Abstract
Staphylococcus aureus is the most common cause of skin and soft tissue infections, and rapidly emerging antibiotic-resistant strains are creating a serious public health concern. If immune-based therapies are to be an alternative to antibiotics, greater understanding is needed of the protective immune response against S. aureus infection in the skin. Although neutrophil recruitment is required for immunity against S. aureus, a role for T cells has been suggested. Here, we used a mouse model of S. aureus cutaneous infection to investigate the contribution of T cells to host defense. We found that mice deficient in γδ but not αβ T cells had substantially larger skin lesions with higher bacterial counts and impaired neutrophil recruitment compared with WT mice. This neutrophil recruitment was dependent upon epidermal Vγ5+ γδ T cell production of IL-17, but not IL-21 and IL-22. Furthermore, IL-17 induction required IL-1, TLR2, and IL-23 and was critical for host defense, since IL-17R–deficient mice had a phenotype similar to that of γδ T cell–deficient mice. Importantly, γδ T cell–deficient mice inoculated with S. aureus and treated with a single dose of recombinant IL-17 had lesion sizes and bacterial counts resembling those of WT mice, demonstrating that IL-17 could restore the impaired immunity in these mice. Our study defines what we believe to be a novel role for IL-17–producing epidermal γδ T cells in innate immunity against S. aureus cutaneous infection.
Introduction
Staphylococcus aureus is a Gram-positive extracellular bacterium that is the most common cause of skin and soft tissue infections, such as cellulitis, impetigo, and folliculitis (1, 2). Although these infections usually originate in the skin, invasive and life-threatening infections such as bacteremia, pneumonia, meningitis, endocarditis, and sepsis may ensue (1, 2). In recent years, S. aureus skin infections have become an enormous public health problem, resulting in approximately 11.6 million outpatient and emergency room visits and 464,000 hospital admissions per year in the US (1). Moreover, these infections have been increasingly caused by virulent antibiotic-resistant strains, such as methicillin-resistant S. aureus (MRSA; refs. 2, 3). In 2005, MRSA caused 94,360 severe invasive infections and resulted in 18,650 deaths in the US, exceeding the annual number of deaths in the US caused by HIV/AIDS, tuberculosis, and viral hepatitis combined (4, 5). Given this rapidly emerging epidemic and the decreased efficacy of currently available antibiotic therapy, immune-based antimicrobial therapy may provide a novel alternative approach to combat these infections. For this to be possible, a clear understanding of the protective immune response against S. aureus infection in the skin is vital.
A key correlate to an effective immune response against S. aureus involves neutrophil recruitment and abscess formation, which are required for bacterial clearance (6, 7). The importance of neutrophils in host defense against S. aureus infection has been demonstrated by recurrent S. aureus infections in patients with chronic granulomatous disease, who have a defect in neutrophil NADPH oxidase and respiratory burst (8). In addition, a large study found that neutropenic patients with cancer were particularly susceptible to S. aureus infections, which resulted in increased morbidity and mortality (9). The essential role of neutrophils in S. aureus infections has also been observed in mouse models of S. aureus infection: neutrophil-depleted mice developed nonhealing skin lesions and were unable to clear the bacteria (6, 7).
In addition to neutrophil recruitment, a number of different observations in humans have provided evidence that T cells play a critical role in host defense against S. aureus skin infections. First, patients with HIV disease, especially those with low peripheral blood CD4+ T cell counts, are highly susceptible to S. aureus colonization and skin infection (10, 11). Second, patients with the inflammatory skin disease atopic dermatitis have increased colonization and superinfection with S. aureus (12), which has been attributed to the Th2 cytokine profile (i.e., IL-4, IL-13, and IL-10) and decreased antimicrobial peptides observed in this disease (13, 14). Finally, human subjects with hyper-IgE syndrome (also known as Job’s syndrome), who suffer from recurrent and severe S. aureus skin infections (15), have STAT3 mutations that render them deficient in Th17 cells (16–18).
In mouse models, there have also been reports that T cells contribute to host defense against S. aureus cutaneous infection. A previous study found that αβ T cell–deficient mice had defective neutrophil abscess formation compared with WT mice after subcutaneous S. aureus challenge (19). However, contrary to the well-established importance of neutrophils in host defense against S. aureus infections, the defective neutrophil abscess formation in the αβ T cell–deficient mice was associated with decreased bacterial burden within the infected lesions through an unknown mechanism (19). Another study demonstrated that γδ T cell–deficient mice had substantially higher bacterial counts within the infected skin lesions than did WT mice after intradermal S. aureus challenge, but the mechanism was not well defined (20).
Taken together, the results of these studies in humans and mice suggest a role for T cells in host defense against S. aureus; nevertheless, the mechanism by which T cells promote protective immunity against S. aureus skin infection is not clear. Thus, we chose to evaluate the contribution and mechanism by which αβ and γδ T cells participate in cutaneous host defense using an in vivo mouse model of S. aureus cutaneous infection.
Results
γδ T cell–deficient mice, but not αβ T cell–deficient mice, developed markedly larger skin lesions with higher bacterial counts compared with WT mice in response to cutaneous challenge with S. aureus.
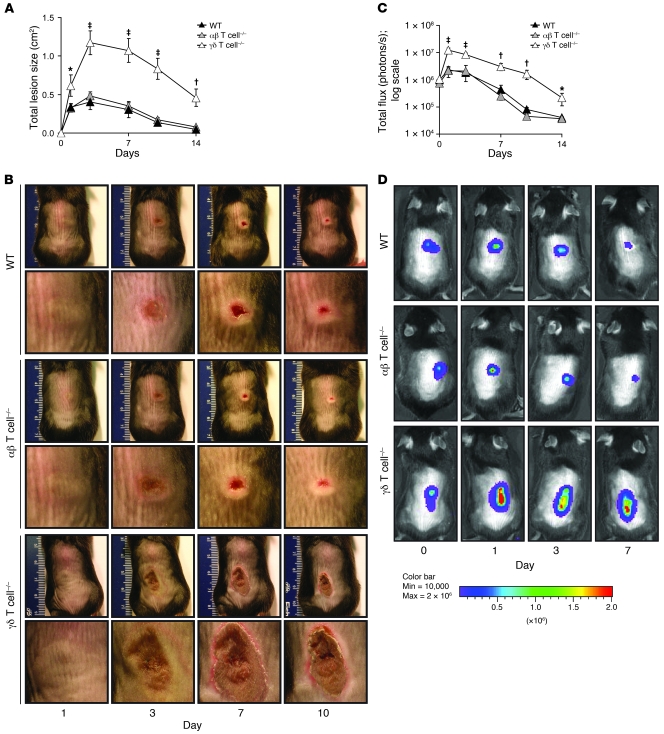
As a first step toward identifying mechanisms by which T cells contribute to host defense against S. aureus infection in skin, we investigated the contribution of αβ and γδ T cells using an in vivo mouse model. This model uses a bioluminescent SH1000 strain of S. aureus and techniques of in vivo bioluminescence imaging to track the bacterial burden within the infected lesions of anesthetized mice in real time (21, 22). The S. aureus SH1000 strain was chosen for this study because it has a complete virulence repertoire due to a restored rsbU gene in the sigB stress response pathway of S. aureus (23), and it has been previously used to study S. aureus cutaneous infection in mouse models in vivo and in human culture systems (21, 24). WT mice and mice deficient in either γδ or αβ T cells were inoculated intradermally with the bioluminescent S. aureus SH1000 strain (unless otherwise indicated, inoculations consisted of 2 × 106 CFUs in 100 μl saline). Lesion sizes and in vivo bioluminescence of live actively metabolizing bacteria within the lesions over time were evaluated (Figure 1). WT mice developed visible skin lesions, which had a maximum size of 0.41 ± 0.05 cm2 by day 3, and healed by day 14 (Figure 1, A and B). αβ T cell–deficient mice developed skin lesions that did not significantly differ in size from those of WT mice. In contrast, γδ T cell–deficient mice developed lesions approximately 3-fold larger than those of WT or αβ T cell–deficient mice that failed to completely heal by day 14 after inoculation.
