SUMMARY
Both pro- and anti-oncogenic properties have been attributed to EphA2 kinase. We report that a possible cause for this apparent paradox is diametrically opposite roles of EphA2 in regulating cell migration and invasion. While activation of EphA2 with its ligand ephrin-A1 inhibited chemotactic migration of glioma and prostate cancer cells, EphA2 overexpression promoted migration in a ligand-independent manner. Surprisingly, the latter effects required phosphorylation of EphA2 on serine 897 by Akt, and S897A mutation abolished ligand-independent promotion of cell motility. Ephrin-A1 stimulation of EphA2 negated Akt activation by growth factors and caused EphA2 dephosphorylation on S897. In human astrocytoma, S897 phosphorylation was correlated with tumor grades and Akt activation, suggesting that the Akt-EphA2 crosstalk may contribute to brain tumor progression.
Keywords: EphA2, Akt, PTEN, glioma, prostate cancer, cell migration
INTRODUCTION
Chemotactic cell migration plays an important role in tumor invasion by directing the spread of tumor cells towards growth factors. Tumor cells can move while attached to each other, which often occurs at early stage of cancer progression. During malignant progression, tumor cells can undergo epithelial to mesenchymal transition and adopt fibroblast-like cell migration or amoeboid movement, by which they migrate as individual cells (Friedl and Wolf, 2003). Although distinct in many aspects, different migration modes share similar signaling mechanisms. Phosphoinositide 3-kinases (PI3Ks) and Rho family of GTPases have been identified as key molecules in regulating cell migration. By generating PI(3,4,5)P3 at the proximity of chemoattractant, PI3Ks activity defines the leading edge of the migrating cell (Charest and Firtel, 2006). Whereas Rho GTPases regulates the cytoskeletal reorganization that drives cell translocation (Hall, 1998; Ridley et al., 2003; Charest and Firtel, 2007).
As a primary target of PI3Ks, Akt has well-documented roles in promoting cell survival, proliferation and growth (Engelman et al., 2006; Manning and Cantley, 2007; Vivanco and Sawyers, 2002; Shaw and Cantley, 2006). Recent cancer genome analyses revealed Akt activation in vast majority of glioblastoma multiforme (GBM) through inactivation of Phosphatase/Tensin homolog deleted on chromosome 10 (PTEN), activation of receptor tyrosine kinases (RTKs) or amplification of Akt (TCGA Research Network, 2008; Parsons et al., 2008). PTEN loss and Akt activation also frequently occur in human prostate cancer (Tomlins et al., 2006). Increasing evidence show that Akt signaling also regulates migration of many types of cells (Kim et al., 2001; Ju et al., 2007; Park et al., 2001; Meng et al., 2006; Shukla et al., 2007; Vasko et al., 2004; Kim et al., 2005; Higuchi et al., 2001). Akt has been proposed to modulate cell migration through several mechanisms including activation and recycling of several integrins (Li et al., 2005; Somanath et al., 2007) and phosphorylation of an actin crosslinking protein Girdin at the cell leading edge (Enomoto et al., 2005; Jiang et al., 2008).
The 16 members of vertebrate Eph kinases constitute the largest subfamily of receptor tyrosine kinase (RTK) superfamily. Interaction of Eph receptors and their membrane-bound ligands called ephrins leads to contact-dependent bidirectional signaling into the opposing cells, which regulates diverse developmental and physiological processes (Kullander and Klein, 2002; Miao and Wang, 2008; Pasquale, 2008; Poliakov et al., 2004). Perturbation of Eph/ephrin systems has been documented in different types of human cancer (Nakamoto and Bergemann, 2002; Pasquale, 2005; Pasquale, 2008). The exact role of Eph kinase in tumor etiology and progression has remained controversial. A case in point is EphA2 kinase that is among the most frequently affected Eph kinases in human cancer. It is overexpressed in a variety of human malignancies, and is associated with poor prognosis in several different tumor types including GBM and cancers of prostate, kidney, and lung (Ireton and Chen, 2005). In several studies, overexpression of EphA2 has been linked to malignant progression (Fang et al., 2005; Zelinski et al., 2001). Paradoxically, activation of EphA2 kinase on tumor cells can trigger signaling events that are more consistent with a tumor suppressor. Thus ligand stimulation of EphA2 inhibits integrin signaling, Ras/ERK pathway and Rac GTPase activation, which is correlated with inhibition of cell proliferation and migration (Miao et al., 2000; Miao et al., 2001; Miao et al., 2003). Furthermore EphA2 is found to be a target gene for p53 family of proteins and causes apoptosis when overexpressed (Dohn et al., 2001). Recent data also show that EphA2 is a key mediator of UV-induced apoptosis independent of p53 (Zhang et al., 2008). Further supporting tumor suppressor role of EphA2, we recently report dramatically increased susceptibility to skin carcinogenesis in EphA2 KO mice (Guo et al., 2006). The seemingly conflicting role of EphA2 kinase in the literature, either as an oncoprotein or a tumor suppressor, is an outstanding dilemma in cancer research today.
It is reported recently that EphA2 overexpression is frequently accompanied by the loss of its cognate ligands (Dodelet and Pasquale, 2000; Hafner et al., 2004; Wykosky et al., 2005; Macrae et al., 2005). In human breast cancer and mouse skin tumors, for example, there is an inverse relationship between EphA2 and ephrin-A1 expression (Macrae et al., 2005; Guo et al., 2006). This unbalanced expression pattern of EphA2 and ligands led us to investigate whether the unligated EphA2 can be selected during tumor progression due to a pro-oncogenic role.
RESULTS
Ligand-independent stimulation and ligand-dependent inhibition of cell migration by EphA2 kinase
We investigated how EphA2 overexpression may regulate chemotactic cell migration and invasion in the absence and presence of its ligand ephrin-A1 using glioma cells as a model system. Examination of public microarray database also revealed overexpression of EphA2 at mRNA levels in GBM (www.oncomine.org). To mimic the in vivo situation, we overexpressed EphA2 in U373 glioma cells. Fig. 1A shows that U373 cells express moderate levels of endogenous EphA2 (Fig. 1A), and infection with EphA2-expressing retrovirus increased the level by about one fold. In keeping with the lack of ligand expression, both endogenous and ectopic EphA2 in U373 cells showed low basal activation. Stimulation with exogenous ephrin-A1 caused rapid EphA2 activation, which was followed by degradation of the receptor itself, characteristic of most RTKs including Eph kinases.
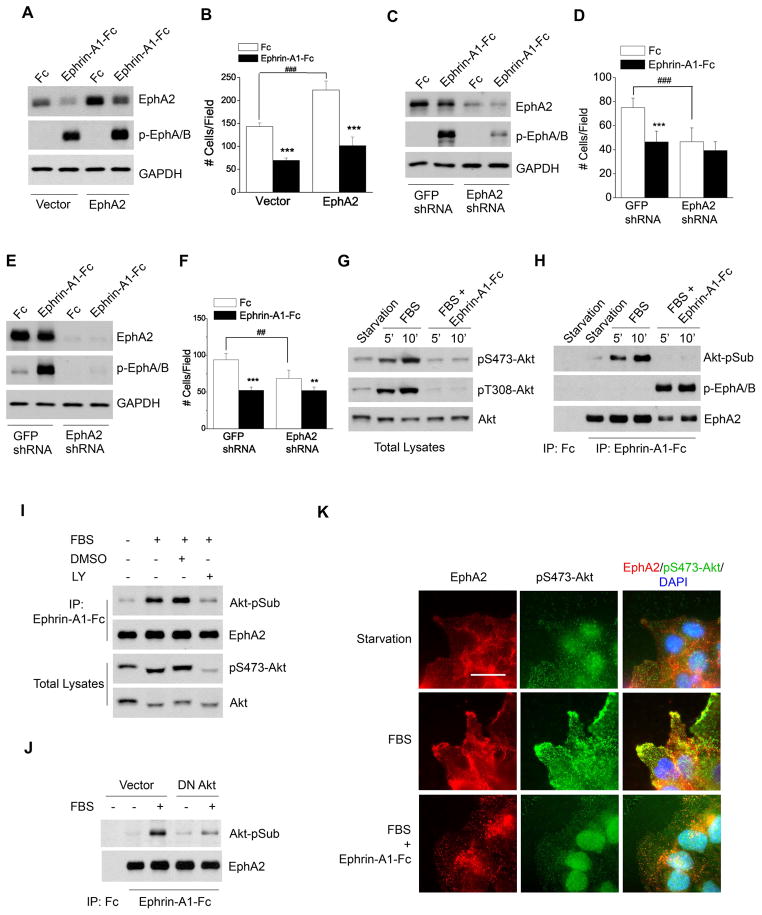
Figure 1. EphA2 possesses both ligand-independent and ligand-dependent functions in regulating growth factor-induced chemotaxis.
