Abstract
Objectives/Hypothesis
To demonstrate the feasibility of optical coherence tomography in microstructural imaging of the porcine cochlea.
Study Design
Ex vivo, porcine model.
Methods
Optical coherence tomographic images of the porcine cochlea were obtained by thinning the bone from the basal turn of the cochlea leaving the endosteum intact. The images were compared with the corresponding histological sections.
Results
In the areas of thinned bone, images were obtained of the stria vascularis, Reissner’s membrane, basilar membrane, tectorial membrane, scala media, scala tympani, and scala vestibuli. The bone was too thick for adequate light penetration in the areas where it was not thinned. Good histological correlation was obtained.
Conclusions
Cochlear and vestibular microanatomic structures of the pig cochlea were clearly identified with histological confirmation, suggesting the potential application of this noninvasive imaging modality for in vivo imaging of the human cochlea.
Keywords: Optical coherence tomography, cochlea, cochlear implantation, porcine
INTRODUCTION
Optical coherence tomography (OCT) is an emerging, noninvasive imaging modality that uses low-coherence interferometry to produce a two-dimensional image of internal tissue microstructures.1 It uses light to discern intrinsic differences in tissue structure and uses coherence gating to localize the origin of the reflected optic signal. Internal tissue microstructures can be visualized using OCT with excellent axial and lateral spatial resolution on the order of 10 μm, and of a depth of penetration of approximately 2 to 3 mm depending on tissue translucency.
Over the last two decades since its inception, OCT has become widely-established for its clinical applications in the fields of ophthalmology and dermatology for visualizing the more translucent tissues of the eye2 and the more superficial tissues of the skin,3 respectively. Clinical use of OCT has since been extended to the fields of cardiology and gastroenterology in visualizing deeper structures such as coronary vessels4 and the gastrointestinal tract.5 Most recently it has been used in the field of otolaryngology to visualize the larynx,6 but use of OCT clinically has largely been limited by its relatively limited depth of penetration, due to the turbidity of most biological tissues. Novel delivery systems and routes of access have broadened the scope of clinical application for OCT.
In this study, we aim to extend the clinical use of OCT to the cochlea. Successful OCT imaging of the cochlear microstructures has recently been demonstrated in the rat;7,8 however, these studies posit that thick human bone would pose a technical challenge for this technology. This will be the first study to date to provide anatomic information on the microstructural features of the pig cochlea using OCT. By successfully performing intracochlear OCT imaging on the pig cochlea, which more closely approximates the human cochlea in bone thickness, we have shown this technology to be of potential value in guiding microsurgical procedures on the human cochlea, such as cochlear implantation (CI) or other inner ear surgeries in the future. Specifically we intend to show that OCT has the potential of providing neurotologists intraoperative feedback on the correct placement of the cochleostomy, before opening the endostium in hearing-preservation CI.
MATERIALS AND METHODS
Three fresh porcine heads were obtained within 1 hour of sacrifice from a commercial supplier (Sierra, Irvine, CA). Soft tissue was removed from the cranium. Six intact otic capsules were extracted from the temporal bones, isolated, and placed in cold saline. Three of the otic capsules were drilled to expose the basal turn of the cochlea, while the stapes was removed from the other three to expose the oval window. The bone over the cochlea was drilled with a high-speed diamond burr until only a thin bony lamina or endosteum remained. The endosteum was not penetrated.
OCT uses near-infrared light to produce real-time, high-resolution (1–10 μm), cross-sectional images of in situ tissue microstructure without ionizing radiation. OCT imaging was performed under a University of California, Irvine, Institutional Review Board-approved protocol. The OCT device consists of a noncontact probe positioned over an imaging stage that can be adjusted in the vertical and horizontal planes (Fig. 1). The specimen was placed on the stage and the OCT beam moved over the specimen at eight frames per second, producing two-dimensional OCT images. Image intensity was proportional to the amount of reflected light in a given region of interest. Image size was 1.87 mm laterally (10 μm resolution) and 2.56 mm in depth (7 μm resolution). After imaging, the cochleae were fixed (10% formalin), embedded, and sectioned for histological comparison.
Fig. 1.
Optical coherence tomography imaging stage.
RESULTS
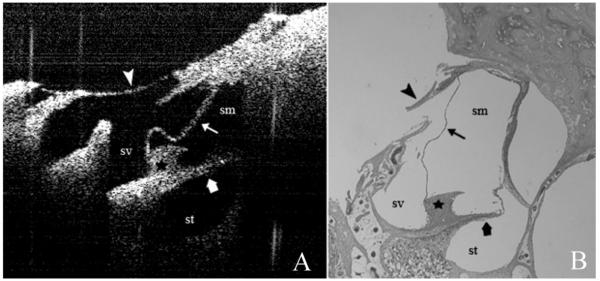
In the areas of thinned bone, images were obtained of the spiral ligament, stria vascularis, Reissner’s membrane, basilar membrane, tectorial membrane, scala media (SM), scala tympani, and scala vestibule (SV) (Fig. 2A) and compared with the corresponding histological section (Fig. 2B). The bone was too thick for adequate light penetration in the areas where it was not thinned.
Fig. 2.
(A) Optical coherence tomography image of the basal turn of the cochlea as seen through the thinned promontory. (B) Corresponding hematoxylin and eosin stained histological section (please note that due to sectioning artifact, there is a disruption of the thinned bone of the promontory). Arrow head, thinned bone of the promontory; thin arrow, Reissner membrane; wide arrow, osseous spiral lamina; star, spiral limbus; sv, scala vestibule; sm, scala media; st, scala tympani.