Figure 1. γδ but not αβ T cell–deficient mice develop markedly larger skin lesions compared with WT mice in response to cutaneous challenge with S. aureus.
γδ T cell–deficient, αβ T cell–deficient, and WT mice were inoculated intradermally with S. aureus SH1000 strain. (A) Mean total lesion size (cm2) ± SEM. (B) Representative lesions for each mouse strain. Shown are entire dorsal backs (top, mm ruler shown for scale) and close-ups of lesions (bottom). (C) Mean total flux (photons/s) ± SEM. (D) Representative in vivo bioluminescence. Data are from 2 experiments with at least 6 mice/group per experiment. *P < 0.05, †P < 0.01, ‡P < 0.001, γδ T cell–deficient versus WT (Student’s t test).
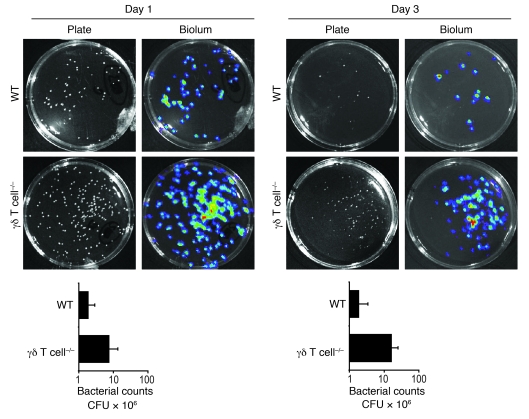
To determine whether the larger lesions of γδ T cell–deficient mice were associated with impaired bacterial clearance, we anesthetized mice and determined bacterial counts within the lesions in real time (Figure 1, C and D) using in vivo bioluminescence imaging techniques (see Methods). We previously demonstrated that in vivo bioluminescence signals closely estimate bacterial CFUs harvested from the skin lesions at various time points after infection (21). After S. aureus infection, WT and αβ T cell–deficient mice had similar bioluminescent signals that decreased over 14 days. In contrast, γδ T cell–deficient mice had increased bioluminescent signals (Figure 1D) that were up to 20-fold higher than those of WT mice (Figure 1C) at all time points after inoculation. Similar to in vivo bioluminescence, numbers of S. aureus CFUs recovered from 8-mm punch biopsies were up to 10-fold higher in γδ T cell–deficient mice than in WT mice on days 1 and 3 after infection (Figure 2). Thus, γδ T cell–deficient mice have a marked defect in bacterial clearance, which likely explains the increased size and persistence of the skin lesions. These data suggest that γδ T cells, but not αβ T cells, are required for mediating host defense and bacterial clearance against cutaneous S. aureus infection.
Figure 2. γδ but not αβ T cell–deficient mice develop higher bacterial counts compared with WT mice in response to cutaneous challenge with S. aureus.
Representative bacterial culture plates after overnight culture with or without bioluminescence, and CFUs of S. aureus recovered from 8-mm lesional punch biopsies on days 1 and 3 (n = 5 per group).
γδ T cell–deficient mice have impaired neutrophil recruitment compared with WT mice in response to cutaneous challenge with S. aureus.
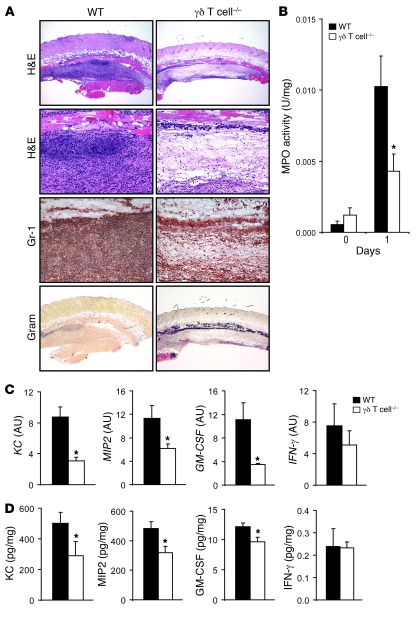
Since the increased lesions sizes and bioluminescent signals of the γδ T cell–deficient mice were observed soon after infection, histology and myeloperoxidase (MPO) activity were evaluated in lesional skin biopsies performed 1 day after inoculation (Figure 3). WT mice developed large neutrophilic abscesses in both H&E-stained and anti–Gr-1 (neutrophil marker) mAb–labeled histologic sections (Figure 3A). In addition, S. aureus bacteria were barely detectable by Gram stain in WT mice, likely because of phagocytosis and clearance of the bacteria by the neutrophils within the abscess. In contrast, lesions of γδ T cell–deficient mice had severely decreased numbers of neutrophils with impaired abscess formation and contained a blue-staining band of Gram-positive bacteria spanning the entire horizontal length of the section. There was also significantly less MPO activity, a marker of neutrophil function, in homogenized lesional punch biopsy specimens of γδ T cell–deficient mice compared with WT mice 1 day after inoculation (Figure 3B). Thus, γδ T cell–deficient mice had impaired recruitment of neutrophils to the site of infection. Furthermore, the readily detectable Gram-positive bacteria in lesions of γδ T cell–deficient mice corroborate the results obtained with in vivo bioluminescence and CFU data in Figures 1 and 2, which demonstrated that γδ T cell–deficient mice had impaired bacterial clearance. Taken together, these data suggest that γδ T cells play an important role in mediating neutrophil recruitment to the site of S. aureus skin infection.
Figure 3. γδ T cell–deficient mice have markedly impaired neutrophil recruitment and production of neutrophil chemokines and granulopoiesis factors in response to cutaneous challenge with S. aureus.
γδ T cell–deficient and WT mice were inoculated intradermally with S. aureus. (A) Representative photomicrographs of sections labeled with H&E stain and anti–Gr-1 mAb (immunoperoxidase method) and Gram stain of lesional skin at 1 day after inoculation. Original magnification, ×40 (H&E, top, and Gram); ×200 (H&E, bottom, and Gr-1). (B) Mean MPO activity (U/mg tissue weight) ± SEM of lesional skin. (C) Mean level of mRNA (AU) ± SEM of KC, MIP2, GM-CSF, and IFN-γ from lesional skin homogenates of skin biopsies performed by Q-PCR 8 hours after S. aureus inoculation. (D) Mean protein levels (pg/mg tissue weight) ± SEM of KC, MIP2, GM-CSF, and IFN-γ from lesional skin homogenates of skin biopsies performed at 8 hours after S. aureus inoculation. Data are from 2 experiments with at least 5 mice/group per experiment. *P < 0.05, γδ T cell–deficient versus WT (Student’s t test).
To further elucidate the mechanism of impaired neutrophil recruitment observed in γδ T cell–deficient mice, levels of soluble factors known to have direct neutrophil chemotactic activity, including the neutrophil chemokines KC and MIP2 and the granulopoiesis factor GM-CSF, were evaluated by performing quantitative real-time PCR (Q-PCR) and protein array analysis on homogenized 8-mm lesional punch biopsies performed 8 hours after skin challenge (Figure 3, C and D). Lesions of γδ T cell–deficient mice had significantly decreased mRNA and protein levels of KC, MIP2, and GM-CSF compared with lesions of WT mice. In contrast, there was no difference in mRNA and protein levels of IFN-γ, an important T cell–derived cytokine involved in cell-mediated immunity, between these mice. Protein levels of other T cell–derived cytokines, including IL-2, IL-4, and IL-13, were undetectable (data not shown). Thus, the impaired neutrophil recruitment in γδ T cell–deficient mice is likely caused, at least in part, by an early decrease in production of chemokines involved in neutrophil recruitment and factors that promote granulopoiesis.
