Abstract
The chemokine, lymphotactin (LTN), was tested as a molecular adjuvant using bicistronic DNA vaccines encoding the protective Yersinia capsular (F1) antigen and virulence antigen (V-Ag) as a F1-V fusion protein. The LTN-encoding F1-V or V-Ag vaccines were given by the intranasal (i.n.) or intramuscular (i.m.) routes, and although serum IgG and mucosal IgA antibodies (Abs) were induced, F1-Ag boosts were required for robust anti-F1-Ag Abs. Optimal efficacy against pneumonic plague was obtained in mice i.m.-, not i.n.-immunized with these DNA vaccines. These vaccines stimulated elevated Ag-specific Ab-forming cells and mixed Th cell responses, with Th17 cells markedly enhanced by i.m. immunization. These results show that LTN can be used as a molecular adjuvant to enhance protective immunity against plague.
Keywords: mucosal, IgA, vaccines, pneumonic plague, lymphotactin
1. Introduction
Plague is a zoonotic disease caused by Yersinia pestis and assumes three forms of disease in humans: bubonic, septicemic, and pneumonic. Bubonic and septicemic plague arise from flea bites in which this vector has previously fed on infected animals [1,2]. Without treatment, even bubonic plague results in high mortality, as does septicemic plague, and also causes secondary pneumonic plague [3]. Pneumonic plague is considered the most infectious form because this disease can be readily transmitted from person to person via inhalation of contaminated airborne droplets, and because of its rapid disease progression, there is a high mortality rate [3]. Throughout history, three major pandemics of plague disease have resulted in an estimated 200 million deaths, and plague still remains endemic in regions of Africa, Asia, and North and South America [1,2]. Therefore, development of efficacious vaccines for plague is warranted.
At present, there are no licensed plague vaccines in the United States. For development of a subunit vaccine to plague, efforts have focused on two primary Y. pestis antigens (Ags), the outer capsule protein (F1-Ag), which is believed to help avoid phagocytosis [4,5], and the low calcium response (LcrV) protein, V-Ag, which has been implicated in mediating a suppressive effect upon Th1 cells via the stimulation of IL-10 [6]. These individual vaccine candidates are protective against bubonic and pneumonic plague [7,8]; however, these vaccines, when applied in combination or in a fusion form, act synergistically in conferring protection [9-12]. Although the observed protective immunity is largely Ab-dependent, Y. pestis is an intracellular pathogen, and new data have shown that during early infection events cellular immunity can contribute to effective protective immunity against plague [13-15].
Lymphotactin (LTN; XCL1) is a member of the chemokine superfamily and classified into the C chemokine family as a single C motif-1 chemokine in both mice and humans [16,17]. LTN is produced mainly by CD8+ T cells and NK cells and has chemotactic activity for lymphocytes, CD4+ and CD8+ T cells, and NK cells upon binding to its specific receptor, XC chemokine receptor-1 (XCR1) [18-22]. In addition, Boismenu et al. reported that TCRγδ TCR+ intraepitheral lymphocytes (IELs) also produce LTN and induce innate and adaptive immunity via chemotaxis for T cells and NK cells [19,23]. Thus, we hypothesize that LTN can enhance recruitment of lymphocytes to react to the encoded plague DNA vaccines, which should result in improved vaccine efficacy when given either by the mucosal or parenteral routes similar to that previously shown [24].
To develop an effective vaccine against pneumonic plague, we constructed LTN-based DNA vaccines that co-express V-Ag or F1-V fusion protein, using a bicistronic eukaryotic expression vector, and assessed their vaccine efficacy against pneumonic plague challenge. This is the first example of using an immunization approach with LTN DNA vaccines for plague. These DNA vaccines did effectively prime and, with subsequent nasal F1-Ag protein boosts, were able to confer variable protection against pneumonic plague. Thus, the LTN DNA vaccine can be used to prime for protection against plague.
2. Materials and Methods
2.1 Plasmids
To develop the lymphotactin (LTN) DNA vaccines, the LTN cDNA was PCR-amplified from pGT146-mLTN (Invivogen, San Diego, CA) as a template similar to that previously described [25]. Primers contained restriction sites for HindIII at the 5′-teminus and BamHI at the 3′-terminus. After TA cloning (TOPO cloning kit, Invitrogen Corp., Carlsbad, CA) and verification of the PCR products' sequence, the LTN fragment was excised from the TA vector and inserted into the pBudCE4.1 vector (Invitrogen Corp.) cut with HindIII and BamHI, resulting in the plasmid pBud-LTN. The V and F1-V fusion Ags were then amplified by PCR from a synthetic gene (GenScript, Piscataway, NJ), which was optimized for mouse codon usage similar to that previously described [26], using primers which contained sequences for NotI at the 5′-terminus and for KpnI at the 3′-terminus. The F1-V fusion protein contained a linker sequence, Pro-Gly-Gly, between the F1 and V-Ag. Following sequence confirmation of the TA cloned (TOPO cloning kit) PCR products, each fragment was excised and inserted into the vectors, resulting in pBud-LTN/V and pBud-LTN/F1-V. These DNA plasmids were purified with a commercially available plasmid purification kit (Qiagen, Inc., Valencia, CA) and resuspended with DNase-free water.
2.2 Transfection
To evaluate the expression of LTN, V-Ag, and F1-V fusion protein, we used supernatants and lysates of 293A cells (ATCC, Manassas, VA) that were transfected with each DNA plasmid using Lipofectamine LTX (Invitrogen). The 293A cells were cultured in a complete medium (CM): RPMI-1640 (Invitrogen) containing 10% FBS (Atlanta Biologicals, GA), 10 mM HEPES buffer, 10 mM nonessential amino acids, 10 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cell culture supernatants and lysates were subjected to ELISA and immunoblotting 2 days after transfection, respectively, as described below.