A–B. Ectopic overexpression of EphA2 enhances serum-induced chemotaxis in a ligand-independent manner. U373 cells were infected with retroviral vector expressing EphA2. Cells were stimulated with ephrin-A1-Fc or Fc, and lysates were blotted for active (p-EphA/B) or total EphA2 (A). The infected cells were subjected to Boyden chamber cell migration assay (B) with 5% FBS in lower chamber as chemoattractant. Cell numbers from 6 random fields were counted. Numbers represent mean ± S.D. ***, p<0.001; ###, p<0.001.
C–F. Downregulation of EphA2 by shRNA reduces serum-induced chemotaxis of U373 and PC-3M cells. EphA2 shRNA or control GFP shRNA was introduced into U373 (C–D) and PC-3M cells (E–F) via Lentiviral infection. Stable cell lines were subjected to immunoblot (C, E) or chemotactic cell migration assay (D, F) as described for A and B. Numbers represent mean ± S.D. **, p<0.01; ***, p<0.001; ##, p< 0.01; ###, p<0.001.
G–H. Serum stimulation results in S/T phosphorylation of EphA2 downstream from Akt activation, which is inhibited by ephrin-A1 co-stimulation. Serum-starved cells were scratch-wounded and stimulated with FBS for the indicated times in absence and presence of ephrin-A1-Fc. Total cell lysates (G) or EphA kinase precipitates (H) were subjected to immunoblot with the indicated antibodies.
I. Serum-induced EphA2 phosphorylation at Akt substrate sites is abolished by pretreating cells with LY294002. Experiments were performed as described in G–H except that 10 μM of LY294002 was added to the cells 1 hour prior to serum stimulation.
J. Serum-induced EphA2 phosphorylation at Akt substrate sites is inhibited by DN-Akt. U373 cells that stably expressed DN-Akt or control vector were stimulated and analyzed as described in G–H.
K. EphA2 kinase activation by ephrin-A1 inhibits leading edge localization of phosphorylated Akt. Cells were starved and wounded as descried above. After stimulation for 10 min with serum in the absence and presence of ephrin-A1-Fc, cells were fixed and stained as described in the methods. Scale bar, 25 μm.
Overexpression of EphA2 alone, in the absence of ligand stimulation, significantly enhanced serum-induced migration of U373 cells in a Boyden chamber cell migration assay (Fig. 1B). In contrast, activation of EphA2 with its ligand ephrin-A1 significantly inhibited the chemotaxis of both vector control and EphA2-overexpressing cells. These data suggest that EphA2 has both ligand-independent stimulatory effects and ligand-dependent inhibitory effects on chemotactic cell migration. The diametrically opposite properties of EphA2 in regulating cell migration were also observed in other cell types, including HEK 293, U87, A172 and PC-3M cells (see below).
In a reverse experiment, shRNA knocking down of EphA2 expression in U373 cells (Fig. 1C) led to a significant reduction in ligand-independent chemotaxis toward serum (Fig. 1D). The residual EphA2 on U373 cells was still able to mediate inhibition of cell migration upon ligand stimulation (Fig. 1D). Similarly, shRNA knockdown of EphA2 in PC-3M prostate cancer cells significantly reduced ligand-independent chemotaxis (Fig. 1E,F), whereas overexpression of EphA2 promoted it (see below).
Extensive previous investigations have established that a major function of Eph kinases is the ligand-dependent repulsion of migrating cells and axons both in vitro and in vivo (Pasquale, 2005). Our data demonstrate that EphA2 receptor can also promote cell migration in cooperation with growth factors in a ligand-independent manner.
EphA2 is both a substrate and a negative regulator of Akt
In exploring the molecular mechanisms that mediate ligand-independent promotion of chemotaxis by EphA2, we screened several signaling pathways. Among them, PI3K/Akt pathways stood out by robustly responding to serum in migrating U373 cells. As shown in Fig. 1G, Akt became highly phosphorylated at both T308 and S473 sites upon serum stimulation. Cotreatment with ephrin-A1 completely blocked Akt activation. To test possible direct crosstalk between Akt and EphA2, we precipitated EphA2 and probed it with an antibody recognizing the consensus Akt substrate sites (Akt-pSub). The antibody detected a strong band at 125 kDa on EphA2 precipitates prepared from serum-stimulated cells (Fig. 1H), indicating that EphA2 could be a substrate for Akt. To confirm this unexpected finding, immunoprecipitation was performed with Akt-pSub antibody, and the precipitates were immunoblotted for EphA2. EphA2 could be readily detected in the Akt-pSub immunoprecipitates (Fig. S1A). Ephrin-A1 cotreatment led to complete inhibition of EphA2 phosphorylation at Akt substrate sites (Fig. 1H), which correlated with the abolishment of Akt activation (Fig. 1G).
To verify the specificity of serum-induced S/T phosphorylation of EphA2 to Akt activity, we performed the same experiments on cells pretreated with PI3K inhibitor, LY294002. Inhibition of PI3K/Akt pathway by LY294002 significantly reduced the phosphorylation of EphA2 at Akt substrate sites (Fig. 1I). To further demonstrate the Akt-EphA2 crosstalk, dominant negative (DN) Akt was expressed in U373 cells. As shown in Fig. 1J, serum-induced S/T phosphorylation of EphA2 was attenuated by the expression of DN Akt. Taken together, these results identify a reciprocal regulatory loop between EphA2 and Akt, with unligated EphA2 functioning as a downstream substrate and effector of Akt kinase, but the ligand-activated EphA2 being an upstream negative regulator to turn off Akt and cause dephosphorylation of EphA2 at the Akt substrate site.
To examine where Akt-EphA2 crosstalk may take place in migrating cells, we investigated the subcellular localization of EphA2 relative to p-Akt. Fig. 1K shows that under serum-starved condition, p-Akt was low and uniformly distributed in cells with no obvious colocalization with EphA2 (Fig. 1K, top panels). Within 10 min after serum treatment, p-Akt was upregulated and became colocalized with EphA2 in lamellipodia at the leading edge. Cotreatment with ephrin-A1 caused retraction of lamellipodia and disappearance of p-Akt from the leading edge. Akt-pSub was also localized at the migrating front together with EphA2, but not at cell-cell junction sites where EphA2 was also present at high levels (Fig. S1B), suggesting that Akt-phosphorylated EphA2 is preferentially targeted to migrating front. Thus, in response to growth factor stimulation, the unligated EphA2 is phosphorylated at the leading edge by the active Akt; upon addition of exogenous ligand, EphA2 is activated, leading to “repulsion” of the migrating cells.
Serine 897 of EphA2 is the major substrate site for Akt
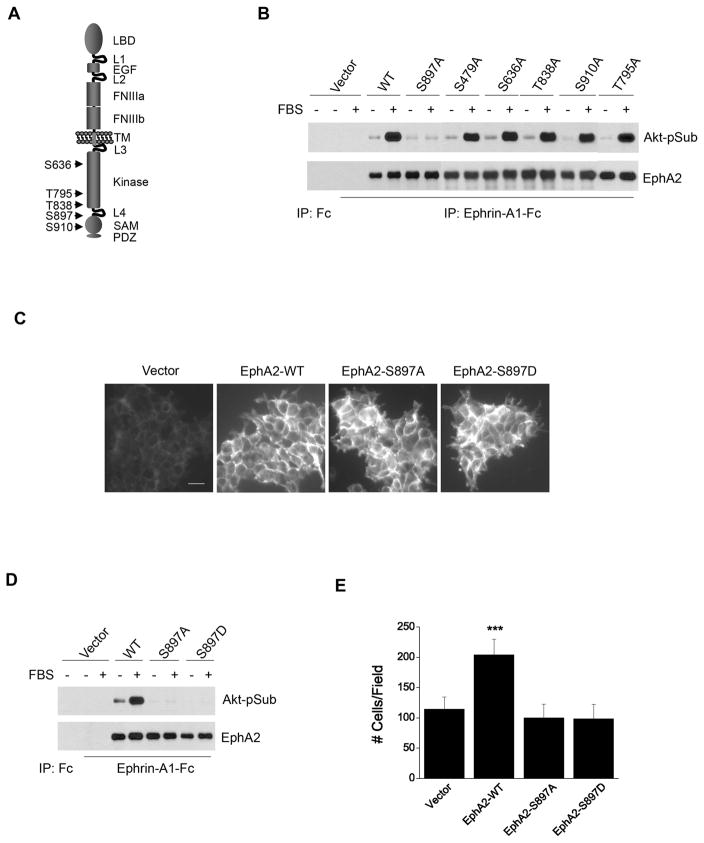
Sequence analysis of EphA2 revealed several potential S/T sites that resemble the consensus motif of known Akt substrates (Alessi et al., 1996; Manning and Cantley, 2007) (Fig. 2A). To map the Akt phosphorylation site(s) in EphA2, site-directed mutagenesis was carried out to replace all five potential S/T residues with alanine in the cytoplasmic tail. A site in the ectodomain (S479) was also included as a control. The resultant mutants were initially tested in HEK 293 cells. As we have reported previously, HEK 293 cells express very low levels of endogenous EphA2 (Miao et al., 2001), providing a low background to evaluate the mutants. In response to serum stimulation, cells expressing wild type (WT)-EphA2 or EphA2 with single mutation at S479, S636, T795, T838, and S910 were equally phosphorylated at Akt substrate sites. In contrast, S897A mutation rendered EphA2 completely resistant to serum-induced phosphorylation at Akt substrate sites (Fig. 2B). Identical results were obtained from three independent transfections. Therefore, our studies mapped serine 897 as the major site for Akt kinase phosphorylation in EphA2. Structurally, serine 897 is located in the linker region between EphA2 kinase domain and sterile α motif (SAM), which is likely to be exposed and accessible to Akt phosphorylation.
