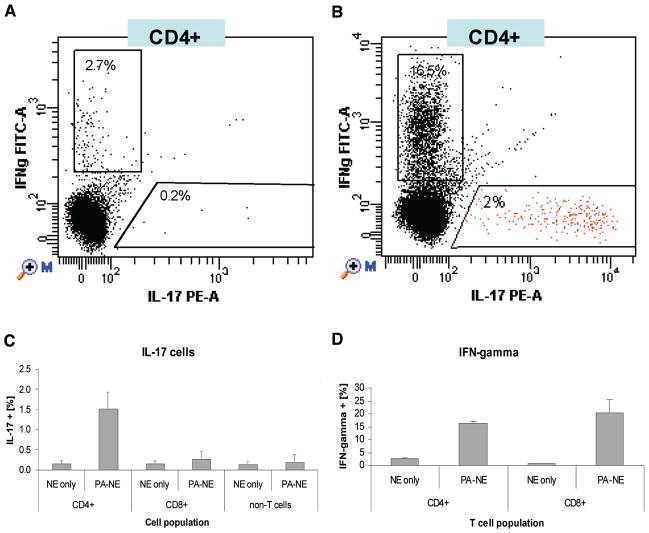
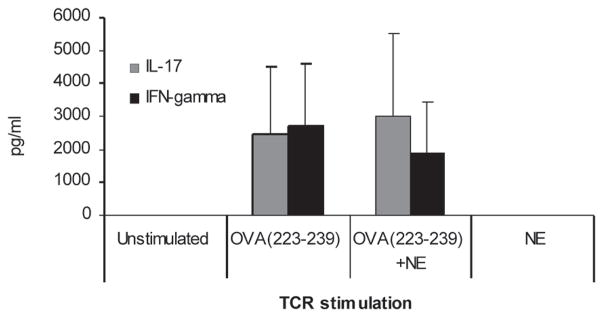
Splenocytes isolated from PA-NE-immunized mice were stimulated with PA and analyzed by flow cytometry. Intracellular cytokine staining demonstrated IL-17 staining in 2% of CD4+ T cells (Fig. 2A), as compared with 0.2% of IL-17-positive cells in PA-stimulated CD4+ T cells from mock-immunized animals (Fig. 2B). IL-17 and IFN-γ were produced by the distinct populations of CD4+ T cells (Fig. 2A and 2C), while IFN-γ was expressed by both CD4+ and CD8+ T cells (Fig. 2D). Consistent with the cytokine expression assays, there was no increase in IL-17-expressing cells in mice immunized with PA-alum (data not shown). As a control, we also assessed the nonspecific NE effect on antigen-specific T-cell activation in vitro. We examined splenic lymphocytes from DO11.10 mice that carry an MHCII (major histocompatibility complex II)-restricted OVA (ovalbumin)-specific T-cell receptor (TCRα/β) transgene. Stimulation of splenocytes with the MHC II-restricted OVA223–239 peptide induced a robust expression of IL-17 and IFN-γ, which was not affected by the presence of NE in vitro (Fig. 3). In addition, the exposure of splenocytes to NE alone did not induce production of either cytokine. These results demonstrate that IL-17 is a result of the normal activation of T cells through the TCR12 and is not an epiphenomenon of NE exposure in vitro.
FIGURE 2.
Antigen-specific induction of Th17 T cells. Intracellular staining detection of IL-17 and IFN-γ in CD4+ T cells in PA-stimulated splenocytes from C57BL/6 mice immunized intranasally in (A) control mice treated with 20% NE or (B) PA-NE immunized mice. The FACS (fluorescence-activated cell sorter) analysis is shown in two representative dot plots of gated CD4+ T cells. C, Antigen-specific induction of IL-17 expression is shown to be limited to CD4+ T cells from PA-NE immunized mice. Splenic lymphocytes obtained from three mice (per group) were stimulated in vitro with 10 μg/mL of recombinant PA protein. Intracellular IL-17 and IFN-γ expression was measured in CD4+, CD8+, and splenic non-T cells. Data, presented as means ± SD, were obtained from three animals and were only significantly different in the CD4 population (p < 0.05). In contrast, significant INFγ production is observed in both CD4 and CD8 populations after stimulation with PA (D).
FIGURE 3.