2.3 Plasmid LTN production
To measure LTN expression in collected cell supernatants from transfected 293A cells, a sandwich ELISA was used. Briefly, the anti-mouse XCL/lymphotactin mAb (8 μg/ml; R&D Systems, MN) in sterile PBS was coated onto Maxisorp Immunoplate II microtiter plates (Nunc, Roskilde, Denmark) at 50 μl/well. After overnight incubation at room temperature, wells were blocked with PBS containing 1% BSA for 2 hour at 37 °C. Cell supernatants from DNA vaccine-transfected 293A cells were loaded to individual wells, and to determine the amount of LTN present in these supernatants, serially diluted recombinant mouse LTN (R&D Systems, MN) was used to generate a standard curve. After overnight incubation at 4 °C, captured LTN was reacted with 0.4 μg/ml of biotinylated goat anti-mouse lymphotactin Ab (R&D Systems, MN) for 1 hr at 37 °C. The specific reactions were detected by anti-biotin HRP conjugated Ab (Vector Laboratories, CA) with incubation for 90 min at room temperature. To visualize the specific reactions, ABTS substrate (Moss, Inc., Pasadena, CA) was used, and absorbance was measured at 415 nm after 1 hour incubation at room temperature using Bio-Tek Instruments ELx808 microtiter plate reader (Winooski, VT).
2.4 Immunoblotting
Transfected 293A cells were lysed in Milli-Q water; 30 μg of total protein were electrophoresed on a 12% SDS-polyacrylamide gel, and then transferred onto a nitrocellulose membrane (Bio-Rad Lab., Hercules, CA). The membrane was incubated with anti-V-Ag rabbit serum [27] overnight at 4 °C and then with HRP-conjugated goat anti-rabbit IgG (Southern Biotechnology Associates, Birmingham, AL) for 90 min at room temperature. The reaction was visualized using the substrate 4-chloro-1-naphtol chromogen and H2O2 (Sigma-Aldrich, St. Louis, MO).
2.5 Immunizations and challenge
Female BALB/c mice were obtained from the National Cancer Institute (Frederick Cancer Research Facility, Frederick, MD). Mice were maintained at Montana State University Animal Resources Center under pathogen-free conditions in individual ventilated cages under HEPA-filtered barrier conditions and were fed sterilized food and water ad libitum.
For intranasal (i.n.) immunization study, mice at 8-10 wks of age were immunized with each DNA vaccine (80 μg/dose) on wks 0, 1, and 2 with each dose administered over a two-day period. On wks 8 and 9, mice were nasally boosted with 25 μg of recombinant F1-Ag protein [27] plus 2.5 μg of cholera toxin (CT; List Biological Laboratories, Campbell, CA) adjuvant. Before challenge, a final boost of DNA vaccine (100 μg) and F1-Ag protein (25 μg) plus CT adjuvant was given i.n. on week 12. One group of mice was immunized only with Fl-Ag, as described.
For intramuscular (i.m.) immunization study, mice were immunized i.m. with each DNA vaccine on wks 0, 1, and 2. For i.m. immunizations, 100 μg DNA were administered with a needle into the tibialis anterior muscles of the two hind legs, as previously described [28]. On wks 8 and 9, mice were nasally boosted with 25 μg of F1-Ag protein plus 2.5 μg of CT (List Biological Laboratories) adjuvant. Before challenge, a final boost of DNA vaccine (100 μg) i.m. and F1-Ag protein (25 μg) plus CT adjuvant was given i.n. on week 12.
To test the efficacy of the LTN DNA vaccines against pneumonic challenge, immunized mice were transported to Colorado State University, acclimated for at least 7 days, and subjected to nasal challenge with 100 LD50 of Y. pestis Madagascar strain (MG05) >2 weeks after the last immunization, as previously described [25,27]. All mice care and procedures were in accordance with institutional policies for animal health and well-being.
2.6 Collection of serum and mucosal samples
Blood was collected from the saphenous vein. Fresh fecal pellets from individual mice were solubilized in sterile PBS containing 50 μg/ml of soybean trypsin inhibitor (Sigma-Aldrich) by vortexing for 10 minutes at 4 °C. After microcentrifugation, supernatants were collected and frozen at -30 °C until assay.
2.7 Measurement of anti-F1- and V-Ag Abs titers by ELISA
Serum and fecal Ab titers were determined by ELISA. Briefly, recombinant F1- or V-Ag [27] in sterile PBS was coated onto Maxisorp Immunoplate II microtiter plates (Nunc) at 50 μl/well. After overnight incubation at room temperature, wells were blocked with PBS containing 1% BSA for 1 hour at 37 °C; individual wells were loaded with serially diluted mouse serum or fecal samples in ELISA buffer (PBS containing 0.5 % BSA and 0.5 % Tween 20) overnight at 4 °C. Ag-specific Abs were reacted with HRP-conjugated goat anti-mouse IgG, IgA, IgG1, IgG2a, or IgG2b Abs (Southern Biotechnology Associates) for 90 minutes at 37 °C. The specific reactions were detected with soluble enzyme substrate, 50 μl of ABTS (Moss), and absorbance was measured at 415 nm after 1 hour incubation at room temperature using Bio-Tek Instruments ELx808 microtiter plate reader. Endpoint titers were determined to be an absorbance of 0.1 OD above negative controls after 1 hour at room temperature.