Figure 2. Serine 897 of EphA2 is the major substrate site for Akt activity, which is essential for ligand-independent promotion of cell migration.
A. Schematic illustration of relative positions of putative Akt substrate sites in EphA2 cytoplasmic tail.
B. S897A mutation abolishes serum-induced S/T phosphorylation of EphA2. HEK 293 cells were infected with retroviral vectors expressing WT or mutant EphA2. EphA kinases were precipitated and immunoblotted for Akt-pSub and total EphA2.
C. Immunofluorescence staining of infected HEK 293 cells shows that the exogenous EphA2 receptors were homogeneously expressed. Scale bar, 25 μm.
D. Both S897A and S897D mutations completely abolish the serum-induced phosphorylation of EphA2 on Akt substrate sites. Cells were stimulated and analyzed as described in B.
E. Overexpression of WT-EphA2 but not S897A-EphA2 or S897D-EphA2 enhances serum-stimulated cell migration. Cell migration assay was performed as described in Fig. 1. Numbers represent mean ± S.D. ***, p<0.001.
Akt-mediated serine 897 phosphorylation is the major mechanism responsible for ligand-independent stimulation of cell migration by EphA2
To determine the functional significance of phosphorylation at S897, HEK 293 cells expressing WT-, S897A-EphA2 or control vector were characterized. A phospho-mimetic mutation was also created by replacing S897 with aspartic acid (S897D) and tested. Immunofluorescence and biochemical analysis showed that the exogenous WT-, S897A- and S897D-EphA2 were homogenously overexpressed in HEK 293 cells (Fig. 2C, D). Similar to what we observed in U373 glioma cells (Fig. 1B), overexpression of WT-EphA2 increased serum-induced chemotaxis of HEK 293 cells (Fig. 2E). Interestingly, both S897A and S897D mutations completely abolished the cell migration-promoting effect by EphA2 (Fig. 2E). These results suggest that Akt phosphorylation of S897-EphA2 is largely responsible for the ligand-independent stimulation of cell motility by EphA2 in HEK 293 cells. The fact that phospho-mimetic mutation (S897D) had the same effects as S897A indicates that phosphorylation, but not the negative charge-induced conformational change is needed to promote cell migration.
Catalytic activity of EphA2 is required for ligand-dependent inhibition, but not for ligand-independent promotion of cell migration
To investigate the role of the intrinsic tyrosine kinase activity of EphA2, a kinase deficient EphA2 with D739N mutation was tested in HEK 293 cells. The mutant was still fully capable of simulating chemotaxis toward serum when overexpressed (Fig. S2). However, it lost the ligand-dependent inhibition of cell migration. Thus the intrinsic catalytic activity of EphA2 is required for ligand-dependent inhibition but not ligand-independent promotion of cell migration. These and additional data described below show that the two processes are mutually exclusive and involve distinct signaling mechanisms. In the context of ligand-independent promotion of cell migration, EphA2 is serving as a substrate of Akt kinase when and if its own catalytic function is kept inactive. To keep the dual functions distinct, the term “kinase” will be avoided when ligand-independent functions of EphA2 are under discussion.
Ligand-independent promotion of cell migration by EphA2 in glioma and prostate cancer cells requires S897 phosphorylation and is sensitized by PTEN deletion
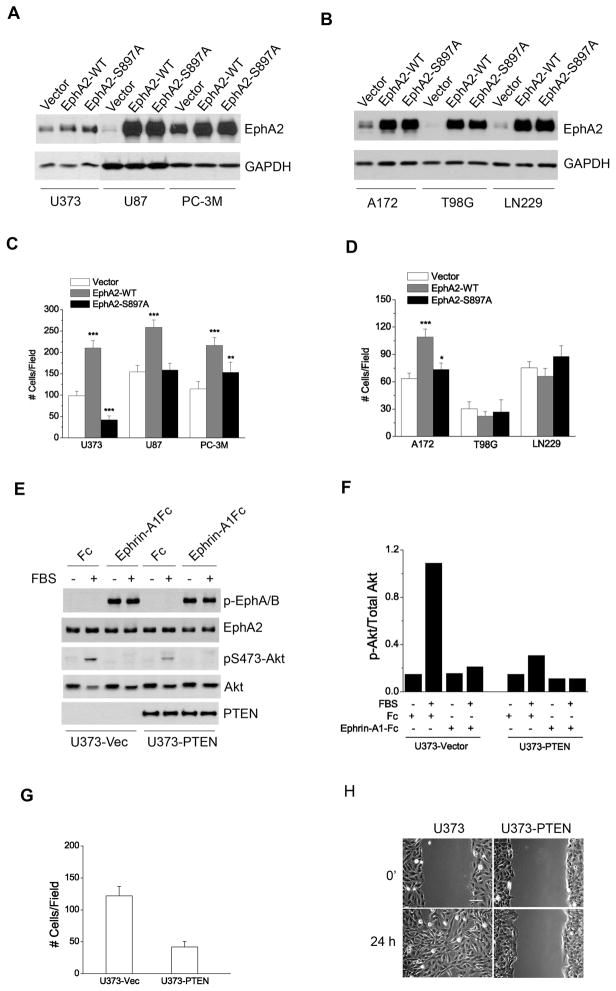
Next we investigated the effects of S897A on ligand-independent chemotaxis in a panel of human tumor cell lines, including U373, U87, A172, LN229 and T98G glioma cells, as well as PC-3M prostate cancer cells. As expected, the PTEN-null U373, U87, and A172 cells generally had more active Akt than PTEN-wild type (WT) T98G and LN229 cells (Fig. S3). Hyperactive Akt was also observed in the PTEN-null PC-3M cells (Fig. S4) as well as in parental PC-3 cells (Shukla et al., 2007). WT- and S897A-EphA2 were introduced into these cells via retroviral infection (Fig. 3A,B). Overexpression of WT-EphA2 enhanced serum-stimulated chemotaxis compared to vector control in PTEN-null U373, U87, A172 and PC-3M cells (Fig. 3C,D). S897A mutation blocked most of the stimulatory effects in these cells. Interestingly, U373 cells expressing S897A-EphA2 displayed lower basal levels of serum-induced chemotaxis than vector control cells. The seemingly dominant negative effects were not observed in U87, A172 and PC-3M cells. The differences may reflect the cell type-specific signaling networks regulating chemotaxis.
Figure 3. Serum-induced chemotaxis in glioma and prostate cancer cells is dependent on Akt-mediated phosphorylation of EphA2 at S897.
A–B. Overexpression of WT-EphA2 and S897A-EphA2 in the indicated cell lines following retroviral infection.
C–D. Chemotaxis towards serum was promoted by overexpression of WT-EphA2 but not S897A-EphA2 in PTEN-null tumor cells. Numbers represent mean ± S.D. from 6 random fields. *, p<0.05; **, p<0.01; ***, p<0.001.
