Abstract
The bandwidth of the nitrile (C≡N) stretching vibration of 5-cyanotryptophan shows a significant broadening upon hydration. Thus, it has been proposed to be a useful infrared probe of the local hydration environment of proteins. However, the molecular mechanism underlying this hydration-induced spectral broadening is not known, making interpretation of the experimental results difficult. Herein, we investigate how interactions of water with various sites of 5-cyanoindole, the sidechain of 5-cyanotryptophan, affect its C≡N stretching vibration via a combined electronic structure/molecular dynamics approach. It is found that, besides those interactions with the nitrile group, interactions of water with the indole ring also play a significant role in mediating the C≡N stretching frequency. Thus, this study provides a molecular basis for understanding how hydration affects the C≡N stretching band of 5-cyanotryptophan. In addition, an empirical model, which includes interactions of water with both the nitrile and indole groups, is developed for predicting the C≡N stretching vibrational band via molecular dynamics simulations.
Keywords: 5-cyanoindole, 5-cyanotryptophan, nitrile stretching vibration, infrared probe, electrostatic map, spectral modeling
It is well known that the C≡N stretching vibration of a nitrile group is sensitive to its environment.1–4 Thus, several nitrile-derivatized, non-natural amino acids, including p-cyanophenylalanine, cyanoalanine, and cyanylated cyteine, have been used as infrared (IR) reporters of local conformation and/or electrostatics of peptides5–9 and proteins.10–12 These applications are primarily based on the premise that the C≡N stretching frequency shifts upon a change in the immediate environment of the corresponding non-natural amino acid. However, recently we have shown that in the case of 5-cyanotryptophan it is the bandwidth of the C≡N stretching vibrational band, instead of its peak frequency, that is more sensitive to the hydration status of the IR reporter.13 In fact, the bandwidth of the C≡N stretching vibrational band of 5-cyanoindole (Figure 1), which is the sidechain of 5-cyanotryptophan, is increased from ∼8.7 cm−1 to ∼15.6 cm−1 when the solvent is changed from tetrahydrofuran (THF) to water, whereas the band frequency shows only a slight blue shift (∼4 cm−1). In addition, the C≡N stretching band of 5-cyanoindole in water is also significantly broader than those of other model nitrile compounds (i.e., acetonitrile1,2,5, p-tolunitrile5, and methyl cyanate14,15).
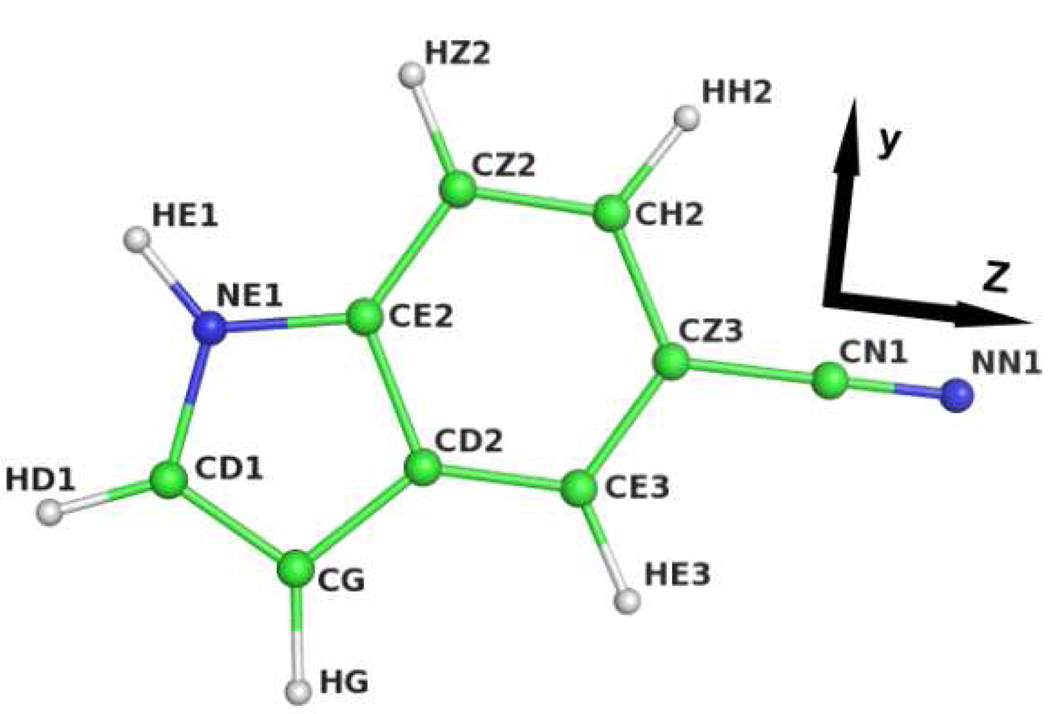
Figure 1.
Atom names in 5-cyanoindole and definition of molecular coordinate system. The x unit vector (not shown) is pointing out of the molecular plane, towards the reader.