2.8 Lymphocyte isolation
Lymphocytes were isolated from nasal-associated lymphoid tissues (NALT), nasal passages (NPs), head and neck lymph nodes (HNLNs), submaxillary glands (SMGs), spleens, small intestinal lamina propria (iLP), Peyer's patches (PPs), lumbar lymph nodes (LLNs), sciatic lymph nodes (SLNs), and popliteal lymph nodes (PopLNs). HNLN, splenic, PP, LLN, SLN, and PopLN mononuclear cells were isolated by conventional methods using Dounce homogenization [26,27]. To isolate the mononuclear cells from NALT, NPs, SMGs, and iLP, the tissues were minced and digested using 300 units/ml of Clostridium histolyticum Type IV collagenase (Worthington, Freehold, NJ) for 30 min at 37 °C in spinner flasks [26]. After incubation, the digestion mixtures were passed through Nitex mesh (FairviewFabrics, Hercules, CA) to remove undigested tissues. Mononuclear cells were separated by Percoll (Pharmacia, Uppsala, Sweden) density gradient centrifugation with cells interfacing between 40 % and 60 % Percoll. Greater than 95 % viability was obtained for all lymphocytes isolated from each tissue, as determined by trypan blue exclusion.
2.9 Ab ELISPOT
On wk 14, sets of studies were terminated to collect NALT, NP, HNLN, SMG, splenic, iLP, PP, LLN, and PopLN mononuclear cells from the immunized mice. NALT, NP, HNLN, SMG, splenic, iLP, and PP mononuclear cells were used from i.n.-immunized mice, and NP, HNLN, splenic, iLP, LLN, and PopLN mononuclear cells were used from i.m.-immunized mice. Ag-specific Ab-forming cell (AFC) responses by the ELISPOT method were detected, using mixed cellulose ester membrane-bottom microtiter plates (MultiScreen-HA; Millipore, Bedford, MA) by coating with 5 μg/ml F1- or V-Ag in sterile PBS, as previously described [27]. For total IgA or IgG AFC responses, wells were coated with 5 μg/ml goat anti-mouse IgA or IgG Abs (Southern Biotechnology Associates) in sterile PBS.
2.10 Cytokine analysis
On wk 7 or 14, groups of i.n.- or i.m.-immunized mice, respectively, were evaluated for cytokine responses to F1- and V-Ags. I.m.-immunized mice were boosted nasally with F1-Ag protein at 8 and 9 wks and with both DNA and nasally dosed with F1-Ag protein at 12 wks. From i.n.-immunized mice, HNLN, splenic, and PP mononuclear cells were obtained, and HNLN, splenic, and peripheral lymph nodes (PLNs), containing LLN, SLN, and PopLN mononuclear cells, were obtained from i.m.-immunized mice. Total mononuclear cells from each lymph tissue were resuspended in CM. Mononuclear cells were restimulated with 10 μg of recombinant F1-Ag, V-Ag, or with media as control in the presence of 10 U/ml human IL-2 (PeproTech) for 2 days at 37 °C in a humidified 5 % CO2 incubator. Cells were washed and resuspended in CM, and then these stimulated lymphocytes were evaluated by IFN-γ-, IL-4-, IL-5-, IL-10-, and IL-13-specific ELISPOT assays, as described previously [24,25,27].
To determine cytokine responses to F1- and V-Ags, on wk 7 or 14, groups of immunized i.n. or i.m. mice were used, respectively. Cell cultures supernatants were collected from the restimulated lymphocytes used in cytokine ELISPOT assay. These supernatants were used to quantify the presence of IFN-γ, IL-6, IL-10, IL-17, and TGF-β by sandwich ELISA, as previously described [25,29,30].
2.11 Statistical analysis
An ANOVA followed by Tukey's method was used to evaluate differences in expression of LTN, Ab titers, and IgG subclass responses; the Mann-Whitney U-test was used to evaluate differences in AFC and CFC responses. The Kaplan-Meier method (GraphPad Prism, GraphPad Software, Inc., San Diego, CA) was applied to obtain the survival fractions following pneumonic Y. pestis challenges in LTN DNA vaccine immunized mice. Using the Mantel-Haenszel log rank test, the P-value for statistical differences between surviving plague challenges among the vaccinated groups versus those dosed with PBS was discerned at the 95% confidence interval.
