Abstract
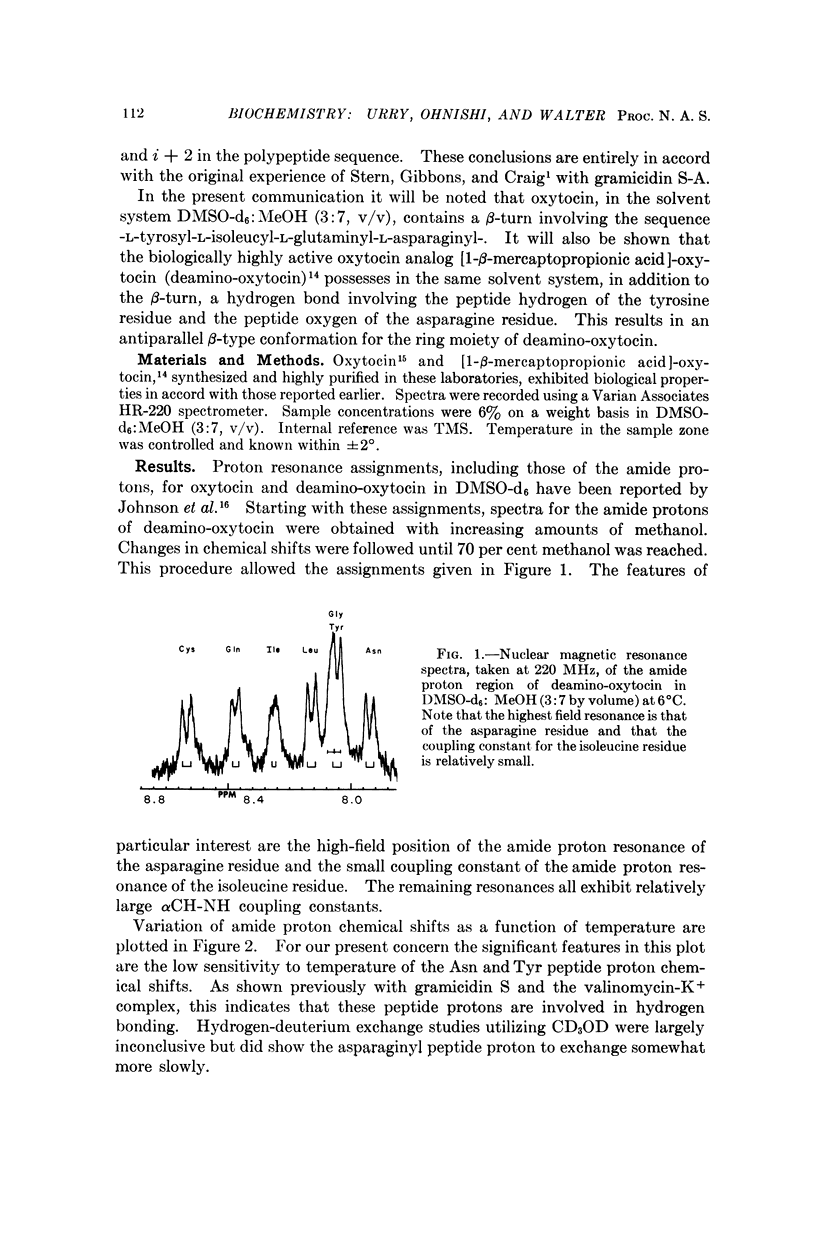
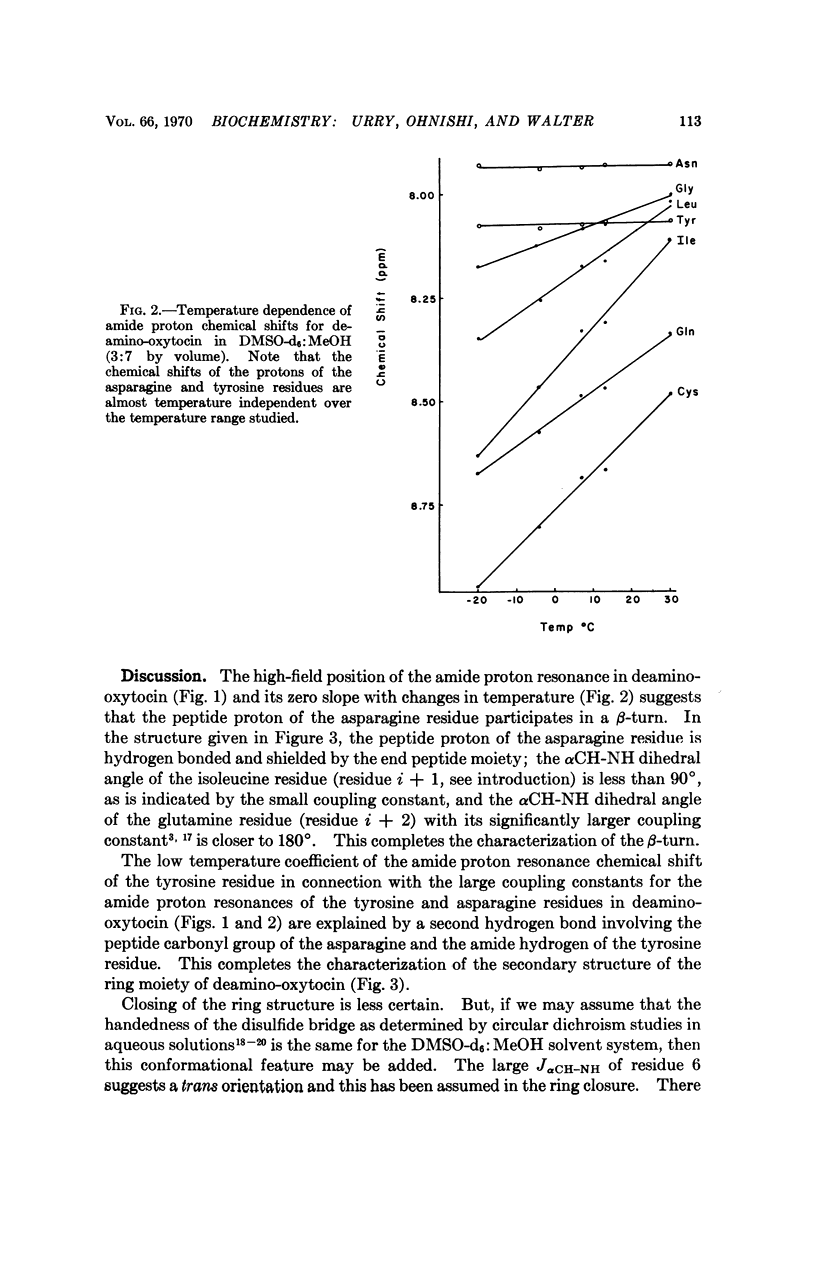
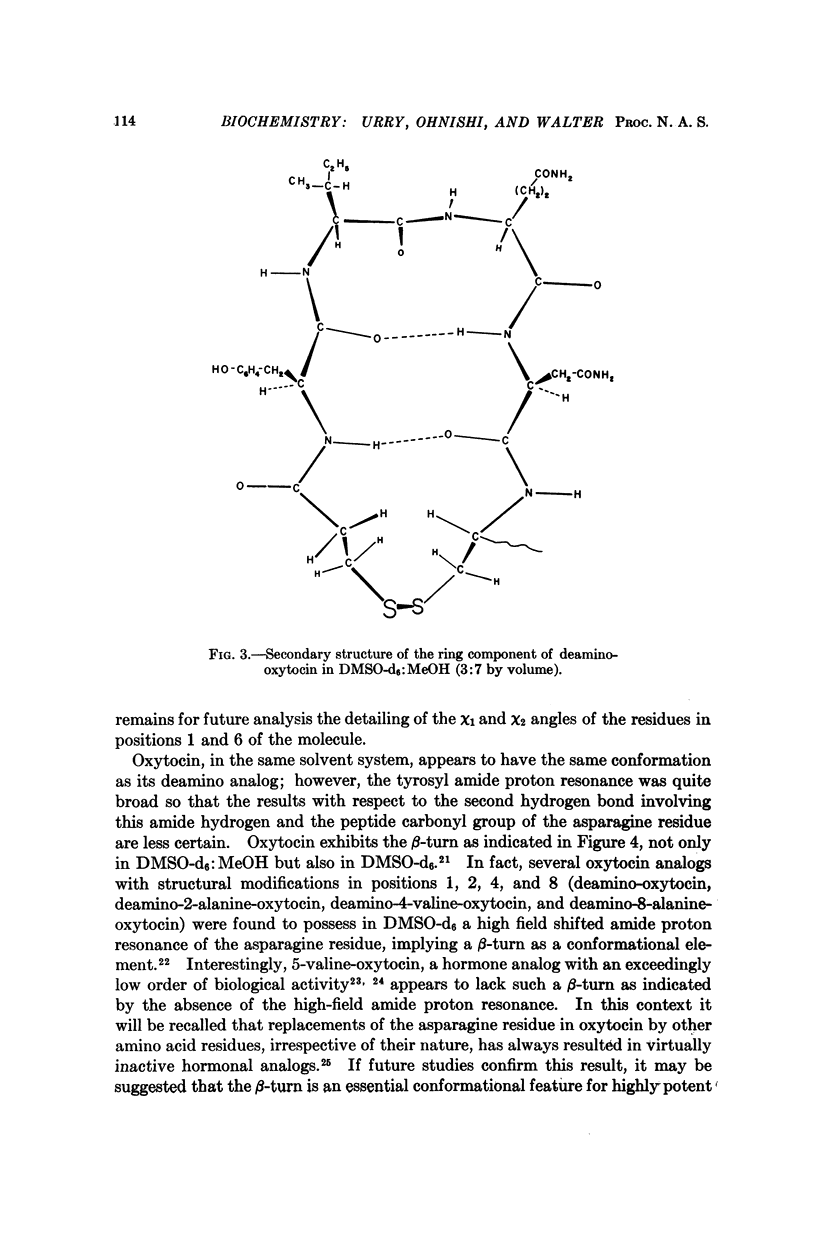
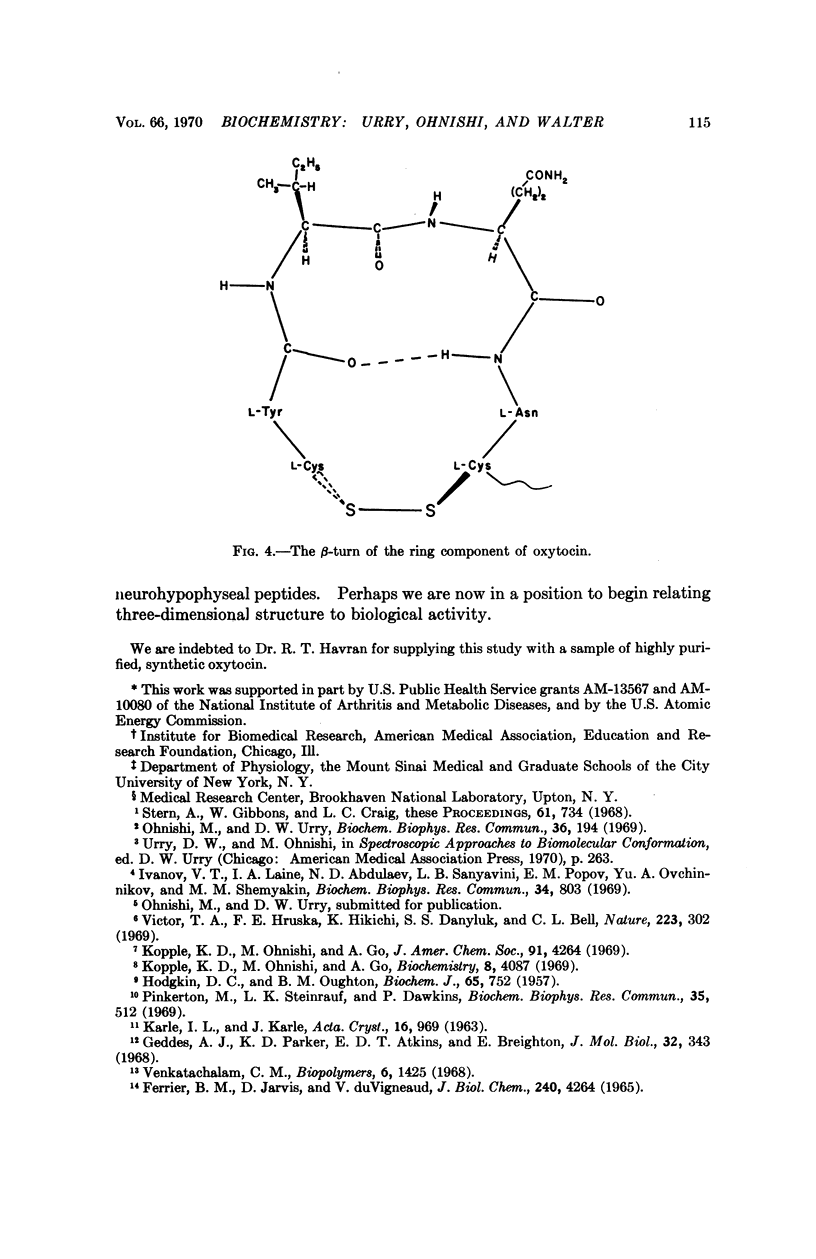
The secondary structure of the cyclic moiety of oxytocin and deamino-oxytocin has been determined by nuclear magnetic resonance spectroscopy (220 MHz). Oxytocin, in a dimethylsulfoxide-methanol mixture, contains a β-turn involving the sequence -L-tyrosyl-L-isoleucyl-L-glutaminyl-L-asparaginyl-. Deamino-oxytocin, in addition to the β-turn, contains a hydrogen bond involving the amide hydrogen of the tyrosine residue and the peptide carbonyl group of the asparagine residue, resulting in an antiparallel β-type conformation for the ring component. An initial attempt has been made to relate conformational features of the hormonal peptides to their biological activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bystrov V. F., Portnova S. L., Tsetlin V. I., Ivanov V. T., Ovchinnikov Y. A. Conformational studies of peptide systems. The rotational states of the NH--CH fragment of alanine dipeptides by nuclear magnetic resonance. Tetrahedron. 1969 Feb;25(3):493–515. doi: 10.1016/s0040-4020(01)83261-0. [DOI] [PubMed] [Google Scholar]
