Abstract
New methods for obtaining metabolic fingerprints of biological samples with improved resolution and sensitivity are highly sought for early disease detection, studies of human health and pathophysiology, and for better understanding systems biology. Considering the complexity of biological samples, interest in biochemical class selection through the use of chemoselective probes for improved resolution and quantitation is increasing. Considering the role of lipids in the pathogenesis of a number of diseases, in this study fingerprinting of lipid metabolites was achieved by 31P labeling using the derivatizing agent 2-chloro-4,4,5,5-tetramethyldioxaphospholane. Lipids containing hydroxyl, aldehyde and carboxyl groups were selectively tagged with 31P and then detected with good resolution using 31P NMR by exploiting the 100% natural abundance and wide chemical shift range of 31P. After standardizing the reaction conditions using representative compounds, the derivatization approach was used to profile lipids in human serum. The results show that the 31P derivatization approach is simple, reproducible and highly quantitative, and has the potential to profile a number of important lipids in complex biological samples.
Keywords: NMR, 31P, metabolite profiling, metabolomics, chemical derivatization, lipids
Introduction
The analysis of lipid constituents in biological samples is a vital part of metabolomics studies due to the important role of lipids in disease diagnosis and human health risk assessment.1 Investigations have shown that alterations in the lipid profile are the result in a number of pathological conditions such as cardiovascular disease,2 certain cancers,3, 4 Alzheimer’s disease5 and some inborn errors of metabolism.6
Mass spectrometry is a commonly used methodology for lipid analysis because hyphenated techniques, such as liquid chromatography- (LC), gas chromatography- (GC) and capillary electrophoresis- (CE) mass spectrometry provide comprehensive profiles of the sample in addition to the potential for relative or absolute quantitation of the components. However, the reproducibility of the mass spectrometry-based metabolite profiling approach can be challenging in complex samples such as biofluids. NMR spectroscopy has also been applied in metabolic profiling studies of a wide range of complex samples such as urine, serum, plasma and tissue, both in in-vivo and in-vitro studies. These studies take advantage of NMR’s experimental versatility, high resolution, reproducibility, and quantitative ability, as well as the fact that it can provide a high degree of information on the biological system of interest, often with limited sample preparation. 7–12 Applications of NMR to the analysis of lipids have been relatively sparse, although a number of lipids such as cholesterol, cholesterol derivatives, glycerophopsholipids and cholines can readily be detected by 1H NMR. The NMR spectral resolution is somewhat diminished in highly viscous lipophilic compounds due to the line broadening that results from restricted molecular motion and a high degree of non-resolved J-couplings among the many methylene protons present. Close structural similarity amongst different lipid categories, combined with their varied concentrations in biological samples, further add to the difficulties in the analysis of lipids by NMR.
An alternative to NMR analysis of the whole biological sample involves a more selective experiment such as class selection. Metabolite class selection has been shown to be a powerful tool in metabolite profiling and biomarker discovery, particularly because it greatly alleviates the problem of peak overlap in biological samples.13–15 In addition, this approach provides the potential for fast and efficient methods which enhance the sensitivity of certain classes of metabolites. Metabolite class selection by chemical derivatization is relatively common in MS and GC analysis.13, 14, 16 However, despite a number of early studies,17 chemical derivatization is less commonly utilized in NMR, especially for metabolomics applications. Recently, we reported a successful chemical derivatization based NMR methodology for metabolomics applications.18 Derivatization of amines and amino acids using 13C labeled acetic anhydride yielded approximately 100-fold enhanced sensitivity for analysis by 13C or heteronuclear 2D NMR. This simple, one-step chemical derivatization reaction is fast, cost effective and requires minimal sample preparation. The spectrum is simplified because only a single peak is detected from the derivatizing agent or tag that reacts with the metabolite of interest. We have recently extended this approach to detect carboxylic acid containing metabolites by tagging them with 15N labeled ethanolamine.19
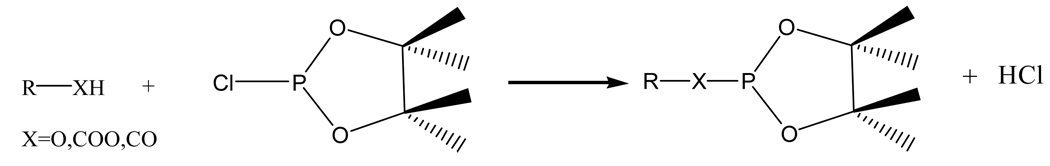
In this study, as a continuation of exploring classes of metabolites in biological systems, we report a simple chemical derivatization procedure for selectively analyzing lipid metabolites with hydroxyl, carboxyl or aldehyde groups in a complex biological fluid, serum. Metabolites with these functionalities have been shown to be altered in a number of diseases. For example, accumulation of free fatty aldehydes and alcohols in the human body is a clinical indication of Sjögren-Larsson syndrome that is due to a genetic enzyme deficiency of fatty aldehyde dehydrogenerase.20 The elevation of total free fatty acids in serum has been reported to be an indication of acute pancreatitis (AP), and more generally, free fatty levels provide an important measure of the physiologic state.21, 22 The 31P containing reagent, 2-chloro-4,4,5,5-tetramethyldioxaphospholane (CTMDP) was used to derivatize the lipid metabolites (Scheme 1). Derivatized metabolites were then detected with enhanced resolution using 31P NMR. Previously, chemical derivatization using this reagent has been performed to quantify fatty acids and glycerides in olive oil for quality control purposes.23, 24 These investigations report CTMDP to be an excellent derivatizing reagent for lipids with hydroxyl, carboxyl or aldehyde functional groups. We find this derivatization approach to be relatively fast and simple; the method requires only a modest amount of sample preparation and can be used to identify a number of lipophilic functionalities in one experiment, without the use of chromatography.