3. Results
3.1 Expression of LTN, V-Ag, and F1-V fusion proteins
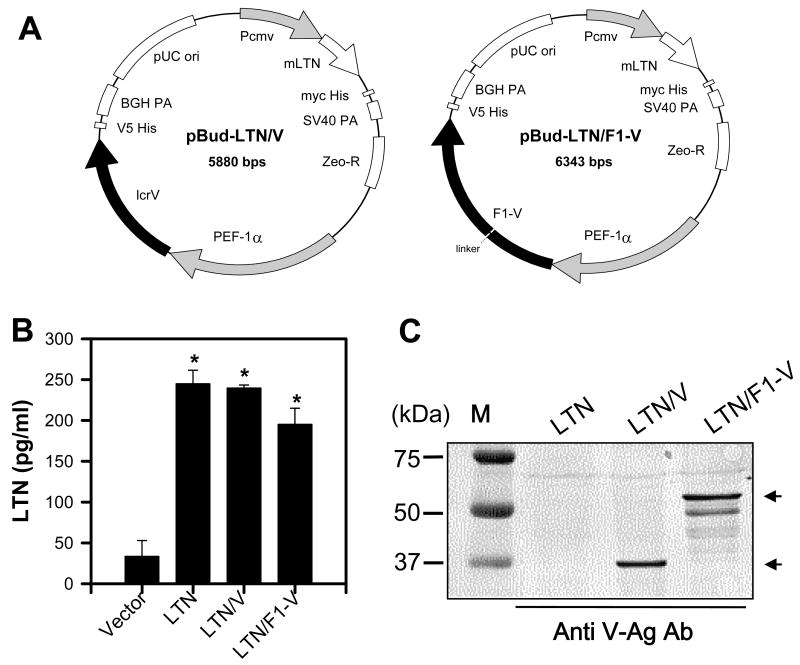
DNA vaccines for plague were generated using a bicistronic expression plasmid carrying the molecular adjuvant, LTN, and under a separate promoter, V-Ag or F1-V fusion protein sequences (Fig. 1A). These are called LTN/V-Ag and LTN/F1-V, respectively. A LTN-based DNA vaccine encoding solely F1-Ag was found to be as immunogenic as the LTN/F1-V vaccine and, thus, was not used for these studies. To verify the expression of LTN, V-Ag, and F1-V fusion proteins, replicate cultures of 293A cells were transfected with each LTN DNA vaccine, and cell culture supernatants and lysates were collected (Fig. 1B and C). LTN could readily be detected in each of the cell supernatants from the transfected 293A cells when compared to supernatants from DNA plasmids lacking LTN using a LTN-specific ELISA (Fig. 1B). To detect the expression of V-Ag and F1-V fusion proteins, cell lysates were used for immunoblotting. The V-Ag and the F1-V could be detected using the anti-V-Ag serum (Fig. 1C). The F1-V protein migrated with an apparent MW of 54 kDa, which represents the expected molecular mass for F1-Ag (17 kDa) plus V-Ag (37 kDa).
Fig. 1.
Plasmid maps and expression determinations for the bicistronic lymphotactin (LTN) DNA vaccines. (A) Circular plasmid maps for LTN/V (pBud-LTN/V) and LTN/F1-V (pBud-LTN/F1-V) DNA vaccines. Pcmv, cytomegalovirus promoter; ori, origin of replication; BGH PA, bovine growth hormone polyadenylation signal; PEF-1α, elongation factor 1α promoter; Zeo-R, zeomycin resistance; SV40 PA, simian virus 40 polyadenylation signal. (B) Expression of LTN was determined by LTN-specific ELISA using supernatants from 293A cells transfected with the pBud-LTN/V or -LTN/F1-V DNA vaccines. Data represent the mean ± SEM of two experiments analyzed using the Mann-Whitney U-test: *, P < 0.05. (C) To detect V-Ag and F1-V fusion Ag expression by the pBud -LTN/V or -LTN/F1-V DNA vaccines, cell lysates from transfected 293A were subjected to Western immunoblot analysis using rabbit anti-V-Ag serum.
3.2 Antibody responses to F1- and V-Ag
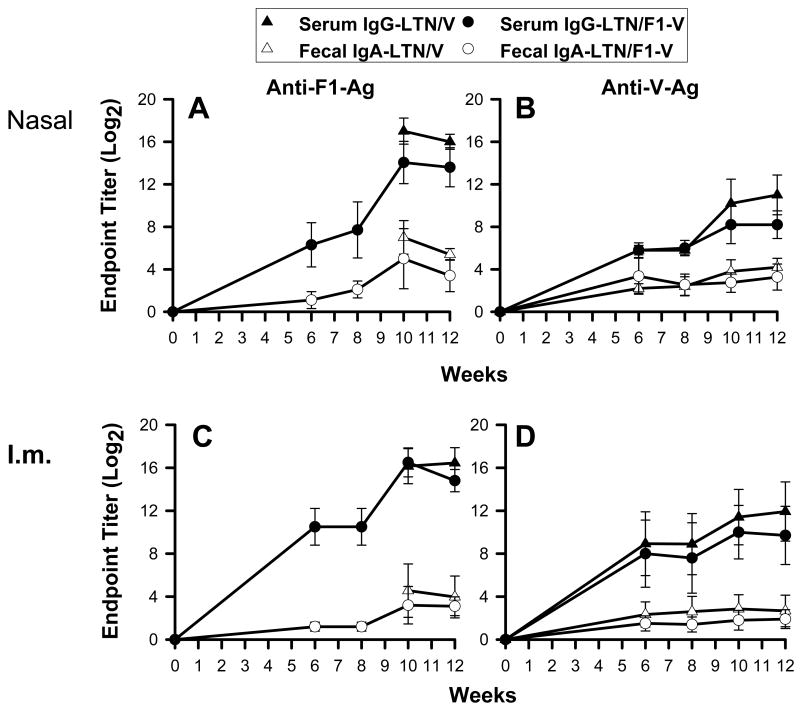
To evaluate the relative immunogenicity of the LTN DNA vaccines, samples were collected at 6 wks post-primary immunization and subsequently at two wk intervals. Past studies with other DNA vaccines show that Ab responses are delayed and peak between 8 and 10 wks post-primary immunization [28]. Ag-specific Ab titers in sera and fecal extracts were measured by ELISA using F1- or V-Ag coated wells (Fig. 2). By 6 wks post-primary immunization to F1- and V-Ag, significant Ab titers were detected in the i.n.- (Fig. 2A and B) and i.m.-immunized groups (Fig. 2C and D), and Ab titers in the i.m.-immunized mice were slightly greater than those in nasally immunized mice on wk 6. While Ab responses to F1-Ag in i.n.-immunized mice steadily increased with time, the anti-F1- or -V-Ag Ab responses in i.m.-immunized mice did not (Fig. 2C and D), nor did the anti-V-Ag Ab responses in nasally immunized mice (Fig. 2B). Thus, to enhance Ab responses, mice were boosted nasally with 25 μg of recombinant F1-Ag plus CT on wks 8 and 9, resulting in robust mucosal IgA and serum IgG titers against both F1- and V-Ags by wk 12 in both i.n.- (Fig. 2A and B) and i.m.-immunized mice (Fig. 2C and D). Before challenge study, a final boost with DNA vaccine, as well as with recombinant F1-Ag plus CT, was given on wk 12.
