Abstract
Mice lacking the pregnancy-associated plasma protein A (PappA) gene exhibit diminished localized IGF-1 bioavailability and a 30% increase in mean life span. However, it is uncertain which tissues exhibit reduced IGF-1 signals in the PappA(−/−) mouse, and whether effects of this mutation parallel those of mutations that diminish IGF-1 in serum. Across a panel of 21 tissues, we used RT-PCR to evaluate the effects of the PappA(−/−) mutation on expression of Igfbp5, which served as an in vivo indicator of IGF-1 signaling. Among these tissues, expression of Igfbp5 was significantly reduced by PappA(−/−) only in kidney. A broader survey of IGF-associated genes in six organs identified five other genes responsive to PappA(−/−) in kidney, with stronger effects in this organ relative to other tissues. Renal expression of Irs1 and Mt1 was increased by PappA(−/−) as well as by mutations that reduce IGF-1 in serum (i.e., Ghr(−/−), Pit1(dw/dw) and Prop1(df/df)), and we demonstrate that expression of these genes is regulated by growth hormone-treatment and calorie restriction. These results provide in vivo data on an important new model of mammalian aging, and characterize both similar and contrasting expression patterns between long-lived mice with reduced local IGF-1 availability and diminished IGF-1 in serum.
Keywords: aging, dwarf, growth hormone, insulin-like growth factor, lifespan, longevity
1. Introduction
Mice that lack the gene encoding pregnancy-associated plasma protein A (PappA) exhibit a substantial increase in lifespan and a delayed rate of age-associated thymic atrophy (Conover et al., 2007; Vallejo et al., 2009). The PappA(−/−) mutation was observed to increase mouse lifespan by 22 – 43%, which is a larger effect than has been observed for any other pro-longevity mutation recently identified (Conover et al., 2007; Swindell, 2009). This strong lifespan effect arises from a narrow endocrine deficiency that involves localized reductions of IGF-1 bioavailability, without significant decline in serum GH or IGF-1 levels (Conover et al., 2007, 2008). This localized endocrine effect contrasts with other long-lived dwarf mouse models, such as the Ghr(−/−), Pit1(dw/dw) and Prop1(df/df) mice, in which circulating IGF-1 levels are diminished, along with other endocrine factors in some cases (e.g., GH, thyroid stimulating hormone, prolactin) (Berryman et al., 2008). The narrow endocrine deficiency associated with the PappA(−/−) mutation, combined with its large effect on lifespan, make the PappA(−/−) mouse an attractive model system from the standpoint of basic aging research. In particular, because the PappA(−/−) mouse lacks any known secondary endocrine deficiency resulting from reduced IGF-1 bioavailability within tissues, dissecting out the relationship between the effects of this mutation and overall lifespan may prove more tractable than is the case for other varieties of long-lived dwarf mice. Moreover, since localized IGF-1 synthesis is GH-dependent in both hepatic and extrahepatic tissues (Phillips et al., 1990), reduced IGF-1 availability within certain tissues could be a feature that PappA(−/−) mice share with long-lived mutants for which GH and/or IGF-1 levels are reduced in circulation (Conover et al., 2008).
The PappA(−/−) mutation regulates localized IGF-1 bioavailability through a mechanism involving the PAPP-A protease and its interaction with insulin-like growth factor binding protein 4 (i.e., IGFBP-4) (Boldt et al., 2007). Within an in vitro context, the PAPP-A protease has been shown to cleave IGFBP-4 in a reaction that requires the presence of either IGF-1 or IGF-2 as cofactors (Lawrence et al., 1999). Because IGFBP-4 binds IGF-1/2 and prevents binding of these IGFs to their receptors, this cleavage reaction should effectively govern the in vivo availability of IGF-1 and 2 within the local tissue environment (Boldt et al., 2007). In the PappA(−/−) mouse, therefore, absence of the PAPP-A protease is believed to promote over-accumulation of IGFBP-4, resulting in reduced bioavailability of IGF-1/2 and diminished signals downstream of IGF-1/2. This model has been supported by the observation that PappA(−/−) mice are, at birth, only 60% the body weight of wild-type littermates, consistent with reduced availability of at least IGF-2 during the course of embryonic development (Conover et al., 2004). Additionally, PappA(−/−) mice have reduced femoral bone mineral density and diminished vascular cell proliferation in response to injury, and both of these effects are consistent with reduced in vivo growth factor availability (Resch et al., 2006; Tanner et al., 2008). The effects of PappA(−/−) on lifespan also appear consistent with previous work, in worms, flies and mice, showing that depression of insulin / insulin-like signals has positive effects on aging and longevity (Berryman et al., 2008; Taguchi et al., 2008). In PappA(−/−) mice, increased survivorship is accompanied by delayed thymic atrophy with age and increased spontaneous physical activity (Conover et al., 2008; Vallejo et al., 2009). Surprisingly, however, metabolic parameters of the PappA(−/−) mice are normal in many respects, and PappA(−/−) mice do not differ from wild type littermates in terms of food intake, total or resting energy expenditure, glucose levels, insulin levels, or sensitivity to glucose and insulin (Conover et al., 2008). At present, specific pathologies influenced by the PappA(−/−) mutation are not completely established, but necropsy of older PappA(−/−) mice has revealed reduced tumor burden (Conover et al., 2007; Vallejo et al., 2009).
The influence of the PappA(−/−) mutation on IGF-1 availability and signaling may vary among cell types and organs. In some tissues, cellular IGF-1 synthesis may be weak, such that localized over-abundance of IGFBP-4 would have little influence on signals associated with activation of the IGF-1 receptor. Additionally, some cell types may express low levels of the PAPP-A protease, or the IGFBP-4 binding protein, or cofactors required for the cleavage of IGFBP-4 by PAPP-A (e.g., IGF-1 and 2). Low expression of any of these components would alter the degree to which PappA(−/−) influences IGFBP-4 over-abundance, IGF-1 availability and IGF-1 signaling. Among all tissues, therefore, the influence of the PappA(−/−) mutation may be heterogeneous, with IGF-1 signaling strongly attenuated in some tissues and weakly attenuated in others. Previous studies have shown that the PappA(−/−) mutation has in vivo effects in bone (femur), pancreas, vascular tissue, and within the thymus and associated immune cell populations (Conover et al., 2004; Tanner et al., 2008; Vallejo et al., 2009). These in vivo effects, however, may or may not reflect loss of IGF-1 signals in the tissue type examined, and could stem from modified IGF-1 signaling in unrelated tissues. Ultimately, establishing the relative effects of the PappA(−/−) mutation on IGF-1 signals across organ systems will advance this model as a tool for exploring the local effects of IGF signals, and for distinguishing between tissue types for which loss of IGF signals has favorable versus deleterious effects on aging and longevity. In the central nervous system, for example, enhanced IGF-1 action has favorable effects on neuronal survival (Chen et al., 2005; Frago et al., 2002; Serbedzija et al., 2009; Subramaniam et al., 2005), and likewise, over-expression of IGF-1 in cardiac tissue was shown to increase mouse survivorship (Li et al., 2007). In vivo effects of the PappA(−/−) mutation may thus, to some degree, be restricted to certain tissues for which loss of localized IGF-1 availability has a favorable influence on longevity. Potentially, such tissues may be similarly affected by other pro-longevity mutations that reduce GH / IGF-1 in serum (Berryman et al., 2008).
The goals of this study were to evaluate in vivo effects of the PappA(−/−) mutation on the expression of IGF-associated genes across a range of mouse tissues, and to evaluate the association between these effects and those observed in long-lived dwarf mice with reduced IGF-1 levels in serum (i.e., Ghr(−/−), Pit1(dw/dw) and Prop1(df/df) mice). With respect to 21 mouse tissues, we used RT-PCR to examine the expression of insulin-like binding protein 5 (Igfbp5) in PappA(−/−) mice and their wild type littermates. The expression of Igfbp5 is regulated by IGF-1 availability and previous studies have used expression of this gene as an indicator of IGF-1 signaling in vivo (Adamo et al., 2006; Bach et al., 1991; Backeljauw et al., 1993; Bartlett et al., 1991; Bondy et al., 1993; Duan et al., 1999; Harrington et al., 2007; Resch et al., 2006; Tanner et al., 2008; Ye et al., 1998). A more comprehensive evaluation of genes encoding IGF system components was also carried out with respect to six major organs (kidney, liver, heart, muscle, lung and neocortex), and in these organs we evaluate whether significant effects of PappA(−/−) on gene expression are also associated with the Ghr(−/−), Pit1(dw/dw) and Prop1(df/df) mutations. These results provide in vivo data on an important model of mouse aging and longevity, and identify gene expression patterns associated with diminished IGF-1 in tissues, in serum, or in both serum and the local tissue environment.