Scheme 1.
Experimental
Chemicals and samples
Lyso lipids, cholesterol, phosphatidyl glycerol were purchased from Avanti polar lipids (Alabaster, AL) and 1,2 dipalmitoyl glycerol was purchased from Sygena (Switzerland). All other compounds (octanoic acid, palmitic acid, oleic acid, 1-hexanol, 1-octanol, spingomyelin, inositol, chromium acetoacetate) including solvents and 2-chloro-4,4,5,5-tetramethyldioxaphospholane, were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. Deuterated solvents were purchased from Cambridge isotope (Andover, MA). Human serum samples were obtained from Innovative Research (Novi, MI).
Lipid extraction
A 0.5 ml sample of serum was mixed with 1.0 ml of methanol, vortexed and kept at −20 °C for 30 min to precipitate serum lipids and proteins. The mixture was subsequently centrifuged at 5000 rpm for 5 min and the supernatant containing aqueous soluble metabolites was separated and discarded. 1.0 ml methanol and 0.5 ml deionized water were then added to the precipitate, vortexed and centrifuged at 5000 rpm for 5 min. The supernatant was again discarded and the residue was treated with 1.0 ml methanol and sonicated (Sonifier cell disruptor w-350, pulse mode, output control 3) for a total of 6 min, with delays of about 15 sec after every 2 min. During sonication the sample container was kept in an ice bath to avoid sample heating. The sample was then treated with an additional 3.0 ml methanol, mixed well and sonicated for another 6 min similarly, and then stored at −4 °C overnight. The sample was sonicated again for 6 min and then passed through a 0.45 µm cellulose filter (Thermo Scientific, Waltham, MA), which had been previously washed multiple times with a methanol/chloroform mixture using a glass syringe. Chloroform and methanol were removed using a dry nitrogen stream, and then the lipid sample was further dried using a speed vac system (Thermo scientific, Waltham, MA).
Chemical derivatization
The chemical derivatization procedure was adapted from the previously described method.23 A stock solution consisting of 6.5 ml pyridine and 3.5 ml deuterated chloroform was mixed in a vial containing molecular sieves to protect the solution from moisture. Cyclohexanol (2 µl) was added as an internal standard and a catalytic amount of chromium acetylacetonate was added as a NMR relaxation agent. The stock solution was kept sealed to limit its exposure to moisture. For standardization and chemical shift calibration, 1–3 mg each (depending on the molecular mass) of a number of representative compounds were dissolved in 500 µl of the stock solution. Subsequently, 60 µl of CTMDP was added. The reaction was allowed to run for 30 min prior to NMR acquisition. For the serum experiments, the dried lipid extract was reconstituted in 500 µl stock solution before chemical derivatization was performed. In a different protocol, aliquots of serum samples were lyophilized to dryness and reconstituted in stock solution before derivatization with CTMDP. The sample was spun down to remove any residual solids prior to transfer to the NMR tube. Duplicate reactions and analyses on a split serum sample were also performed. To assess the quantitative yield and reproducibility, the derivatization reaction was performed in triplicate for a set of representative compounds (palmitic acid, cholesterol, 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate, 1,2-dipalmitoyl glycerol, hexanal and 1-octanol).
NMR acquisition
Measured volumes (500 µl) of the derivatized (serum extract or model compounds) were placed in 5 mm NMR tubes and a concentric capillary insert containing methylenediphosphonic acid (50 µl of 0.1 M or 50 µl of 9.3 mM for the serum experiments) as a separate internal standard was placed in the NMR tube. All NMR experiments were carried out at 25 °C on a Bruker DRX 500 MHz spectrometer equipped with multinuclear broadband observe (BBO) probe. One-dimensional 31P NMR spectra were recorded with inverse-gated proton decoupling using the WALTZ-16 sequence. A total of 1024 (for lipid extracts) or 64 (for model compounds) transients were averaged and 64 K data points acquired for each sample. Appodization corresponding to a line broadening of 1.0 Hz was applied prior to Fourier transformation. A recycle delay of 10 sec was used to analyze the derivatized serum extracts, while 30 or 50 sec was used for the quantification and reproducibility studies.