E–H. Restoration of PTEN expression in PTEN–null U373 cells inhibited basal cell migration. (E) PTEN re-expression significantly reduced FBS-induced Akt activities, which was quantified in F. In both Boyden chamber migration (G) and scratch wound (H) assays, PTEN restoration reduced cell migration. Numbers represent mean ± S.D. from 6 random fields. Scale bar, 100 μm.
Together these data indicate that PTEN-null cells with hyperactive Akt may have become more dependent on Akt-EphA2 crosstalk to promote cell motility. To test this possibility, we restored PTEN expression in U373 cells, which caused a significant reduction in serum-induced Akt activities (Fig. 3E,F). In both chemotactic cell migration and scratch wound assays, restoration of PTEN expression dramatically inhibited cell migration (Fig. 3G,H), supporting important roles of PTEN loss in promoting malignant behaviors of glioma cells. However, other mechanisms in addition to loss of PTEN may also permit EphA2 to promote chemotactic cell migration, since in PTEN-WT HEK 293 cells EphA2 overexpression was still capable of promoting migration (Fig. 2E). Notably hyperactivation of Akt can be achieved by a variety of mechanisms including loss of PTEN, RTK amplification/mutation, Akt amplification, and Ras activation. Further studies are needed to identify the alternative mechanisms.
Serum-induced glioma cell invasion through MatriGel also requires Akt phosphorylation of EphA2 on S897
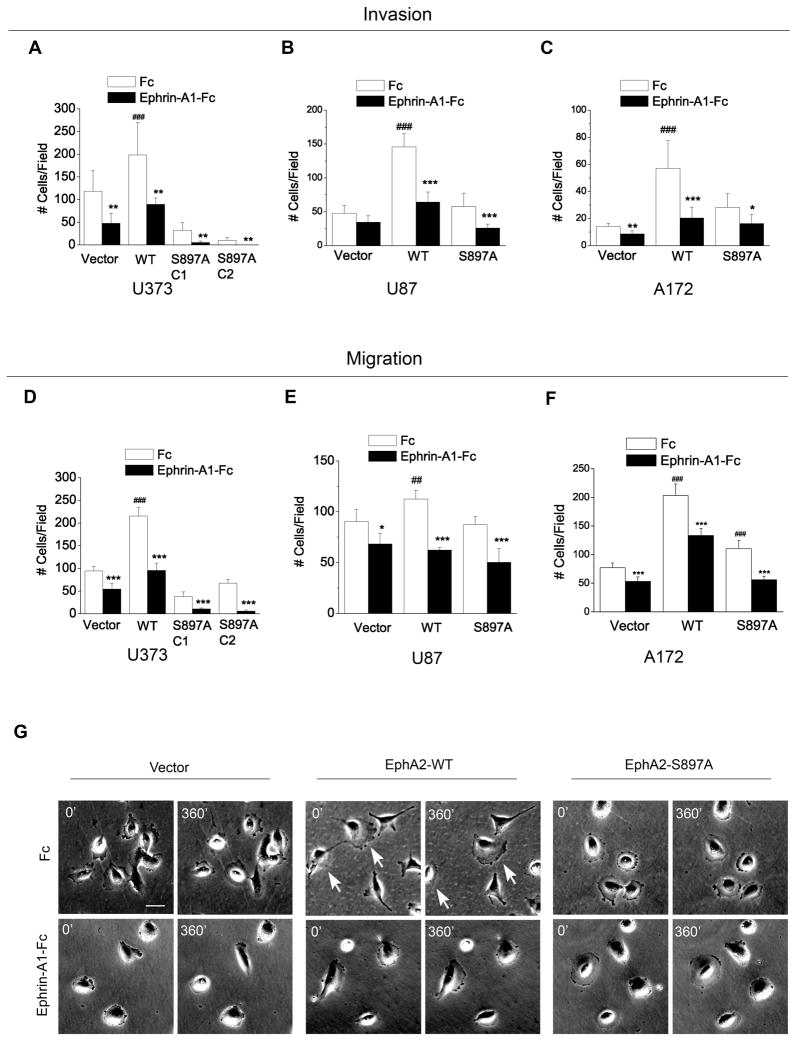
Invasion through the surrounding normal tissues is a hallmark of malignant progression, which is particularly deadly for glioma patients. We used U87, U373 and A172 cell lines to test how EphA2 might regulate serum-induced glioma cell invasion. The multiplicity of the cell lines with a shared genetic alteration in PTEN status makes them good models for assessing general applicability of our findings. We found that, in the absence of ligand stimulation, overexpression of WT-EphA2 strongly promoted MatriGel invasion by all three cell lines (Fig. 4A–C, open bars). The S897A mutation largely abolished the stimulatory effects. Together with the data from the last section, we conclude that EphA2 functions as a ligand-independent positive regulator of chemotactic migration as well as invasion, and Akt-mediated phosphorylation of EphA2 on S897 is required for both effects.
Figure 4. EphA2 promotes MatriGel invasion, and S897A mutation does not affect ligand-dependent inhibition of cell migration and invasion.
(A–C) EphA2 promotes MatriGel invasion in a ligand-independent manner, which is abolished by S897A mutation (open bars). (A–F) S897A mutation of EphA2 does not affect ligand-dependent inhibitory effect on migration or invasion of U373, U87, and A172 cells. In both migration and invasion assays, 5% FBS was used as chemoattractants. Numbers represent mean ± S.D. from 6 random fields. *, p<0.05; **, p<0.01; ***, p<0.001 compared to Fc control of the same cell line. ##, p< 0.01; ###, p<0.001 compared to Fc control of vector cells.
G. Overexpression of WT-EphA2 induces a polarized morphology and enhanced chemokinetic motility, which is inhibited by S897A mutation. Cells were plated at low density in 6-well dishes, and subject to time-lapse analysis. The still images at 0 and 360 min are shown. Arrows indicate cells that have gained net translocation. Scale bar, 50 μm.
Ligand-dependent inhibition of cell migration and invasion by EphA2 is not affected by S897A mutation
Next we investigated if S897 of EphA2 is involved in ligand-dependent inhibition of cell migration and invasion. Similar to what was found in U373 cells (Fig. 1B), chemotactic migration of A172 and U87 cells expressing WT-EphA2 or vector control was significantly inhibited by ephrin-A1 (Fig. 4D–F). Interestingly, S897A EphA2 was still capable of mediating ephrin-A1-induced inhibition of migration in all three cell lines, despite its ability to abolish most ligand-independent promotion of cell migration. Indeed, the degrees of ligand-dependent inhibition were indistinguishable between WT- and S897A-EphA2 cells. Similarly, ephrin-A1 treatment also significantly blocked MatriGel invasion of cells expressing either WT- or S897A-EphA2 (Fig. 4A–C). One consistent difference across all three cell lines was that both ligand-independent promotion and ligand-dependent inhibition were more pronounced in the invasion assay than migration assay (Fig. 4D–F).
Time-lapse imaging was employed to monitor cell morphology and chemokinetic movement of cells on two-dimensional culture plate. In keeping with the increased cell motility, U373 cells expressing WT-EphA2 displayed elongated and polarized morphology with a large protruding lamellipodium (Fig. 4G), and were more motile compared with vector control cells (Supplementary Movies). In contrast, cells expressing S897A-EphA2 showed a “pancake-like” morphology in culture dishes and were largely stationary during the course of the observation. Ephrin-A1 treatment minimized chemokinetic movement in all three cell types.