Fig. 2.
Antibody responses in sera and fecal extracts to F1- and V-Ags. BALB/c (10/group) were first dosed with DNA vaccines (A,B) nasally or (C,D) intramuscularly on wks 0, 1, and 2 followed by nasal boosts with recombinant F1-Ag plus 2.5 μg CT on wks 8 and 9. A kinetic analysis was performed using the LTN/V DNA and LTN/F1-V DNA vaccines. Serum IgG and fecal IgA Ab titers to F1- and V-Ags were followed for 12 wks. Data depict the mean ± SD.
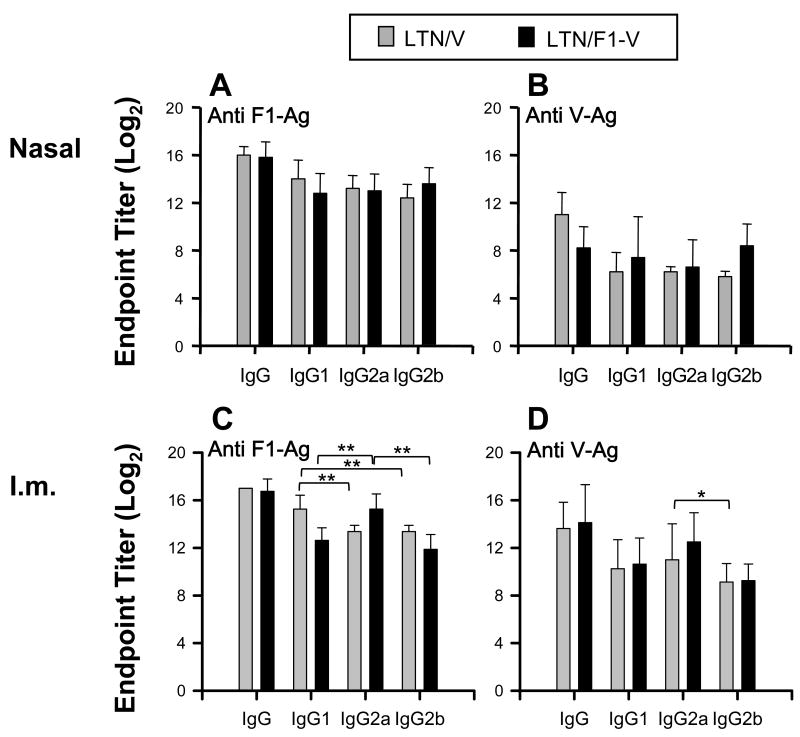
3.3 IgG subclasses responses with i.n. or i.m. LTN DNA vaccinations
IgG subclass responses were determined using serum samples from i.n. or i.m. LTN DNA vaccine immunized mice on wk 12 (Fig. 3). Nasal LTN DNA vaccinations induced equivalent IgG1, IgG2a, and IgG2b anti-F1-Ag and -V-Ag Ab responses (Fig. 3A and B). In the i.m. LTN DNA-immunized mice, significant differences were shown in responses between each IgG subclass (Fig. 3C and D). LTN/V-Ag DNA vaccination induced greater IgG1 anti-F1-Ag responses than IgG2a or IgG2b responses. The LTN/F1-V DNA vaccine stimulated greater IgG2a endpoint titers than IgG1 or IgG2b anti-F1-Ag endpoint titers (Fig. 3C). These results show that LTN DNA vaccinations could induce mixed IgG subclass responses, but these differences were influenced by the route and composition of the LTN DNA vaccine.
Fig. 3.
IgG subclass responses to F1-Ag and V-Ag from LTN DNA vaccinated mice. Sera (the same as those from those in Fig. 2) from LTN/V-Ag DNA and LTN/F1-V DNA vaccinated mice by the (A,B) nasal or (C,D) i.m. route on week 12. Endpoint titers depicted as the mean ± SD for 10 mice/group. * P < 0.05 and ** P < 0.01 are significant differences between each IgG subclass response.