Impaired induction of IL-17, but not IL-22 or IL-21, in γδ T cell–deficient mice after S. aureus cutaneous challenge.
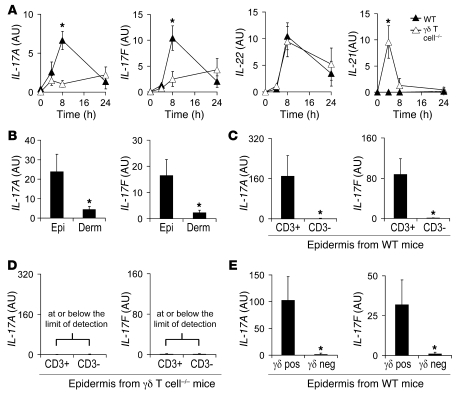
To further elucidate the mechanism by which γδ T cells promote neutrophil recruitment against S. aureus skin infection, we investigated the production of T cell–derived cytokines known to promote neutrophil recruitment. These include IL-17 (i.e., IL-17A and IL-17F) and IL-22, which trigger epithelial cells and fibroblasts to produce neutrophil recruitment factors, including the neutrophil chemokines KC and MIP2 and the granulopoiesis factor GM-CSF (25–30). For comparison, we also evaluated levels of IL-21, which, along with IL-17 and IL-22, is produced by Th17 cells (31, 32). We found that WT mice exhibited an early burst of both IL-17A and IL-17F gene expression, which peaked at 8 hours and returned to baseline by 24 hours after challenge in vivo (Figure 4A). In contrast, γδ T cell–deficient mice lacked this early induction of IL-17A and IL-17F. IL-22 was induced in both γδ T cell–deficient and WT mice, which suggests that IL-22 is produced by cells other than γδ T cells. WT mice did not induce IL-21 at all time points evaluated. However, γδ T cell–deficient mice had detectable levels of IL-21 at 4 hours after infection.
Figure 4. Impaired induction of IL-17, but not IL-22 or IL-21, in γδ T cell–deficient mice after S. aureus cutaneous challenge.
Mice were inoculated intradermally with S. aureus, and the mean level of mRNA (AU) ± SEM of IL-17A and IL-17F by Q-PCR were determined from (A) lesional skin homogenates of skin biopsies from γδ T cell–deficient and WT mice performed 0, 4, 8, and 24 hours after inoculation (levels of IL-22 and IL-21 also evaluated); (B) epidermal and dermal split specimens of skin biopsies from WT mice performed 8 hours after inoculation; (C and D) positively selected cells (CD3+) and negative fractions (CD3–) of epidermal cell suspensions after enrichment for CD3+ cells (MACS) from skin biopsies taken from WT (C) or γδ T cell–deficient (D) mice performed 8 hours after inoculation; and (E) positively selected cells (γδ pos) and negative fractions (γδ neg) of epidermal cell suspensions after enrichment for γδ T cells (MACS) from skin biopsies taken from WT mice 8 hours after inoculation. Values in D were at or below the limit of detection. Data are from 2 experiments with at least 5 mice/group per experiment. *P < 0.05 between experimental groups (Student’s t test).
Because γδ T cell–deficient mice had a defect in IL-17 induction, but not IL-21 or IL-22 induction, we investigated the cellular source of IL-17 in lesional skin samples of WT mice harvested 8 hours after inoculation (Figure 4B). IL-17A and IL-17F were highly expressed in the epidermal compartment and were virtually absent from the dermal compartment. Furthermore, at 8 hours after infection, IL-17A and IL-17F induction was detected exclusively in the T cell fraction (CD3+ cells) after enrichment of epidermal T cells using a specific mAb directed against the pan–T cell marker CD3 (Figure 4C). In contrast, at 8 hours after infection, IL-17A and IL-17F induction was not observed in either the T cell–enriched fractions or the negative fractions from the epidermis of γδ T cell–deficient mice (Figure 4D). Thus, there was no evidence of IL-17–producing compensatory cells in γδ T cell–deficient mice after infection. Finally, at 8 hours after infection, IL-17A and IL-17F induction was detected exclusively in the γδ T cell–positive fraction after enrichment of epidermal γδ T cells using the γδ T cell–specific mAb GL3 (Figure 4E). Taken together, these findings demonstrate that epidermal γδ T cells are the major source of IL-17 production in response to S. aureus cutaneous challenge. Furthermore, epidermal γδ T cells are not required for induction of IL-22 or IL-21.
γδ T cells expressing the Vγ5 chain are present in the epidermis of WT mice after cutaneous challenge with S. aureus and produce IL-17 after in vitro stimulation.
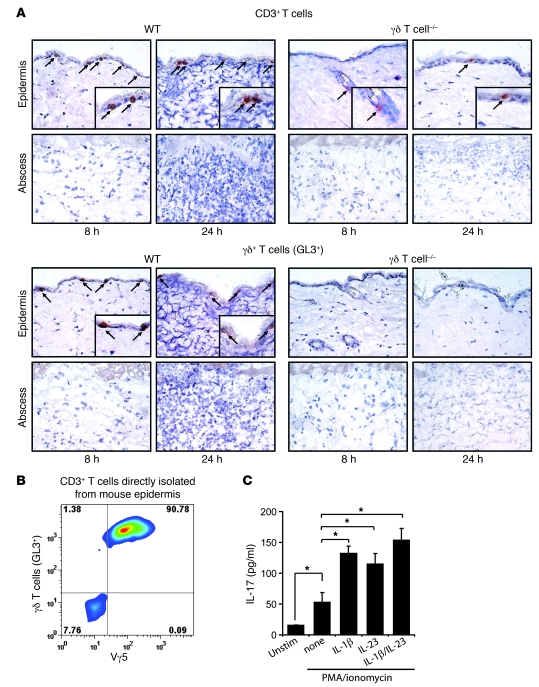
To determine the precise location of the γδ T cells within the infected skin lesions, we evaluated histological sections of lesional biopsy specimens taken 8 and 24 hours after skin inoculation (Figure 5A; isotype controls are provided in Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI40891DS1). We observed CD3+ T cells within the epidermal compartment of WT mice at 8 and 24 hours after inoculation. The vast majority of these T cells were positively labeled in serial sections with GL3. In contrast, only rare CD3+ T cells (0–1 cells per ×400 high-power field) were detected within the epidermis of γδ T cell–deficient mice. T cells were not observed in the dermis or within the abscess of skin specimens from WT or γδ T cell–deficient mice at either 8 or 24 hours after infection. To determine the percentage of γδ T cells that express the known skin-specific Vγ5 chain, epidermal cells were isolated from uninfected WT mouse epidermis. We found that approximately 90% of the CD3+ T cells from WT mouse epidermis expressed the Vγ5 chain, as measured by flow cytometry (Figure 5B). In addition, the total proportion of γδ T cells in whole skin tissue was approximately 2.3% (Supplemental Figure 2). Importantly, the Vγ5 γδ T cells represented virtually all of the γδ T cell population in the skin at these early time points (data not shown), and the expression of the Vγ5 TCR remained unchanged 8–24 hours after cutaneous challenge with S. aureus (Supplemental Figure 3).
Figure 5. γδ T cells expressing the Vγ5 chain are present in the epidermis of WT mice after skin challenge with S. aureus and produce IL-17 after in vitro stimulation.