Dendritic cell-independent, TCR-dependent IL-17 expression in vitro. IL-17 expression was analyzed in splenocytes from transgenic TCR Tg DO11.10 Balb/c mice. Cells were stimulated with either a MHCII-restricted ovalbumin peptide (Ova223–239, 10 μg/mL), Ova223–239 mixed with 0.001% NE (Ova223–239 + NE), or with 0.001% NE alone. Concentrations of IL-17 and IFN–γ in cell-culture supernatants are presented in pg/mL (± SEM). No statistical difference was detected in cells treated with or without NE, suggesting the need for antigen-presenting cells to induce specific an IL17 phenotype immune response.
C. NE-Mediated Th17 Response Involves IL-6, Co-stimulatory Molecules CD80/CD86 and CD40, and Innate Receptors TLR2 and TLR4
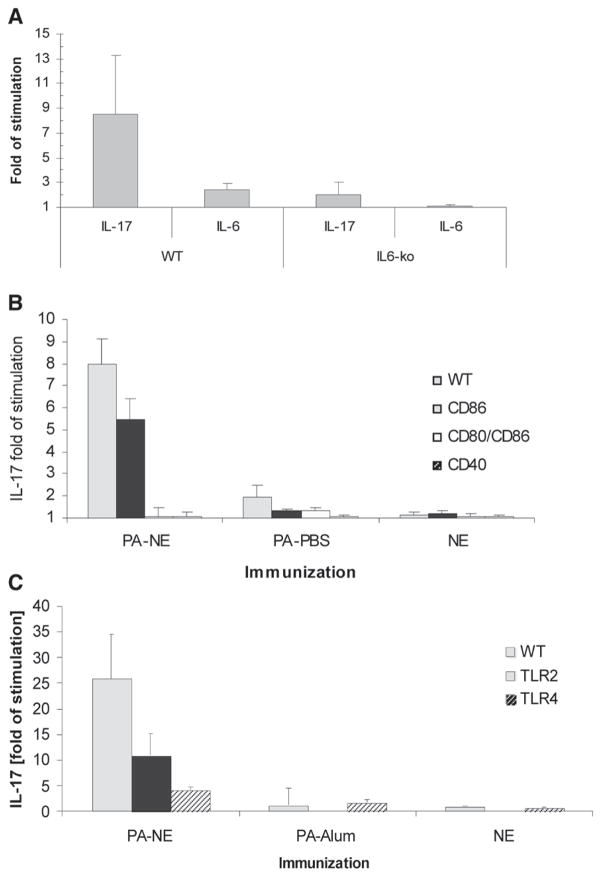
IL-6 has been identified as a critical cytokine for the development of Th17 T cells.2 To assess the role of IL-6 in NE adjuvant-induced IL-17 responses, IL-6-knockout (IL6-ko) mice and their wild-type counterparts (C57BL/6) were immunized intranasally with PA-NE. Antigen-specific induction of IL-17 expression was measured in the activated splenocytes in vitro. IL-17 production was severely diminished in splenocytes from PA-NE-immunized IL6-ko mice, while wild-type animals demonstrated robust IL-17 induction with only weak antigen-specific IL-6 expression (Fig. 4A). This suggested a central role for IL-6 in this activation process.
FIGURE 4.
IL-17 splenocyte recall response to PA in IL6-ko, CD80/86, CD40, TLR2, and TLR4 mutant mice. All mice were immunized intranasally with PA-NE, PA in PBS, or NE alone. Splenocytes were obtained at 10 to 12 weeks after primary immunization. Representative results from two experiments are presented as -fold of IL-17 cytokine induction in antigen-stimulated splenocytes over nonstimulated cultures (± SEM). Antigen-specific IL-17 expression was analyzed in a wild-type C57BL/6 and compared with IL6-ko mice (A). A statistically significant difference in IL-17 expression was detected between wild-type and IL6-ko mice, p value = 0.0305. B, CD86, double CD80/CD86, and CD40 mutant mice were analyzed for the response to PA. Statistically significant differences in IL-17 expression were detected after nasal PA-NE immunization, as wild-type animals produced significantly more IL-17 than either CD80/CD86 or DC40 mutant mice with p values of 0.006 and 0.006, respectively. No statistical difference was detected between wild-type and the single CD86 mutants (p = 0.0502). C, TLR2 and TLR4 mutant mice show differences in IL-17 expression, as wild-type mice produced much greater amounts of IL-17 after NE immunization than TLR mutant mice, with p values of 0.0278 and 0.0249 for TLR2 and TLR4, respectively. No statistical difference was detected in the PA-alum and the NE groups (C, data not shown for A and B).
