Abstract
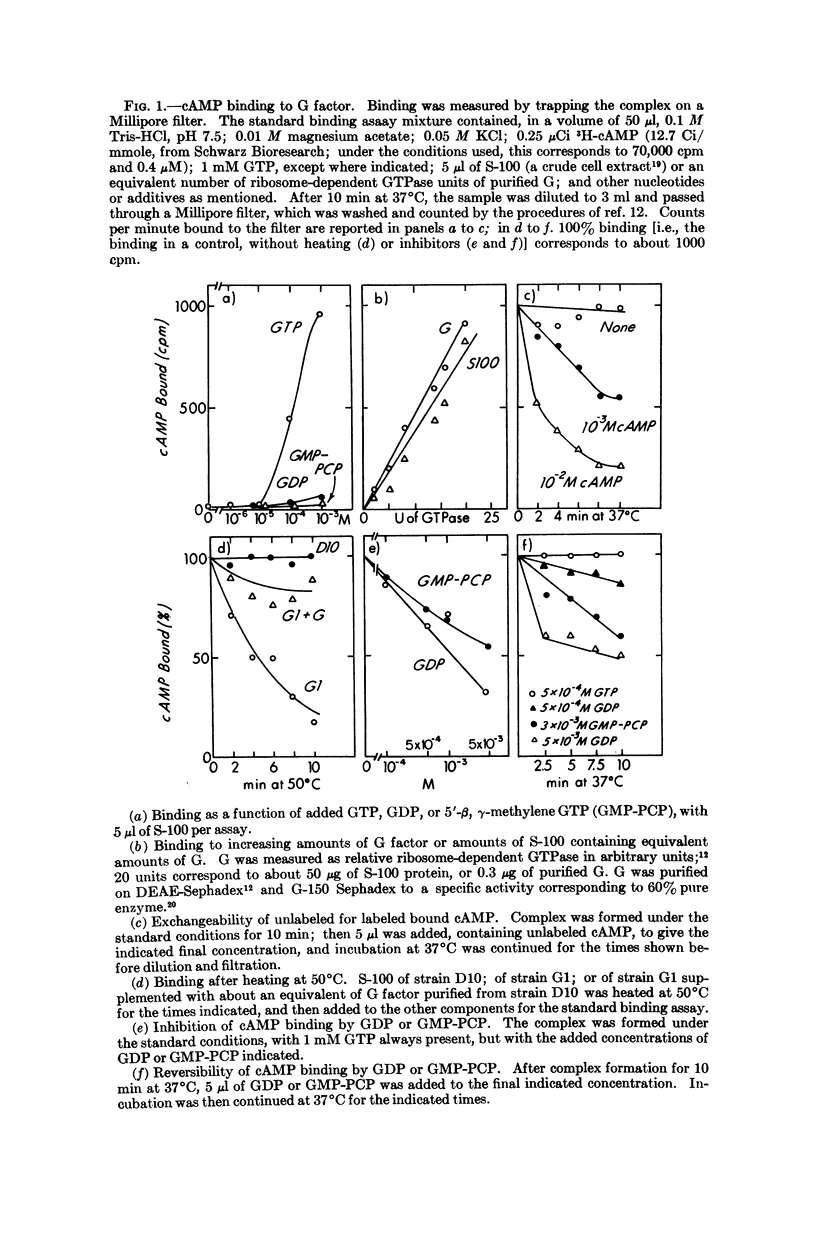
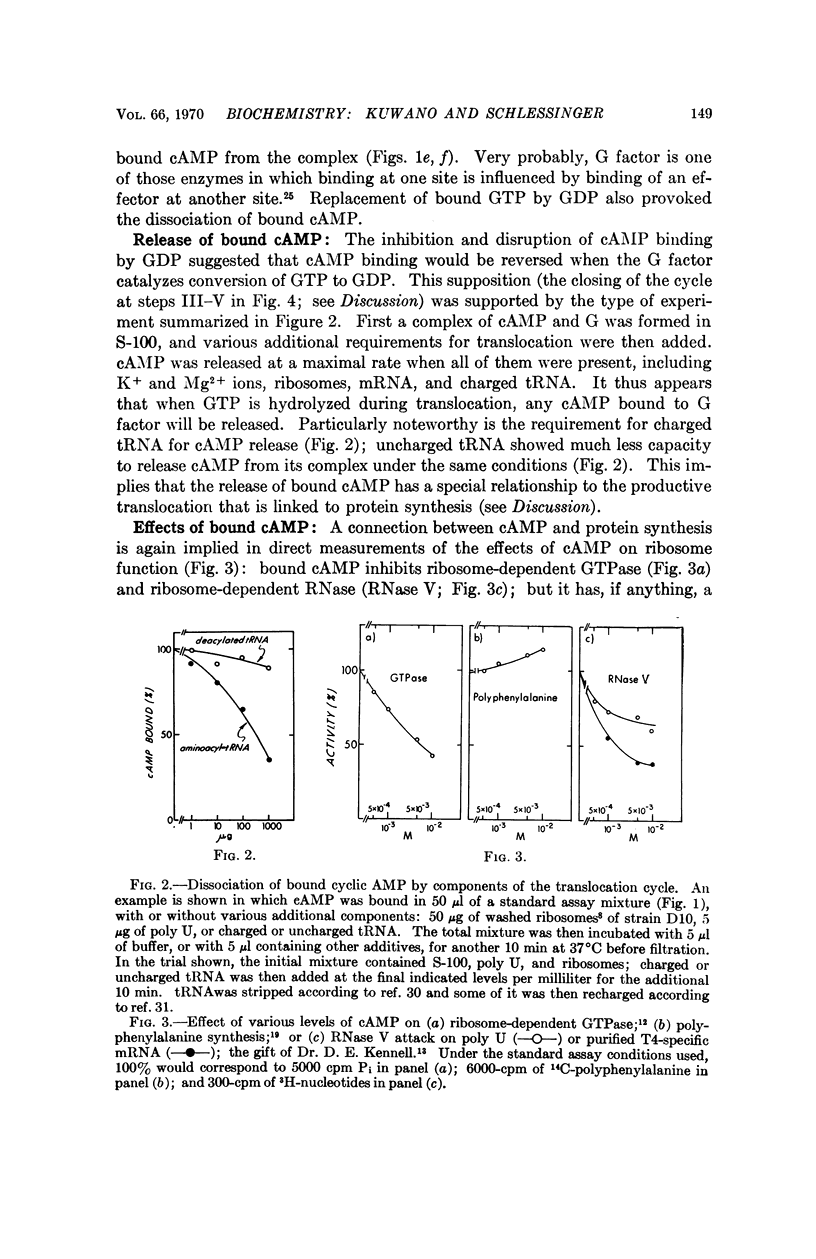
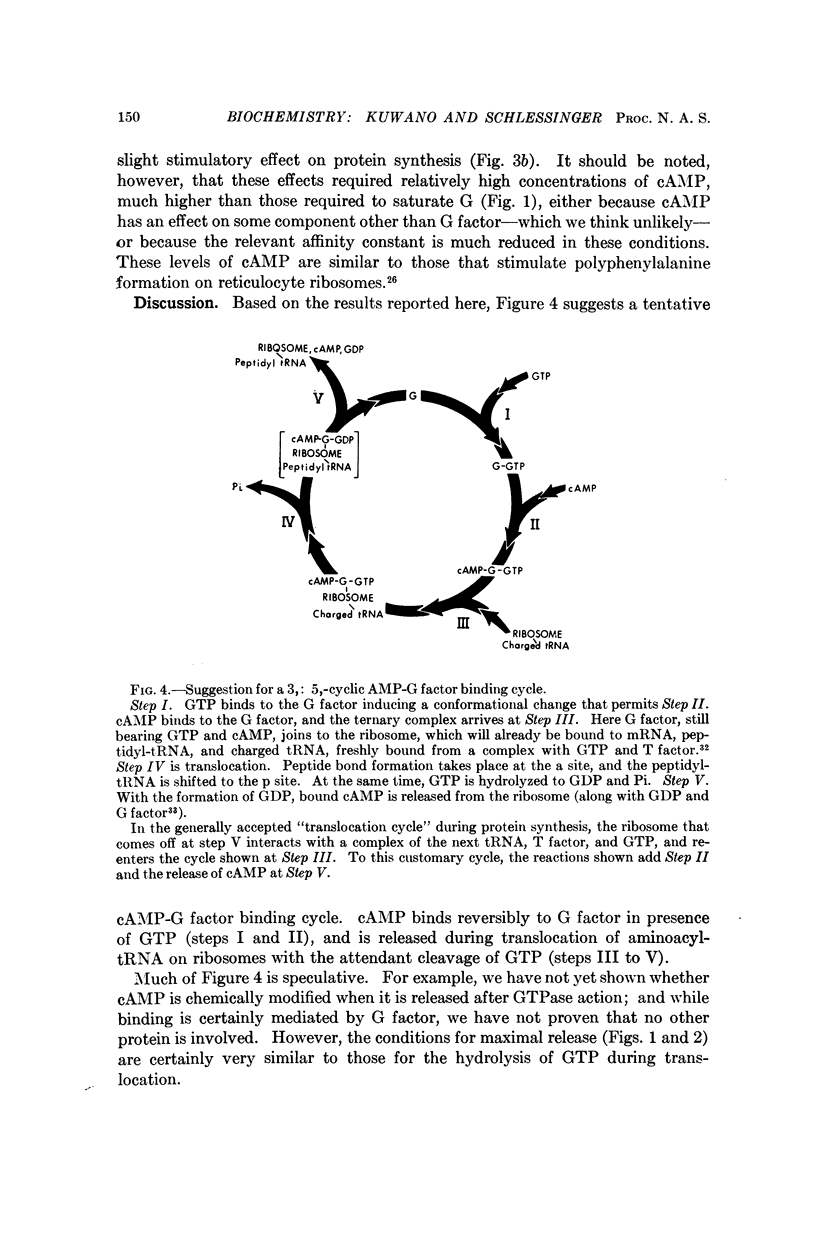
Unique among adenine nucleotides tested by filter binding assays, 3′:5′-cyclic AMP binds to the G translocation factor. Binding is dependent on the presence of GTP, and is inhibited by GDP, by the analog 5′-β,γ-methylene GTP, and by the antibiotic fusidic acid. The cAMP seems to be released during the ribosome-dependent translocation of charged tRNA catalyzed by G factor. Bound cAMP inhibits GTPase and ribosome-associated degradation of messenger RNA, but does not inhibit protein synthesis. cAMP might thereby regulate the ratio of productive to degradative transits of ribosomes on messenger RNA, and this may account for some part of its profound effect on levels of specific bacterial messenger RNA species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONWAY T. W., LIPMANN F. CHARACTERIZATION OF A RIBOSOME-LINKED GUANOSINE TRIPHOSPHATASE IN ESCHERICHIA COLI EXTRACTS. Proc Natl Acad Sci U S A. 1964 Dec;52:1462–1469. doi: 10.1073/pnas.52.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY T. W. ON THE ROLE OF AMMONIUM OR POTASSIUM ION IN AMINO ACID POLYMERIZATION. Proc Natl Acad Sci U S A. 1964 Jun;51:1216–1220. doi: 10.1073/pnas.51.6.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Gussin G. N. Suppression in vitro: Identification of a Serine-sRNA as a "Nonsense" Suppressor. Science. 1965 Jul 23;149(3682):417–422. doi: 10.1126/science.149.3682.417. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Perlman R. L., Varmus H. E., Pastan I. Regulation of inducible enzyme synthesis in Escherichia coli by cyclic adenosine 3', 5'-monophosphate. J Biol Chem. 1969 Nov 10;244(21):5828–5835. [PubMed] [Google Scholar]