Results and Discussion
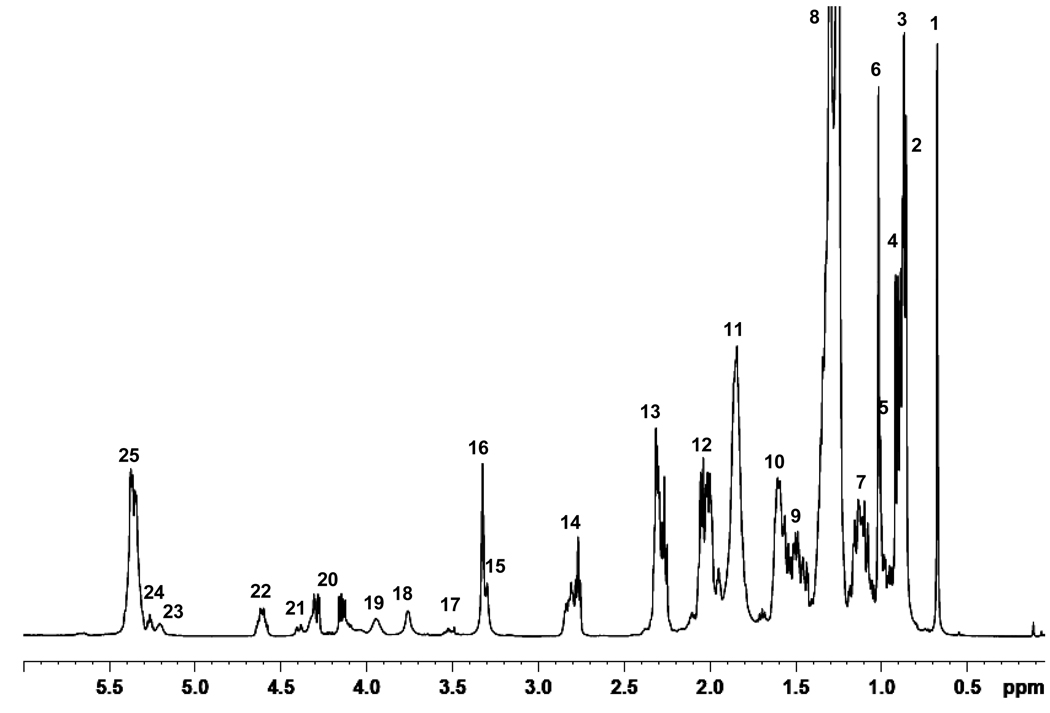
The NMR spectrum of serum lipids typically shows a number of reasonably well resolved peaks in chloroform solution. The 1H NMR spectrum (Figure 1) of a reconstituted serum lipid extract 8 was obtained prior to derivatization for comparison with features post-derivatization. Table 1 lists the observed peaks and their assignments based on literature reports6, 12, 25 and NMR databases;26, 27 assignments were subsequently confirmed via COSY (data not shown). Typically, the 1H NMR spectrum of serum is complex as a consequence of severe spectral overlap from numerous low mass metabolites and macromolecular lipophilic compounds and proteins. However, as is apparent in Figure 1, exclusion of the proteins and hydrophilic low mass metabolites, especially the highly abundant glucose, glycogen and amino acids in serum, as well as the use of deuterated chloroform, leads to improved resolution and good line shape and to an unambiguous identification of individual compounds. The spectrum shows intense resonances from aliphatic fatty chains as well as cholesterol, cholesterol derivatives, cholines, phospholipids, glycerides, spingomyelin, etc. Aliphatic fatty chain resonances can be assigned to sub-groups such as methyl, methylene, olefin, and allylic features but it is not possible to identify individual lipophilic components. For example, it is not possible to distinguish between monacyl- and diacyl phospholipids. Despite the detection of 25 resonances, many of these are from the same category of lipid, and thus only 6 or so lipid categories are identified in the spectrum.
Figure 1.
1H NMR spectrum of a serum extract from a healthy individual (commercial sample). Assignments for the numbered peaks are listed in Table 1.
Table 1.
Resonance assignments for signals identified in the lipid extract of serum
| Peak | Assignment |
|---|---|
| 1 | Total cholesterol C-18 CH3 |
| 2 | Total cholesterol C-26 and C-27 CH3 |
| 3 | Fatty acid terminal CH3 |
| 4 | Total cholesterol C-21 CH3 |
| 5 | Free cholesterol C-19 CH3 |
| 6 | Esterified cholesterol C-19 CH3 |
| 7 | Free and esterified cholesterol protons |
| 8 | Fatty acyl chain CH2 |
| 9 | Free and esterified cholesterol protons |
| 10 | Fatty acyl chain CH2CH2CO |
| 11 | Free and esterified cholesterol protons |
| 12 | Fatty acyl allyl -CH2- |
| 13 | Fatty acyl CH2CO |
| 14 | Fatty acyl diallylic proton |
| 15 | Sphingomyelin |
| 16 | Choline |
| 17 | Free cholesterol C-3 H |
| 18 | Phosphatidylcholine N-CH2 |
| 19 | Glycerophospholipid backbone |
| 20 | Glycerophospholipid backbone |
| 21 | Phosphatidylcholine PO-CH2 |
| 22 | Esterified cholesterol CHOCOR |
| 23 | Glycerophospholipid backbone |
| 24 | Glycerol backbone |
| 25 | Fatty acyl CH=CH |
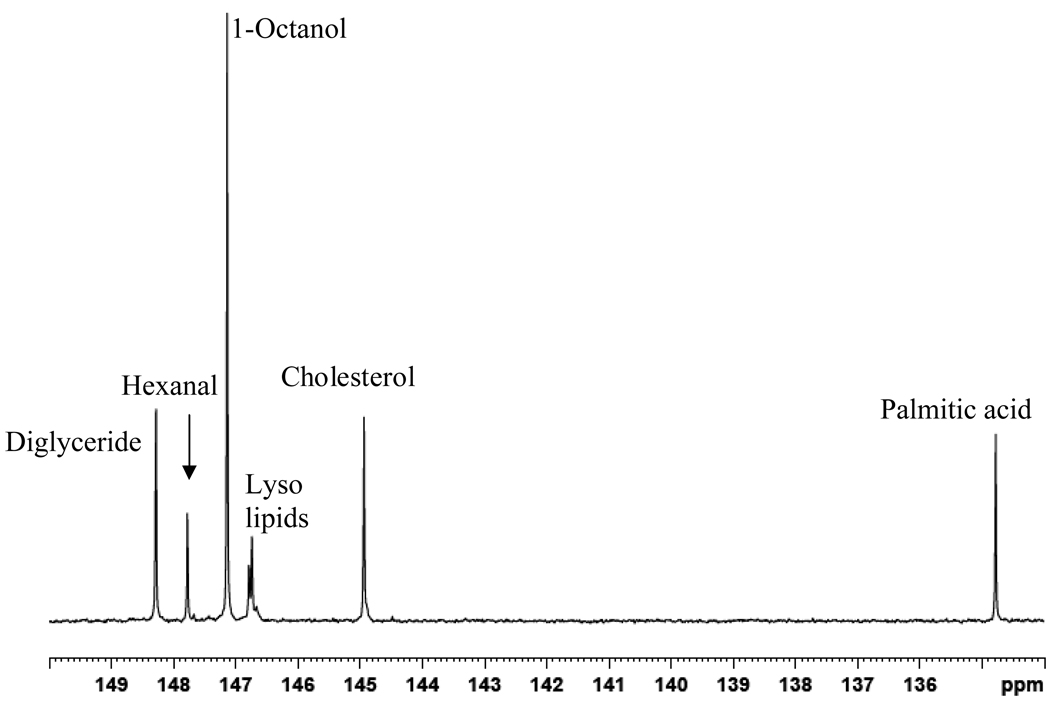
To expand on the number of identifiable and quantifiable lipid components, we examined the reactions of representative lipophilic compounds with CTMDP to ascertain whether these compounds could be differentiated in the 31P NMR spectrum. Model compounds such as mono- and diacylglycerides, long chain fatty acids, fatty alkanols, lyso lipids, sphingomyelin, and steroidal compounds were derivatized to examine the applicability of this methodology and to build a reference library of chemical shift values. These compounds were chosen because they mimic a set of biologically relevant compounds found in human serum. 