(A) Representative photomicrographs labeled with specific mAbs for CD3+ (arrows) and GL3+ (i.e., γδ T) cells (arrows; immunoperoxidase method) of histologic sections from skin biopsies from γδ T cell–deficient and WT mice performed 8 and 24 hours after skin inoculation with S. aureus. Insets show positively labeled cells. Isotype controls are shown in Supplemental Figure 2. Original magnification, ×400; ×800 (insets). (B) Epidermal cell suspensions of skin biopsies from normal uninfected WT mouse skin were labeled with specific mAbs for CD3, γδ T cells (GL3+), and Vγ5, and expression was analyzed by flow cytometry. Cells were gated on CD3+ cells, and the percentage of cells in each quadrant is indicated. (C) Purified GL3+ epidermal γδ T cells (106 cells/ml) from uninfected C57BL/6 mice were left unstimulated or were activated with PMA/ionomycin and cultured in the presence or absence of 20 ng/ml IL-1β, 20 ng/ml IL-23, or 20 ng/ml of both IL-1β and IL-23. Supernatants were collected after 24 hours for analysis of IL-17 protein levels (pg/ml) by ELISA. Data are from 2 experiments with at least 4 mice/group per experiment. *P < 0.05 (Student’s t test).
To confirm the ability of resident epidermal γδ T cells to produce IL-17 protein, we isolated γδ T cells from the epidermis of uninfected mice. These γδ T cells were enriched using positive selection and then stimulated with PMA/ionomycin (Figure 5C). PMA/ionomycin stimulation resulted in a 3-fold induction of IL-17 protein compared with unstimulated cells in culture supernatants, as determined by ELISA. In addition, since previous reports have demonstrated that IL-1β and IL-23 promote production of IL-17 by CD4+ αβ Th17 cells (33–39), we also evaluated whether addition of exogenous recombinant IL-1β and/or IL-23 could promote IL-17 production by epidermal γδ T cells. We found that addition of IL-1β, IL-23, or both in combination all resulted in 2- to 3.5-fold enhancement of IL-17 protein production over PMA/ionomycin alone, demonstrating that IL-1β and/or IL-23 is required for optimal production of IL-17 by epidermal γδ T cells. Taken together, these data indicate that the resident epidermal γδ T cells expressing the Vγ5 chain represent the predominant T cell subset present in mouse skin at baseline and during the early immune response to S. aureus skin infection. Furthermore, these resident epidermal γδ T cells have the capacity to readily produce IL-17 upon activation in vitro.
It should be noted that we also examined whether other CD3+ T cells present in normal skin of γδ T cell–deficient mice have the capacity to produce IL-17. After enriching CD3+ T cells from the skin of WT and γδ T cell–deficient mice, CD3+ cells were cultured (5 × 105 cells/ml) in the presence of PMA/ionomycin with or without IL-1β and IL-23 (20 ng/ml each, in combination), and IL-17 protein levels in culture supernatants were measured by ELISA (Supplemental Figure 4). IL-17 was only slightly induced in cultures of CD3+ T cells from γδ T cell–deficient mice compared with the substantial production observed in cultures of CD3+ T cells from WT mice. Because approximately 90% of the T cells from the skin of WT mice were γδ T cells (Figure 5B), this finding further demonstrates that the major population of cells responsible for IL-17 production after S. aureus cutaneous infection is epidermal γδ T cells, not other compensatory T cells present in the skin of γδ T cell–deficient mice.
IL-17 production in response to S. aureus cutaneous challenge is dependent upon IL-1, TLR2, and IL-23.
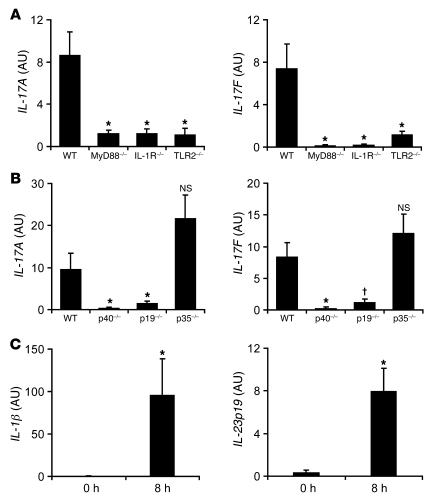
We next evaluated whether IL-1 and IL-23 are required for IL-17 production in response to S. aureus cutaneous challenge in vivo. In addition, we also evaluated whether TLR2 is required, since TLR2 has recently been shown to be expressed by γδ T cells (40). To determine whether the induction of IL-17A and IL-17F was dependent upon IL-1 and/or TLR2, we inoculated WT mice and mice deficient in IL-1R, TLR2, or MyD88, the signaling adapter for IL-1R and TLR family members (Figure 6A). At 8 hours after challenge, IL-1R–, TLR2-, and MyD88-deficient mice had impaired induction of IL-17A and IL-17F compared with WT mice. The impaired induction of IL-17 in IL-1R– or MyD88-deficient mice was not caused by reduced numbers of γδ T cells, since similar numbers of Vγ5+ γδ T cells were found in the epidermis between WT mice and mice deficient in IL-1R or MyD88 (Supplemental Figure 5). In addition, to determine whether IL-17A and IL-17F induction in the skin was also dependent upon IL-23, we inoculated WT mice and mice deficient in p40 (the shared subunit for IL-12 and IL-23), p19 (the IL-23–specific subunit), and p35 (the IL-12–specific subunit). At 8 hours after inoculation, IL-12/23p40– and IL-23p19–deficient mice, but not IL-12p35–deficient mice, had impaired induction of IL-17A and IL-17F compared with WT mice (Figure 6B), demonstrating an important role for IL-23, but not IL-12, in induction of IL-17A and IL-17F. Finally, to confirm that IL-1β and IL-23 were induced after infection, IL-1β and IL-23p19 gene induction was measured at 0 and 8 hours after infection (Figure 6C). We found that both IL-1β and IL-23p19 were significantly upregulated in response to infection. Taken together, these findings suggest that the induction of IL-17A and IL-17F by γδ T cells is dependent upon IL-1 and IL-23, which corresponds to our findings with purified epidermal γδ T cells in vitro and is similar to the characteristics of CD4+ αβ Th17 cells.
Figure 6. IL-17A and IL-17F mRNA production in response to S. aureus skin challenge is dependent upon IL-1R, TLR2, and IL-23.
Mice were inoculated intradermally with S. aureus, and the mean level of mRNA (AU) ± SEM of IL-17A and IL-17F by Q-PCR were determined from lesional skin homogenates of skin biopsies from (A) MyD88-deficient, IL-1R–deficient, TLR2-deficient, and WT mice and (B) IL-12/23p40–deficient, IL-23p19–deficient, IL-12p35–deficient, and WT mice, performed 8 hours after inoculation. (C) Mean mRNA level (AU) ± SEM of IL-1β and IL-23p19 from lesional skin homogenates of skin biopsies performed 8 hours after inoculation by Q-PCR. Data are from 2 experiments with at least 5 mice/group per experiment. *P < 0.05, †P < 0.01 versus appropriate WT or 0-hour control (Student’s t test).
IL-17 receptor–deficient mice, but not IFN-γ receptor–deficient mice, have defects in immunity against S. aureus infection similar to those of γδ T cell–deficient mice.