Given the range of lifetimes (∼1–5 ps) measured for the C≡N stretching vibration of several related nitrile compounds,16,17 it seems unlikely that lifetime broadening plays a dominant role in the broadening of the C≡N stretching band of 5-cyanoindole. Instead, it seems more likely that the broadening is caused by the heterogeneous interactions between the bulky indole ring and water molecules. Such a picture is supported by the studies of the groups of Eaton, Reimers, and Cho, which showed that even for acetonitrile (CH3CN) the C≡N stretching frequency is determined by the precise configuration of water molecules surrounding the nitrile group.1,2,15,18 For example, a linear arrangement of CH3CN--HOH (termed σ-bonding with nitrogen lone-pair) results in a blue-shift of the C≡N stretching frequency as compared to its gas phase value, whereas interactions of water molecules with the π-orbital of the C≡N group (termed π-bonding) lead to a red-shift. However, for 5-cyanoindole, besides those interactions between water molecules and the C≡N group, one might expect that the solvation status of the parent molecule, which is comparatively much larger than a methyl group and also contains an extended π-electron system that is electronically coupled to the triple bond of the nitrile group, also plays an important role in determining the peak frequency and band shape of the C≡N stretching vibration. In order to provide a more quantitative description, herein we carry out a theoretical investigation of how interactions of water molecules with 5-cyanoindole influence its C≡N stretching vibration.
The details of the frequency calculations, which are based on methods used in similar studies,3,15,18–30 are described in the Supporting Information. Briefly, a molecular mechanics (MM) model of 5-cyanoindole was first developed, and subsequently used in a molecular dynamics (MD) simulation of 5-cyanoindole in water. The Born-Oppenheimer potentials of the C≡N stretch were then determined for a set of 5-cyanoindole-water clusters, which were randomly selected from a MD trajectory. These potentials were then fitted to a Morse potential function in order to obtain their quantum mechanical (QM) vibrational frequencies. Finally, an empirical model, which relates the C≡N stretching frequency to its electrostatic environment, was developed.
Specifically, the atomic charges of 5-cyanoindole (Figure 1) were parameterized by achieving a satisfactory agreement between the MM and QM solvent-solute interaction energies. To do so, the internal parameters of 5-cyanoindole (i.e., bond-, angle-, and dihedral-force constants, as well as Urey-Bradley parameters) were based on those of its structural analogs, indole31 and 3-cyanopyridine.32 Its bond lengths were determined based on the MPW1B95/6−31++G(d,p) optimized 5-cyanoindole molecule, while its Lennard-Jones parameters were taken from those of indole and acetonitrile.33 Using the Gaussian 03 software package34, the initial MM atomic charges were then obtained via a CHelpG35 analysis of the electron density of 5-cyanoindole at the MPW1B95/6−31++G(d,p) level of theory, under the constraint that the atomic charges are to reproduce the QM permanent dipole moment of the molecule. The results are summarized in Table S1 (Supporting Information).
To further optimize the initially obtained atomic charges so that the MM interaction energies agree with the QM interaction potentials, a fitting procedure using the following harmonic penalty function36 was then carried out.
| (1) |
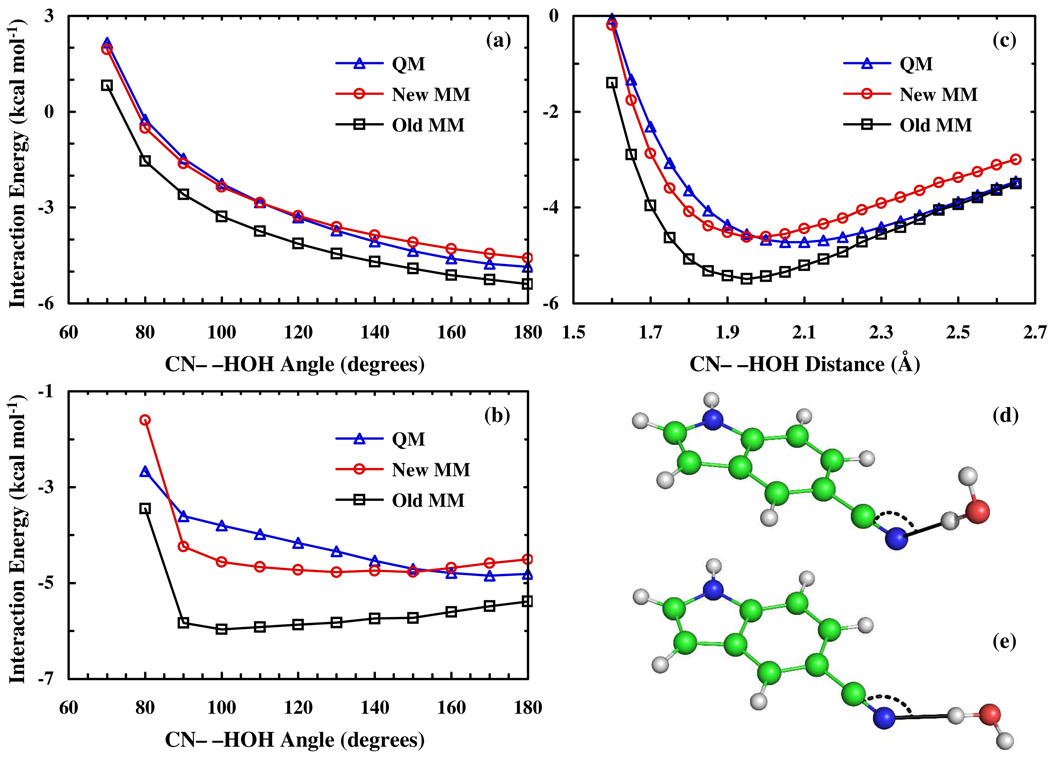
Where and represent the MM and QM interaction energies at geometry α of the interaction potential and and are the MM charge of atom i and the corresponding CHelpG reference charge, respectively. The weighting factor, ρ, of the penalty function was set to a value of 15. Three QM interaction potentials, defined as IE = E(complex) – E(5-cyanoindole) – E(water), were calculated at the MPW1B95/6−311++G(3df,2dp) level of theory. Specifically, the optimization was achieved by considering the following three interaction potentials: (A) A water molecule was placed 2.0 Å away from the nitrile nitrogen with one H–O bond pointing towards the C≡N bond and the other pointing out of the indole ring plane (Figure 2d). The C≡N--H angle was then stepped in 10° increments, moving the water out of the indole ring plane. (B) A water molecule was placed 2.0 Å away from the nitrile nitrogen, within the plane defined by the indole ring (Figure 2e) and with one H–O bond pointing towards the C≡N bond. The C≡N--H angle was then stepped in 10° increments, moving the water within the indole ring plane. (C) A water molecule was placed 1.6 Å from the nitrile nitrogen with one H–O bond pointing towards the C≡N bond and the other pointing out of the indole ring plane. The C≡N--HOH distance was than stepped in 0.05 Å increments from 1.60 to 2.65 Å.