31P NMR chemical shift values for 16 derivatized compounds are summarized in Table 2. Cyclohexanol was used as an internal standard as it readily reacts with CTMDP to produce a derivatized species that appears at 145.2 ppm.28 In addition, a capillary containing methylenediphosphonic acid (17.9 ppm) was used as a separate internal reference in order to ensure that the signals produced by cyclohexanol and other derivatized metabolites were accurate. The derivatization reaction yields, evaluated using the 31P peak integrals of the representative lipids reacted in triplicate were highly reproducible. A mixture of six different compounds carrying labile hydrogens (palmitic acid, cholesterol, 1-octanol, hexanal, 1,2-dipalmitoyl glycerol and 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine) was used to evaluate the reproducibility and reaction yield individually and in a mixture. The yields were quite reproducible, with coefficients of variation varying between 4 and 9% for the different chemical species. Reaction yields varied by class: long chain alcohols showed the highest reactivity, producing reaction yields over 95%; glycerides and cholesterol yielded over 90% product; lyso lipids and fatty acids produced ~65% and ~75% reaction yields; while aldehydes gave the lowest yield, 40%. These reaction yields for pure compounds and mixtures are similar.
Table 2.
31P resonance assignments for representative compounds after CTMDP derivatization
| Compound | 31P NMR chemical shift |
|---|---|
| 5-cholesten-3β-ol-7-one | 144.93 |
| Cholesterol | 144.93 |
| Monoglyceride | 147.6 (primary) |
| L-α-Phosphatidyl-DL-glycerol | 146. 80 |
| 1,2 dipalmitoyl glycerol | 148.26 |
| 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate | 146.74, 146.68 |
| 1-1auroyl-2-hydroxy-sn-glycero-3-phosphocholine | 146.74, 146.68 |
| 1-behenoyl-2-hydroxy-sn-glycero-3-phosphocholine | 146.74, 146.68 |
| Sphingomyelin | Multiple resonances |
| Inositol | 146.78 |
| 1-hexanol | 147.18 |
| 1-octanol | 147.18 |
| Hexanal | 147.70 |
| Octanoic acid | 134.78 |
| Palmitic acid | 134.78 |
| Oleic acid | 134.78 |
The 31P chemical shift values obtained in this study for mono- and diglycerides, free fatty acids and cholesterol are comparable to those previously reported in two studies focused on food analysis.23, 24 The assignments were verified by running proton coupled experiments in which triplets and doublets were observed for the derivatized primary and secondary hydroxyl groups, respectively. Derivatized long chain fatty acids appear at 134.78 ppm; not surprisingly, there appears to be little or no chemical shift sensitivity to the length of carbon chain and degree of unsaturation. Chemical shifts for derivatized octanoic acid (8:0), palmitic acid (16:0) and oleic acid (18:1 9Z) are exactly the same separately and in a mixture of all three, indicating that the 10 structural similarity at the reactive carboxylic acid head is dominant in determining the 31P chemical shift value. The straight-chain fatty alcohols 1-octanol and 1-hexanol also showed the same chemical shifts (147.12 ppm).
Cholesterol, an important constituent in human serum, appeared at 144.93 ppm after CTMDP derivatization. As there are several similar forms of cholesterol found in human serum, such as free and esterified cholesterol and cholestenol, we compared the derivatization of free cholesterol to 5-cholesten-3β-ol-7-one. Both derivatized cholesterol and 5-cholesten-3β-ol-7-one gave the same peak, suggesting that free cholesterol and structurally similar compounds will show essentially the same 31P chemical shift. As esterified forms of cholesterol will not be derivatized by CTMDP, the total non-esterified cholesterol can be evaluated by this methodology.