2. Materials and methods
2.1. Mice
All PappA(−/−) and Pit1(dw/dw) mice used in this study were generated from breeding colonies at the University of Michigan, while all Ghr(−/−) and Prop1(df/df) mice were generated from colonies at Southern Illinois University. The colony of PappA(−/−) mice at University of Michigan was initiated from a breeding pair generously provided by Dr. Cheryl A. Conover. The generation of these PappA(−/−) mice using targeted disruption of the PappA gene (exon 4) has been described previously (Conover et al., 2004). The PappA(−/−) and Pit1(dw/dw) mutations were generated on mixed C57BL/6-129SV/E and DW/J Pit1dw × C3H/HeJ Pit1dw-J genetic backgrounds, respectively. The Ghr(−/−) mutation was generated on a mixed background generated by crossing mice of 1290la, BALB/c, C57BL/6 and C3H strains, as described by Panici et al. (2009). The Prop1(df/df) mice are also maintained on a heterogeneous genetic background (Dhahbi et al., 2007). Genotypes of PappA(−/−) mice and wild-type littermates were confirmed by PCR analysis of DNA extracted from tail snip biopsies at the time of euthanasia. All experimental animals were raised in specific-pathogen free facilities and provided ad libitum access to water. The pathogen-free status of colonies was verified by exposing sentinel mice to bedding and evaluating sentinels for antibodies and parasites, with no positive test results obtained during the study period. All procedures were approved by animal care and use boards at University of Michigan and Southern Illinois University.
2.2. Total RNA extraction and RT-PCR
Tissues were dissected from euthanized mice and immediately flash-frozen in liquid nitrogen or submerged in RNAlater solution (Ambion cat. no. 7020). Tissues stored in RNAlater were placed at 4°C for at least 24 hours prior to extraction of total RNA (or extended storage at −20°C). Flash-frozen tissues were initially stored at −80°C and later transitioned to −20°C with incubation in RNAlater ICE solution (Ambion cat. no. AM7030). Immediately prior to extraction of total RNA, tissues were placed in small vessels containing RLT buffer and homogenized using a rotor-stator apparatus. The extraction of RNA was then carried out using columns provided in Qiagen RNeasy kits (Qiagen cat. no. 74104), with on-column DNase digestion to limit the possibility of DNA contamination (Qiagen cat. no. 79254). Extracted RNA was diluted in 60 – 100 µL of RNAase-free water and quantification was carried out by dispensing 2 µL of extracted RNA onto the optical pedestal of a NanoDrop spectrophotometer. Working RNA dilutions of 2 – 4 ng/µl were later prepared for use in one-step RT-PCR reactions. All reactions were set up on ice in 0.1 mL strip tubes at a volume of 20 µl with 4 – 8 ng of template RNA per reaction. Master and RT mix reagents used in RT-PCR reactions were obtained from Quantitect SYBR Green RT-PCR kits (Qiagen cat. no. 204243). Thermocycling was performed using the Rotor-Gene 3000 (Corbett) with samples arranged in a 72-well block that rotates throughout the reaction procedure, which limits block effects and ensures uniformity of temperature among samples. Melting curve analyses were performed following the completion of each run and we inspected log-derivative plots to ensure specific amplification of target and reference genes. All genes evaluated in this analysis were amplified using bioinformatically validated primer assays (Qiagen; see Supplemental Data File).
2.4. GH-treatment of ad lib and CR-fed mice
Mice of the (Ames) Prop1(df/df) genotype and their wild-type littermates (approximately 7 months of age) were maintained on a caloric restriction (CR) or ad lib diet and treated with daily injections of growth hormone (GH) or saline. The caloric restriction regime corresponded to a 30% decrease in calories relative to the ad lib-fed group and was maintained for a period of 8 weeks. The GH-treatments were carried out over a six week period at a dose of 4 µg/gram of body weight per day (porcine GH, Sigma, St. Louis, MO). Over the six week treatment period, we observed an average body weight increase in GH-treated Prop1(df/df) mice of 30%, with an average increase of only 5% in saline-treated Prop1 (df/df) mice. Additionally, we observed significant responses in blood chemistry measures during the treatment period (insulin, IGF-1, glucose, triglycerides, FFA; data not shown).
2.4. Statistical Analysis
The ratio of gene expression in mutant mice relative to wild-type littermates was calculated using the ΔΔCt method with expression of either 18S ribosomal RNA (Rn18s) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as an internal reference gene. All experiments were based upon at least 6 biological replicates per treatment, with 2–3 technical replicates performed for each biological replicate. For each biological replicate, ΔCt values were calculated as the difference in the mean Ct value obtained for the target gene and the mean Ct value obtained for the reference gene. Statistical analyses were based upon ΔCt values, corresponding to relative expression on a logarithmic scale. For two-treatment comparisons involving mutant and wild-type littermates, a two-sample t-test (two-sided) was used to evaluate whether the average ΔCt value among mutant mice differed significantly from the average ΔCt value among wild-type littermates. In a small number of cases, one or more outlying observations was present, and we applied a non-parametric Kolmogorov-Smirnov test, which evaluates the null hypothesis that ΔCt values associated with experimental and control mice are drawn from the same continuous distribution.