- Felicetti L., Tocchini-Valentini G. P., Di Matteo G. F. The role of G factor in protein synthesis. Studies on a temperature-sensitive Escherichia coli mutant with an altered G factor. Biochemistry. 1969 Aug;8(8):3428–3432. doi: 10.1021/bi00836a044. [DOI] [PubMed] [Google Scholar]
- Gallant J., Harada B. The control of ribonucleic acid synthesis in Escherichia coli. 3. The functional relationship between purine ribonucleoside triphosphate pool sizes and the rate of ribonucleic acid accumulation. J Biol Chem. 1969 Jun 25;244(12):3125–3132. [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Hershey J. W., Monro R. E. A competitive inhibitor of the GTP reaction in protein synthesis. J Mol Biol. 1966 Jun;18(1):68–76. doi: 10.1016/s0022-2836(66)80077-3. [DOI] [PubMed] [Google Scholar]
- Ippen K., Miller J. H., Scaife J., Beckwith J. New controlling element in the Lac operon of E. coli. Nature. 1968 Mar 2;217(5131):825–827. doi: 10.1038/217825a0. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Kawano G., Tanaka N. Association of fusidic acid sensitivity with G factor in a protein-synthesizing system. Biochem Biophys Res Commun. 1968 Dec 9;33(5):769–773. doi: 10.1016/0006-291x(68)90226-x. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Kwan C. N., Apirion D., Schlessinger D. Ribonuclease V of escherichia coli. I. Dependence on ribosomes and translocation. Proc Natl Acad Sci U S A. 1969 Oct;64(2):693–700. doi: 10.1073/pnas.64.