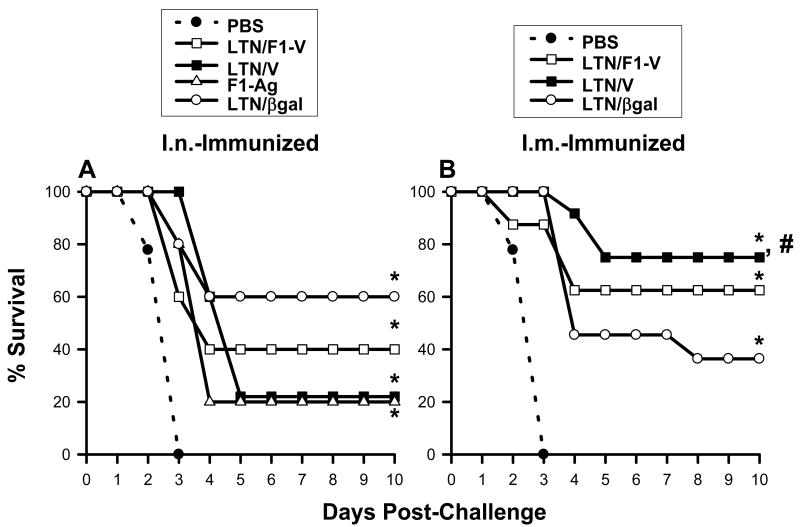
3.4 Vaccinations with LTN DNA vaccine protects against pneumonic plague
To test the efficacy of these nasal or i.m. DNA vaccines against pneumonic plague, LTN DNA plus F1-Ag-immunized mice were challenged nasally with 100 LD50 Y. pestis Madagascar strain >2 wks after the final boost, and the mean survival rates were determined (Fig. 4A and B). All mice dosed with PBS succumbed to challenge within 3 days (Fig. 4A and B). Mice nasally vaccinated with LTN/βgal, LTN/V-Ag, or LTN/F1-V DNA showed partial protection, 60% (P < 0.001), 20% (P < 0.001) and 40% (P < 0.005) survival, respectively (Fig. 4A). Mice vaccinated i.m. with LTN/V-Ag or LTN/F1-V showed better efficacy, 75% (P < 0.001) and 62.5 % (P < 0.001) survival, respectively (Fig. 4B). Mice i.m.-vaccinated with LTN/βgal showed only partial protection, 36.5% (P < 0.001). The efficacy conferred by the nasal LTN/V DNA vaccine plus F1-Ag protein-dosed mice was similar to the efficacy obtained with mice nasally dosed with F1-Ag protein only (20% survival; P < 0.005) (Fig. 4A), and this level of protection was significantly less than that conferred in i.m.-immunized mice (P < 0.05) (Fig. 4B). Thus, the nasal LTN/V-Ag DNA vaccine was minimally protective. These results show that the LTN DNA vaccines contribute to optimal protection against pneumonic plague when given by the parenteral route rather than the mucosal route.
Fig. 4.
LTN DNA vaccines confer effective protection against pneumonic plague. (A) Mice were nasally dosed on wks 0, 1, 2, and 12 with LTN/βgal (n = 5), LTN/F1-V (n = 5), or LTN/V (n = 5) DNA and boosted on wks 8, 9, and 12 with recombinant F1-Ag plus CT adjuvant. An additional group received recombinant F1-Ag (n = 5) plus CT adjuvant only on wks 8, 9, and 12; a negative control group received PBS only (n = 14). All mice were challenged 2-6 weeks after the last immunization. Survival fractions obtained from vaccinated-mice were compared to PBS-dosed mice, and significance was determined: *P < 0.005. (B) Similar immunization regimen using LTN DNA vaccine plus recombinant F1-Ag, as described, “A” was applied to i.m.-dosed mice using the LTN/βgal (n = 11), LTN/V-Ag (n = 12) or LTN/F1-V (n = 8) DNA vaccines. A negative control group received PBS only (n = 14). Survival fractions obtained from vaccinated mice were compared to PBS-dosed mice as well as between routes of immunization, and significance was determined: *P < 0.001 and #P < 0.05, respectively.
3.5 Nasal immunization with LTN vaccine stimulates weak mucosal IgA anti-V-Ag Abs
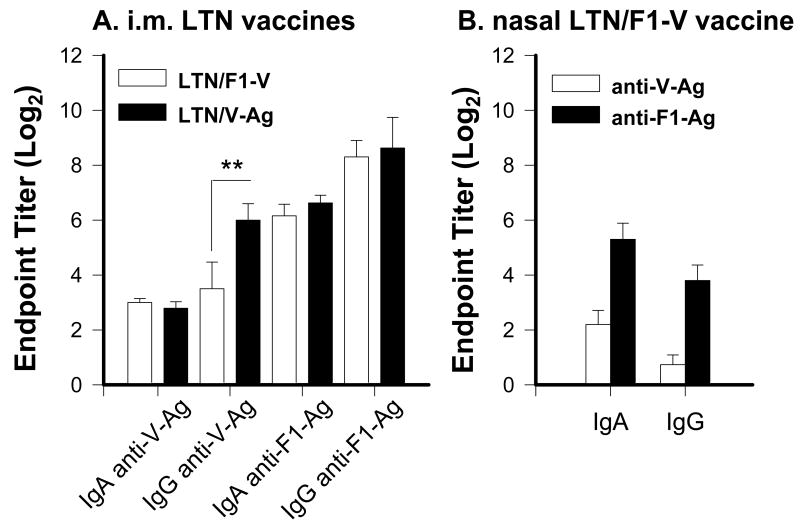
To assess the differences between parenteral and nasal immunizations with LTN vaccines, nasal washes from mice immunized with the vaccine regimen were used for the challenge studies (Fig. 5). As evident from the challenge studies, i.m. immunization showed the protective responses, and both LTN/F1-V and LTN/V-Ag vaccines elicited similar nasal IgA and IgG Ab titers to V-Ag and F1-Ag, except the LTN/V-Ag mice induced significantly enhanced nasal IgG anti-V-Ag Ab titers (Fig. 5A). Since the nasal LTN/F1-V vaccine showed the best protection against pneumonic plague challenge, analysis of nasal washes from mice immunized with this vaccine regimen still showed weak nasal IgA or IgG anti-V-Ag Abs, although IgA and IgG anti-F1-Ag Abs were induced (Fig. 5B), but with diminished magnitude when compared to i.m. vaccinated mice. Thus, i.m. DNA priming produced more robust nasal Ab responses to V-Ag and F1-Ag.
Fig. 5.