The repertoire of the co-stimulatory signals necessary for Th17 immune induction is not yet known.1,13,14 We examined the role of the antigen-presenting cell (APC) co-stimulatory molecules CD80, CD86, and CD40 in the NE-mediated differentiation of IL-17-producing T cells. Mice deficient in CD86, mice double mutant for CD80/CD86, and CD40-deficient mice were intranasally immunized with PA-NE. The results demonstrated that a single, CD86-only defect resulted in only in somewhat lower antigen-stimulated IL-17 expression in splenocytes (Fig. 4B). In contrast, antigen-specific IL-17 was absent in the CD80/CD86 double mutant and in the CD40-deficient mice. This suggests that activation through CD40 and CD80/CD86 signaling is critical for NE-mediated induction of Th17 immunity.15,16
To assess the role of innate PPR receptors in the NE-mediated Th17 response, splenic lymphocytes from mice defective in either TLR2 or TLR4 were tested for antigen-specific IL-17 expression in vitro. Nasal immunization with PA-NE produced a T-cell response with a significantly diminished IL-17 expression in either TLR4- (over 5-fold) or TLR2- (approximately 2-fold) deficient mice compared with wild-type controls (Fig. 3C). No Th17 response was induced in either the TLR mutants or in the wild-type mice after intramuscular immunization with PA-alum. These data suggest that TLR2 and TLR4 may contribute to the development of the NE adjuvant-mediated antigen-specific Th17 response.17,18
IV. DISCUSSION
For more than two decades, adaptive cellular immune responses have been divided into a Th1/Th2 paradigm. Th1 immunity is characterized by CD4+ T cells secreting IL-2 and IFN-γ, and this response drives various aspects of cell-mediated immunity. In contrast, Th2 responses have CD4+ T cells producing IL-4, IL-5, and IL-13, and mediating predominantly humoral immune responses and atopy.19 In recent years, a third type of inducible T-cell immunity involving IL-17-producing CD4+ T cells (Th17 cells), has been identified as a distinct response that does not share developmental pathways with either Th1 or Th2 cells.4,20–21 While the presence of the Th17 cells is now definitive, the role of these cells in the immune response is just being clarified.
IL-17 was first described as a cytokine important in the pathogenesis of autoimmune and chronic inflammatory diseases.2,3 More recently, it has also been recognized that IL-17 is important in normal immune responses to pathogens. Many strains of bacteria, mycobacteria, and fungi induce strong Th17 responses.2,22 IL-17 has also been demonstrated to contribute to the efficacy of immune responses induced by vaccination. An IL-17-induced influx of neutrophils is important in the immune response to Heliobacter pylori,23 and is responsible for a decrease in pneumococcal colonization in response to pneumococcal vaccine.24 IL-17 induction by whole-cell pertussis vaccines is involved in cell-mediated immunity by enhancing the bactericidal activity of macrophages.25 Finally, IL-17 induction in mice vaccinated with a peptide derived from Mycobacterium tuberculosis is important for chemokines that attract CD4+/IFN-γ-producing cells.26 Together, these findings suggest an important role for IL-17 in the protection from infection, especially on mucosal surfaces.
Our studies address the role of a novel mucosal adjuvant on the development of Th17 immunity. We investigated whether NE adjuvants could induce an IL-17 response in mice, in part because most other adjuvants, such as alum, do not induce Th17 immunity. We have demonstrated antigen-specific IL-17 expression in splenocytes isolated from mice immunized intranasally with a variety of antigens mixed in NE adjuvant, while intramuscular immunization of the same antigens with alum did not induce an IL-17 response. Furthermore, in vitro IL-17 expression is related to the production of IFN-γ and not IL-4, suggesting an association with Th1 immunity. The coexistence of Th1 and Th17, as well as regulatory mechanisms involving differential IFN-γ effects on APC, was recently described in humans.27 This may be important in vaccines for which a combination of Th1 and Th17 responses may be necessary for protection from infection, potentially with pathogens such as M. tuberculosis.26
We believe that the NE adjuvant may stimulate a Th17 response independent of the source of antigen or the antigen’s molecular properties. For example, recombinant PA and EGFP are soluble monomeric proteins of 83- and a 29-kDa molecular weight, respectively, while recombinant HBs antigen is an aggregate of an HBV (hepatitis B virus) surface polypeptide and lipids and exists as 22-nm virus-like particles.28 In each case, however, NE immunization produces Th17 responses to each antigen. In addition, the induction of Th17 after NE immunization was independent of the mouse model used, as this response occurred in either outbred or different strains of inbred mice. This is surprising because mucosal immunization with NE adjuvant produces none of the local pro-inflammatory cytokines, chemokines, or metalloproteinases that are hallmarks of Th17 immunity.27,29 However, NE immunization may be unique in that it clearly induces distinct subsets of antigen-specific IL-17- and IFN-γ-producing T cells. In contrast, co-expression of IL-17 and Th1 cytokines IFN-γ, TNF-α, and/or IL-2 has been previously reported in inflammatory and autoimmune Th17 models.30–32 Populations of CD4+ T cells expressing both IFN-γ and IL-17 are also found in the gut of patients with Crohn’s disease33 and cytotoxic CD8+ T cells producing both cytokines are recruited during antigen sensitization in the skin.34 The IL-17 cytokine detected only in unique CD4+ T-cell populations suggests that the effects of NE adjuvant may be different from the Th17 response in autoimmunity, allergy, and inflammatory diseases.2,3 This is consistent with the induction of IL-17 expression in anti-CD3-activated CD4+ T cells.35–37 In other reports, IL-17 expression in Th17 cells was both TCR and antigen independent, but required IL-1 and IL-18.38 In this regard, it is important to note that exposure to NE alone does not induce IL-17 either in vitro or in vivo.