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Malkin M., Lipmann F. A stimulation by cyclic 3',5'-adenosine monophosphate of amino acid activation and polymerization in reticulocyte hemolysates. Proc Natl Acad Sci U S A. 1969 Nov;64(3):973–980. doi: 10.1073/pnas.64.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmeggiani A. Crystalline transfer factors from Escherichia coli. Biochem Biophys Res Commun. 1968 Mar 27;30(6):613–619. doi: 10.1016/0006-291x(68)90556-1. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Stimulation of tryptophanase synthesis in Escherichia coli by cyclic 3',5'-adenosine monophosphate. J Biol Chem. 1969 Apr 25;244(8):2226–2232. [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. The role of the lac promotor locus in the regulation of beta-galactosidase synthesis by cyclic 3',5'-adenosine monophosphate. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1336–1342. doi: 10.1073/pnas.61.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Regulation of beta-galactosidase synthesis in Escherichia coli by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1968 Oct 25;243(20):5420–5427. [PubMed] [Google Scholar]
- Ravel J. M. Demonstration of a guanosine triphosphate-dependent enzymatic binding of aminoacyl-ribonucleic acid to Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1811–1816. doi: 10.1073/pnas.57.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Tao M., Lipmann F. Isolation of adenyl cyclase from Escherichia coli. Proc Natl Acad Sci U S A. 1969 May;63(1):86–92. doi: 10.1073/pnas.63.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Berg P. Recognition of tRNA by aminoacyl tRNA synthetases. J Mol Biol. 1967 Sep 28;28(3):479–490. doi: 10.1016/s0022-2836(67)80098-6. [DOI] [PubMed] [Google Scholar]