Multiple growth factors stimulate S897 phosphorylation of EphA2
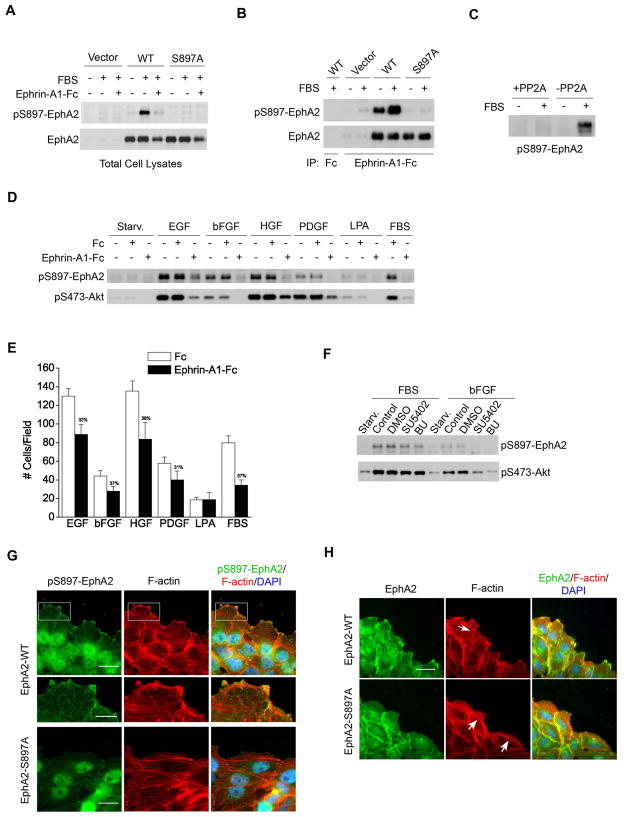
To better understand the function of S897 phosphorylation of EphA2, we generated and characterized a rabbit polyclonal antibody against a synthetic phospho-peptide surrounding S897. We validated the specificity of the antibody in HEK 293 cells expressing WT- or S897A-EphA2. Immunoblot of total cell lysates with the antibody detected a strong band at the same molecular weight as EphA2 in the serum-stimulated WT-EphA2-expressing cells, but not in cells expressing S897A-EphA2 (Fig. 5A). Costimulation with serum and ephrin-A1 abolished the phospho-S897 (pS897)-EphA2 signal. A robust pS897 band co-migrating with EphA2 was also detected in EphA2 immunoprecipitates (Fig. 5B). Conversely, EphA2 was detected in the materials precipitated with the anti-pS897-EphA2 antibody (Fig. S5). Treatment of cell lysates with recombinant S/T protein phosphatase 2A (PP2A) caused dephosphorylation of pS897-EphA2 (Fig. 5C). Finally treatment of cells with SH-5, an Akt inhibitor, suppressed S897 phosphorylation (Fig. S6A); as did LY294002, an inhibitor of PI3K (Fig. S6B). Taken together, this series of experiments confirmed specificity of the antibody. Moreover, the results further established EphA2 as an effector molecule for PI3K/Akt signaling cascade.
Figure 5. Multiple growth factors can induce S897 phosphorylation of EphA2, which is required for EphA2 localization to the leading edge and for cell polarization.
A–B. Characterization of a rabbit polyclonal antibody against S897 phospho-peptide. Serum-starved HEK 293 cells that express vector, WT-EphA2, or S897A-EphA2 were stimulated with FBS in the absence and presence of ephrin-A1-Fc for 10 min. Total cell lysates (A) and EphA kinase precipitates (B) were analyzed by immunoblotting with polyclonal anti-pS897-EphA2 and total EphA2.
C. PP2A treatment causes dephosphorylation of serum-induced pS897-EphA2. Serum-stimulated U373 cell lysates were subjected to PP2A phosphatase assay followed by immunoblot with polyclonal anti-pS897-EphA2.
D–E. Multiple growth factors induce S897 phosphorylation of EphA2 and migration. (D) Serum-starved U373 cells were stimulated with 10 ng/ml of EGF, bFGF, PDGF, HGF, 10% FBS, or 10 μM LPA alone or in combination with Fc or ephrin-A1-Fc for 10 min. Total cell lysates were analyzed as in A. (E) U373 cell migration toward same concentrations of growth factors and LPA or 5%FBS. Numbers represent mean ± S.D. from 6 random fields. The numbers over solid bars show the percentage of inhibition by ephrin-A1.
F. Inhibition of FGFR is not sufficient for inhibiting serum-induced pS897-EphA2. Serum-starved U373 cells were pretreated with 10 μM SU5402 or 100 nM 1-(2-Amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)-3-tert-butyl urea (BU) for 30 min. Cells were then stimulated with FBS for 10 min. Cell lysates were analyzed by immunoblotting with polyclonal anti-pS897-EphA2 and pS473-Akt.
G. Phospho-S897-EphA2 is localized to the leading edge and the tips of actin stress fibers. Serum-starved cells were treated and processed for immunofluorescent staining as described in Fig. 1K. Scale bar, 25 μm in top and bottom row, 10 μm in the zoomed images.
H. Cells expressing mutant S897A-EphA2 show defective cell polarization, which was correlated with mislocalization of the mutant proteins to cell-cell junctions. Scale bar, 25 μm.
To determine which growth factors induce phosphorylation of EphA2 on S897, we stimulated U373 glioma cells with a panel of growth factors including EGF, bFGF, PDGF, and HGF, which have all been implicated in human GBM. Serum was used as a positive control. We found that all growth factors were capable of inducing pS897-EphA2, which was correlated with Akt activation (Fig. 5D). In cell migration assays, all four growth factors were potent chemoattractants for U373 cells (Fig. 5E). The number of migrating cells correlated with the degree of S897 phosphorylation and Akt activation. Costimulation of cells with ephrin-A1-Fc significantly inhibited all growth factor-induced pS897-EphA2 and chemotaxis, concomitant with Akt inactivation (Fig. 5D,E). The degree of ephrin-A1-dependent inhibition of Akt varied among different growth factors, with strongest inhibition for bFGF; significant but less inhibition was seen with EGF and HGF (Fig. 5D,E). These results suggest that phosphorylation of EphA2 at S897 is a common mediator of growth factor-induced cell migration, which could have important implications in understanding the molecular basis of malignant progression for GBM and other tumor types.
LPA in serum is known to activate Akt and induce cell migration. However, in U373 cells, LPA is a weaker inducer of Akt activation and chemotactic cell migration, which was not affected by ephrin-A1 (Fig. 5D,E). Thus LPA in serum did not contribute significantly to either Akt activation or S897 phosphorylation in this system.
Next we examined whether kinase activities of the growth factor receptors are required for activation of Akt and phosphorylation of EphA2 on S897. Two inhibitors of FGFR abolished the stimulatory effects of bFGF (Fig. 5F). Although a small decrease was observed, serum-induced activation of Akt and pS897-EphA2 was not significantly reduced by FGFR inhibitors (Fig. 5F), consistent with the presence of multiple growth factors in serum.
S897 phosphorylation of EphA2 is required for dendritic actin cytoskeleton assembly and lamellipodia formation at the leading edge of migrating cells
Immunofluorescence staining of U373 expressing WT-EphA2 revealed that pS897-EphA2 was localized to the migrating front with dendritic actin in lamellipodia or tips of F-actin fibers that ran perpendicular to the wound (Fig. 5G). As expected, cells expressing mutant S897A-EphA2 showed little staining for phospho-S897 (Fig. 5G, lower panel). Staining for total EphA2 showed that the mutant S897A-EphA2 was primarily localized to the cell-cell junctions instead (Fig. 5H). Interestingly, prominent actin stress fibers were detected in cells expressing mutant S897A-EphA2 that ran parallel to the wound (Fig. 5G,H). This is analogous to rearrangement of F-actin following stimulation with ephrin-A1, that caused F-actin to switch from a predominantly dendritic pattern in lamellipodia to stress fibers that aligned with the direction of wounding (Fig. S7). These results suggest that phosphorylation of EphA2 at S897 by Akt is critical for EphA2 localization at cell leading edge, which is required to promote assembly of actin cytoskeleton and extension of lamellipodia.