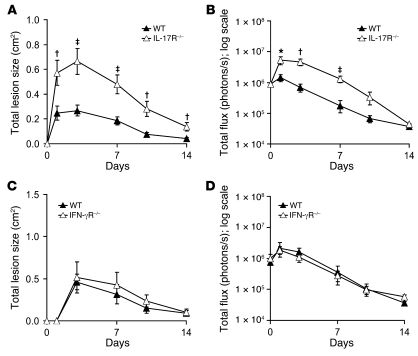
To evaluate whether IL-17 contributes directly to the impaired neutrophil recruitment and host defense against S. aureus cutaneous challenge observed in γδ T cell–deficient mice, WT mice and mice deficient in the IL-17 receptor (IL-17R) were inoculated intradermally with S. aureus. IL-17R–deficient mice developed significantly larger lesions and higher bacterial counts (as assessed by in vivo bioluminescence signals) compared with WT mice (Figure 7, A and B). For comparison, WT and IFN-γ receptor–deficient (IFN-γR–deficient) mice were also inoculated intradermally. In contrast to IL-17R–deficient mice, IFN-γR–deficient mice had lesion sizes and bacterial burdens similar to those of WT mice (Figure 7, C and D), which suggests that IFN-γ is not required for cutaneous host defense against S. aureus skin infection in our model. Taken together, these data demonstrate that IL-17R–deficient mice have an impairment in host defense against S. aureus cutaneous challenge similar to that of γδ T cell–deficient mice. These data provide additional evidence that IL-17–producing γδ T cells are required for host defense against S. aureus skin infection.
Figure 7. IL-17R–deficient mice, but not IFN-γR–deficient mice, develop increased lesion sizes and bacterial counts resembling those of γδ T cell–deficient mice in response to S. aureus cutaneous challenge.
IL-17R–deficient, IFN-γR–deficient, and WT mice were inoculated intradermally with S. aureus. (A and C) Mean total lesion size (cm2) ± SEM. (B and D) Mean total flux (photons/s) ± SEM. Data are from 2 experiments with at least 6 mice/group per experiment. *P < 0.05, †P < 0.01, ‡P < 0.001, IL-17R–deficient versus WT (Student’s t test).
Administration of recombinant murine IL-17A with the S. aureus inoculum rescues γδ T cell–deficient mice.
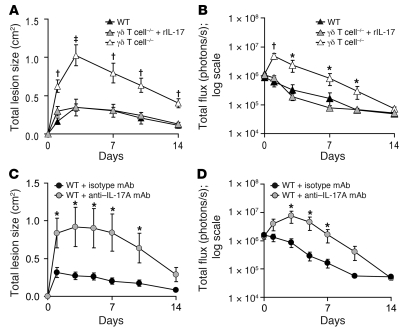
Given the critical role of γδ T cells and IL-17 in host defense against cutaneous S. aureus challenge, we evaluated whether the addition of exogenous recombinant murine IL-17A (rIL-17) can rescue the increased lesion sizes and bacterial counts observed in γδ T cell–deficient mice. Administration of a single dose of rIL-17 (1,000 ng in 100 μl saline) given with the S. aureus inoculum resulted in significantly reduced lesion sizes and bioluminescent signals compared with γδ T cell–deficient mice inoculated with S. aureus plus vehicle alone (Figure 8, A and B). In addition, administration of rIL-17 to γδ T cell–deficient mice resulted in lesion sizes and bacterial counts that were indistinguishable from those of WT mice, demonstrating the rIL-17 alone was sufficient to restore the immune defects in these mice. Thus, these data indicate that IL-17 is the essential mediator by which γδ T cells contribute to host defense against S. aureus cutaneous challenge. To confirm the requirement of IL-17 for clearance of infection, a neutralizing IL-17A mAb (50 μg) or an isotype control mAb was administered to WT mice along with the bacterial inoculum (Figure 8, C and D). Consistent with our demonstration that IL-17R–deficient mice had impaired bacterial clearance (Figure 7, A and B), administration of a single dose of a neutralizing IL-17A mAb with the inoculum to WT mice resulted in larger lesions and higher bacterial counts similar to those of γδ T cell–deficient mice, further demonstrating the key role for IL-17 in host defense against cutaneous S. aureus infection.
Figure 8. Administration of exogenous rIL-17 with the S. aureus inoculum restores an effective immune response against S. aureus in γδ T cell–deficient mice.
(A and B) γδ T cell–deficient and WT mice were inoculated intradermally with S. aureus along with administration of a single dose of rIL-17 (1,000 ng/100 μl) or vehicle alone to γδ T cell–deficient mice. (A) Mean total lesion size (cm2) ± SEM. (B) Mean total flux (photons/s) ± SEM. Data are from 2 experiments with at least 6 mice/group per experiment. (C and D) WT mice (n = 7 mice/group) were inoculated intradermally with S. aureus along with an anti–IL-17A neutralizing mAb or an isotype control mAb. (C) Mean total lesion size (cm2) ± SEM. (D) Mean total flux (photons/s) ± SEM. *P < 0.05, †P < 0.01, ‡P < 0.001 versus all other groups (Student’s t test).
It should be mentioned that we attempted to administer rIL-17 to WT mice to evaluate whether additional IL-17 has any impact for host defense in animals with a normal endogenous IL-17 response. We found no difference in bacterial counts, as assessed by in vivo bioluminescence, between the IL-17–treated and –untreated mice (data not shown). Despite this finding, the fact that a single dose of IL-17 was sufficient to rescue the immune defects of the γδ T cell–deficient mice suggests that immunomodulatory therapy or vaccine development to enhance IL-17 responses may help in the treatment of S. aureus cutaneous infections in patients with altered T cell function with deficient or dysregulated Th17 responses.
Discussion
Neutrophil recruitment is an essential component of the host innate immune response against S. aureus skin infection and is required for bacterial clearance (6, 7). However, humans with diseases characterized by altered T cell function, including HIV/AIDS, atopic dermatitis, and hyper-IgE syndrome, are highly susceptible to S. aureus cutaneous infection (10, 12, 15), which suggests that T cells also contribute to host defense. Studies in mouse models have also indicated a key role for T cells, although the mechanism by which they contribute to host defense is not clear (19, 20). In this study, we report that resident epidermal γδ T cells, but not αβ T cells, were required for neutrophil recruitment in host defense against S. aureus cutaneous challenge, directly linking cutaneous T cell function with neutrophil recruitment. Furthermore, we demonstrated a mechanism by which skin γδ T cells contributed to host defense, via the IL-1–, TLR2-, and IL-23–dependent production of IL-17, which was required to control the infection. Importantly, the role of γδ T cells in combating S. aureus was restored by a single dose of rIL-17 administered with the bacterial inoculum, which suggests that immunomodulatory or vaccine therapy aimed at inducing IL-17 responses in the skin may provide a potential therapeutic intervention for humans susceptible to S. aureus skin infections.
Our data do not exclude a role for other Th17 cytokines, such as IL-22, which has antimicrobial activity (28, 29), and IL-21, which promotes differentiation of T cells and inflammatory responses (31, 32, 41). However, IL-22 induction was not affected by the lack of γδ T cells, which suggests it is made by other cell types, such as αβ T cells, NKT cells, and/or NK cells (42–44). In addition, IL-21 was not induced at early time points, which indicates that it may not participate in the early host response. Interestingly, an almost 10-fold induction of IL-21 gene expression was observed in γδ T cell–deficient mice after cutaneous S. aureus challenge. The significance of this is unknown, but the induction of IL-21 mRNA, which is known to be expressed by CD4+ T cells, NK cells, B cells, macrophages, and dendritic cells during infection (45), did not impact the defective neutrophil recruitment observed in the γδ T cell–deficient mice. Our results indicate that the predominant function of IL-17 is to promote neutrophil recruitment via induction of chemokines and granulopoiesis factors (25–27, 30), which is ultimately required for S. aureus clearance (6, 7). However, it is also possible that IL-17 and/or IL-22 may contribute to host defense against S. aureus cutaneous infection via production of antimicrobial peptides (e.g. β-defensins and cathelicidin) or by inducing epithelial proliferation, thereby increasing epithelial resistance to infection (28, 29, 41, 46, 47).