Figure 2.
(a) QM and MM interaction energies between water and 5-cyanoindole as a function of the CN--H angle when the water is bent out of the indole plane (b) and when the water is kept in the indole ring plane. (c) QM and MM interaction energies as a function of the CN--HOH distance with one of the H–O bonds aligned with the nitrile group. Example geometries (CN--H angle = 130°) corresponding to the scans in (a) and (b) are shown in panels (d) and (e), respectively.
Once the charges on the water molecule (O = −0.834 e; H = 0.417 e) and the Lennard-Jones term are given, the Coulomb part of the interaction potential can be optimized by adjusting the MM atomic charges of 5-cyanoindole via minimization of the χ2 (eq. 1). As shown (Figure 2), the new MM interaction energies of the optimized force field match the QM interaction potential significantly better than the CHelpG based force field energies, while the optimized charges deviate only slightly from those obtained via QM calculations (Table S1, Supporting Information). Thus, this optimization procedure (i.e., eq. 1) produced a set of charges that is expected to better describe the interactions between water and the C≡N moiety, a pivotal requirement for the correct prediction of its stretching frequency shift upon hydration.15,18,29,30
Having developed a MM model of 5-cyanoindole, we next sought to establish an empirical model that allows us to determine the localized C≡N stretching frequency in water from MD simulations based on the notion that the vibrational frequency of a localized vibrational mode is related to the local electric field (or potential).15,18–28 Specifically, we employed a computational procedure that is similar to those used by Skinner and coworkers21–23 as well as by Corcelli and coworkers.29,30 First, we randomly selected 40 5-cyanoindole-water clusters from a 1 ns MD simulation at 300 K and 1 atm (details of the NAMD simulation37 are given in the Supporting Information). As shown (Figure S1, Supporting Information), these clusters contain 5–17 water molecules (12 on average) and were further divided into two subsets, Set-A and Set-B. For Set-A, wherein the indole ring is considered dehydrated, only those water molecules that have at least one atom located within 4.5 Å of the NN1 atom of the indole ring are included in each cluster. On the other hand, the clusters in Set-B are composed of water molecules that have at least one atom located within 3.5 Å of the NN1, NE1, or CD2 atom of the indole ring (atom names are given in Figure 1). Thus, the indole ring in Set-B is regarded as partially hydrated. In the following we will consider Set-A and Set-B as a common set. Second, DFT calculations were carried out on these clusters using the Zhao-Truhlar hybrid meta functional MPW1B9538 in conjunction with the 6−31++G(d,p) basis set. It has been shown that this level of theory adequately describes the weak interactions between water and the indole ring.39 For each cluster, the potential energy of the system as a function of the C≡N bond length (from 1.0 to 1.4 Å in increments of 0.05 Å) was determined via DFT calculations wherein all other atomic coordinates were fixed. Third, the obtained potential energy curves were then fitted to a Morse oscillator model, allowing analytical determination of the underlying anharmonic frequencies. Fourth, an empirical model relating the vibrational frequency to the electrostatic field components on the nitrile group was obtained by least-square fitting the following equation to the ab initio frequencies: , where is the Cartesian electric field component i at a site a on the molecule, is a constant of proportionality, and ωgas corresponds to the gas phase value of the C≡N stretching frequency of 5-cyanoindole, which was calculated to be 2392.3 cm−1 using an isolated 5-cyanoindole molecule and the procedure described above. Finally, the electric fields arising from the partial charges of water were calculated using an in-house written code within the VMD software environment,40 and the proportionality constants were determined by the multivariate least-square fitting method.