In mice, IL-6 in addition to TGF-β is required for the differentiation of T lymphocytes into Th17 cells.33,39 We have demonstrated that immunization of the IL6-ko mice did not produce a Th17 recall response, while IL-6 is produced locally in the wild-type mice mucosa in response to NE (P. Makidon, personal communication). This clearly shows that NE-induced Th17 immunity depends on IL-6 activation. Interestingly, NE treatment does not induce TGF-β in either APCs or in splenic lymphocytes in vitro, which suggests that the TGF-β signal may be generated independently from the NE.
In contrast to the rich body of research on the co-stimulatory requirements necessary for Th1/Th2 responses, the role of co-stimulation in the induction of Th17 cells is not fully understood.1,14 T-cell activation requires not only engagement of the antigen-specific TCR, but also the engagement of antigen nonspecific, co-stimulatory molecules. The co-stimulatory molecules CD80, CD86, and CD40 are extensively characterized for their role in adaptive immunity,40 but their exact function in the regulation of Th17 development remains unclear. We found that CD86-deficient mice immunized with NE adjuvant were capable of mounting Th17 immunity comparable to that of wild-type animals; however, mice lacking both CD80 and CD86 molecules did not produce IL-17 in response to NE and antigen. Consistent with our findings, the requirement for both CD80 and CD86 has been demonstrated as essential for the IL-17 response in mice immunized with keyhole limpet hemocyanin in complete Freund’s adjuvant. 36 In contrast, CD86 but not CD80 blockade in vivo led to significant IL-17 suppression in inflammatory antigen-induced arthritis.13 CD80 and CD86 share approximately 27% external domain homology and activate the bidirectional signaling by engaging either the immunostimulatory CD28 or the inhibitory CTLA-4 receptors on T cells.40,41 It has been shown that human T cells rapidly produce IL-17 in response to TCR and CD28 stimulation,29,36 and CTLA-4 blockade using antibody increased Th17 cell in patients with a metastatic melanoma.42 There are significant differences between inflammation, autoimmunity, and tumor immunity in humans and the mucosal immune response in a mouse model, and the discrepancies in these data open the question of whether there is more than one mechanism that induces the Th17 response via co-stimulation.
Recent reports underline the importance of CD40-CD40L signaling in IL-6-mediated Th17 development.14 CD40 is a member of the tumor necrosis factor receptor family that is expressed on a broad variety of cells, including dendritic cells, B cells, monocytes, endothelial cells, and epithelial cells. Induction of the immune response is mediated by bidirectional signaling via CD40 and its ligand, CD154 (CD40L), which is expressed primarily on activated CD4+ T cells.16,43 Similar to our results with mucosal NE adjuvant, the CD40-deficient mice immunized with Freund’s complete adjuvant failed to induce Th17 cells in autoimmune encephalitis.44 The lack of Th17 immunity underscores the role of CD40 in the mechanism of NE adjuvant.