For experiments evaluating expression of insulin receptor substrate 1 (Irs1), metallothionein 1 (Mt1) and insulin-like growth factor binding protein acid labile subunit (Igfals) in response to GH-treatment within ad lib and CR-fed mice, five sets of RT-PCR reactions were performed, where each set included target and reference gene reactions for n = 2 mice from each of the eight experimental groups. We calculated ΔCt values for each set of reactions, and adjusted these ΔCt values to have a mean of zero in each of the five sets (n = 10 per treatment in total). A three-factor analysis of variance was applied to adjusted ΔCt values, with genotype (Prop1(df/df) or wild-type), diet (ad lib or CR) and treatment (GH or saline-injections) as fixed effects. Post-hoc comparisons among treatments were performed using the Tukey honest significant difference method, or Dunnett's approach for multiple comparisons to a common control treatment.
3. Results
3.1. In vivo effects of PappA(−/−) on the expression of IGF-associated genes
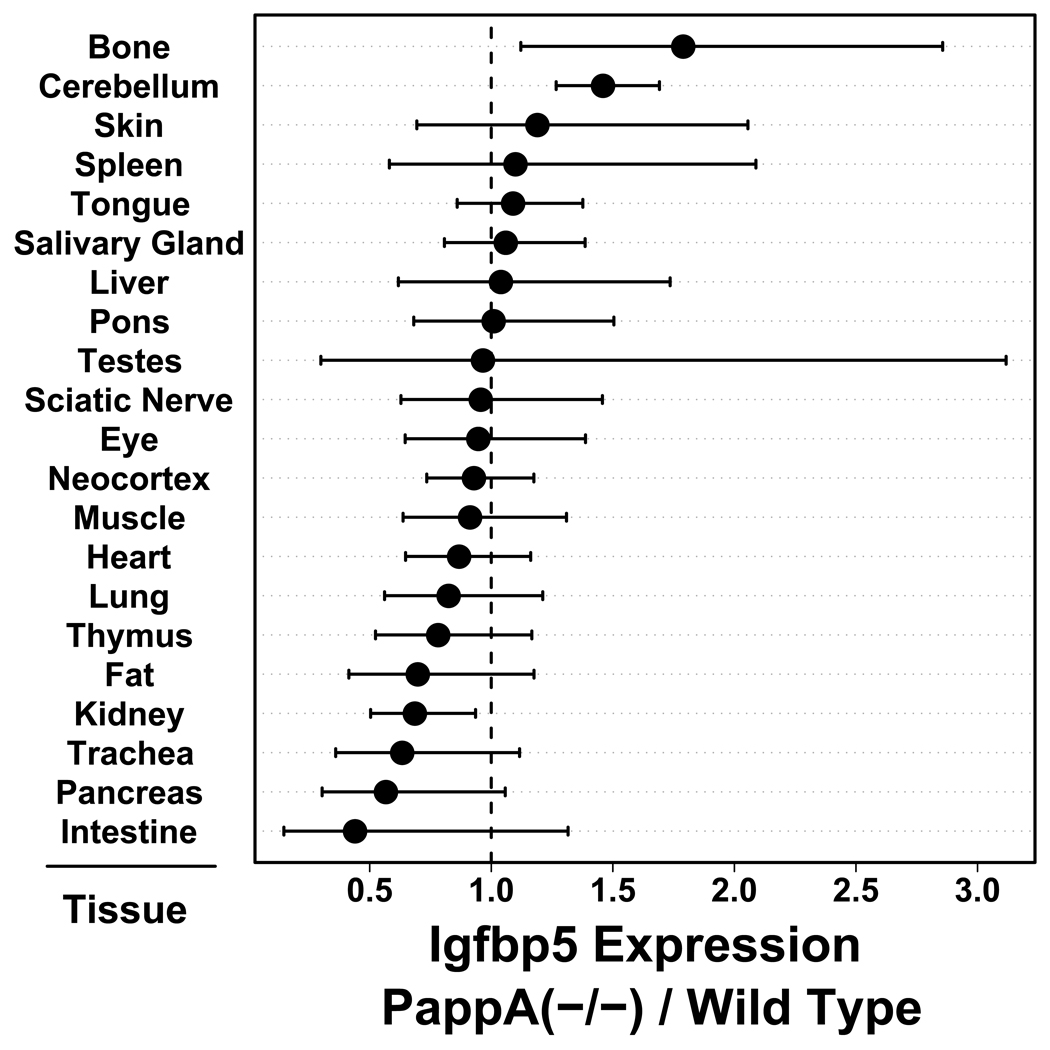
The expression of Igfbp5 is positively regulated by IGF-1 signals and Igfbp5 expression has previously been used as an in vivo indicator of IGF-1 signaling (Adamo et al., 2006; Bach et al., 1991; Backeljauw et al., 1993; Bartlett et al., 1991; Bondy et al., 1993; Duan et al., 1999; Harrington et al., 2007; Resch et al., 2006; Tanner et al., 2008; Ye et al., 1998). To identify tissues in which IGF-1 signals may be attenuated, therefore, we evaluated the expression of Igfbp5 in PappA(−/−) mice and wild type littermates across a panel of 21 tissues (males, 3 – 6 months of age, n = 6 per treatment). It was expected that Igfbp5 expression would be reduced in PappA(−/−) mice relative to wild-type littermates, and for 13 of the 21 tissues, Igfbp5 expression was indeed lower among PappA(−/−) mice relative to controls (Fig. 1) (P = 0.047; sign-test with one-sided alternative hypothesis). However, in most cases, effects of PappA(−/−) were weak, and on average among all tissues, the average reduction of Igfbp5 expression was only 5%. Additionally, the influence of PappA(−/−) on Igfbp5 expression was heterogeneous among tissues, and in contrast to the expectation, Igfbp5 expression was increased in bone (P = 0.022) and cerebellum (P < 0.001) (Fig. 1). The expected decrease in Igfbp5 expression was only observed to a significant degree in kidney (P = 0.024), although the expected trend was also evident in intestine (P = 0.125), pancreas (P = 0.070) and trachea (P = 0.101) (Fig. 1). The effect of PappA(−/−) on Igfbp5 expression thus varied continuously and was heterogeneous among tissues, but was consistent with localized reduction of IGF-1 signals in the kidney of PappA(−/−) mice.
Figure 1.
Insulin-like growth factor binding protein 5 (Igfbp5) expression in 21 tissues of PappA(−/−) mice and wild type littermates. The chart shows 95% confidence intervals associated with the estimated ratio of expression in PappA(−/−) mice relative to wild type littermates (n = 6 mice per tissue; RT-PCR). Estimates larger than one indicate increased expression of Igfbp5 in PappA(−/−) mice relative to controls, while estimates less than one indicate decreased Igfbp5 expression in PappA(−/−) mice relative to controls. Significant effects are indicated by confidence intervals that do not overlap with the dotted vertical line, which corresponds to an estimated ratio of one (i.e., equal expression in PappA(−/−) and control mice). Calculations were performed using the ΔΔCt method and expression of either 18S ribosomal RNA (Rn18s) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal reference gene.
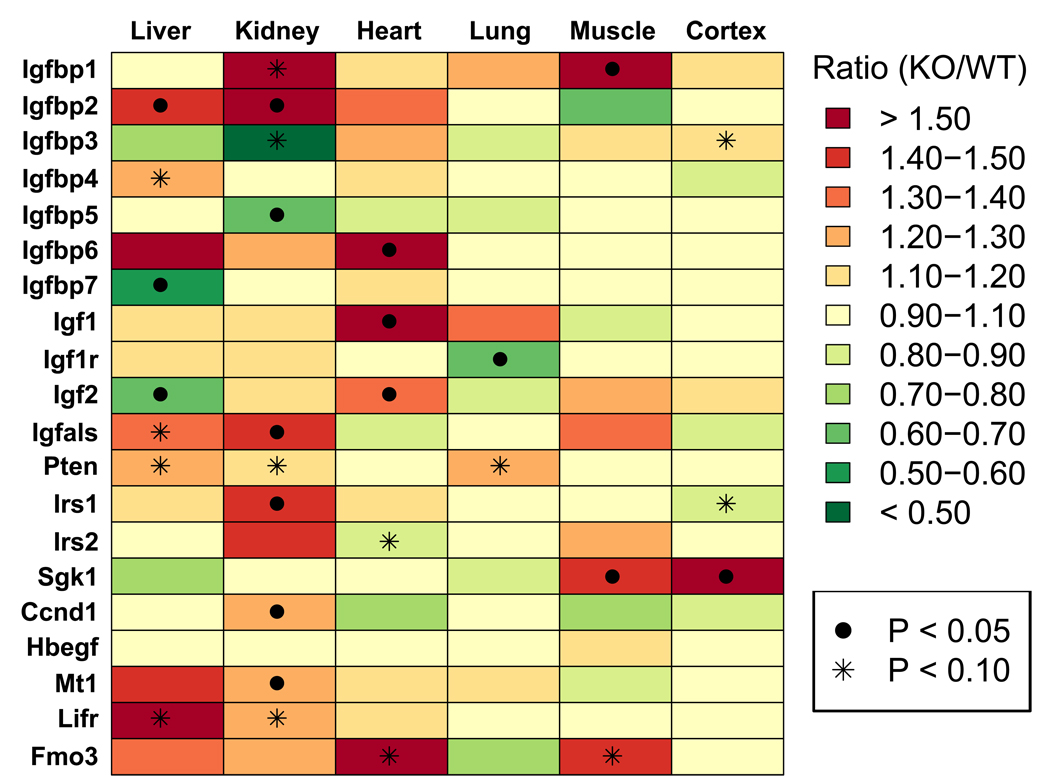
The effects of PappA(−/−) on the expression of other IGF-associated genes was evaluated in six major organ types (i.e., kidney, liver, muscle, heart, neocortex and lung). We examined genes that encoded components of the IGF-1 system (i.e., Igfbp1, Igfbp2, Igfbp3, Igfbp4, Igfbp5, Igfbp6, Igfbp7, Igf1, Igf1r, Igf2, Irs1, Irs2, Igfals, Pten), as well as genes that do not encode components of the IGF-1 system, but were viewed as possible targets of the IGF-1 pathway (i.e., Sgk1, Ccnd1, Hbegf, Mt1, Lifr and Fmo3) (see Supplemental Data File 1). These analyses again suggested that IGF-1 signals were most strongly perturbed with respect to the PappA(−/−) kidney (Fig. 2). In kidney, PappA(−/−) significantly increased the expression of Igfbp2, Igfals, Irs1, Mt1, and Ccnd1, and had marginally significant effects on the expression of several other genes (i.e., Igfbp1, Igfbp3, Pten and Lifr) (Fig. 2). Relative to kidney, effects of PappA(−/−) on gene expression were weaker in other tissues. In cardiac tissue, PappA(−/−) significantly increased expression of Igf1, Igf2 and Igfbp6, while in liver, PappA(−/−) increased expression of Igfbp2 and decreased expression of Igfbp7 and Igf2 (Fig. 2). In muscle, PappA(−/−) increased expression of Igfbp1 and Sgk1 (Fig. 2), and with respect to lung and neocortex, only one examined gene was significantly altered by the PappA(−/−) mutation (Fig. 2). Taken together, these data are consistent with the pattern of Igfbp5 expression and point to the kidney as an organ in which the PappA(−/−) mutation has strong effects relative to other tissues.
Figure 2.
Relative expression of IGF-associated genes in PappA(−/−) mice and wild-type littermates with respect to six organs (liver, heart, kidney, muscle, lung and neocortex) (RT-PCR). Colors correspond to the estimated ratio of expression in PappA(−/−) relative to wild type littermates (n = 6 mice per treatment). Red colors correspond to increased expression in PappA(−/−) mice relative to controls, while green colors correspond to decreased expression in PappA(−/−) mice relative to controls (see color chart). Significant effects of the PappA(−/−) mutation are indicated by the ● symbol (P < 0.05; two-sample t-test or rank-sum test). Marginally significant effects of the PappA(−/−) mutation are indicated by the * symbol (0.05 < P < 0.10; two-sample t-test or rank-sum test). Calculations were performed using the ΔΔCt method with expression of either 18S ribosomal RNA (Rn18s) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) used as an internal reference gene. The full gene name associated with each gene symbol is provided in Supplemental Data File 1.