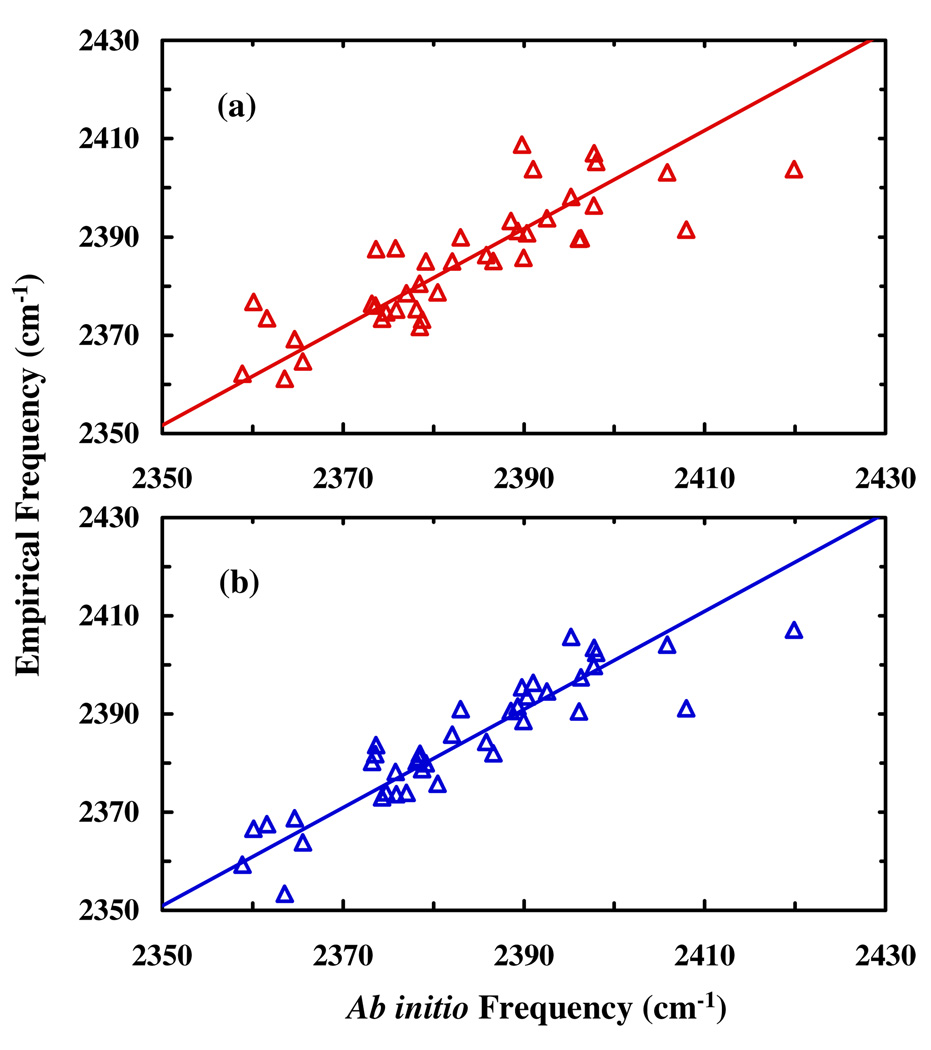
As shown (Figure 3a), fitting the 40 ab initio frequencies obtained from the vibrational analysis of the clusters with a model that only includes the electric field components on the nitrile group in the direction of the vibrational transition dipole, i.e.,
| (2) |
yields a relatively poor correlation coefficient (R2) of 0.64 and a root mean square deviation (rmsd) of 7.6 cm−1 between empirical and ab initio frequencies. However, when the model was refined to include the z-component of the electric field at the center of the six-membered ring, , i.e.,
| (3) |
the resultant correlation (Figure 3b) is significantly improved, yielding a correlation coefficient of 0.81 and a rmsd of 5.8 cm−1 with , , and Taken together, these results indicate that the electric field component at a point on the ring but distant from the nitrile group could have a significant effect on its vibrational frequency. While a model including only the electric field localized at the C≡N group could also yield a good correlation, it requires at least six electric field components to produce a comparable correlation (Table S2, Supporting Information). Thus, these findings show that by considering the electric field away from the C≡N group it is possible to fit the ab initio frequencies with a minimum number of parameters.
Figure 3.
Ab initio frequency versus the empirical frequency determined from (a) the two-component and (b) the three-component empirical models. The correlation coefficients (R2) are 0.64 and 0.81 for the data presented in (a) and (b), respectively.
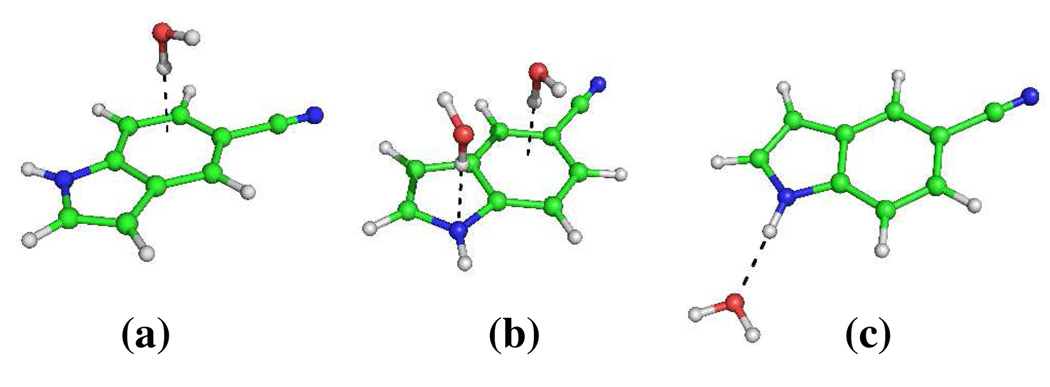
To further verify that the indole ring indeed plays a significant role in mediating the stretching frequency of the nitrile group, we have investigated the specific effect of one or two water molecules placed at different positions with respect to the ring, as shown in Figure 4. As expected, we found that the C≡N stretching frequency of all three clusters shows a significant shift (i.e., Δω = ωcluster −ωgas ≠ 0) from that of the unsolvated ring with Δω = 3.9, 8.8 , and −4.0 cm−1 for (a), (b) and (c), respectively. Thus, these results, which indicate that Δω is sensitive to the position and orientation of the water molecule(s), corroborate the notion that the solvent-indole interaction is a significant determinant of the C≡N stretching frequency.
Figure 4.