Nasal wash anti-F1-Ag and anti-V-Ag from (A) i.m.-immunized mice with LTN/F1-V or LTN/V-Ag and (B) nasally immunized mice with LTN/F1-V DNA vaccines at wk 14. Mice were dosed via i.n. or i.m. route with DNA and nasally boosted with recombinant F1-Ag, as described in Fig.4. IgA and IgG anti-V-Ag and anti-F1-Ag Ab titers are depicted. (A) I.m. immunization stimulated a weak IgA, but robust IgG anti-V-Ag Ab responses and robust IgA and IgG anti-F1-Ag Ab responses. (B) Nasal immunization with LTN/F1-V vaccine stimulated a blunted local anti-V-Ag Ab response, but a robust anti-F1-Ag Ab response. The endpoint titers are depicted as the mean ± SEM for 10-24 mice/group. ** P = 0.009 represents significant difference in IgG anti-V-Ag titers between i.m. LTN/F1-V- and LTN/V-Ag-immunized mice.
3.6 Distribution of immune B cells following immunization
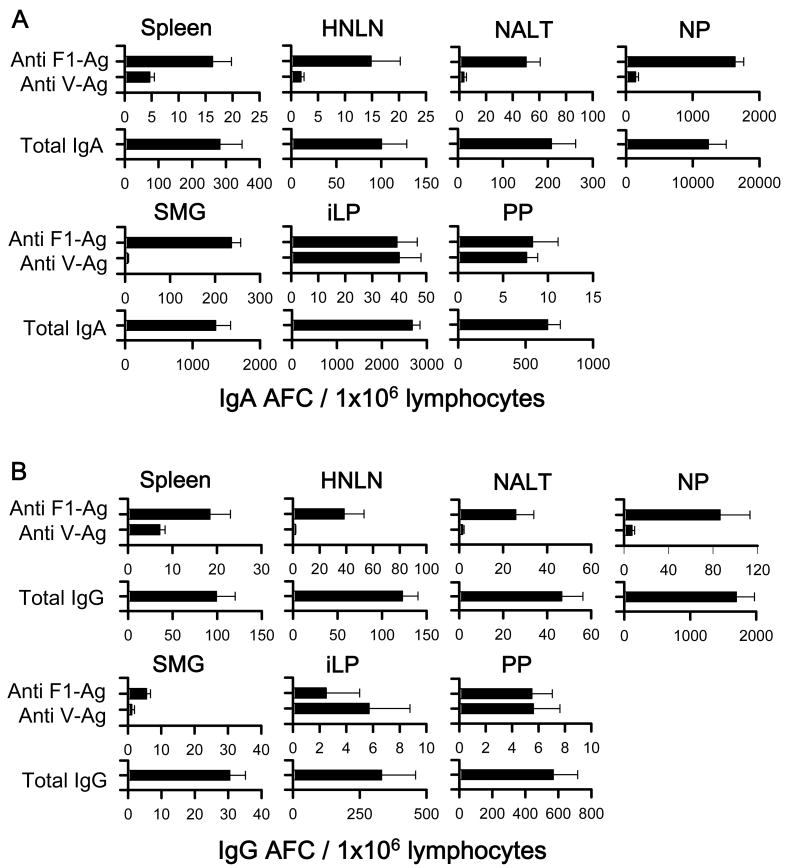
To assess the magnitude and distribution of Ab-forming cell (AFC) responses induced by the LTN DNA vaccines, a B cell ELISPOT was performed using lymphocytes of various lymphoid tissues at 14 wks post-primary immunization. For the i.n. immunization study, since LTN/F1-V DNA vaccine showed best efficacy against pneumonic plague challenge, only these mice were evaluated, and elevated F1- and V-Ag-specific IgA and IgG AFC responses were detected in the spleens, HNLNs, NALT, NPs, SMGs, iLP, and PPs from nasally LTN/F1-V DNA immunized mice (Fig. 6). Anti-F1- and -V-Ag specific IgA and IgG AFC responses were detected in the spleens and peripheral lymph nodes, as well as in mucosal tissues, HNLNs, NALT, NPs, SMGs, iLP, and PPs. These results showed that the nasal LTN DNA vaccine stimulated elevated immune B cells in both the mucosal and peripheral immune compartments.
Fig. 6.
IgA (A) and IgG (B) Ab-forming cell (AFC) responses by mice nasally vaccinated with LTN/F1-V DNA vaccine. Mice were nasally dosed and boosted with DNA and F1-Ag protein vaccines, as described in Fig. 4, and lymphoid tissues were isolated on wk 14. Total splenic, head and neck lymph node (HNLN), NALT, nasal passage (NP), submaxillary gland (SMG), small intestinal lamina propria (iLP), and Peyer's patch (PP) mononuclear cells were isolated from each DNA vaccine group (5 mice/group/experiment) and evaluated in a B cell ELISPOT assay to assess F1-Ag- and V-Ag-specific (A) IgA and (B) IgG AFCs, as well as total (A) IgA and (B) IgG AFCs. Depicted is the mean ± SEM of AFC responses taken from two experiments.