3.2. Relationship between PappA(−/−) and mutations that reduce IGF-1 in serum
A previous analysis of microarray experiments identified genes for which expression in liver tissue was altered, in a similar direction, by multiple mutations that promote dwarfism and longevity, including the (GHRKO) Ghr(−/−) and (Snell) Pit1(dw/dw) mutations (Swindell, 2007). Both of these mutations are associated with decreased IGF-1 in serum (Berryman et al., 2008). In the case of Ghr(−/−), decreased IGF-1 in circulation is due to GH-insensitivity (Coschigano et al., 2003), and with respect to Pit1(dw/dw), serum IGF-1 levels are diminished as a secondary consequence of reduced GH levels (Flurkey et al., 2001). In both mutants, low serum IGF-1 reflects decreased synthesis of IGF-1 in liver, with localized reduction of IGF-1 within at least some liver cells possessing endocrine function. We therefore considered whether genes characteristically altered in liver tissue of Ghr(−/−) and Pit1(dw/dw) mice were correspondingly influenced by the PappA(−/−) mutation (Table 1). The genes examined included some encoding IGF-system components (e.g., Igf1, Igfals), along with others for which an association to GH / IGF-1 signals was primarily based upon prior whole-genome array data (e.g., Lifr, Keg1, Fmo3) (Swindell, 2007). Our RT-PCR analyses confirmed, for nearly all genes, the expected expression patterns for selected genes in Ghr(−/−) and Pit1(dw/dw) mice (Table 1) (Swindell, 2007). However, we observed little or no association between these expression patterns and those from PappA(−/−) mice. In 4 of 12 cases, PappA(−/−) had a marginally significant effect on hepatic expression, but in three of these cases (Igfals, Lifr, Hao3) the weak effect of PappA(−/−) was opposite to that associated with the Ghr(−/−) and Pit1(dw/dw) mutations. The one similar effect among all three mutations was decreased expression of kidney expressed gene 1 (Keg1), which was markedly observed in Ghr(−/−) and Pit1(dw/dw) mice (P < 0.001; Table 1), and observed to a much lesser degree in PappA(−/−) mice (P = 0.062, two-sided t-test; P = 0.031, one-sided t-test; Table 1).
Table 1.
Hepatic gene expression patterns in PappA(−/−), Ghr(−/−) and Pit1(dw/dw) mice relative to wild-type controls. RT-PCR was used to evaluate relative expression of genes associated with loss of GH / IGF-1 signals, which had been identified in a prior meta-analysis of microarray experiments (Swindell, 2007). The table lists estimated ratios of expression in mutant mice relative to wild-type littermates (n = 6 per mice treatment), with values larger than one indicating increased expression in mutants. Calculations were performed using the ΔΔCt method with expression of 18S ribosomal RNA (Rn18s) as an internal reference gene. P-values in parentheses are based upon results from two-sample t-tests applied to the estimated ΔCt values. Significant results are shown in bold-faced type. The full gene name associated with each gene symbol is provided in Supplemental Data File 1.
| Gene |
PappA(−/−) / WT (P-value) |
Ghr(−/−) / WT (P-Value) |
Pit1(dw/dw) / WT (P-Value) |
|---|---|---|---|
| Igf1 | 1.16 (0.319) | 0.01 (< 0.001) | 0.02 (< 0.001) |
| Igfals | 1.35 (0.073) | 0.03 (< 0.001) | 0.04 (< 0.001) |
| Socs2 | 0.96 (0.927) | 0.25 (0.002) | 0.12 (< 0.001) |
| Lifr | 1.59 (0.070) | 0.74 (0.147) | 0.32 (< 0.001) |
| Egfr | 0.87 (0.358) | 0.11 (< 0.001) | 0.08 (< 0.001) |
| Keg1 | 0.76 (0.062) | 0.03 (< 0.001) | 0.05 (< 0.001) |
| Mup3 | 1.08 (0.627) | 0.09 (< 0.001) | 0.00 (< 0.001) |
| Sult2a2 | 0.92 (0.686) | 2070 (< 0.001) | 696 (< 0.001) |
| Spink3 | 1.92 (0.224) | 104 (< 0.001) | 187 (< 0.001) |
| Fmo3 | 1.34 (0.401) | 28.0 (< 0.001) | 921 (< 0.001) |
| Mt1 | 1.42 (0.451) | 4.91 (0.071) | 30.1 (< 0.001) |
| Mt2 | 1.12 (0.818) | 7.46 (0.080) | 40.3 (< 0.001) |
| Hao3 | 0.59 (0.069) | 8.84 (0.003) | 18.9 (< 0.001) |
| Cyp2a4 | 0.937 (0.795) | 5.27 (< 0.001) | 2.74 (< 0.001) |
Our evaluation of kidney, liver, heart, muscle, neocortex and lung in PappA(−/−) mice identified 16 significant effects of the PappA(−/−) mutation (Fig. 2), and we evaluated whether these effects were shared by the Ghr(−/−) and Pit1(dw/dw) mutations (Table 2). In heart, lung, muscle and neocortex, genes for which expression was altered in PappA(−/−) mice were not similarly altered Ghr(−/−) or Pit1(dw/dw) mutants (Table 2). In muscle, PappA(−/−) increased expression of Sgk1 (P = 0.028), while the opposite effect was observed in Pit1(dw/dw) mice (P =0.041). In cardiac tissue, PappA(−/−) and Pit1(dw/dw) had similar effects on the expression of Igf2 (P < 0.026), but contrasting effects on the expression of Igf1 (P < 0.045), with no significant effect of the Ghr(−/−) mutation on expression of either Igf1 or Igf2 (P > 0.393) (Table 2). Interestingly, while both PappA(−/−) and Pit1(dw/dw) had similar effects on Igf2 expression in heart, these mutations had contrasting effects on Igf2 expression in liver, with PappA(−/−) decreasing hepatic Igf2 expression and Pit1(dw/dw) increasing Igf2 expression (Table 2). All three mutations, PappA(−/−), Girl(−/−) and Pit1(dw/dw), increased Igfbp2 expression in liver, although the effects of Ghr(−/−) were marginally significant (P = 0.051) (Table 2).
Table 2.
Comparison between significant effects of the PappA(−/−) mutation in six organs and gene expression patterns in Ghr(−/−) and Pit1(dw/dw) mice. Our analysis of IGF-associated genes in six organs from the PappA(−/−) mouse identified 16 significant effects of the PappA(−/−) mutation. For each gene and tissue in which a significant effect was found, we evaluated effects of the Ghr(−/−) and Pit1(dw/dw) mutations. The table lists estimated ratios of expression in mutant mice relative to wild-type littermates (n = 6 per mice treatment), with values larger than one indicating increased expression in mutants. Calculations were performed using the ΔΔCt method with expression of either 18S ribosomal RNA (Rn18s) or glyceraldehyde-3-phosphate dehydrogenase (Gapdh) used as an internal reference gene. Unless otherwise indicated, p-values in parentheses are based upon results from two-sample t-tests applied to the estimated ΔCt values. Significant results are shown in bold-faced type. The full gene name associated with each gene symbol is provided in Supplemental Data File 1.
| Tissue | Gene |
PappA(−/−) / WT (P-value) |
Ghr(−/−) / WT (P-Value) |
Pit1(dw/dw) / WT (P-Value) |
|---|---|---|---|---|
| Kidney | Igfbp2 | 1.65 (0.026†) | 1.76 (0.114) | 2.66 (0.213) |
| Kidney | Igfbp5 | 0.69 (0.024) | 0.78 (0.311) | 1.19 (0.389) |
| Kidney | Igfals | 1.46 (0.001) | 0.29 (< 0.001) | 0.16 (< 0.001) |
| Kidney | Irs1 | 1.42 (0.020) | 1.90 (0.004) | 3.23 (< 0.001) |
| Kidney | Ccnd1 | 1.23 (0.017) | 0.80 (0.154) | 0.69 (0.275) |
| Kidney | Mt1 | 1.23 (0.040) | 1.69 (0.002) | 2.72 (0.024) |
| Liver | Igf2 | 0.64 (0.021) | 1.57 (0.129) | 6.76 (< 0.001) |
| Liver | Igfbp2 | 1.48 (0.026†) | 3.65 (0.051) | 6.24 (< 0.001) |
| Liver | Igfbp7 | 0.57 (0.032) | 1.51 (0.022) | 0.74 (0.095) |
| Heart | Igfbp6 | 1.77 (0.003) | 1.53 (0.061) | 1.17 (0.548) |
| Heart | Igf1 | 1.62 (0.009) | 0.97 (0.932) | 0.37 (0.045) |
| Heart | Igf2 | 1.40 (0.026†) | 0.76 (0.393) | 2.66 (0.009) |
| Lung | Igf1r | 0.66 (0.011) | 0.90 (0.776) | 2.21 (< 0.001) |
| Muscle | Igfbp1 | 1.54 (0.003) | 0.99 (0.954) | 0.75 (0.575) |
| Muscle | Sgk1 | 1.47 (0.028) | 0.88 (0.638) | 0.55 (0.041) |
| Cortex | Sgk1 | 1.57 (0.038) | 0.80 (0.154) | 1.09 (0.583) |
P-value generated from non-parametric Kolmogorov-Smirnov test
The strongest correspondence among the three mutations was observed with respect to certain genes for which expression was modified by PappA(−/−) in kidney (Table 2). In this organ, the expression of Mt1 and Irs1 was increased by PappA(−/−), Ghr(−/−) and Pit1(dw/dw) (P < 0.04), and each of these mutations also tended to increase renal Igfbp2 expression as well (P < 0.213). In contrast to these points of consistency, however, PappA(−/−) increased expression of Igfals in kidney (P < 0.001), while both Ghr(−/−) and Pit1(dw/dw) decreased expression of this gene (P < 0.001).