We also examined several lyso lipids with different chain lengths, unsaturation and head groups: 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine, 1-behenoyl-2-hydroxy-sn-glycero-3-phosphocholine, 1-1auroyl-2-hydroxy-sn-glycero-3-phosphocholine and 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate. All of these derivatized lyso lipids had the same 31P chemical shift and produced two peaks at 146.68 ppm and 146.74 ppm. This is likely due to the chirality of the sn-2 carbon atom bearing the –OH group. If inversion of the 5-membered phospholane ring is slow there would be two diostereomers present. This suggestion is supported by the proton coupled 31P NMR spectrum, which shows three peaks (a triplet-like structure) with a 3JP-H coupling of 9.25 Hz, suggesting two overlapped doublets. Phospholane derivatization of lyso lipids have not been reported before and this may be an area for further studies. Derivatized 1,2-dipalmitoyl glycerol produced a resonance at 148.26, which was comparable with the chemical shift value reported in literature for diglycerides.23 3-Palmitoyl-sn-glycerol produced two peaks at 146.4 ppm and 147.58 ppm, corresponding to derivatized secondary and primary hydroxyl groups, respectively.
Although sphingomyelin carried only one hydroxyl functional group, it produced multiple resonances after derivatization which was consistent in several trials. This may be again attribute to slow inversion of phospholane ring with respect to two adjacent carbon centers and spacial arrangement in solution. The possibility of sphingomyelin caring any impurities were ruled out by 1H and 31P NMR spectra of authentic compounds.
Human serum
The 31P NMR spectrum of human serum was recorded prior to derivatization to identify the naturally occurring phosphorous signals. The aqueous fraction shows mainly inorganic phosphate, which appears at −0.596 ppm, along with small amounts of phosphocholines, sugar phosphates and phosphoethanolamine. The lipophilic fraction mainly consists of phosphocholines/lysophosphocholines located at 0.5 ppm. The 31P NMR spectrum did not show any additional resonances in an extended region of −40 ppm to 220 ppm in the lipid extract samples. After derivatization with CTMDP, newly formed P-O groups derived from hydroxyl groups appeared in the 144.5 – 150 ppm region, while P-O groups derived from carboxylic acids appeared at 134.5 – 135.5 ppm.
Human serum was derivatized according to two protocols. In the first procedure, lipids were extracted from serum as described above, while in the second procedure serum aliquots were lyophilized to dryness and reconstituted in the pyridine/deuterated chloroform stock solution. There were two distinct differences seen in the 31P NMR spectra of samples from these two protocols. The concentration of cholesterol was found to be higher in extracted samples than in reconstituted samples. This can be attributed to the high solubility of cholesterol in 100% chloroform compared to its solubility in stock solution (4:1(v/v) pyridine: chloroform). On the other hand, free fatty acids were less abundant in the extracted samples than in the lyophilized samples. This may be attributed to a lower extraction efficiency of some short chain fatty acids in chloroform or loss of material during the extraction procedure. Of the two protocols, lyophilization followed by derivatization is recommended for clinical applications. The optimal quantity of serum for detection by 31P NMR was assessed and samples sizes from 10 ml to 0.5 ml were studied. It was found that even 0.5 ml of serum could produce useable spectra for the study. However, 1.0 ml serum samples were used for a majority of the experiments. Because these are extracted samples, there is a potential for further analyte pre-concentration and detection using, for example microcoil NMR.29–31