- Eggena P., Schwartz I. L., Walter R. A sensitive hydroosmotic toad bladder assay. Affinity and intrinsic activity of neurohypophyseal peptides. J Gen Physiol. 1968 Sep;52(3):465–481. doi: 10.1085/jgp.52.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier B. M., Jarvis D., Du Vigneaud V. Deamino-oxytocin. Its isolation by partition chromatography on Sephadex and crystallization from water, and its biological activities. J Biol Chem. 1965 Nov;240(11):4264–4266. [PubMed] [Google Scholar]
- Geddes A. J., Parker K. D., Atkins E. D., Beighton E. "Cross-beta" conformation in proteins. J Mol Biol. 1968 Mar 14;32(2):343–358. doi: 10.1016/0022-2836(68)90014-4. [DOI] [PubMed] [Google Scholar]
- HODGKIN D. C., OUGHTON B. M. Possible molecular models for gramicidin S and their relationship to present ideas of protein structure. Biochem J. 1957 Apr;65(4):752–756. doi: 10.1042/bj0650752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V. T., Laine I. A., Abdulaev N. D., Senyavina L. B., Popov E. M. The physicochemical basis of the functioning of biological membranes: the conformation of valinomycin and its K+ complex in solution. Biochem Biophys Res Commun. 1969 Mar 31;34(6):803–811. doi: 10.1016/0006-291x(69)90251-4. [DOI] [PubMed] [Google Scholar]