TLRs comprise a signaling system that recognizes a pathogen-associated molecule pattern and plays a pivotal role in the innate and adaptive immune response.45 This study examined the role of TLR2 and TLR4 in NE-mediated Th17 immunity. Both TLR2- and TLR4-defective mice had a diminished IL-17 splenocyte recall response compared with wild-type mice; however, TLR4 deficiency appeared to have a more profound effect. A critical role of TLR4 in vaccine-induced Th17 immunity has been documented for whole cell, but not for acellular, pertussis vaccine.25 Interestingly, in a mouse autoimmune arthritis model with IL-1 receptor antagonist-knockout (IL1rn−/−), TLR4 contributed to more severe disease by modulating the Th17-cell population, while TLR2 seemed to inhibit arthritis progression through down-regulation of IFN-γ-producing Th1 cells.46 In contrast to our results, TLR2 deficiency led to increased Th17 cells infiltrates (mainly γδ T cells) in experimental Staphylococcus aureus brain abscesses47 and in a pulmonary model of fungal infection.48 Again, these differences in TLR2 effects suggest that NE-induced Th17 immunity on the respiratory surface may have distinct features from the Th17 response in inflammatory disease models.
In summary, our data document that mucosal immunization with NE adjuvant produces innate immune cell activation that helps to direct the induction of Th1 and Th17 cells. This may be important in vaccination for protective cellular immunity against intracellular pathogens, especially on mucosal surfaces.
Acknowledgments
The authors wish to thank Dr. Ilona Kryczek for her help with FACS analysis. This project has been funded in whole or in part from the Bill and Melinda Gates Foundation, under award 37868 and a subcontract from NIAID-NIH, Great Lakes Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (U54 AI57153-02).
ABBREVIATIONS
- APC
antigen-presenting cell
- EGFP
enhanced green fluorescent protein
- EU
endotoxin units
- HBs
hepatitis B surface antigen
- IgG
immunoglobulin G
- IL
interleukin
- NE
nanoemulsion
- PA
protective antigen of anthrax
- PBS
phosphate-buffered saline
- Th
helper T cell
References
- 1.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 3.Cox CA, Shi G, Yin H, Vistica BP, Wawrousek EF, Chan C-C, Gery I. Both Th1 and Th17 are immunopathogenic but differ in other key biological activities. J Immunol. 2008;180:7414–22. doi: 10.4049/jimmunol.180.11.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 5.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–7. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyaka PN, Iwakura Y, Chaplin DD. Role of IL-17 in the mucosal adjuvant activity of cholera toxin for epicutaneous vaccines. J Immunol. 2009;182:134–53. [Google Scholar]
- 7.Bielinska AU, Chepurnov AA, Landers JJ, Janczak KW, Chepurnova TS, Luker GD, Baker JR., Jr A novel, killed-virus nasal vaccinia virus vaccine. Clin Vaccine Immunol. 2008;15:348–58. doi: 10.1128/CVI.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielinska AU, Janczak KW, Landers JJ, Makidon P, Sower LE, Peterson JW, Baker JR., Jr Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge infect. Immun. 2007;75:4020–9. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielinska AU, Janczak KW, Landers JJ, Markovitz DM, Montefiori DC, Baker JR., Jr Nasal immunization with a recombinant HIV gp120 and nanoemulsion adjuvant produces Th1 polarized responses and neutralizing antibodies to primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2008 Feb;24(2):271–81. doi: 10.1089/aid.2007.0148. [DOI] [PubMed] [Google Scholar]
- 10.Makidon PE, Bielinska AU, Nigavekar SS, Janczak KW, Knowlton J, Scott AJ, Mank N, Cao Z, Rathinavelu S, Beer MR, Wilkinson JE, Blanco LP, Landers JJ, JR, Baker JR., Jr Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS ONE. 2008;3:e2954. doi: 10.1371/journal.pone.0002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myc A, Kukowska-Latallo JF, Bielinska AU, Cao P, Myc PP, Janczak K, Sturm TR, Grabinski MS, Landers JJ, Young KS, Chang J, Hamouda T, Olszewski MA, Baker JR. Development of immune response that protects mice from viral pneumonitis after a single intranasal immunization with influenza A virus and nanoemulsion. Vaccine. 2003;21:3801–14. doi: 10.1016/s0264-410x(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 12.Shimamura M, Huang Y-Y, Kobayashi M, Goji H. Altered production of immunoregulatory cytokines by invariant V{alpha}19 TCR-bearing cells dependent on the duration and intensity of TCR engagement. Int Immunol. 2009;21:179–85. doi: 10.1093/intimm/dxn136. [DOI] [PubMed] [Google Scholar]
- 13.Odobasic D, Michelle TL, Jin RX, Stephen RH. Distinct in vivo roles of CD80 and CD86 in the effector T-cell responses inducing antigen-induced arthritis. Immunology. 2008;124:503–13. doi: 10.1111/j.1365-2567.2007.02802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perona-Wright G, Jenkins SJ, O’Connor RJ, Zienkiewicz D, McSorley HJ, Maizels RM, Anderton SM, MacDonald AS. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–15. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998;161:2762–71. [PubMed] [Google Scholar]