Although these human subjects with diseases with altered T cell responses have increased susceptibility to S. aureus colonization and infection (10–15), the mechanisms have been thought to be unrelated. However, emerging data suggests that these unrelated clinical conditions share in common a deficiency in Th17 cells. For example, there is significant loss of Th17 cells in the blood and mucosa of HIV-infected patients (48). Similarly, skin lesions from patients with atopic dermatitis have recently been shown to be deficient in Th17 cells (49). Finally, human subjects with hyper-IgE syndrome have STAT3 mutations that render them deficient in Th17 cells (16–18). Our findings demonstrating that IL-17 was required for host defense against S. aureus skin infections thus point to a mechanism by which Th17 dysregulation leads to increased susceptibility to S. aureus skin infections among these different human diseases.
We found that IL-17–producing resident epidermal γδ T cells were required for host defense against S. aureus skin infection. In contrast, humans with altered T cell function and susceptibility to S. aureus skin infections have been shown to be deficient in αβ Th17 cells (16–18, 48, 49). In human skin, a large proportion of αβ Th17 cells are present in inflamed skin (50), and γδ T cells have been reported to be recruited to a site of skin infection (51). However, it is not clear whether αβ Th17 cells, γδ T cells, or other functionally equivalent cells are important in producing IL-17 during S. aureus infections in human skin. In the mouse, our findings are supported by previous studies demonstrating that γδ T cell–deficient mice had a severe defect in their ability to clear intradermal S. aureus inoculation (20), that mice deficient in both IL-17A and IL-17F develop spontaneous S. aureus skin infections (52), and that a large proportion of the resident epidermal γδ T cells constitutively express the retinoid-related orphan receptor–γt (RORγt) transcription factor, which is required for IL-17 production (53).
In the present study, we found that after S. aureus cutaneous challenge, there was little IFN-γ production, which was equivalent in γδ T cell–deficient and WT mice. Furthermore, IFN-γR–deficient mice had no impairment in host defense against S. aureus skin infection. In contrast, in a surgical thigh muscle wound S. aureus infection mouse model, IFN-γ promoted neutrophil chemokine production and neutrophil recruitment (19, 54). In addition, in response to intravenous S. aureus challenge, IFN-γ production was associated with natural or vaccine-induced protection (55, 56). However, since these studies did not involve epithelial sites, the role of IL-17–producing intraepithelial γδ T cells would be diminished. Thus, the type of cytokine response required for protection against S. aureus may depend on the site of infection: IL-17 for skin infections (and perhaps other epithelia; refs. 52, 57), and IFN-γ for invasive and systemic infections (55, 56). This key role for IL-17 against S. aureus skin infection has direct implications in future vaccine development, which may depend on the generation of IL-17–producing memory T cells at cutaneous sites. The critical role of vaccine-induced IL-17 at epithelial sites has been previously demonstrated against Helicobacter pylori gut infection and Mycobacterium tuberculosis, Streptococcus pneumonia, and Pseudomonas aeruginosa lung infections (58–61). However, both IL-17– and IFN-γ–producing memory T cells may be required for vaccine protection against S. aureus at all sites of infection. It should be mentioned that any therapeutic strategy aimed at modulating IL-17 responses would need to be tightly controlled, since a previous study demonstrated that a neutrophil-rich environment, which may be induced by IL-17, could potentiate S. aureus pathogenesis by facilitating bacterial survival within the neutrophils (54).
Finally, we have demonstrated that IL-17 production by skin γδ T cells required IL-1R/MyD88 signaling, TLR2/MyD88 signaling, and IL-23. We have previously demonstrated that IL-1R/MyD88 is a more critical determinant than TLR2/MyD88 for neutrophil recruitment to a site of S. aureus cutaneous infection (21). Therefore, we have defined a key host defense circuit, whereby IL-1 (along with TLR2 and IL-23) promotes neutrophil recruitment to a site of cutaneous S. aureus infection by inducing IL-17 production by resident skin γδ T cells. A similar host defense circuit may exist in humans, since pediatric patients deficient in signaling molecules downstream of IL-1R and TLR2, including MyD88 and IRAK4, are highly susceptible to S. aureus skin infection (62, 63). These human subjects and patients with hyper-IgE syndrome all share in common increased susceptibility to S. aureus skin infection, providing further evidence of a possible link among IL-1, TLR2, and IL-17 in the human immune response against S. aureus cutaneous infection. From a therapeutic point of view, augmentation of the downstream IL-17 portion of this host defense circuit may provide a basis for novel therapeutic strategies to combat S. aureus cutaneous infections. This may be particularly relevant for providing new immunomodulatory therapy or vaccine development to treat S. aureus infections in humans, especially in patients with altered T cell function with deficient or dysregulated Th17 responses.
Methods
S. aureus strains.
All data were obtained using the bioluminescent S. aureus SH1000 strain ALC2906, which possesses the shuttle plasmid pSK236 with the penicillin-binding protein 2 (pbp2) promoter fused to the luxABCDE reporter cassette from Photorhabdus luminescens as previously described (21, 22). This strain emits bioluminescent signals from live, actively metabolizing bacteria in all stages of the S. aureus life cycle (21, 22).
Preparation of S. aureus for skin inoculation.
The S. aureus SH1000 strain has a chloramphenicol resistance plasmid selection marker, and all cultures were performed in the presence of chloramphenicol (10 μg/ml; Sigma-Aldrich). S. aureus was streaked onto tryptic soy agar (tryptic soy broth plus 1.5% bacto agar; BD Biosciences) and grown overnight at 37°C in a bacterial incubator. Single colonies of S. aureus were placed into tryptic soy broth and grown overnight at 37°C in a bacterial shaking incubator. Midlogarithmic phase bacteria were obtained after a 3-hour subculture of a 1:50 dilution of the overnight culture. Bacterial cells were pelleted, resuspended, and washed 3 times in PBS. Bacterial concentrations were estimated by measuring the absorbance at 600 nm (A600; DU 640B spectrophotometer; Beckman Coulter). CFUs were verified by plating dilutions of the inoculum overnight.
Mice.
Male congenic mice on a C57BL/6 genetic background were used in all experiments. γδ T cell–deficient mice (Tcrd–/–; B6.129P2-Tcrdtm1Mom/J), αβ T cell–deficient mice (Tcrb–/–; B6.129P2-Tcrbtm1Mom/J), IL-1R–deficient mice (B6.129S7-Il1r1tm1Imx/J), IL-12p35–deficient mice (B6.129S1-Il12atm1Jm/J), IL-12/23p40–deficient mice (B6.129S1-Il12btm1Jm/J), and WT C57BL/6J control mice were obtained from Jackson Laboratories. MyD88-deficient mice were a gift from S. Akira (Osaka University, Osaka, Japan). IL-23p19–deficient mice were obtained from N. Ghilardi (Genentech Inc., San Francisco, California, USA). IL-17R–deficient mice (Il17ra–/–) have been previously described (64). All mouse colonies were maintained at UCLA in autoclaved cages under specific pathogen–free conditions.
Mouse model of cutaneous S. aureus infection.
All procedures were approved by the UCLA Animal Research Committee. The mice were shaved on the back and inoculated intradermally with midlogarithmic growth phase S. aureus SH1000 strain (2 × 106 CFUs) in 100 μl sterile pharmacy-grade saline (0.9%) using a 27-gauge insulin syringe (Abbott Laboratories). Measurements of total lesion size (cm2) were made by analyzing digital photographs (Nikon Coolpix 5400) of mice taken every 1–3 days using the software program Image J ( http://rsbweb.nih.gov/ij/) and a millimeter ruler as a reference.
Quantification of in vivo S. aureus by in vivo bioluminescence and CFUs.