3.3. Transcriptional regulation of Irs1, Mt1 and Igfals in kidney by circulating GH / IGF-1 levels
Comparison of expression patterns among long-lived mutants revealed that, in kidney, expression of insulin receptor substrate 1 (Irs1), metallothionein 1 (Mt1) and insulin-like growth factor acid labile subunit (Igfals) is altered by each of three mutations affecting GH / IGF-1 signaling (i.e., PappA(−/−), Ghr(−/−) and Pit1(dw/dw)). This suggested that expression of these genes was regulated by local IGF-1 availability or plasma GH / IGF-1 levels. To further evaluate the role of GH / IGF-1 in transcriptional regulation, we treated GH-deficient (Ames)Prop1(df/df) and wild-type littermates with GH or saline for a period of six weeks, and evaluated expression of Irs1, Mt1 and Igfals in kidney. It was expected that expression of these genes would be altered in Prop1(df/df) mice relative to wild-type littermates, and that such effects of Prop1(df/df) would be countered in GH-treated Prop1(df/df) mice. The experiment was carried out using both ad lib mice and mice provided a calorie restriction (CR) diet (6 weeks; 30% CR), because CR reduces serum IGF-1 in mice and also because effects of the Prop1(df/df) mutation on longevity are diet-dependent (Garcia et al., 2008). Additionally, previous analyses of whole-genome expression data have indicated that expression of Mt1 is sensitive to CR in several organs (Swindell, 2008a).
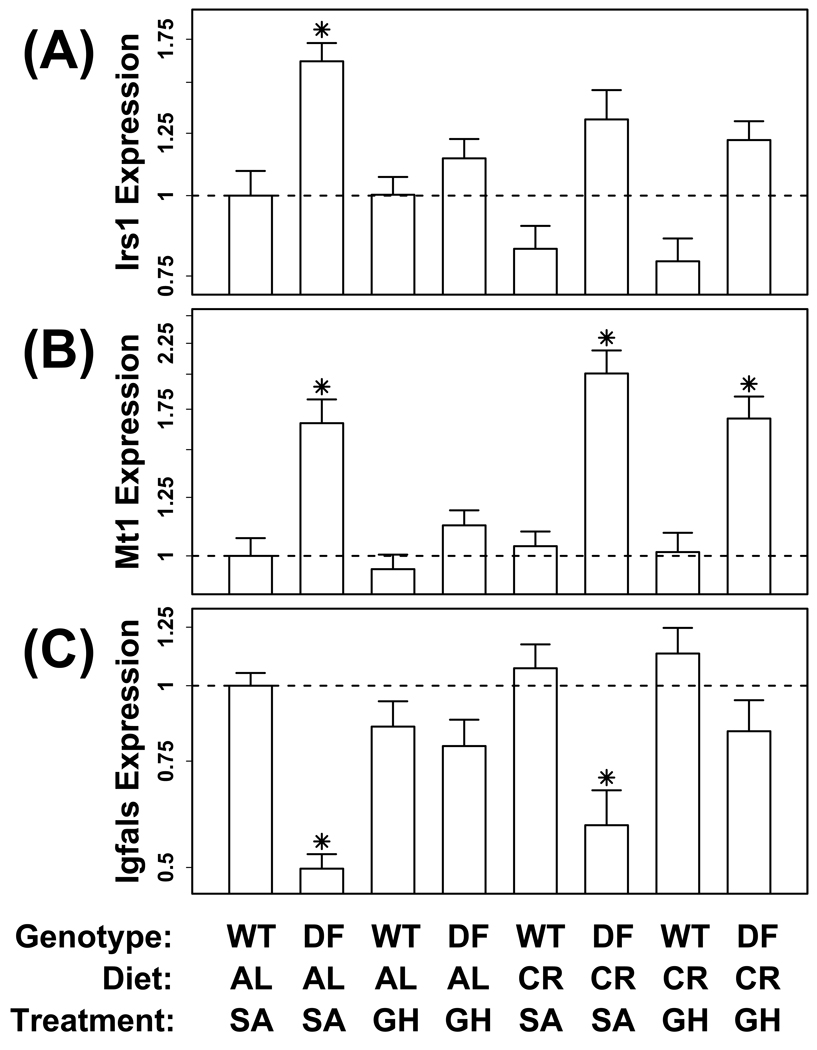
Renal expression of Irs1 was regulated by circulating GH / IGF-1 levels in ad lib fed Prop1(df/df) mice, as expected, but was also decreased by CR, and the effect of CR interfered with the influence of GH-treatment on Irs1 expression (Fig. 3A). Our analysis of long-lived mutants had indicated that expression of Irs1 was significantly increased in kidney by the PappA(−/−), Ghr(−/−) and Pit1(dw/dw) mutations (P ≤ 0.02) (Table 2). Consistent with these results, Irs1 expression was also significantly increased in kidney tissue from ad lib-fed Prop1(df/df) mice (P < 0.001; Dunnett post-hoc comparison) (Fig. 3A). Following GH-treatment of Prop1(df/df) mice on the ad lib diet, expression of Irs1 was decreased and comparable to that observed in wild-type mice (P = 0.002, two-sample t-test; P = 0.051, Tukey post-hoc comparison) (Fig. 3A). Interestingly, in contrast to observed effects of GH / IGF-1 mutations, the effect of CR was to decrease Irs1 expression (P = 0.007; analysis of variance; see Table 3). Additionally, whereas GH-treatment of ad lib fed mice reduced Irs1 expression, this effect was not observed in CR-fed mice (P = 0.562; two-sample t-test) (Fig. 3A). Overall, no significant diet × treatment interaction was observed (P = 0.319; Table 3), although this interaction effect was marginally significant when analyses were limited to the Prop1(df/df) mice (P = 0.09).
Figure 3.
Transcriptional regulation of insulin receptor substrate 1 (Irs1), metallothionein 1 (Mt1) and insulin-like growth factor binding protein acid labile subunit (Igfals) by growth hormone (GH) and/or calorie restriction (CR) in kidney. GH-deficient (Ames) Prop1(df/df) and wild-type mice were injected with either GH or saline for a six-week period. The experiment was performed with mice maintained on either a ad lib or 30% CR diet. The chart shows the relative expression of each gene in each of the eight experimental treatments (n = 10 mice per treatment). The asterisk (*) symbol is used to denote treatments that differ significantly from the baseline treatment (i.e., ad lib fed wild-type mice; P < 0.05, Dunnett post-hoc comparison). Calculations were performed using the ΔΔCt method with expression of 18S ribosomal RNA (Rn18s) used as an internal reference gene.
Table 3.