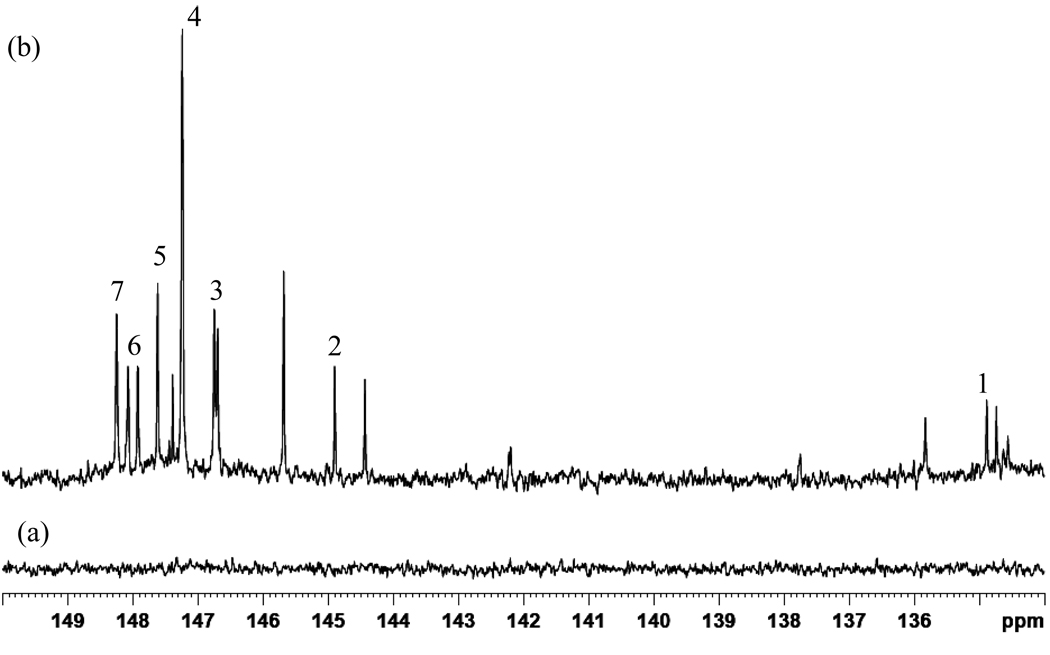
Figure 3(b) shows the spectral region between 134 and 150 ppm. Ten hydroxyl-containing metabolites were clearly detected, while three metabolites and some non-resolved metabolites were seen in the carboxylic acid region. In comparison, non-derivatized human serum (Figure 3(a)) does not show any 31P NMR resonances in this region. Based on the data in Table 2 as well as other literature sources, seven metabolites could be identified in this region. Derivatized cholesterol appears at 144.93 ppm and this signal represents all unesterified cholesterol species in serum. In contrast, individual identification and quantification of esterified and non-esterified cholesterol using 1D 1H NMR is ambiguous due to the strong spectral overlap of proton signals. In this study distinct identification and quantification of the total non-esterified fraction of cholesterol is possible. Mono and diglycerides can also be distinguished by this methodology, whereas derivatized 1,2 diglycerides in serum appear at 148.26 ppm, while for mono glycerides, derivatized primary and secondary hydroxyl groups appear at 147.6 and 146.54 ppm, respectively. Human serum carries a considerably high content of triglycerides; however, due to lack of hydroxyl groups, triglycerides cannot be detected by this methodology. Regardless of the length of the carbon chain, polar head group or unsaturation, all lyso lipids produced two resonances at 146.68 ppm and 146.74 ppm in serum. Serum free fatty carboxylic acids appeared at 134.78 ppm. In addition, two other distinct phosphorous resonances and several unidentified resonances can be seen in the derivatized carboxylic acid region. Compounds such as derivatized syringic acids can appear in this region.23 Further studies are needed to identify these unknown resonances in this region.
Figure 3.
A portion of the 31P NMR spectrum of derivatized serum from a healthy individual. Derivatized compounds include: free fatty acids 1; cholesterol 2; lyso lipids 3; possible fatty aldehydes 4; phosphotidyl mono glycerides (primary) 5; free n-alkanol 6; 1,2-diacylglycerol 7.
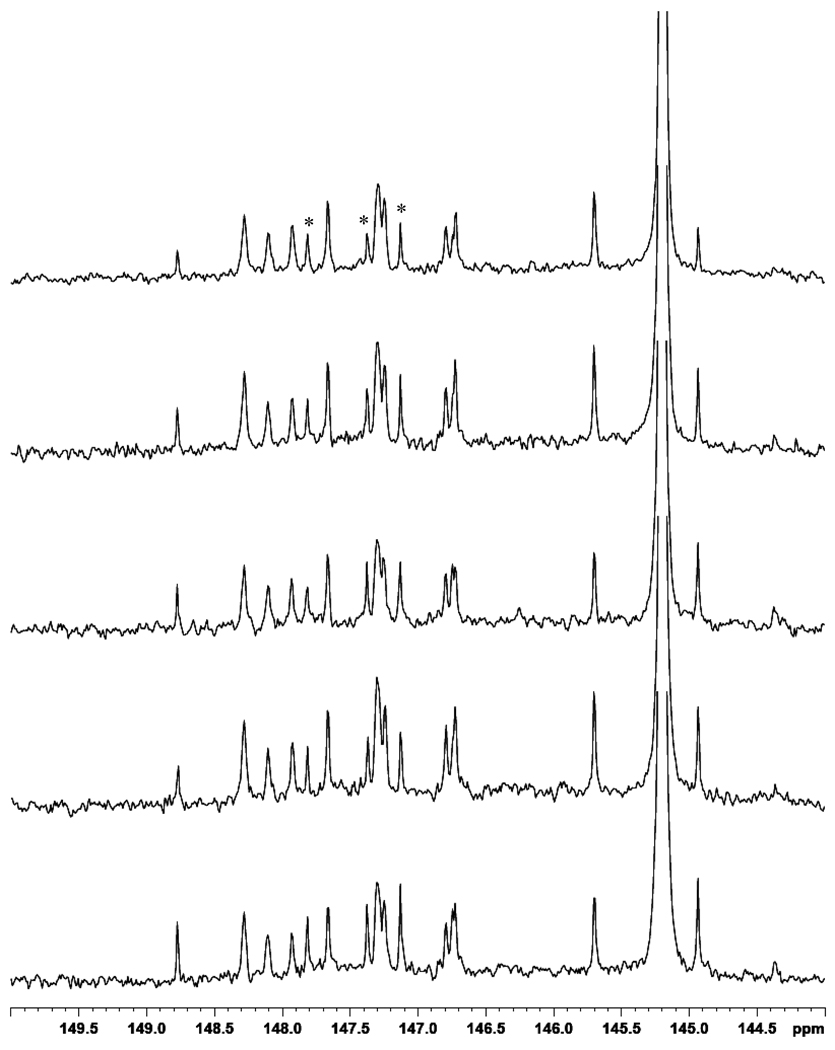
Reproducibility studies were performed by derivatizing the same split human serum sample in triplicate. This methodology showed high reproducibility with standard deviation of ≤8% for all peaks using the 3 replicates. Figure 4 shows the 144 – 150 ppm region of the 31P NMR spectra for five derivatized serum samples from healthy individuals. All spectra showed good consistency with some intensity variations. We would anticipate significantly larger variations in samples from individuals with lipid pathologies. This set of samples was analyzed with cyclohexanol as an internal standard, which is evident in the spectra at 145.2 ppm. The components marked with an asterisk are impurities arising from cyclohexanol. The average concentration of several compounds was calculated using sample triplicates, and these results are tabulated in Table 3.