Mice were anesthetized via inhalation of 2% isoflurane, and in vivo bioluminescence imaging was performed using the Xenogen IVIS imaging system (Caliper Life Sciences) at the Crump Institute for Molecular Imaging, UCLA, as previously described (21, 22). Data are presented on color scale overlaid on a grayscale photograph of mice and were quantified as total flux (photons/s) within a circular region of interest (1 × 103 pixels) using Living Image software (Xenogen), with a lower limit of detection of 1 × 104 photons/s. In some experiments, to confirm that the in vivo bioluminescence signals accurately reflected bacterial burden in vivo, S. aureus CFUs were determined after overnight cultures of homogenized (Pro200 Series homogenizer; Pro Scientific) 8-mm punch biopsy (Acuderm) specimens of lesional skin taken at days 1 and 3 after inoculation.
Tissue embedding and staining.
For histological analysis, lesional 8-mm punch biopsy (Acuderm) skin specimens were bisected; one-half was fixed in 10% formalin and embedded in paraffin, and the other half was embedded in Tissue-Tek OTC compound (Sakura Finetek) and frozen in liquid nitrogen. H&E and Gram stains were performed on paraffin sections (4 μm) by the Tissue Procurement and Histology Core Laboratory and Histopathology Laboratory, UCLA, according to guidelines for clinical samples.
Immunoperoxidase labeling.
Detection of Gr-1–positive (Ly-6G+), CD3+, and γδ T cell–positive (GL3+) cells on frozen cryostat specimens of lesional skin punch biopsy specimens was performed using the immunoperoxidase method, as previously described (21, 22), with the following mAbs: biotinylated rat anti-mouse Gr-1 (1 μg/ml; clone RB6-8C5, IgG2b isotype; BD Biosciences — Pharmingen), hamster anti-mouse CD3ε (5 μg/ml; clone 145-2c11, IgG1 isotype; BD Biosciences — Pharmingen), hamster anti-mouse TCRγδ complex (5 μg/ml; clone GL3, IgG2 isotype; BD Biosciences — Pharmingen), and corresponding isotype controls. In some cases, primary antibody binding was detected using biotin-labeled mouse anti-hamster IgG cocktail (5 μg/ml; clone G70-204, G94-56, IgG1, and IgG2b isotypes; BD Biosciences — Pharmingen).
MPO assay.
MPO enzymatic activity was determined from homogenized (Pro200 Series homogenizer; Pro Scientific) 8-mm punch biopsy (Acuderm) lesional skin specimens using an MPO assay kit according to the manufacturer’s recommendations (Cytostore). The amount of MPO activity was normalized to the weight of the tissue specimen, and data were presented as U/mg tissue.
Protein arrays.
Protein levels of KC, MIP2, GM-CSF, and IFN-γ were determined from homogenized (Pro200 Series homogenizer; Pro Scientific) 8-mm punch biopsy (Acuderm) lesional skin specimens using SearchLight Chemiluminescent Assays according to the manufacturer’s recommendations (Aushon Biosystems). The levels of these cytokines and chemokines were normalized to the weight of the tissue specimen, and data were presented as pg/mg tissue.
Separation of epidermis and dermis and ex vivo epidermal CD3+ T cell or γδ T cell enrichment.
To separate the epidermis from the dermis, whole skin biopsy specimens were incubated in RPMI containing dispase II (2.4 U/ml; Roche) for 1 hour at 37°C, according to the manufacturer’s recommendations. For Q-PCR analysis, epidermal and dermal specimens were separated and placed into TRIzol (Invitrogen) for RNA extraction as described below. In some experiments, epidermal cell suspensions were generated by incubation of the epidermal specimens in 0.05% trypsin-EDTA (Invitrogen) for 30 minutes at 37°C. A single-cell suspension was obtained by mechanical disruption of tissues on 70-μm nylon cell-strainer filters (BD Biosciences — Falcon). CD3+ or γδ T cells were positively selected from cell suspensions by labeling with a biotinylated anti-CD3 mAb or a γδ T cell–specific mAb (GL3; BD Biosciences), respectively, followed by separation with anti-biotin conjugated magnetic beads on a MACS MS column, according to the manufacturer’s instructions (Miltenyi Biotec). Positive fractions contained greater than 90% CD3+ T cells or GL3+ γδ T cells, and negative epidermal fractions contained less than 1% CD3+ T cells or GL3+ T γδ cells, as determined by flow cytometry (data not shown).
RNA extraction and mRNA quantification.
Total RNA from homogenized (Pro200 Series homogenizer; Pro Scientific) 8-mm lesional skin biopsy (Acuderm) specimens, dermal and epidermal specimens, and ex vivo epidermal cell suspensions was extracted by TRIzol reagent (Invitrogen) according to the manufacturer’s recommendations. Q-PCR reactions were performed as previously described (21, 22). TaqMan Gene Expression Assays primer and probes sets for KC, MIP2, GMCSF, IFN-γ, IL-21, IL-22, and the normalizer GAPDH were purchased from Applied Biosystems, and TaqMan primers and probes for IL-17A and IL-17F were previously published (65). The relative quantities of mRNA per sample were determined using the ΔΔCt formula as previously described (21).
Flow cytometry.
Epidermal single-cell suspension preparations are described above. In certain experiments, whole skin single-cell suspensions were prepared with collagenase digestion as previously described (66). Cell surface expression for CD3, TCRγδ complex, and the Vγ5 chain on epidermal cell suspensions or whole skin specimens was performed using specific fluorescently conjugated mAbs, including the following: PE-conjugated hamster anti-mouse CD3ε (5 μg/ml; clone 145-2c11, IgG1 isotype; BD Biosciences — Pharmingen), APC-conjugated hamster anti-TCRγδ complex (5 μg/ml; clone GL3, IgG2 isotype; BD Biosciences — Pharmingen), FITC-conjugated hamster anti-Vγ5 (5 μg/ml; clone 536, IgG1 isotype; BD Biosciences) and corresponding fluorescently conjugated isotype controls. Two- and 3-color flow cytometry was performed on a BD FACSCalibur Flow Cytometer (BD Biosciences) and analyzed with FlowJo software (Treestar).
ELISA.
GL3+ epidermal γδ T cells were positively selected from epidermal cell suspensions as described above and then stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 750 ng/ml ionomycin (Sigma-Aldrich) with or without rIL-1 (20 ng/ml), rIL-23 (20 ng/ml), or both rIL-1 and rIL-23 (all from R&D Systems) for 24 hours. Culture supernatants were collected, and IL-17 levels in the supernatants were determined by IL-17 DuoSet ELISA (R&D Systems) according to the manufacturer’s instructions.
Fluorescence microscopy of epidermal sheets.
Epidermal sheets from WT, IL-1R–deficient, and MyD88-deficient mice were prepared from mouse ears as previously described (67). Briefly, mouse ears were split into dorsal and ventral sides and floated dermal side–down for 2 hours at 37°C in 20 mM EDTA. Epidermal sheets were separated from the dermis, washed in PBS, and fixed in cold acetone for 20 minutes at –20°C. Epidermal sheets were subsequently washed in PBS, blocked for 1 hour at 25°C with 2% (w/v) BSA in PBS, and stained overnight at 4°C with a FITC-conjugated mAb specific for TCR Vγ5 (clone 536; BD Biosystems). Sheets were washed thoroughly in PBS, and bound antibodies were detected for 90 minutes at 37°C with an anti-FITC Alexa Fluor 488–conjugated antibody (Invitrogen). After extensive washing, epidermal sheets were mounted onto slides with Prolong Gold mounting medium (Invitrogen) and were examined with a Leica Microsystems inverted confocal microscope equipped with a ×63 oil immersion objective.
Administration of rIL-17.