Regulation of Irs1, Mt1 and Igfals in kidney by growth hormone (GH) and/or calorie restriction (CR). The tables lists p-values generated from an analysis of variance applied to the expression data shown in Figure 3. The analysis of variance included three factors, including genotype (Prop1(df/df) or wild-type), diet (CR or ad lib) and treatment (GH or saline injection). For each of the three genes examined, the three-way interaction between genotype, diet and treatment was non-significant, and was therefore excluded from each model. Significant p-values are shown in bold-faced type.
| Effect | P-value (Irs1) | P-Value (Mt1) | P-Value (Igfals) |
|---|---|---|---|
| Genotype (G) | < 0.001 | < 0.001 | < 0.001 |
| Diet (D) | 0.007 | 0.907 | 0.219 |
| Treatment (T) | 0.430 | 0.275 | 0.525 |
| G*D | 0.208 | 0.019 | 0.654 |
| G*T | 0.096 | 0.020 | 0.001 |
| D*T | 0.319 | 0.236 | 0.708 |
The expression of Mt1 was also regulated by circulating GH / IGF-1 levels in ad lib fed Prop1(df/df) mice (Fig. 3B). Interestingly, Mt1 expression was increased by CR only in GH-injected Prop1(df/df) mice, and CR also interfered with the influence of GH-treatment on Mt1 expression in Prop1(df/df) mice (Fig. 3B). Among mutations we examined, expression of Mt1 in kidney was increased by PappA(−/−), Ghr(−/−) and Pit1(dw/dw) (Table 2), and this was also true of the Prop1(df/df) mutation (P < 0.001; Dunnett post-hoc comparison) (Fig. 3B). Expression of Mt1 was GH-sensitive in ad lib-fed Prop1(df/df) mice, and decreased significantly following GH-treatment (P = 0.007, Tukey post-hoc comparison) (Fig. 3B). However, the effect of GH-treatment was weaker and non-significant in CR-fed mice (P = 0.174, two-sample t-test) (Fig. 3B). There was a significant diet × treatment interaction effect, with CR having no significant effect in wild-type mice, but increasing Mt1 expression in Prop1(df/df) mice. For instance, CR increased Mt1 expression of in GH-treated Prop1(df/df) mice (P = 0.004, Tukey post-hoc comparison), but this effect was not observed in GH-treated wild-type mice (P = 0.489, two-sample t-test; P = 0.998, Tukey post-hoc comparison). Across the same treatment groups, Mt2 exhibited a very similar pattern of gene expression (data not shown), which was expected because Mt1 and Mt2 are co-expressed in most tissues (Swindell, 2008b).
The expression of Igfals in kidney was regulated by circulating GH / IGF-1 levels in ad lib fed Prop1(df/df) mice, but there was little effect of CR on expression of this gene (Fig. 3C). Both Ghr(−/−) and Pit1(dw/dw) significantly decreased Igfals expression, while the PappA(−/−) mutation had an opposite effect (Table 2). Consistent with effects of Ghr(−/−) and Pit1(dw/dw), expression of Igfals was decreased in Prop1(df/df) mice (P < 0.001; Tukey post-hoc comparison) (Fig. 3C). This appeared to be a consequence of circulating GH levels, since GH-treatment restored normal levels of Igfals expression in Prop1(df/df) mice (P = 0.021; Tukey post-hoc test). As with Irs1 and Mt1, this effect was weakened in CR-fed mice, but was nonetheless marginally significant (P = 0.060, two-sample t-test; 0.163, Tukey post-hoc comparison) (Fig. 3C).
4. Discussion
Mice lacking the pregnancy associated plasma protein A (PappA) gene exhibit a considerable increase in lifespan that arises from a narrow endocrine deficiency involving localized reductions of IGF-1 availability. These characteristics make the PappA(−/−) mouse an important model for the study of mammalian aging and a valuable tool for exploring local effects of IGF-1 availability independently of GH / IGF-1 levels in serum. This study provided an in vivo characterization of IGF-associated gene expression patterns in multiple tissues from PappA(−/−) mice and wild-type littermates. Among 21 mouse tissues, PappA(−/−) had weak and variable effects on the expression of insulin-like growth factor binding protein 5 (Igfbp5), which served as an in vivo indicator of IGF-1 signals in our analysis (Adamo et al., 2006; Bach et al., 1991; Backeljauw et al., 1993; Bartlett et al., 1991; Bondy et al., 1993; Duan et al., 1999; Harrington et al., 2007; Resch et al., 2006; Tanner et al., 2008; Ye et al., 1998). Expression of Igfbp5 was decreased by PappA(−/−) only kidney, and we identified several other IGF-associated genes for which expression was modified by PappA(−/−) in this organ (e.g., Igfbp2, Igfals, Irs1, Ccnd1, Mt1). This result highlights the kidney as an organ of potential importance in future investigations of the PappA(−/−) mutation, and as a site at which loss of IGF signals could have favorable effects on mouse longevity. Overlap between effects of PappA(−/−) and GH / IGF-1 endocrine mutations on gene expression was limited, but was most marked with respect to kidney. Renal expression of Irs1 and Mt1 was elevated in PappA(−/−), Ghr(−/−), Pit1(dw/dw) and Prop1(df/df) mice, and we show that expression of these genes is regulated by GH and also sensitive to caloric restriction. These expression patterns represent a common feature among four mutations that increase mouse lifespan by an IGF-mediated mechanism, and warrant further investigation as downstream elements of pathways sensitive to both loss of localized IGF-1 availability and depression of the GH/IGF-1 endocrine axis.
Reduction or loss of insulin / insulin-like signals (IIS) increases lifespan in worms, flies and mice and thus the IIS pathway appears to have conserved effects on longevity across species (Berryman et al., 2008; Taguchi et al., 2008). In C. elegans, there have been attempts to identify cell types that mediate effects of IIS inhibition on survival (Libina et al., 2003; Wolkow et al., 2000). For instance, in long-lived daf-2 mutant worms, it was found that restoration of daf-2 signals in neurons abolished increased lifespan, whereas this was not the case with respect to muscle or intestine (Wolkow et al., 2000). However, a later study indicated that, in short-lived daf-2(−); daf-16(−) mutants, restoration of daf-16 activity in adipose tissue (also intestine of C. elegans) increased lifespan, but such effects were not observed with respect to neurons or muscle (Libina et al., 2003). These results conflict, but have highlighted a possible role for nervous, intestinal and adipose tissues as sites for which loss of IIS could have favorable effects, and indeed, insulin receptor knockout mutations specific to brain and adipose tissue were shown to increase mouse lifespan by a small margin (Blüher et al., 2003; Taguchi et al., 2007). In this study, PappA(−/−) decreased Igfbp5 expression in intestine and fat by 56% and 30%, respectively, but in both cases the effect was non-significant (P > 0.126, n = 6 per treatment). There was no significant indication, therefore, that PappA(−/−) altered IGF-1 signals in either fat or intestine, although we cannot rule out that significant effects would have been observed based upon a larger sample size, or that other indicators of IGF-1 signaling might have yielded significant results (e.g., phosphorylation of the IGF-1 receptor, expression of other IGF-sensitive genes). With regard to the nervous system, we evaluated the effects of PappA(−/−) in three brain regions (i.e., neocortex, cerebellum and pons) as well as in an element of the peripheral nervous system (i.e., sciatic nerve), but found that PappA(−/−) did not decrease Igfbp5 expression significantly in any of these tissues. Hypothalamus was not included among the brain components evaluated in our analysis, although this may be an informative target for future work, given the potential role of this gland in the regulation of GH secretion. Interestingly, PappA(−/−) significantly increased Igfbp5 expression in cerebellum, which is suggestive of elevated IGF-1 signals in this region of the central nervous system (Ye et al., 1998). Evidence of increased IGF-1 signaling within the central nervous system (hippocampus) has also been reported in long-lived (Ames) Prop1(df/df) mice that lack GH in circulation (Sun et al., 2005), and this counterintuitive feature of GH/IGF-1-deficient mice could be neuroprotective in some respects (Chen et al., 2005; Frago et al., 2002; Serbedzija et al., 2009; Subramaniam et al., 2005), but might also augment accumulation of betaamyloid (Freude et al., 2009).