Figure 4.
A portion of the 31P NMR spectrum of derivatized serum from 5 healthy individuals with added cyclohexanol (2µl; 145.2 ppm) as an internal standard in the stock solution. Impurities arising from cyclohexanol are marked with an asterisk.
Table 3.
Concentrations of lipid species in healthy human serum measured after derivatization.
| Compound | Concentration (µM) |
|---|---|
| Free carboxylic acids | 53.1 |
| Free cholesterol | 77 |
| Lyso lipids | 252 |
| Fatty alcohols | 354.5 |
| 1,2-diglycerides | 147 |
Conclusions
Chemical derivatization of human serum with 2-chloro-4,4,5,5-tetramethyldioxaphospholane provides an approach complimentary to that of conventional NMR methods for the identification and quantitation of a number of lipophilic compounds. The method provides sufficient sensitivity and spectral resolution, and derivatized species have unique and well-resolved resonances located in the 135–150 ppm region of the 31P NMR spectrum. The number of lipid components identified is comparable to those identified by 1H NMR. The addition of sample pre-concentration and microcoil NMR detection would provide an avenue for sensitivity enhancement and further metabolite identification. In addition, 2D 1H-31P NMR would be useful for resolving lipid components which overlap in the 1D spectra. This methodology offers the possibility of identification of a number of additional lipophilic compounds that contain hydroxyl and carboxylic acid functionalities in different chemical environments. This approach is consistent with the requirements for a fast screening method for lipid pathologies in human serum making possible the efficient use of 31P NMR spectroscopy alongside 1H NMR spectroscopy in metabolomics studies.
Figure 2.
A portion of the 31P NMR spectrum of a mixture of six derivatized representative compounds.
Acknowledgements
This work was supported by a grant from the NIH/NIGMS (Grant 1R01GM085291-01).
References Cited
- 1.Gowda GAN, Zhang SC, Gu HW, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Review of Molecular Diagnostics. 2008;8(5):617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of Plasma-Lipoproteins by Proton Nuclear-Magnetic-Resonance Spectroscopy. Clinical Chemistry. 1991;37(3):377–386. [PubMed] [Google Scholar]
- 3.Shah FD, Shukla SN, Shah PM, Patel HRH, Patel PS. Significance of alterations in plasma lipid profile levels in breast cancer. Integrative Cancer Therapies. 2008;7(1):33–41. doi: 10.1177/1534735407313883. [DOI] [PubMed] [Google Scholar]
- 4.Andreotti G, Chen JB, Gao YT, Rashid A, Chang SC, Shen MC, Wang BS, Han TQ, Zhang BH, Danforth KN, Althuis MD, Hsing AW. Serum lipid levels and the risk of biliary tract cancers and biliary stones: A population-based study in China. International Journal of Cancer. 2008;122(10):2322–2329. doi: 10.1002/ijc.23307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tukiainen T, Tynkkynen T, Makinen VP, Jylanki P, Kangas A, Hokkanen J, Vehtari A, Grohn O, Hallikainen M, Soininen H, Kivipelto M, Groop PH, Kaski K, Laatikainen R, Soininen P, Pirttila T, Ala-Korpela M. A multi-metabolite analysis of serum by H-1 NMR spectroscopy: Early systemic signs of Alzheimer's disease. Biochemical and Biophysical Research Communications. 2008;375(3):356–361. doi: 10.1016/j.bbrc.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Oostendorp M, Engelke UFH, Willemsen M, Wevers RA. Diagnosing inborn errors of lipid metabolism with proton nuclear magnetic resonance spectroscopy. Clinical Chemistry. 2006;52(7):1395–1405. doi: 10.1373/clinchem.2006.069112. [DOI] [PubMed] [Google Scholar]
- 7.Wevers RA, Engelke U, Heerschap A. High-Resolution H-1-NMR Spectroscopy of Blood-Plasma for Metabolic Studies. Clinical Chemistry. 1994;40(7):1245–1250. [PubMed] [Google Scholar]
- 8.Daykin CA, Foxall PJD, Connor SC, Lindon JC, Nicholson JK. The comparison of plasma deproteinization methods for the detection of low-molecular-weight metabolites by H-1 nuclear magnetic resonance spectroscopy. Analytical Biochemistry. 2002;304(2):220–230. doi: 10.1006/abio.2002.5637. [DOI] [PubMed] [Google Scholar]
- 9.Duarte IF, Stanley EG, Holmes E, Lindon JC, Gil AM, Tang HR, Ferdinand R, McKee CG, Nicholson JK, Vilca-Melendez H, Heaton N, Murphy GM. Metabolic assessment of human liver transplants from biopsy samples at the donor and recipient stages using high-resolution magic angle spinning H-1 NMR spectroscopy. Analytical Chemistry. 2005;77(17):5570–5578. doi: 10.1021/ac050455c. [DOI] [PubMed] [Google Scholar]