In human astrocytoma, S897 phosphorylation of EphA2 is correlated with malignant progression and overlaps with active Akt
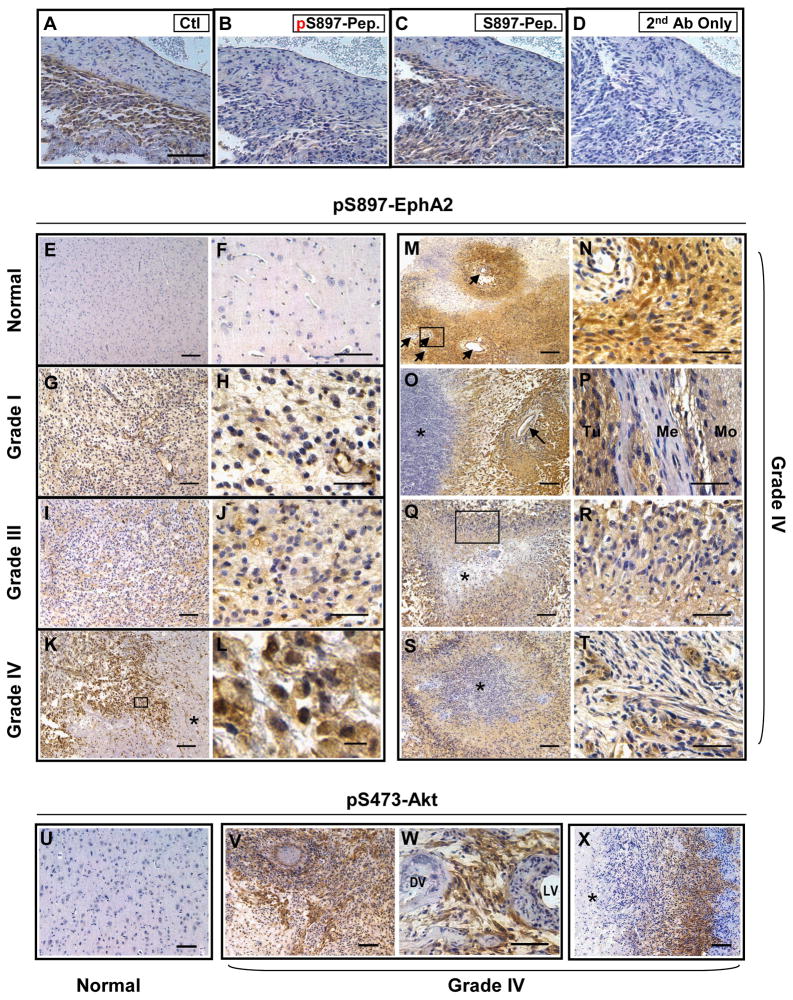
PI3K/Akt is activated in the vast majority of human GBM (TCGA Research Network, 2008; Parsons et al., 2008), and glioma cells induced to migrate are known to have further elevated Akt activities (Joy et al., 2003). We hypothesize that if Akt phosphorylation of S897-EphA2 facilitates glioma cell migration in vitro, S897 phosphorylation of EphA2 could be elevated during malignant progression of human brain tumors. To test this, we performed immunohistochemical (IHC) staining on paraffin-embedded sections from normal human brains and human gliomas of different grades using the phospho-specific antibody characterized above. We first tested the specificity of the antibody in IHC staining. The antibody detected pS897 signals in a grade IV GBM. On adjacent sections, the signal was blocked by the immunizing phospho-peptide but not the unphosphorylated peptide (Fig. 6A–D), suggesting the antibody specifically recognized p897-EphA2 in tumor sections.
Figure 6. Levels of pS897-EphA2 in human glioma specimens are correlated with tumor grades and overlap with active Akt.
A–D. Rabbit polyclonal anti-pS897-EphA2 specifically recognizes pS897-EphA2 in GBM specimens. Paraffin sections from a human astrocytoma were stained with the anti-pS897-EphA2 antibody preincubated with vehicle control (A), phospho-S897 peptide (B) or unphosphorylated S897 peptide (C). Sections processed with omission of primary antibody served as the negative control (D). Scale bar, 50 μm.
E–F. Normal brains were negative for pS897-EphA2. Scale bar, 200 μm in E, 100 μm in F.
G–J. Grade I (G, H) and grade III (I, J) astrocytomas showed low immunoreactivity for pS897-EphA2. Scale bar, 100 μm in G and I, 50 μm in H and J.
K–T. Grade IV astrocytomas (or GBM) frequently showed high levels of pS897-EphA2. (K) Positive tumor cells localized to a necrotic area indicated by an asterisk (K, scale bar, 100 μm). High power inset (L, scale bar, 10 μm) shows membrane expression pattern of pS897-EphA2.
(M–N) Perivascular staining pattern of pS897-EphA2. N, inset from M. scale bar, 200 μm in M, 50 μm in N. Blood vessels are indicated by arrows.
(O) pS897-EphA2 was detected abundantly at the interface between necrosis (asterisk) and vasculature (arrow). Scale bar, 100 μm.
(P) pS897-EphA2 was present at high levels in the meningeal invasion. Positive tumor cells (Tu) invaded meninges (Me) and further infiltrated the molecular layer of adjacent side of the gyrus (Mo). Scale bar, 50 μm.
(Q–S) pS897-EphA2 was detected in the pseudopalisading cells surrounding necrotic foci (Asterisk indicates necrosis). R, High power inset from Q. Scale bar, 100 μm in Q and S, 50 μm in R.
(T) S897 phosphorylation of EphA2 was also abundant in microvasculature proliferation, another distinguishing feature of GBM. Scale bar, 50 μm.
U–X. Phospho-S473-Akt expression in GBM. pS473-Akt was most abundant in tumor cells surrounding blood vessels (V, W) or necrotic regions (X) where pS897-EphA2 presented at high levels. DV, dead vessel. LV, live vessel. Asterisk indicates necrosis. Scale bar, 100 μm in U, V and X; 50 μm in W.
As shown in Fig. 6E and F, normal brains from five individuals were largely negative for pS897-EphA2, whereas one case of grade I (Fig. 6G,H) and two cases of grade III astrocytomas (Fig. 6I,J) showed low to moderate pS897-EphA2 levels. In contrast, 19 of 21 cases of grade IV astrocytomas (GBM) samples contained regions displaying high levels of pS897-EphA2 (Fig. 6K–T). The areas affected varied among different tumors, comprising 5% to over 90% of the non-necrotic regions. The 90% positive rate is consistent with a recent large scale study where Akt is activated in 88% of human GBM cases (TCGA Research Network, 2008).
Interestingly the strongest staining for pS897-EphA2 was frequently localized in regions known to be enriched for growth factors and invasive cells in grade IV astrocytomas or GBM. Clusters of strongly positive tumors cells were often observed next to necrotic areas, one of the WHO-designated hallmarks of grade IV astrocytomas (Fig. 6K) (Miller and Perry, 2007; Furnari et al., 2007). The distinctive membrane staining pattern of pS897-EphA2 could be visualized in the high magnification images (Fig. 6L). Phospho-S897-EphA2 was also abundantly detected in the perivascular regions (Fig. 6M,N), or at the interface between vasculature and necrosis (Fig. 6O). Invasive glioma cells often migrate along blood vessels, which is referred to as perivascular satellitosis, one of morphological signatures of invasive GBM collectively known as Scherer’s secondary structures (Scherer, 1938; Furnari et al., 2007). Phospho-S897-EphA2 was also detected in a tumor that has infiltrated meninges adjacent to normal brain (Fig. 6P), further supporting its role in promoting tumor cell migration. On multiple sections pS897-EphA2 was found at high levels in cells comprising palisading necrosis (Fig. 6Q–S). In GBMs, necrotic foci are often surrounded by pseudopalisading cells, which are thought to be actively migrating and are critical for tumor progression (Brat et al., 2004; Wippold et al., 2006). Finally, pS897-EphA2 was found in microvascular proliferation resulting from active angiogenesis in GBM (Fig. 6T), suggesting that pS897-EphA2 could also play a role in promoting endothelial cell migration and invasion during neo-angiogenesis.
One prediction based on our data is that pS897-EphA2 and pS473-Akt are likely to reside in the same cells in vivo. To directly examine this possibility, we stained the same set of GBM specimens for pS473-Akt. Interestingly, in most regions where there was strong pS473-Akt, there was also robust staining for pS897-EphA2 on adjacent sections (Fig. 6U–X). The spatial colocalization was most pronounced in tumor cells surrounding blood vessels (Fig. 6V, W on adjacent slides with Fig. 6M,N) or necrotic regions (Fig. 6X). In about a third of the specimens, pS473-Akt signal was detectable but much weaker than pS897-EphA2, possibly due to the more labile nature of the pS473-Akt epitope, suggesting that p897-EphA2 could serve as surrogate marker for Akt activation. Taken together, the results demonstrate that phosphorylation of EphA2 at S897 by Akt is correlated with malignant progression of human astrocytoma. We propose that the crosstalk between RTK/PI3K/Akt and EphA2 may contribute to malignant progression of GBM and potentially other types of human cancers.
DISCUSSION
We report here that EphA2 kinase has diametrically opposite roles in regulating chemotactic cell migration: ligand-independent promotion and ligand-dependent inhibition. A reciprocal regulatory loop between EphA2 and Akt was characterized whereby unligated EphA2 is a substrate for Akt that in turn is negatively regulated by the ligand-activated EphA2 (see Fig. 7). We further demonstrate that phosphorylation of a single serine residue, S897, by Akt is responsible for ligand-independent promotion of cell migration and invasion by EphA2, and the site becomes dephosphorylated upon ligand stimulation. The ligand-dependent inhibition and ligand-independent stimulation of cell migration and invasion may represent one possible mechanism responsible for the hitherto conflicting roles attributed to EphA2 in tumorigenesis. Our data also suggest that EphA2 is an important effector molecule of Akt in promoting malignant progression, making it an attractive target for therapeutic intervention.