- 16.Harnett MM. CD40: A growing cytoplasmic tale. Sci STKE. 2004:pe25. doi: 10.1126/stke.2372004pe25. [DOI] [PubMed] [Google Scholar]
- 17.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci U S A. 2007;104:17034–9. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliahmadi E, Gramlich R, Grützkau A, Hitzler M, Krüger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009 May;39(5):1221–30. doi: 10.1002/eji.200838742. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 20.Ghilardi N, Ouyang W. Targeting the development and effector functions of TH17 cells. Semin Immunol. 2007 Dec;19(6):383–93. doi: 10.1016/j.smim.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Harrington LE. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 22.Scriba TJ, Kalsdorf B, Abrahams D-A, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–70. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeLyria E, Raymond WR, Thomas GB. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 2009;136:247–56. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins SC, Jarnicki AG, Lavelle EC, Mills KHG. TLR4 mediates vaccine-induced protective cellular immunity to bordetella pertussis: Role of IL-17-producing T cells. J Immunol. 2006;177:7980–9. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 26.Khader SA. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 27.Kryczek I, Wei S, Gong W, Shu X, Szeliga W, Vatan L, Chen L, Wang G, Zou W. Cutting edge: IFN-{gamma} enables APC to promote memory Th17 and abate Th1 cell development. J Immunol. 2008;181:5842–6. doi: 10.4049/jimmunol.181.9.5842. [DOI] [PubMed] [Google Scholar]
- 28.Vassileva A, Chugh DA, Swaminathan S, Khanna N. Expression of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris using the GAP promoter. J Biotechnol. 2001 Jun 1;88(1):21–35. doi: 10.1016/s0168-1656(01)00254-1. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–46. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 31.Amadi-Obi A, Yu C-R, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WS, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron J-C, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 33.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008 Nov;20(11):1361–8. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 34.Kish DD, Li X, Fairchild RL. CD8 T cells producing IL-17 and IFN-{gamma} initiate the innate immune response required for responses to antigen skin challenge. J Immunol. 2009;182:5949–59. doi: 10.4049/jimmunol.0802830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naundorf S, Schröder M, Höflich C, Suman N, Volk HD, Grütz G. IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. Eur J Immunol. 2009 Apr;39(4):1066–77. doi: 10.1002/eji.200838773. [DOI] [PubMed] [Google Scholar]
- 36.Park H. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, Netea MG. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host & Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Lee YK, Weaver CT. TCR-independent induction of IL-17 production in TH17 Cells. Cell Host Microbe. 2009 Apr 23;5(4):329–40. [Google Scholar]
- 39.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–9. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 41.Carreno BM, Collins M. The B7 family of ligands and its receptors. New pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 42.von Euw E, Chodon T, Attar N, Jalil J, Koya R, Comin-Anduix B, Ribas A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009 May 20;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pilon C, Levast B, Meurens F, Le Vern Y, Kerboeuf D, Salmon H, Velge-Roussel F, Lebranchu Y, Baron C. CD40 engagement strongly induces CD25 expression on porcine dendritic cells and polarizes the T cell immune response toward Th1. Mol Immunol. 2009 Jan;46(3):437–47. doi: 10.1016/j.molimm.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 44.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009 Jan 20;106(3):876–81. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 46.Abdollahi-Roodsaz S, Joosten LA, Helsen MM, Walgreen B, van Lent PL, van den Bersselaar LA, Koenders MI, van den Berg WB. Shift from toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 2008 Dec;58(12):3753–64. doi: 10.1002/art.24127. [DOI] [PubMed] [Google Scholar]
- 47.Nichols JR, Aldrich AL, Mariani MM, Vidlak D, Esen N, Kielian T. TLR2 deficiency leads to increased Th17 infiltrates in experimental brain abscesses. J Immunol. 2009;182:7119–30. doi: 10.4049/jimmunol.0802656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loures FV, Pina A, Felonato M, Calich VL. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol. 2009 Jul 15;183(2):1279–90. doi: 10.4049/jimmunol.0801599. [DOI] [PubMed] [Google Scholar]