- Johnson L. F., Schwartz I. L., Walter R. Oxytocin and neurohypophyseal peptides: spectral assignment and conformational analysis by 220 MHz nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1269–1275. doi: 10.1073/pnas.64.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopple K. D., Ohnishi M., Go A. Conformations of cyclic peptides. IV. Nuclear magnetic resonance studies of cyclo-pentaglycyl-L-leucyl and cyclo-diglycyl-L-histidyldiglycyl-L-tyrosyl. Biochemistry. 1969 Oct;8(10):4087–4095. doi: 10.1021/bi00838a028. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Urry D. W. Temperature dependence of amide proton chemical shifts: the secondary structures of gramicidin S and valinomycin. Biochem Biophys Res Commun. 1969 Jul 23;36(2):194–202. doi: 10.1016/0006-291x(69)90314-3. [DOI] [PubMed] [Google Scholar]
- Pinkerton M., Steinrauf L. K., Dawkins P. The molecular structure and some transport properties of valinomycin. Biochem Biophys Res Commun. 1969 May 22;35(4):512–518. doi: 10.1016/0006-291x(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Stern A., Gibbons W. A., Craig L. C. A conformational analysis of gramicidin S-A by nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1968 Oct;61(2):734–741. doi: 10.1073/pnas.61.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Quadrifoglio F., Walter R., Schwartz I. L. Conformational studies on neurohypophyseal hormones: the disulfide bridge of oxytocin. Proc Natl Acad Sci U S A. 1968 Jul;60(3):967–974. doi: 10.1073/pnas.60.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Victor T. A., Hruska F. E., Hikichi K., Danyluk S. S., Bell C. L. Nuclear magnetic resonance study of the structure and interactions of actinomycin D: temperature and solvent effects on the N--H and NH2 groups. Nature. 1969 Jul 19;223(5203):302–303. doi: 10.1038/223302a0. [DOI] [PubMed] [Google Scholar]
- Walter R., Rudinger J., Schwartz I. L. Chemistry and structure-activity relations of the antidiuretic hormones. Am J Med. 1967 May;42(5):653–677. doi: 10.1016/0002-9343(67)90087-3. [DOI] [PubMed] [Google Scholar]
- Walter R., Schwartz I. L. 5-Valine-oxytocin and 1-deamino-5-valine-oxytocin. Syntheses and some pharmacological properties. J Biol Chem. 1966 Dec 10;241(23):5500–5503. [PubMed] [Google Scholar]