DISCUSSION
Hearing preservation approach for CI is becoming more and more prevalent. When performing the cochleostomy in a hearing preservation approach, it is important to not penetrate the SM. It has been found that the position of the spiral ligament in the basal turn and the hook region is variable.9 Localization of the spiral ligament before opening the basal turn endosteum in the soft technique will help to prevent inadvertent SM or SV electrode insertion. Potential misdirection of the electrode can lead to SM, SV, vestibular,10 extracochlear, intrameatal, or carotid canal11 array insertion, or injuring the spiral ligament or stria vascularis thus leading to total hearing loss.
At present, there are no imaging modalities that can provide tomographic information on cochlear microstructure. The purpose of this study was to evaluate OCT, which uses safe, low energy, nonionizing light, for imaging of the cochlear microanatomy before cochleostomy to allow accurate placement of cochlear implant arrays. The previous studies on OCT of the cochlea were conducted in rats and posited that the thick bone of the promontory in humans would cause near total loss of the backscattered light signal and thus prevent useful imaging. Porcine cochleae were chosen for this study because the osseous thickness more closely approaches that of humans. After drilling, intracochlear and vestibular microanatomic structures were clearly identified. This is the first study to date to provide anatomic information on the structural features of the pig cochlea using OCT, and the images reflect the ability of OCT to provide micron scale information of internal cochlear structure in real time. Our results suggest that OCT can be a useful adjunct in hearing preservation CI or the future of neurotology and inner ear surgery. OCT probes small enough to image small to medium sized blood vessels already exist. Therefore, the technology will just have to be adapted to probes that are suitable for microsurgery of the ear.4
As stated above, the radiation is near the infrared portion of the electromagnetic spectrum, and, therefore, is nonionizing. The energy content is very low and does not lead to heating at the exposure times needed to obtain the necessary information.6 As an example, it is used safely to image the delicate tissue of the retina,2 and, thus, should also be safe for the cochlea.
CONCLUSION
This study demonstrates the viability of visualizing the porcine cochlear microstructural anatomy. As this imaging modality is noncontact and uses a safe near-infrared light source, OCT may in the future provide a means to image microstructural changes in the inner ear in vivo while preserving inner ear integrity. It has the potential to become an indispensable surgical tool whenever detailed information about microanatomy is essential for accurate surgical guidance, for example in accurate electrode placement during hearing-preservation CI. This study was conducted in otic capsules that were extracted from the remainder of the temporal bone. Further research is needed to evaluate the feasibility of OCT assisted CI through transmastoid surgical exposure of the middle ear.
Acknowledgment
The authors thank Lih-Huei Liaw for performing the histology preparation.
Supported by National Institutes of Health (DC 006026, CA 91717, EB 00293, RR 01192, M01-RR00827-28), Flight Attendant Medical Research Institute (32456), State of California Tobacco Related Disease Research Program (12RT-0113), and Air Force Office of Scientific Research (F49620-00-1-0371).
Footnotes
Presented at the Annual Meeting and OTO EXPO, Washington, DC, September 16–19, 2007.
This work was done at Beckman Laser Institute and Medical Clinic, University of California—Irvine, Irvine, California.
BIBLIOGRAPHY
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112:1584–1589. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 3.Welzel J. Optical coherence tomography in dermatology: a review. Skin Res Technol. 2001;7:1–9. doi: 10.1034/j.1600-0846.2001.007001001.x. [DOI] [PubMed] [Google Scholar]
- 4.Jang IK, Bouma BE, Kang DH, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 5.Shen B, Zuccaro G., Jr Optical coherence tomography in the gastrointestinal tract. Gastrointest Endosc Clin North Am. 2004;14:551–571. doi: 10.1016/j.giec.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Wong BJ, Jackson RP, Guo S, et al. In vivo optical coherence tomography of the human larynx: normative and benign pathology in 82 patients. Laryngoscope. 2005;115:1904–1911. doi: 10.1097/01.MLG.0000181465.17744.BE. [DOI] [PubMed] [Google Scholar]
- 7.Wong BJ, de Boer JF, Park BH, et al. Optical coherence tomography of the rat cochlea. J Biomed Opt. 2000;5:367–370. doi: 10.1117/1.1310165. [DOI] [PubMed] [Google Scholar]
- 8.Wong BJ, Zhao Y, Yamaguchi M, et al. Imaging the internal structure of the rat cochlea using optical coherence tomography at 0.827 microm and 1.3 microm. Otolaryngol Head Neck Surg. 2004;130:334–338. doi: 10.1016/j.otohns.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Adunka OF, Radeloff A, Gstoettner WK, et al. Scala tympani cochleostomy II: topography and histology. Laryngoscope. 2007;117:2195–2200. doi: 10.1097/MLG.0b013e3181453a53. [DOI] [PubMed] [Google Scholar]
- 10.Tange RA, Grolman W, Maat A. Intracochlear misdirected implantation of a cochlear implant. Acta Otolaryngol. 2006;126:650–652. doi: 10.1080/00016480500445206. [DOI] [PubMed] [Google Scholar]
- 11.Gastman BR, Hirsch BE, Sando I, et al. The potential risk of carotid injury in cochlear implant surgery. Larnygoscope. 2002;112:262–266. doi: 10.1097/00005537-200202000-00012. [DOI] [PubMed] [Google Scholar]