- 10.Keun HC, Ebbels TMD, Antti H, Bollard ME, Beckonert O, Schlotterbeck G, Senn H, Niederhauser U, Holmes E, Lindon JC, Nicholson JK. Analytical reproducibility in H-1 NMR-based metabonomic urinalysis. Chemical Research in Toxicology. 2002;15(11):1380–1386. doi: 10.1021/tx0255774. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Granados B, Monleon D, Martinez-Bisbal MC, Rodrigo JM, del Lmo J, Lluch P, Ferrandez A, Marti-Bonmati L, Celda B. Metabolite identification in human liver needle biopsies by high-resolution magic angle spinning H-1 NMR spectroscopy. NMR in Biomedicine. 2006;19(1):90–100. doi: 10.1002/nbm.1005. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson JK, Foxall PJD, Spraul M, Farrant RD, Lindon JC. 750-Mhz H-1 and H-1-C-13 NMR Spectroscopy of Human Blood-Plasma. Analytical Chemistry. 1995;67(5):793–811. doi: 10.1021/ac00101a004. [DOI] [PubMed] [Google Scholar]
- 13.Li YL, Su X, Stahl PD, Gross ML. Quantification of diacylglycerol molecular species in biological samples by electrospray ionization mass spectrometry after one-step derivatization. Analytical Chemistry. 2007;79(4):1569–1574. doi: 10.1021/ac0615910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han XL, Yang K, Cheng H, Fikes KN, Gross RW. Shotgun lipidomics of phosphoethanolamine-containing lipids in biological samples after one-step in situ derivatization. Journal of Lipid Research. 2005;46(7):1548–1560. doi: 10.1194/jlr.D500007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nature Biotechnology. 2003;21(6):660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 16.Christie WW. Preparation of ester derivatives of fatty acids for chromatographic analysis. Advances in lipid methodology- Two. 1993:69–111. [Google Scholar]
- 17.Sleevi P, Glass TE, Dorn HC. Trifluoroacetyl Chloride for Characterization of Organic Functional-Groups by F-19 Nuclear Magnetic-Resonance Spectrometry. Analytical Chemistry. 1979;51(12):1931–1934. [Google Scholar]
- 18.Shanaiah N, Desilva MA, Gowda GAN, Raftery MA, Hainline BE, Raftery D. Class selection of amino acid metabolites in body Fluids using chemical derivatization and their enhanced C-13 NMR. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(28):11540–11544. doi: 10.1073/pnas.0704449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye TMH, Shanaiah N, Gowda GAN, Zhang S, Raftery D. A Chemoselective 15N Tag for Sensitive and High Resolution NMR Profiling of the Carboxyl-Containing Metabolome. 2009 doi: 10.1021/ac900539y. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haug S, Braun-Falco M. Restoration of fatty aldehyde dehydrogenase deficiency in Sjogren-Larsson syndrome. Gene Therapy. 2006;13(13):1021–1026. doi: 10.1038/sj.gt.3302743. [DOI] [PubMed] [Google Scholar]
- 21.Sztefko K, Panek J. Serum free fatty acid concentration in patients with acute pancreatitis. Pancreatology. 2001;1(3):230–236. doi: 10.1159/000055816. [DOI] [PubMed] [Google Scholar]
- 22.Richieri GV, Kleinfeld AM. Unbound Free Fatty-Acid Levels in Human Serum. Journal of Lipid Research. 1995;36(2):229–240. [PubMed] [Google Scholar]
- 23.Spyros A, Dais P. Application of P-31 NMR spectroscopy in food analysis. 1. Quantitative determination of the mono- and diglyceride composition of olive oils. Journal of Agricultural and Food Chemistry. 2000;48(3):802–805. doi: 10.1021/jf9910990. [DOI] [PubMed] [Google Scholar]
- 24.Dais P, Spyros A. P-31 NMR spectroscopy in the quality control and authentication of extra-virgin olive oil: A review of recent progress. Magnetic Resonance in Chemistry. 2007;45(5):367–377. doi: 10.1002/mrc.1985. [DOI] [PubMed] [Google Scholar]
- 25.Lindon JC, Nicholson JK, Everett JR. Annual Reports on NMR Spectroscopy, Vol 38. San Diego: Academic Press, Inc.; 1999. NMR spectroscopy of biofluids; pp. 1–88. [Google Scholar]
- 26. http://www.bmrb.wisc.edu/metabolomics/
- 27. http://www.hmdb.ca/
- 28.Jiang ZH, Argyropoulos DS, Granata A. Correlation-Analysis of P-31 NMR Chemical-Shifts with Substituent Effects of Phenols. Magnetic Resonance in Chemistry. 1995;33(5):375–382. [Google Scholar]
- 29.Djukovic D, Appiah-Amponsah E, Shanaiah N, Gowda GAN, Henry I, Everly M, Tobias B, Raftery D. Ibuprofen metabolite profiling using a combination of SPE/column-trapping and HPLC-micro-coil NMR. Journal of Pharmaceutical and Biomedical Analysis. 2008;47(2):328–334. doi: 10.1016/j.jpba.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Djukovic D, Liu S, Henry I, Tobias B, Raftery D. Signal enhancement in HPLC/microcoil NMR using automated column trapping. Analytical Chemistry. 2006;78(20):7154–7160. doi: 10.1021/ac0605748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kc R, Henry ID, Park GHJ, Raftery D. Design and construction of a versatile dual volume heteronuclear double resonance microcoil NMR probe. Journal of Magnetic Resonance. 2009;197(2):186–192. doi: 10.1016/j.jmr.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]