Three model 5-cyanoindole-water clusters. In (a) and (b), the distance between the closest water hydrogen and the indole ring plane is 2.4 Å, whereas in (c) the H2O--HN distanceis 2.0 Å.
Having determined a set of parameters , eq. 3 was then used to calculate the C≡N stretching frequency of individual snapshots along the 1 ns MD trajectory of 5-cyanoindole in water. The resultant frequencies show a Gaussian distribution with a standard deviation of 13.5 cm−1 and a mean that is −9.7 cm−1 red-shifted with respect to ωgas. The latter suggests that the current model correctly predicts the trend of the frequency shift of the C≡N stretching vibration upon hydration as the nitrile stretching frequency is typically red-shifted with respect to the gas-phase value. For example, the C≡N stretching frequency of benzonitrile is ∼2244 cm−1 in the gas-phase,41 which shifts to ∼2235 cm−1 in water.16 Unfortunately, for the present case a more quantitative assessment of the predicted shift cannot be made at this time because an experimental value for ωgas for 5-cyanoindole is currently not available.
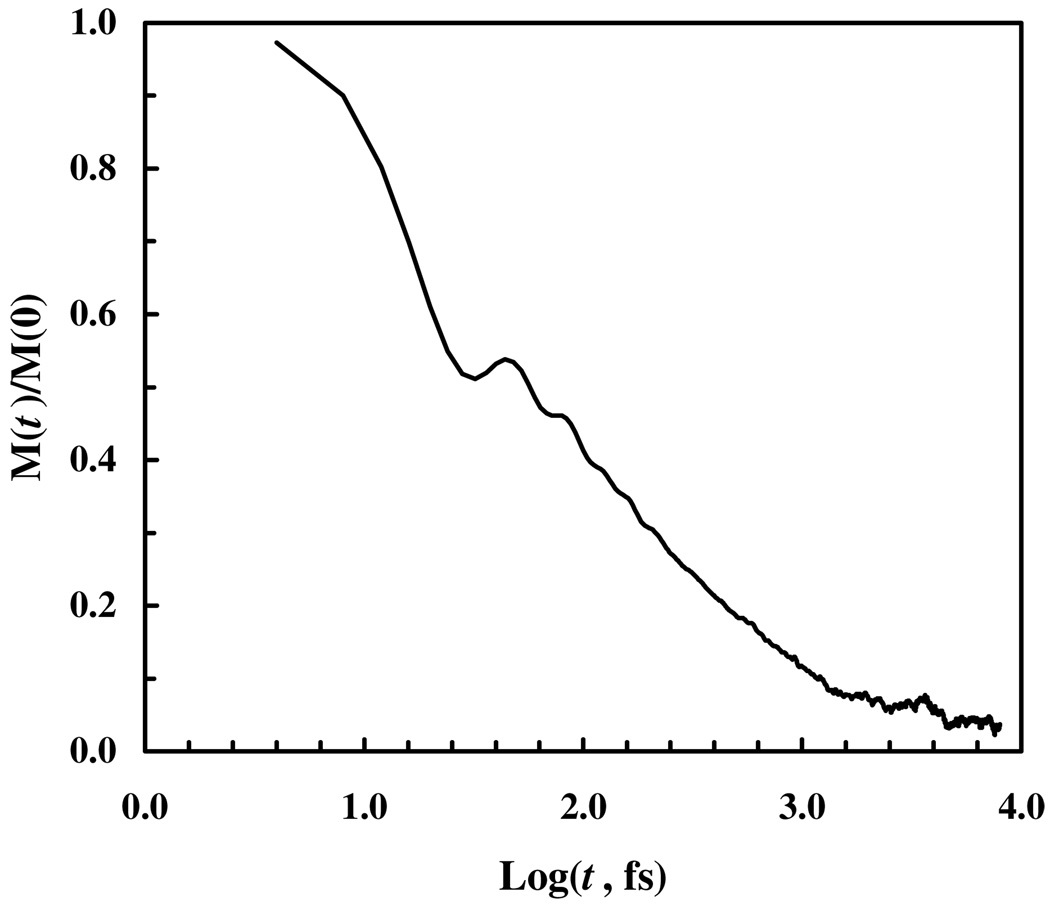
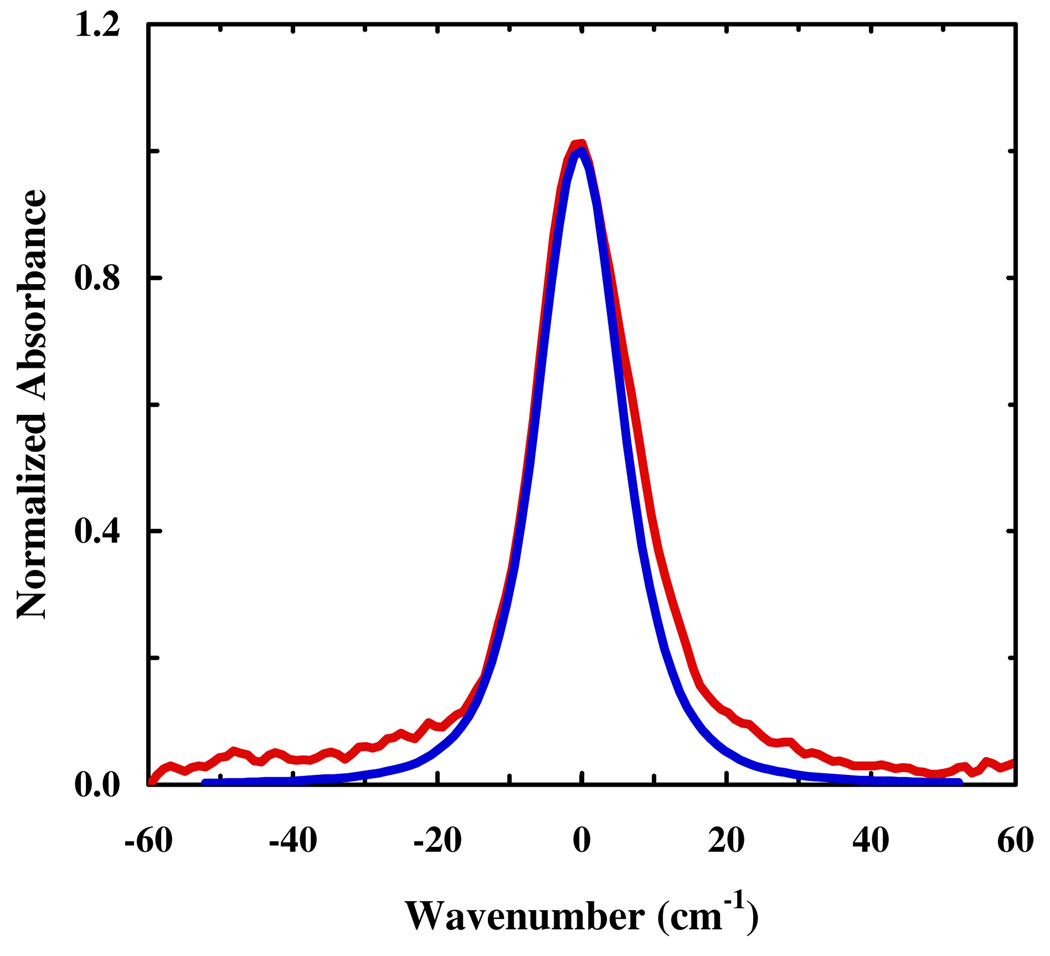
Finally, we calculated the IR band of the C≡N stretching vibration from the frequency-frequency correlation function, with . As shown (Figure 5), the (normalized) autocorrelation function decays rapidly in a bimodal fashion, similar to that observed for other nitrile compounds.15,16,17,30 The vibrational pure dephasing constant, , was estimated to be 0.25 ps. The variance, , and correlation time, , of the C≡N stretching frequency fluctuations of 5-cyanoindole were found to be 2.53 ps−1 and 0.61 ps, respectively. Thus, the spectrum is neither in the homogeneous nor in the inhomogeneous regime. Therefore, the vibrational spectrum was calculated using a semiclassical approximation42–44 to , where μ is the quantum mechanical dipole operator (see Supporting Information for details). As shown (Figure 6), the bandwidth of the simulated spectrum is in very good agreement