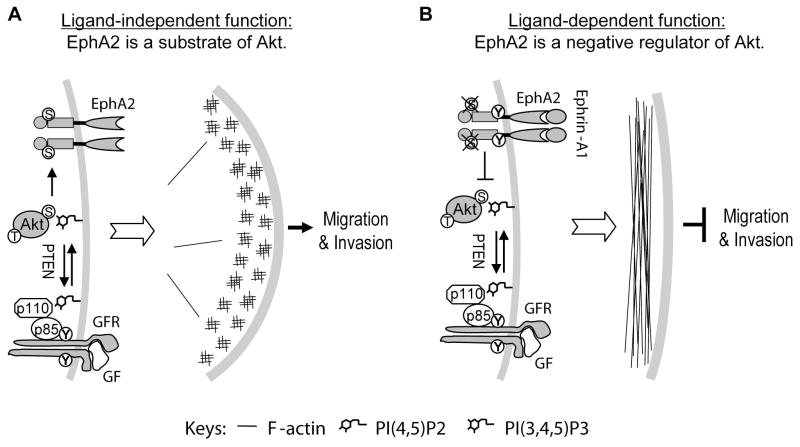
Figure 7. A model depicting ligand-dependent inhibition and ligand-independent stimulation of cell migration and invasion by EphA2.
A. In the absence of ligand, EphA2 is a substrate of Akt activated in tumor cells due to growth factor receptor activation or loss of PTEN. EphA2 phosphorylated on S897 promotes cell polarization, lamellipodium protrusion and cell migration. S897A mutant of EphA2 is sufficient to block chemotactic cell migration.
B. Ephrin-A1 stimulation causes tyrosine phosphorylation in the juxtamembrane domain and serine dephosphorylation on S897 of EphA2, which prevents lamellipodium protrusion and inhibits cell migration.
To phosphorylate or to dephosphorylate: S897 of EphA2 as a binary switch to control cell motility
In addition to the well-characterized tyrosine phosphorylation events, proteomic analyses in vitro and in vivo demonstrate extensive serine/threonine phosphorylation of Eph kinases as well (Kalo and Pasquale, 1999; Kalo et al., 2001). However, neither the responsible kinases nor the physiological relevance for any of the phospho-S/T sites has been documented. We present cellular, biochemical, pharmacological, and pathological evidence to demonstrate that EphA2 is an unexpected substrate for Akt S/T kinase in vitro and in vivo. The phosphorylation occurs predominantly if not exclusively at a single residue S897. It is rapidly induced upon stimulation with serum and all growth factors tested, suggesting EphA2 as a common downstream effector molecule for growth factor signaling. The propensity of EphA2 to serve as a substrate for Akt is dictated by the presence or absence of its cognate ligands. In fact, ligand stimulation of EphA2 caused rapid dephosphorylation of phospho-S897 concomitant with dephosphorylation and inactivation of Akt kinase. The reciprocal crosstalk with RTK/PI3K/Akt pathway established in these studies adds another layer of intricacy to EphA2 kinase signaling. Moreover, it intimately interconnects EphA2 with one of the central nodes of cell signaling network. Through the PI3K/Akt pathway, EphA2 becomes an integral part of the large cellular signaling network both as a substrate and as a negative regulator.
S897 of EphA2 is located in a linker region between the kinase and SAM domains. Sequence alignments show that the linker is not well conserved among different Eph kinases. The putative recognition sequence for Akt is only conserved between EphA2 (RLPS897TS) and EphA1 (RLPS906LS) kinases. In all other mammalian Eph kinases, the amino acid corresponding to S897 is replaced by aspartic or glutamic acid, except for EphB6 and EphA8. EphB6 does have a threonine; however, the requisite basic residue (R/K) at -3 position for Akt phosphorylation is replaced with a leucine. EphA8 has a unique SAM linker completely different from that of EphA2. Therefore, only EphA1 and EphA2 can serve as substrates for Akt at this site. First cloned from an erythropoietin-producing hepatoma cell line in 1987, EphA1 was the namesake and founding member of Eph kinases (Hirai et al., 1987). It will be interesting to determine if EphA1 can indeed serve as a substrate for Akt, and how the phosphorylating event may contribute to cell migration/invasion and malignant transformation. The lack of the consensus sequence in other 12 mammalian Eph kinases indicates that EphA1 and EphA2 are functionally distinct. At a minimum, the differences could limit the role of other Eph kinases in promoting cell migration and invasion in cooperation with PI3K/Akt pathway.
A model for ligand-dependent tumor suppressive and ligand-independent pro-oncogenic functions of EphA2
As reviewed in the Introduction, an outstanding paradox in Eph field is whether EphA2 is an oncogene or a tumor suppressor gene. Upon examinations of previous studies and the data herein, a model emerges for both anti- and pro-oncogenic functions for EphA2. We propose that the ligand-dependent inhibition of cell proliferation and migration by EphA2 may help maintain homeostasis in normal tissues and suppress tumor development at early stages of tumorigenesis (Miao and Wang, 2008). On the other hand, ligand-independent stimulation of cell migration by EphA2 promotes tumor progression and fulfils an oncogenic function by partnering with the activated Akt. Consistent with this model, 22 of 29 breast cancer derived cell lines have lost EphA2 expression compared with a relatively normal breast epithelial cell line MCF10A, suggesting a tumor suppressor function of EphA2 in breast epithelial cells (Macrae et al., 2005). In the remaining seven cell lines that still retain EphA2 expression, ephrin-A1 expression is invariably lost, leading to a loss of ligand-dependent tumor suppressor functions. Moreover, the unligated EphA2 can be hijacked by PI3K/Akt pathway frequently activated in breast cancer to promote malignant progression.
EphA2-Akt crosstalk in brain tumors and its clinical implications
GBM is the most common primary brain tumor (Miller and Perry, 2007; Furnari et al., 2007). Its dismal prognosis with an average 14 month survival is attributable to difficulties in early detection and to widespread brain invasion at the time of diagnosis. Extensive molecular studies have identified PI3K/Akt signaling cascade as one of the most frequently altered pathways in primary and secondary GBM (Li et al., 1997; Wang et al., 2004; TCGA Research Network, 2008; Parsons et al., 2008). Activation of upstream RTK growth factor receptors and/or loss of the negative regulator, PTEN, are the major causes for PI3K/Akt activation, particularly in primary GBM. The data reported here show that one pathological consequence of Akt activation is promotion of glioma cell migration and invasion through phosphorylation of EphA2. Given that both EphA2 overexpression and Akt activation occur in majority of human GBM, the Akt-EphA2 signaling axis could play a significant role in the malignant progression of GBM in vivo.
The stable immunoreactivity of S897-EphA2 and its correlation with tumor grades in human cancer suggest that it can be used as an easy-to-detect marker for malignant tumor progression. Moreover, because inhibitors of growth factor receptors, PI3K, and Akt could all diminish pS897-EphA2, it may serve as a surrogate marker of measuring therapeutic efficacy for agents targeting various components in the RTK/Ras/PI3K/Akt pathway.
Finally it is important to note that the intrinsic tyrosine kinase activity of EphA2 is not required for its effects on promoting cell migration. Therefore tyrosine kinase inhibitors targeting EphA2, either by design or by off-target effects, will not inhibit tumor cell migration stimulated by the Akt-EphA2 axis. Moreover, such inhibitors may have untoward side effects by inadvertently suppressing the intrinsic tumor suppressor activities of EphA2. We propose that targeting EphA2 kinase with agonists may constitute more attractive therapeutic strategies, which will inhibit malignant progression by suppressing Akt activation, severing pro-oncogenic Akt phosphorylation of EphA2 and reactivating its intrinsic tumor suppressor functions.
MATERIALS AND METHODS
Reagents and cell culture
Ephrin-A1-Fc was produced as described (Miao et al., 2000). Rabbit polyclonal anti-pS374-Akt, anti-pT308-Akt, anti-Akt, and anti-Akt-pS/T-substrates (Akt-pSub) were obtained from Cell Signaling. Rabbit anti-pEphA/B was raised against the conserved phosphopeptides from the juxtamembrane regions. Rabbit polyclonal phospho-S897-specific antibody was raised against phospho-peptide DPRVSIRLP-pS897-TSGSEGVPFR. Other antibodies were purchased from Santa Cruz Biotechnology (rabbit polyclonal anti-EphA2) and Upstate Biotechnology (mouse monoclonal anti-EphA2 and PP2A). LY294002, SU5402, FGFR inhibitor (1-(2-Amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl)-3-tert-butyl urea), and Akt inhibitor II (SH-5) were purchased from Calbiochem. Mutant EphA2 was generated using the QuickChange Kit (Stratagene). HEK293, U87, U373, LN229, A172 and T98G cells were maintained in Dulbecco’s modified MEM medium (DMEM) supplemented with 10% FBS, 10 mg/ml glutamate, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. PC-3M cells were maintained in RPMI-1640 supplemented with the same supplements.