A single dose of rIL-17 (1,000 ng/100 μl; R&D Systems) in sterile pharmacy-grade saline, or vehicle alone (saline), was mixed and administered intradermally along with the inoculum of S. aureus (2 × 106 CFUs in 100 μl PBS) to γδ T cell–deficient mice. Similar doses of rIL-17 (500–1,000 ng) were previously shown to be biologically active in mouse skin in vivo (68, 69). We demonstrated that rIL-17 did not have any direct bactericidal or bacteriostatic activity against S. aureus in vitro (Supplemental Figure 6).
Monitoring growth of S. aureus in the presence of rIL-17.
To determine whether rIL-17 has a direct effect in the growth of S. aureus, the growth responses of S. aureus were monitored in RPMI (with 10% serum) in the presence of rIL-17 at various concentrations as previously described (70). Briefly, RPMI cultures of 106 CFU midlogarithmic growth phase S. aureus were supplemented with 0, 20, 100, and 500 ng rIL-17 and incubated at 37°C in a humidified incubator with 5% CO2 for 5 hours, with intermittent shaking. Culture aliquots were serially diluted and cultured overnight on tryptic soy agar plates (tryptic soy broth plus 1.5% Bacto-agar; BD Biosciences) supplemented with 10 μg/ml chloramphenicol. The resulting bacterial colonies were counted manually and expressed as total CFU number.
Statistics.
Data were compared using 2-tailed Student’s t test. All data are expressed as mean ± SEM as indicated. P values less than 0.05, 0.01, and 0.001 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Shizuo Akira for the gift of MyD88-deficient mice and Nico P. Ghilardi for the gift of the IL-23p19–deficient mice. We also thank Ping Fu and Christopher Creencia (UCLA Tissue Procurement and Histology Core Laboratory) and Saeedeh Shapourifar-Tehrani (UCLA Histopathology Laboratory) for their expertise with embedding, cutting, and H&E and Gram staining of tissue sections. Fluorescence microscopy was performed at the California NanoSystems Institute (CNSI) Advanced Light Microscopy/Spectroscopy Shared Facility at UCLA. This work was supported in part by grants R01 AI078910 and R03 AR054534 (to L.S. Miller), R37 AI047868 (to R.L. Modlin), R01 AI056154 (to G. Cheng), and UCLA Small Animal Imaging Resource Program (SAIRP) R24 CA92865 from the NIH.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(5):1762–1773. doi:10.1172/JCI40891.
References
- 1.McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. . Emerg Infect Dis. 2006;12(11):1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran GJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. . N Engl J Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. . JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Deleo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. . J Clin Invest. 2009;119(9):2464–2474. doi: 10.1172/JCI38226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. . Clin Infect Dis. 2008;46(Suppl 5):S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 6.Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. . Infect Immun. 2000;68(11):6162–6167. doi: 10.1128/IAI.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. . Infect Immun. 1997;65(7):2517–2521. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15(5):578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Barca E, Carratala J, Mykietiuk A, Fernandez-Sevilla A, Gudiol F. Predisposing factors and outcome of Staphylococcus aureus bacteremia in neutropenic patients with cancer. . Eur J Clin Microbiol Infect Dis. 2001;20(2):117–119. doi: 10.1007/s100960000431. [DOI] [PubMed] [Google Scholar]
- 10.Mathews WC, et al. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. . J Acquir Immune Defic Syndr. 2005;40(2):155–160. doi: 10.1097/01.qai.0000179464.40948.b9. [DOI] [PubMed] [Google Scholar]
- 11.Manfredi R, Calza L, Chiodo F. Epidemiology and microbiology of cellulitis and bacterial soft tissue infection during HIV disease: a 10-year survey. . J Cutan Pathol. 2002;29(3):168–172. doi: 10.1034/j.1600-0560.2002.290307.x. [DOI] [PubMed] [Google Scholar]
- 12.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 13.Ong PY, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 14.Cho SH, Strickland I, Tomkinson A, Fehringer AP, Gelfand EW, Leung DY. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. . J Invest Dermatol. 2001;116(5):658–663. doi: 10.1046/j.0022-202x.2001.01331.x. [DOI] [PubMed] [Google Scholar]
- 15.Grimbacher B, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 16.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205(7):1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renner ED, et al. Novel signal transducer and activator of transcription 3 (STAT3) mutations, reduced T(H)17 cell numbers, and variably defective STAT3 phosphorylation in hyper-IgE syndrome. J Allergy Clin Immunol. 2008;122(1):181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLoughlin RM, et al. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. . Proc Natl Acad Sci U S A. 2006;103(27):10408–10413. doi: 10.1073/pnas.0508961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molne L, Corthay A, Holmdahl R, Tarkowski A. Role of gamma/delta T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. . Clin Exp Immunol. 2003;132(2):209–215. doi: 10.1046/j.1365-2249.2003.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller LS, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. . Immunity. 2006;24(1):79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Miller LS, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus In Vivo. . J Immunol. 2007;179(10):6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 23.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. . J Bacteriol. 2002;184(19):5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke SR, et al. The Staphylococcus aureus Surface Protein IsdA Mediates Resistance to Innate Defenses of Human Skin. . Cell Host & Microbe. 2007;1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Fossiez F, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26(6):748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 27.Kawaguchi M, et al. Induction of granulocyte-macrophage colony-stimulating factor by a new cytokine, ML-1 (IL-17F), via Raf I-MEK-ERK pathway. J Allergy Clin Immunol. 2004;114(2):444–450. doi: 10.1016/j.jaci.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 28.Aujla SJ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 30.Laan M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. . J Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 31.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 32.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Sasson SZ, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106(17):7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 36.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8(9):942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 38.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 39.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Caruso R, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009;15(9):1013–1015. doi: 10.1038/nm.1995. [DOI] [PubMed] [Google Scholar]
- 42.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 43.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 44.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10(1):75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 45.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445(7128):648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 47.Hedrick MN, et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest. 2009;119(8):2317–2329. doi: 10.1172/JCI37378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guttman-Yassky E, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181(10):7420–7427. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pene J, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180(11):7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 51.Modlin RL, et al. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- 52.Ishigame H, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205(6):1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McLoughlin RM, Lee JC, Kasper DL, Tzianabos AO. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. . J Immunol. 2008;181(2):1323–1332. doi: 10.4049/jimmunol.181.2.1323. [DOI] [PubMed] [Google Scholar]
- 55.Gaudreau MC, Lacasse P, Talbot BG. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus. . Vaccine. 2007;25(5):814–824. doi: 10.1016/j.vaccine.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 56.Zhao YX, Tarkowski A. Impact of interferon-gamma receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. . J Immunol. 1995;155(12):5736–5742. [PubMed] [Google Scholar]
- 57.Chung DR, et al. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170(4):1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 58.DeLyria ES, Redline RW, Blanchard TG. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 2009;136(1):247–256. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferreira DM, et al. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin Vaccine Immunol. 2009;16(5):636–645. doi: 10.1128/CVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priebe GP, et al. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. . J Immunol. 2008;181(7):4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. . Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 62.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299(5615):2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 63.von BH, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321(5889):691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagata T, McKinley L, Peschon JJ, Alcorn JF, Aujla SJ, Kolls JK. Requirement of IL-17RA in Con A induced hepatitis and negative regulation of IL-17 production in mouse T cells. J Immunol. 2008;181(11):7473–7479. doi: 10.4049/jimmunol.181.11.7473. [DOI] [PubMed] [Google Scholar]
- 66.Schaerli P, et al. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. 2004;199(9):1265–1275. doi: 10.1084/jem.20032177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strid J, et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9(2):146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 68.Harper EG, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129(9):2175–2183. doi: 10.1038/jid.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopez KS, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182(5):3039–3046. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanangat S, et al. Enhanced extracellular growth of Staphylococcus aureus in the presence of selected linear peptide fragments of human interleukin (IL)-1beta and IL-1 receptor antagonist. . J Infect Dis. 2001;183(1):65–69. doi: 10.1086/317645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.