with that determined experimentally. However, this agreement must be cautiously interpreted. First, the vibrational lifetime of 5-cyanoindole is currently not available. Thus, we have assumed a vibrational lifetime of 5 ps. Second, the production run of the simulation was carried out in the NPT ensemble, which may adversely affect the quality of the frequency-frequency time correlation function.
Figure 5.
Normalized frequency-frequency correlation function, calculated from the C≡N frequency trajectory.
Figure 6.
Comparison between the experimentally measured (red) and simulated (blue) C≡N stretching bands of 5-cyanoindole.
Moreover, it is worth noting that the present electrostatic map may not be applicable to 5-cyanotryptophan, especially when it locates in a heterogeneous environment of proteins or membranes, as this map incorporates interactions that are specific to water. Although such “solvent-specific” maps have been used within the context of peptides,45 alternative approaches such as those developed by the groups of Corcelli,29,30 Mukamel,24–26 , Jansen28 and Kurnikova46 may be more appropriate.
In conclusion, we demonstrate that interactions between water and the indole ring make an important contribution to the broadening of the C≡N stretching band of 5-cyanoindole in aqueous solution. Thus, this study provides a molecular basis to support the notion that 5-cyanotryptophan is a useful IR probe of local hydration status of proteins, and also indicates that the chemical moiety to which the C≡N group is attached may need to be considered in the development of electrostatic maps for calculation of the C≡N stretching frequencies.
Supplementary Material
Acknowledgement
We gratefully acknowledge financial support from the National Institutes of Health (GM-065978 and RR-01348) and also the Computer Facility of the Department of Chemistry at the University of Pennsylvania. We thank Professor Jeffery G. Saven for helpful discussions and comments, and Christopher MacDermaid for his assistance in setting up the computer system.