The PappA(−/−) mutation had the strongest effects on IGF-associated gene expression patterns in the kidney and led to a significant (31%) decline in Igfbp5 expression in this organ. There is, at present, no direct evidence to indicate that loss of insulin or IGF-1 signals in the kidney can increase mammalian lifespan, and several potentially important phenotypic characteristics of PappA(−/−) mice are unlikely to be explained in terms of renal IGF-1 signaling alone. For instance, the PappA(−/−) mice exhibit increased locomotion and are reported to have reduced tumor burden in kidney but also several other organ systems (liver, lung and colon) (Conover and Bale, 2007). Additionally, although expression of Igfbp5 may be responsive to localized IGF availability, it is unknown whether expression of this gene coincides with activation of key pathways downstream of IGF that mediate beneficial effects of the PappA(−/−) mutation on longevity. Nevertheless, it is clear that, in both mice and rats, genetic manipulations that alter GH / IGF-1 levels in circulation have large effects on kidney pathology, particularly renal lesions associated with progression of glomerulosclerosis (Chen et al., 1995; Doi et al., 1988, 1990, 2001; Doublier et al., 2000; Gay et al., 1997; Shimokawa et al., 2002; Zha et al., 2006, 2008), and it has been postulated that these effects are indeed consequential for survivorship (Razzaque, 2007). In particular, severity of glomerulosclerosis is enhanced with elevated serum GH / IGF-1 levels (Doublier et al., 2000), and is alleviated with declines in serum GH / IGF-1 levels (Zha et al., 2006, 2008). While the role of plasma IGF-1 levels in these effects is not disputed, circulating GH levels can also have direct effects of local IGF-1 availability within the kidney (Jacobs et al., 1997; Flyvbjerg et al., 1992), which raises the possibility that effects of the GH / IGF-1 endocrine axis on kidney pathology are mediated by both localized and circulating IGF-1 (Doi et al., 1990). In support of this notion, oral administration of rapamycin, a compound that increases mouse lifespan and inhibits mTOR signals downstream of IGF-1 (Harrison et al., 2009), has been shown to inhibit progression of anti-thy1-induced glomerulosclerosis in rats (Krämer et al., 2008). These effects of rapamycin on glomerulosclerosis, and other forms of kidney pathology (Bonegio et al., 2005; Diekmann et al., 2007; Esposito et al., 2009), appear related to inhibition of the IGF-1 / mTOR pathway at the local tissue level, without alternation of endocrine GH / IGF-1 signals. Taken together, these observations suggest that glomerulosclerosis could be among age-associated pathologies attenuated in PappA(−/−) mice, and that such effects may be shared among long-lived mutants carrying GH / IGF-1 endocrine mutations, and possibly rapamycin-treated mice as well. Future biochemical and histological investigations of renal tissue from young and old PappA(−/−) mice may thus provide a useful evaluation of localized effects of IGF-1 availability on glomerulosclerosis, apart from circulating GH / IGF-1 levels.
Our results show that the kidney expression of insulin receptor substrate 1 (Irs1), metallothionein 1 (Mt1) and insulin-like growth factor acid labile subunit (Igfals) are regulated by the GH / IGF-1 endocrine axis. In the case of Irs1 and Mt1, renal expression was inversely related to serum GH / IGF-1 levels, and expression was increased by the PappA(−/−), Ghr(−/−), Pit1(dw/dw) and Prop1(df/df) mutations. Expression of these genes was also sensitive to caloric restriction (CR), although effects of CR on Irs1 expression opposed those of the dwarf mutations, while effects of CR on Mt1 expression were only observed in Prop1(df/df) mice treated with growth hormone. Collectively, these data suggest that several factors combine to modulate the expression of Irs1 and Mt1 in kidney, including serum GH / IGF-1 levels, caloric intake, and possibly localized IGF-1 availability as well. It remains unclear whether regulation of renal Irs1 and Mt1 expression by GH/IGF-1 is direct, or if the effect is indirect and dependent upon certain metabolic consequences of reduced or elevated levels of GH / IGF-1 in circulation (e.g., altered adipose tissue mass and associated cytokine levels). Understanding the transcriptional regulation of these genes will shed light on the in vivo molecular events associated with genetic and environmental manipulations that influence the aging process and increase mouse lifespan. It is of considerable interest, for example, that kidney expression of Mt1, and hepatic expression of both Mt1 and Mt2, was elevated in multiple long-lived dwarf models, since the expression of these genes is also increased in many tissues of CR-fed mice (Swindell, 2008a). The metallothioneins have anti-oxidant properties and their elevated expression could serve to combat accumulation of oxidative stress damage in both dwarf mice and CR-fed mice (Bokov et al., 2009). The influence of GH / IGF-1 signals and CR on Mt1/2 expression could reflect a conserved effect associated with manipulations that promote longevity, since both CR and loss of insulin / insulin-like signals (IIS) in C. elegans have been shown to increase expression of mtl-1 and mtl-2 (Barsyte et al., 2001; Szewczyk et al., 2006), which are orthologous to the mouse Mt1 and Mt2 genes.
The identification of mutations that increase mouse lifespan has considerably bolstered efforts to understand the genetic basis of mammalian aging (Berryman et al., 2008; Taguchi et al., 2008). In recent years, however, the trend has been towards a rapidly increasing number of new pro-longevity mutations, but with relatively small effects on mouse lifespan in nearly every case (Swindell, 2009). The PappA(−/−) mutation represents a notable exception to this trend, with a large effect on lifespan that rivals that of the Pit1(dw/dw) and Prop1(df/df) hypo-pituitary mutations, but with a much narrower endocrine deficiency. There remain substantial questions regarding mechanisms by which PappA(−/−) has in vivo effects, and foremost among these are whether some effects of PappA(−/−) are IGFBP4-independent. This is a possibility because the PAPP-A protease can also degrade both IGFBP-2 and IGFBP-5 (Laursen et al., 2001, 2002; Monget et al., 2003; Rivera et al., 2003), which could have compounded in vivo effects in PappA(−/−) mice, since both IGFBP-2 and −5 have cellular effects that are IGF-independent (Miyakoshi et al., 2001). Moreover, notwithstanding IGFBP4-independent effects of the PAPP-A protease, the view of IGFBP-4 as strictly an inhibitor of IGF signals in vivo requires further study, since mice lacking the Igfbp4 gene exhibit a dwarf phenotype, possibly due to a disruption of IGF-2 action that is mediated by IGFBP-4 during development (Ning et al., 2008). Nevertheless, the long-lived PappA(−/−) mouse may ultimately provide impetus for a conceptual shift, involving increased attention to the role played by localized IGF-1 availability as a regulator of age-associated pathology in mice, with endocrine GH / IGF-1 levels serving primarily to mediate these tissue-specific effects (Conover et al., 2008). This development may advance understanding of the diverse effects of IGF action within different cell types, and contribute towards a more detailed model of mechanisms by which insulin / insulin-like signaling controls mammalian aging and longevity.
Supplementary Material
Acknowledgements
This work was supported by National Institute on Aging grants AG023122, AG024824 and AG198899. WRS was supported by National Institute of Health grants T32-AG00114 and T32-AR007197 during completion of this work. We thank Lynn Winkleman and Lisa Burmeister for technical assistance related to genotyping and animal care. Dr. Richard A. Miller, Dr. Cheryl A. Conover and two anonymous reviewers provided helpful comments and critique of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo ML, Ma X, Ackert-Bicknell CL, Donahue LR, Beamer WG, Rosen CJ. Genetic increase in serum insulin-like growth factor-I (IGF-I) in C3H/HeJ compared with C57BL/6J mice is associated with increased transcription from the IGF-I exon 2 promoter. Endocrinology. 2006;147:2944–2955. doi: 10.1210/en.2005-0742. [DOI] [PubMed] [Google Scholar]
- Bach MA, Shen-Orr Z, Lowe WL, Roberts CT, LeRoith D. Insulin-like growth factor I mRNA levels are developmentally regulated in specific regions of the rat brain. Brain Res. Mol. Brain Res. 1991;10:43–48. doi: 10.1016/0169-328x(91)90054-2. [DOI] [PubMed] [Google Scholar]
- Backeljauw PF, Dai Z, Clemmons DR, D'Ercole AJ. Synthesis and regulation of insulin-like growth factor binding protein-5 in FRTL-5 cells. Endocrinology. 1993;132:1677–1681. doi: 10.1210/endo.132.4.7681763. [DOI] [PubMed] [Google Scholar]
- Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001;15:627–634. doi: 10.1096/fj.99-0966com. [DOI] [PubMed] [Google Scholar]