Cell migration
Chemotactic cell migration was carried out as described (Miao et al., 2003). Briefly, both sides of the filter were coated with 10 μg/ml rat tail collagen type I at 4°C overnight. 1×105 cells in serum-free medium containing 0.1% BSA were plated in the top of insert. Ephrin-A1-Fc or Fc was added to a final concentration of 1 μg/ml to the lower chamber together 5% FBS or various growth factors. Cells were allowed to migrate for 4 hours and then fixed with 4% paraformaldehyde. After staining with 0.5% crystal violet, cells that have passed through the filter and stayed on the undersides of inserts were counted. Time-lapse imaging was performed as described (Miao et al., 2005).
MatriGel invasion assay
Growth factor-reduced MatriGel-coated Transwell inserts (BD Biosciences) were rehydrated with DMEM for 2 hours at 37 °C. 2.5 × 104 cells were plated in the upper chamber. Ephrin-A1-Fc or Fc was added to the lower chamber containing 5% FBS in DMEM. After incubation at 37°C for 16–20 hours, cells were fixed and stained with 0.5% crystal violet. Cells migrating through the MatriGel and the pores of the filter were counted from six randomly selected fields.
Cell stimulation, immunoprecipitation and immunoblot
For stationary cells, sub-confluent cells were stimulated with 1 μg/ml ephrin-A1-Fc for different times. To study the signaling events in migrating cells, the monolayer of freshly confluent cells was wounded by repeated scratch-wounding using a multi-channel pipette. Four hours after wounding cells were stimulated with 1 μg/ml ephrin-A1-Fc for different times. To evaluate the serum-induced signaling, cells were starved in serum-free medium overnight before stimulation or wounding. Both stationary and wounded cells were stimulated with 10% FBS in the presence of 1 μg/ml Fc or ephrin-A1-Fc. Immunoprecipitation and immunoblot were performed as described previously(Miao et al., 2000).
Immunohistochemistry
Studies involving human tissues from normal brain and astrocytomas were approved by the Institutional Review Boards at MetroHealth Medical Center and Case Western Reserve University. Paraffin-embedded sections were deparaffinized and rehydrated. Antigens were retrieved by boiling sections in citrate buffer (pH 6.0) for 10 minutes. The endogenous peroxidase was blocked with 3% H2O2. Sections were blocked with 5% normal goat serum, and incubated with rabbit polyclonal anti-pS897-EphA2 (1:500) or anti-pS473-Akt (1:50) at RT for 1–2 hours. After extensive washing in TBST, sections were detected with biotinylated goat anti-rabbit secondary antibody for 30 minutes followed by amplification with ABC reagents (Vectastain), and visualized with 3, 3′-diaminobenzidine. Sections were counterstained with Hematoxylin. To test the binding specificity of the rabbit polyclonal anti-pS897-EphA2, the antibody was preincubated with 100 μM phospho-S897 peptide or unphosphorylated S897 peptide at RT for 1 hour. IHC staining was performed using untreated or peptide-treated anti-pS897-EphA2 antibodies. A negative control was obtained by omitting primary antibody.
The mice were cared for in accordance with guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the USPHS “Policy on Human Care and Use of Laboratory Animals,” and all studies were approved and supervised by The Case Western Reserve University Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
This work was supported by grants from National Institute of Health to B.W. (CA96533, CA92259, DK077876), and awards to B.W. from Prostate Cancer, FAMRI and Joan’s Legacy Foundations. H.M. is a recipient of the Beginning Grant-In-Aid award from American Heart Association (0465235B). A.S. is supported by grants from the National Institute of Health (CA 101954) and the Ivy Brain Tumor Foundation.
Footnotes
SIGNIFICANCE
Akt is frequently activated in human glioblastoma and prostate cancer due to loss of PTEN or activation of components in PI3K/Akt pathway. We report that EphA2 is both an upstream negative regulator and a downstream effector of Akt. Phosphorylation of EphA2 by Akt promotes cell migration and invasion. In contrast, EphA2 stimulation by ephrin-A1 ligand suppresses Akt activation and inhibits cell migration. Thus activation of PI3K/Akt pathway coupled with the loss of ephrin-As convert EphA2 from a tumor suppressor into a partner with Akt in promoting malignant progression. The data have important implications in developing therapeutic strategies targeting EphA2 for treatment of malignant tumors where PI3K/Akt pathway is activated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- Dohn M, Jiang J, Chen X. Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene. 2001;20:6503–6515. doi: 10.1038/sj.onc.1204816. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Masuyama N, Fukui Y, Suzuki A, Gotoh Y. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr Biol. 2001;11:1958–1962. doi: 10.1016/s0960-9822(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- Jiang P, Enomoto A, Jijiwa M, Kato T, Hasegawa T, Ishida M, Sato T, Asai N, Murakumo Y, Takahashi M. An actin-binding protein Girdin regulates the motility of breast cancer cells. Cancer Res. 2008;68:1310–1318. doi: 10.1158/0008-5472.CAN-07-5111. [DOI] [PubMed] [Google Scholar]
- Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, Berens ME. Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, Zhou J, Turner J, Lisanti MP, Russell RG, Mueller SC, Ojeifo J, Chen WS, Hay N, Pestell RG. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci U S A. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo MS, Pasquale EB. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry. 1999;38:14396–14408. doi: 10.1021/bi991628t. [DOI] [PubMed] [Google Scholar]
- Kalo MS, Yu HH, Pasquale EB. In vivo tyrosine phosphorylation sites of activated ephrin-b1 and ephb2 from neural tissue. J Biol Chem. 2001;276:38940–38948. doi: 10.1074/jbc.M105815200. [DOI] [PubMed] [Google Scholar]
- Kim CS, Vasko VV, Kato Y, Kruhlak M, Saji M, Cheng SY, Ringel MD. AKT activation promotes metastasis in a mouse model of follicular thyroid carcinoma. Endocrinology. 2005;146:4456–4463. doi: 10.1210/en.2005-0172. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling 2. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell. 2005;9:663–673. doi: 10.1016/j.devcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Miao H, Nickel CH, Cantley LG, Bruggeman LA, Bennardo LN, Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J Cell Biol. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of Integrin-mediated Cell Adhesion but Not Directional Cell Migration Requires Catalytic Activity of EphB3 Receptor Tyrosine Kinase: ROLE OF RHO FAMILY SMALL GTPases. J Biol Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int J Biochem Cell Biol. 2008;41:762–70. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131:397–406. doi: 10.5858/2007-131-397-G. [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc Res Tech. 2002;59:58–67. doi: 10.1002/jemt.10177. [DOI] [PubMed] [Google Scholar]
- Park BK, Zeng X, Glazer RI. Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 2001;61:7647–7653. [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Scherer HJ. Structural development in gliomas. Am J Cancer. 1938;34:333–351. [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shukla S, MacLennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Kandel ES, Hay N, Byzova TV. Akt1 signaling regulates integrin activation, matrix recognition, and fibronectin assembly. J Biol Chem. 2007;282:22964–22976. doi: 10.1074/jbc.M700241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins SA, Rubin MA, Chinnaiyan AM. Integrative biology of prostate cancer progression. Annu Rev Pathol. 2006;1:243–271. doi: 10.1146/annurev.pathol.1.110304.100047. [DOI] [PubMed] [Google Scholar]
- Vasko V, Saji M, Hardy E, Kruhlak M, Larin A, Savchenko V, Miyakawa M, Isozaki O, Murakami H, Tsushima T, Burman KD, De Micco C, Ringel MD. Akt activation and localisation correlate with tumour invasion and oncogene expression in thyroid cancer. J Med Genet. 2004;41:161–170. doi: 10.1136/jmg.2003.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- Wippold FJ, Lammle M, Anatelli F, Lennerz J, Perry A. Neuropathology for the neuroradiologist: palisades and pseudopalisades. AJNR Am J Neuroradiol. 2006;27:2037–2041. [PMC free article] [PubMed] [Google Scholar]
- Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- Zhang G, Njauw CN, Park JM, Naruse C, Asano M, Tsao H. EphA2 is an essential mediator of UV radiation-induced apoptosis. Cancer Res. 2008;68:1691–1696. doi: 10.1158/0008-5472.CAN-07-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.