Footnotes
Supporting Information Available: Details of the molecular dynamics simulation, the vibrational frequency analysis, and band shape calculation. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Eaton G, Pena-Nuñez AS, Symons CR. Solvation of Cyanoalkanes [CH3CN and (CH3)3CCN] J. Chem. Soc. Faraday Trans. 1988;1:2181–2193. [Google Scholar]
- 2.Reimers JR, Hall LE. The Solvation of Acetonitrile. J. Am. Chem. Soc. 1999;121:3730–3744. [Google Scholar]
- 3.Lindquist BA, Furse KE, Corcelli SA. Nitrile Groups as Vibrational Probes of Biomolecular Structure and Dynamics: An Overview. Phys. Chem. Chem. Phys. 2009;11:8105–8380. doi: 10.1039/b908588b. [DOI] [PubMed] [Google Scholar]
- 4.Aschaffenburg DJ, Moog RS. Probing Hydrogen Bonding Environments: Solvatochromic Effects on the CN Vibration of Benzonitrile. J. Phys. Chem. B. 2009;113:12736–12743. doi: 10.1021/jp905802a. [DOI] [PubMed] [Google Scholar]
- 5.Getahun Z, Huang C-Y, Wang T, De León B, DeGrado WF, Gai F. Using Nitrile-Derivatized Amino Acids as Infrared Probes of Local Environment. J. Am. Chem. Soc. 2003;125:405–411. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]
- 6.Tucker MJ, Getahun Z, Nanda V, DeGrado WF, Gai F. A New Method for Determining the Local Environment and Orientation of Individual Side Chains of Membrane-Binding Peptides. J. Am. Chem. Soc. 2004;126:5078–5079. doi: 10.1021/ja032015d. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee S, Chowdhury P, DeGrado WF, Gai F. Site-Specific Hydration Status of an Amphipathic Peptide in AOT Reverse Micelles. Langmuir. 2007;23:11174–11179. doi: 10.1021/la701686g. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Strzalka J, Tronin A, Johansson JS, Blasie JK. Mechanism of Interaction between the General Anesthetic Halothane and a Model Ion Channel Protein, II: Fluorescence and Vibrational Spectroscopy Using a Cyanophenylalanine Probe. Biophys. J. 2009;96:4176–4187. doi: 10.1016/j.bpj.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou H, Liu J, Blasie JK. Mechanism of Interaction between the General Anesthetic Halothane and a Model Ion Channel Protein, III: Molecular Dynamics Simulation Incorporating a Cyanophenylalanine Spectroscopic Probe. Biophys. J. 2009;96:4188–4199. doi: 10.1016/j.bpj.2009.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fafarman AT, Webb JL, Chuang JI, Boxer SG. Site-Specific Conversion of Cysteine Thiols into Thiocyanate Creates an IR Probe for Electric Fields in Proteins. J. Am. Chem. Soc. 2006;128:13356–13357. doi: 10.1021/ja0650403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz KC, Supekova L, Ryu Y, Xie J, Perera R, Schultz PG. A Genetically Encoded Infrared Probe. J. Am. Chem. Soc. 2006;128:13984–13985. doi: 10.1021/ja0636690. [DOI] [PubMed] [Google Scholar]
- 12.Sigala PA, Fafarman AT, Bogard PE, Boxer SG, Herschlag D. Do Ligand Binding and Solvent Exclusion Alter the Electrostatic Character within the Oxyanion Hole of an Enzymatic Active Site? J. Am. Chem. Soc. 2007;129:12104–12105. doi: 10.1021/ja075605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waegele MM, Tucker MJ, Gai F. 5-Cyanotryptophan as an Infrared Probe of Local Hydration Status of Proteins. Chem. Phys. Lett. 2009;478:249–253. doi: 10.1016/j.cplett.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maienschein-Cline MG, Londergan CH. The CN Stretching Band of Aliphatic Thiocyanate is Sensitive to Solvent Dynamics and Specific Solvation. J. Phys. Chem. A. 2007;111:10020–10025. doi: 10.1021/jp0761158. [DOI] [PubMed] [Google Scholar]
- 15.Oh K-I, Choi JH, Lee J-H, Han B-J, Lee H, Cho M. Nitrile and Thiocyanate IR Probes: Molecular Dynamics Simulation Studies. J. Chem. Phys. 2008;128:154504. doi: 10.1063/1.2904558. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh A, Remorino A, Tucker MJ, Hochstrasser RM. 2D IR Photon Echo Spectroscopy Reveals Hydrogen Bond Dynamics of Aromatic Nitriles. Chem. Phys. Lett. 2009;469:325–330. doi: 10.1016/j.cplett.2008.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha J-H, Lee K-K, Park K-H, Choi J-H, Jeon S-J, Cho M. Integrated and Dispersed Photon Echo Studies of Nitrile Stretching Vibration of 4-Cyanophenol in Methanol. J. Chem. Phys. 2009;130:204509. doi: 10.1063/1.3140402. [DOI] [PubMed] [Google Scholar]
- 18.Choi J-H, Oh K-I, Lee H, Lee C, Cho M. Nitrile and Thiocyanate IR probes: Quantum Chemistry Calculation Studies and Multivariate Least-Square Fitting Analysis. J. Chem. Phys. 2008;128:134506. doi: 10.1063/1.2844787. [DOI] [PubMed] [Google Scholar]
- 19.Ham S, Kim JH, Lee H, Cho M. Correlation Between Electronic and Molecular Structure Distortions and Vibrational Properties. II. Amide I Modes of NMA–nD2O Complexes. J. Chem. Phys. 2003;118:3491–3498. [Google Scholar]
- 20.Bour P, Keiderling TA. Empirical Modeling of The Peptide Amide I Band IR Intensity in Water Solution. J. Chem. Phys. 2003;119:11253–11262. [Google Scholar]