- Bartlett WP, Li XS, Williams M, Benkovic S. Localization of insulin-like growth factor-1 mRNA in murine central nervous system during postnatal development. Dev. Biol. 1991;147:239–250. doi: 10.1016/s0012-1606(05)80021-1. [DOI] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm. IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm. IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Bondy C, Lee WH. Correlation between insulin-like growth factor (IGF)-binding protein 5 and IGF-I gene expression during brain development. J. Neurosci. 1993;13:5092–5104. doi: 10.1523/JNEUROSCI.13-12-05092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonegio RG, Fuhro R, Wang Z, Valeri CR, Andry C, Salant DJ, Lieberthal W. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J. Am. Soc. Nephrol. 2005;16:2063–2072. doi: 10.1681/ASN.2004030180. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chen NY, Chen WY, Bellush L, Yang CW, Striker LJ, Striker GE, Kopchick JJ. Effects of streptozotocin treatment in growth hormone (GH) and GH antagonist transgenic mice. Endocrinology. 1995;136:660–667. doi: 10.1210/endo.136.2.7835300. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK. Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell. 2007;6:727–729. doi: 10.1111/j.1474-9726.2007.00328.x. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Füchtbauer EM, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;31:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- Conover CA, Mason MA, Levine JA, Novak CM. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J. Endocrinol. 2008;198:599–605. doi: 10.1677/JOE-08-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Dhahbi J, Li X, Tran T, Masternak MM, Bartke A. Circulating blood leukocyte gene expression profiles: effects of the Ames dwarf mutation on pathways related to immunity and inflammation. Exp. Gerontol. 2007;42:772–788. doi: 10.1016/j.exger.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann F, Rovira J, Carreras J, Arellano EM, Bañón-Maneus E, Ramírez-Bajo MJ, Gutiérrez-Dalmau A, Brunet M, Campistol JM. Mammalian target of rapamycin inhibition halts the progression of proteinuria in a rat model of reduced renal mass. J. Am. Soc. Nephrol. 2007;18:2653–2660. doi: 10.1681/ASN.2007010087. [DOI] [PubMed] [Google Scholar]
- Doi SQ, Rasaiah S, Tack I, Mysore J, Kopchick JJ, Moore J, Hirszel P, Striker LJ, Striker GE. Low-protein diet suppresses serum insulin-like growth factor-1 and decelerates the progression of growth hormone-induced glomerulosclerosis. Am. J. Nephrol. 2001;21:331–339. doi: 10.1159/000046270. [DOI] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Gibson CC, Agodoa LY, Brinster RL, Striker GE. Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. Am. J. Pathol. 1990;137:541–552. [PMC free article] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Quaife C, Conti FG, Palmiter R, Behringer R, Brinster R, Striker GE. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am. J. Pathol. 1988;131:398–403. [PMC free article] [PubMed] [Google Scholar]
- Doublier S, Seurin D, Fouqueray B, Verpont MC, Callard P, Striker LJ, Striker GE, Binoux M, Baud L. Glomerulosclerosis in mice transgenic for human insulinlike growth factor-binding protein-1. Kidney Int. 2000;57:2299–2307. doi: 10.1046/j.1523-1755.2000.00090.x. [DOI] [PubMed] [Google Scholar]
- Duan C, Liimatta MB, Bottum OL. Insulin-like growth factor (IGF)-I regulates IGF-binding protein-5 gene expression through the phosphatidylinositol 3-kinase, protein kinase B/Akt, and p70 S6 kinase signaling pathway. J. Biol. Chem. 1999;274:37147–37153. doi: 10.1074/jbc.274.52.37147. [DOI] [PubMed] [Google Scholar]
- Esposito C, Villa L, Grosjean F, Mangione F, Esposito V, Castoldi F, Serpieri N, Arra M, Pertile E, Maggi N, Valentino R, Dal Canton A. Rapamycin reduces proteinuria and renal damage in the rat remnant kidney model. Transplant Proc. 2009;41:1370–1371. doi: 10.1016/j.transproceed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyvbjerg A, Frystyk J, Osterby R, Orskov H. Kidney IGF-I and renal hypertrophy in GH-deficient diabetic dwarf rats. Am. J. Physiol. 1992;262:E956–E962. doi: 10.1152/ajpendo.1992.262.6.E956. [DOI] [PubMed] [Google Scholar]
- Frago LM, Pañeda C, Dickson SL, Hewson AK, Argente J, Chowen JA. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology. 2002;143:4113–4122. doi: 10.1210/en.2002-220261. [DOI] [PubMed] [Google Scholar]
- Freude S, Hettich MM, Schumann C, Stöhr O, Koch L, Köhler C, Udelhoven M, Leeser U, Müller M, Kubota N, Kadowaki T, Krone W, Schröder H, Brüning JC, Schubert M. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer's disease. FASEB J. 2009;23:3315–3324. doi: 10.1096/fj.09-132043. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Calder RB, Dollé ME, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech. Ageing Dev. 2008;129:528–533. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay E, Seurin D, Babajko S, Doublier S, Cazillis M, Binoux M. Liver-specific expression of human insulin-like growth factor binding protein-1 in transgenic mice:repercussions on reproduction, ante- and perinatal mortality and postnatal growth. Endocrinology. 1997;138:2937–2947. doi: 10.1210/endo.138.7.5282. [DOI] [PubMed] [Google Scholar]
- Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ. Res. 2007;100:1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs ML, Chandrashekar V, Bartke A, Weber RF. Early effects of streptozotocininduced diabetes on insulin-like growth factor-I in the kidneys of growth hormonetransgenic and growth hormone-deficient dwarf mice. Exp. Nephrol. 1997;5:337–344. [PubMed] [Google Scholar]
- Krämer S, Wang-Rosenke Y, Scholl V, Binder E, Loof T, Khadzhynov D, Kawachi H, Shimizu F, Diekmann F, Budde K, Neumayer HH, Peters H. Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. Am. J. Physiol. Renal. Physiol. 2008;294:F440–F449. doi: 10.1152/ajprenal.00379.2007. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Nielsen CG, Boldt HB, Hopmann KH, Conover CA, Sottrup-Jensen L, Giudice LC, Oxvig C. Substrate specificity of the metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) assessed by mutagenesis and analysis of synthetic peptides: substrate residues distant from the scissile bond are critical for proteolysis. Biochem. J. 2002;367:31–40. doi: 10.1042/BJ20020831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Søe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc. Natl. Acad. Sci. USA. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ren J. Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell. 2007;6:799–806. doi: 10.1111/j.1474-9726.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. Evidence that IGF-binding protein-5 functions as a growth factor. J. Clin. Invest. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monget P, Mazerbourg S, Delpuech T, Maurel MC, Manière S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT. Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol. Reprod. 2003;68:77–86. doi: 10.1095/biolreprod.102.007609. [DOI] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol. Endocrinol. 2008;22:1213–1225. doi: 10.1210/me.2007-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Wang F, Bonkowski MS, Spong A, Bartke A, Pawlikowska L, Kwok PY, Masternak MM. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LS, Harp JB, Goldstein S, Klein J, Pao CI. Regulation and action of insulin-like growth factors at the cellular level. Proc. Nutr. Soc. 1990;49:451–458. doi: 10.1079/pns19900053. [DOI] [PubMed] [Google Scholar]
- Razzaque MS. Does renal ageing affect survival? Ageing Res. Rev. 2007;6:211–222. doi: 10.1016/j.arr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology. 2006;147:5634–5640. doi: 10.1210/en.2006-0493. [DOI] [PubMed] [Google Scholar]
- Rivera GM, Fortune JE. Selection of the dominant follicle and insulin-like growth factor (IGF)-binding proteins: evidence that pregnancy-associated plasma protein A contributes to proteolysis of IGF-binding protein 5 in bovine follicular fluid. Endocrinology. 2003;144:437–446. doi: 10.1210/en.2002-220657. [DOI] [PubMed] [Google Scholar]
- Serbedzija P, Madl JE, Ishii DN. Insulin and IGF-I prevent brain atrophy and DNA loss in diabetes. Brain Res. 2009;1303:179–194. doi: 10.1016/j.brainres.2009.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am. J. Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Shahani N, Strelau J, Laliberté C, Brandt R, Kaplan D, Unsicker K. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J. Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol. Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity-associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi: 10.1186/1471-2164-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech. Ageing Dev. 2008a;129:138–153. doi: 10.1016/j.mad.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Genes regulated by caloric restriction have unique roles within transcriptional networks. Mech. Ageing Dev. 2008b;129:580–592. doi: 10.1016/j.mad.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Accelerated failure time models provide a useful statistical framework for aging research. Exp. Gerontol. 2009;44:190–200. doi: 10.1016/j.exger.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk NJ, Udranszky IA, Kozak E, Sunga J, Kim SK, Jacobson LA, Conley CA. Delayed development and lifespan extension as features of metabolic lifestyle alteration in C. elegans under dietary restriction. J. Exp. Biol. 2006;209:4129–4139. doi: 10.1242/jeb.02492. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu. Rev. Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- Tanner SJ, Hefferan TE, Rosen CJ, Conover CA. Impact of pregnancy-associated plasma protein-a deletion on the adult murine skeleton. J. Bone Miner. Res. 2008;23:655–662. doi: 10.1359/JBMR.071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc. Natl. Acad. Sci. USA. 2009;106:11252–11257. doi: 10.1073/pnas.0807025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Ye P, D'Ercole J. Insulin-like growth factor I (IGF-I) regulates IGF binding protein-5 gene expression in the brain. Endocrinology. 1998;139:65–71. doi: 10.1210/endo.139.1.5676. [DOI] [PubMed] [Google Scholar]
- Zha Y, Le VT, Higami Y, Shimokawa I, Taguchi T, Razzaque MS. Life-long suppression of growth hormone-insulin-like growth factor I activity in genetically altered rats could prevent age-related renal damage. Endocrinology. 2006;147:5690–5698. doi: 10.1210/en.2006-0302. [DOI] [PubMed] [Google Scholar]
- Zha Y, Taguchi T, Nazneen A, Shimokawa I, Higami Y, Razzaque MS. Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am. J. Nephrol. 2008;28:755–764. doi: 10.1159/000128607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.