- 21.Corcelli SA, Lawrence CP, Skinner JL. Combined Electronic Structure/Molecular Dynamics Approach for Ultrafast Infrared Spectroscopy of Dilute HOD in Liquid H2O and D2O. J. Chem. Phys. 2004;120:8107–8117. doi: 10.1063/1.1683072. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt JR, Corcelli SA, Skinner JL. Ultrafast Vibrational Spectroscopy of Water and Aqueous N-Methylacetamide: Comparison of Different Electronic Structure/Molecular Dynamics Approaches. J. Chem. Phys. 2004;121:8887–8896. doi: 10.1063/1.1791632. [DOI] [PubMed] [Google Scholar]
- 23.Corcelli SA, Skinner JL. Infrared and Raman Line Shapes of Dilute HOD in Liquid H2O and D2O from 10 to 90 °C. J. Phys. Chem. A. 2005;109:6154–6165. doi: 10.1021/jp0506540. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Jansen TlC, Zhuang W, Mukamel S. Collective Solvent Coordinates for the Infrared Spectrum of HOD in D2O Based on an Ab Initio Electrostatic Map. J. Phys. Chem. A. 2005;109:64–82. doi: 10.1021/jp046685x. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Zhuang W, Mukamel S. Electrostatic DFT Map for the Complete Vibrational Amide Band of NMA. J. Phys. Chem. A. 2005;109:9747–9759. doi: 10.1021/jp052324l. [DOI] [PubMed] [Google Scholar]
- 26.Jansen TlC, Hayashi T, Zhuang W, Mukamel S. Stochastic Liouville Equations for Hydrogen-Bonding Fluctuations and Their Signatures in Two-Dimensional Vibrational Spectroscopy of Water. J. Chem. Phys. 2005;123:114504. doi: 10.1063/1.2008251. [DOI] [PubMed] [Google Scholar]
- 27.Watson TM, Hirst JD. Theoretical Studies of the Amide I Vibrational Frequencies of [Leu]-Enkephalin. Mol. Phys. 2005;103:1531–1546. [Google Scholar]
- 28.Jansen TlC, Knoester J. A Transferable Electrostatic Map for Solvation Effects on Amide I Vibrations and Its Application to Linear and Two-Dimensional Spectroscopy. J. Chem. Phys. 2006;124:044502. doi: 10.1063/1.2148409. [DOI] [PubMed] [Google Scholar]
- 29.Lindquist BA, Corcelli SA. Nitrile Groups as Vibrational Probes: Calculations of the CN Infrared Absorption Line Shape of Acetonitrile in Water and Tetrahydrofuran. J. Phys. Chem. B. 2008;112:6301–6303. doi: 10.1021/jp802039e. [DOI] [PubMed] [Google Scholar]
- 30.Lindquist BA, Haws RT, Corcelli SA. Optimized Quantum Mechanics/Molecular Mechanics Strategies for Nitrile Vibrational Probes: Acetonitrile and para-Tolunitrile in Water and Tetrahydrofuran. J. Phys. Chem. B. 2008;112:13991–14001. doi: 10.1021/jp804900u. [DOI] [PubMed] [Google Scholar]
- 31.Macias AT, MacKerell AD., Jr CH/ πInteractions Involving Aromatic Amino Acids: Refinement of the CHARMM Tryptophan Force Field. J. Comput. Chem. 2005;26:1452–1463. doi: 10.1002/jcc.20281. [DOI] [PubMed] [Google Scholar]
- 32.Yin D. Ph. D. Thesis. University of Maryland; Baltimore, MD: 1997. [Google Scholar]
- 33.Grabuleda X, Jaime C, Kollman PA. Molecular Dynamics Simulation Studies of Liquid Acetonitrile: New Six-Site Model. J. Comput. Chem. 2000;21:901–908. [Google Scholar]
- 34.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, et al. Gaussian 03, Revision B.05. Pittsburgh PA: Gaussian, Inc; 2003. [Google Scholar]
- 35.Breneman CM, Wiberg KB. Determining Atom-Centered Monopoles from Molecular Electrostatic Potentials. The Need for High Sampling Density in Formamide Conformational Analysis. J. Comput. Chem. 1990;11:361–373. [Google Scholar]
- 36.Bayly CI, Cieplak P, Cornell WD, Kollman PA. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges: The RESP Model. J. Phys. Chem. 1993;97:10269–10280. [Google Scholar]
- 37.Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, Truhlar DJ. Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative Assessments for Hydrogen Bonding and van der Waals Interactions. J. Phys. Chem. A. 2004;108:6908–6918. [Google Scholar]
- 39.Zhang RB, Somers KRF, Kryachko ES, Nguyen MT, Zeegers-Huyskens T, Ceulemans A. Hydrogen Bonding to π-Systems of Indole and 1-Methylindole: Is There Any OH···Phenyl Bond? J. Phys. Chem. A. 2005;109:8028–8034. doi: 10.1021/jp0525437. [DOI] [PubMed] [Google Scholar]
- 40.Humphrey W, Dalke A, Schulten K. VMD: Visual Molecular Dynamics. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 41.Varsanyi G, Lang L. Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives. New York: Wiley; 1974. [Google Scholar]
- 42.Gordon RG. Correlation Functions for Molecular Motion. Adv. Mag. Resonance. 1968;3:1–42. [Google Scholar]
- 43.Mukamel S. Principles of Nonlinear Spectroscopy. Oxford: Oxford University Press; 1995. [Google Scholar]
- 44.Kwac K, Cho M. Molecular Dynamics Simulation Study of N-Methylacetamide in Water. I. Amide I Mode Frequency Fluctuation. J. Chem. Phys. 2003;119:2247. [Google Scholar]
- 45.Ganim Z, Tokmakoff A. Spectral Signatures of Heterogeneous Protein Ensembles Revealed by MD Simulations of 2DIR Spectra. Biophys. J. 2006;91:2636–2646. doi: 10.1529/biophysj.106.088070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speranskiy K, Kurnikova M. Accurate Theoretical Prediction of Vibrational Frequencies in an Inhomogeneous Dynamic Environment: A Case Study of a Glutamate Molecule in Water Solution and in a Protein-Bound Form. J. Chem. Phys. 2004;121:1516–1524. doi: 10.1063/1.1752887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.