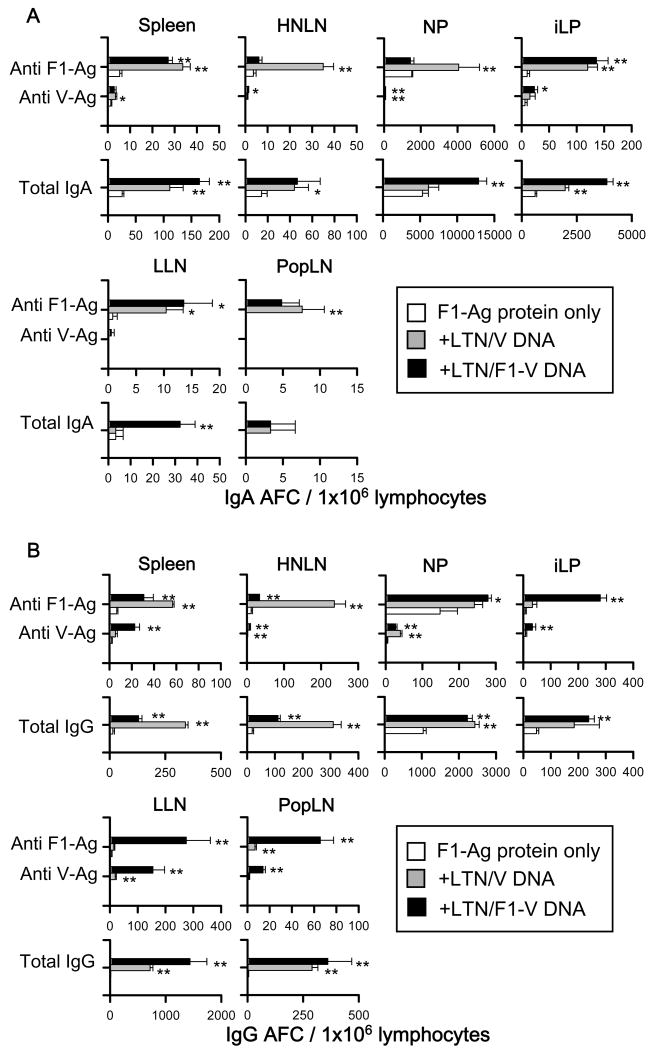
For i.m. immunization study, F1- and V-Ag-specific IgA and IgG AFC responses were detected in the spleen, HNLNs, NPs, iLP, LLNs, and PopLNs from mice immunized with each of the LTN DNA vaccines (Fig.7). In addition to show the priming effect by the LTN DNA vaccines to stimulate protective immunity against plague, AFC responses were also detected from F1-Ag protein-only immunized mice. Significantly greater anti-F1- and -V-Ag specific IgA and IgG AFC responses were detected in each lymphoid tissue from LTN DNA-vaccinated mice compared to mice immunized with F1-Ag protein only. These AFC responses were detected not only in systemic and peripheral tissues, including spleens, PopLNs, and LLNs, but also in mucosal tissues, HNLNs, NPs, and iLP. These results suggest that i.m. priming with LTN DNA vaccine followed by nasal F1-Ag boosts induced Ag-specific B lymphocytes in both the systemic and mucosal immune compartments.
Fig. 7.
IgA (A) and IgG (B) Ab-forming cell (AFC) responses by mice i.m. vaccinated with LTN/F1-V or LTN/V DNA vaccine. Mice were i.m. immunized with DNA vaccines and nasally boosted with F1-Ag protein, as described in Fig. 4, and lymphoid tissues were isolated on wk 14. Total splenic, HNLN, NP, iLP, lumbar lymph node (LLN), and popliteal lymph node (PopLN) mononuclear cells were isolated from each DNA vaccine group (5 mice/group/experiment) and evaluated in a B cell ELISPOT assay to assess F1-Ag- and V-Ag-specific and total (A) IgA and (B) IgG AFCs. Depicted is the mean ± SEM of AFC responses taken from two experiments. Significant differences comparing with F1-Ag protein immunization only group are shown as *P < 0.05 and **P < 0.01.
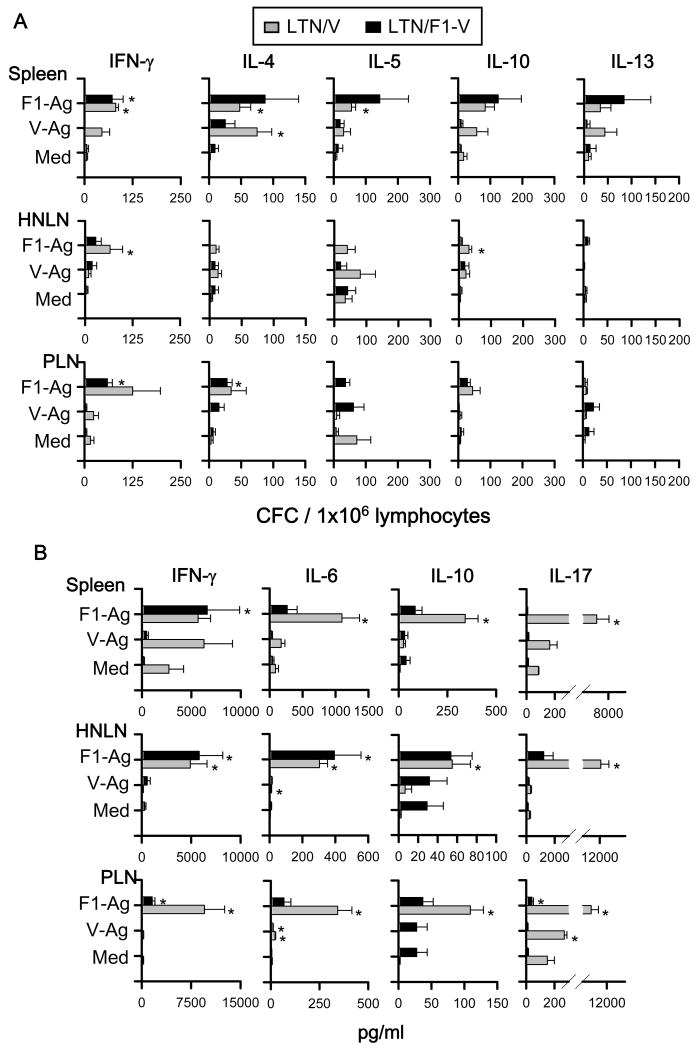
3.7 DNA priming elicits a mixed Ag-specific Th cell response
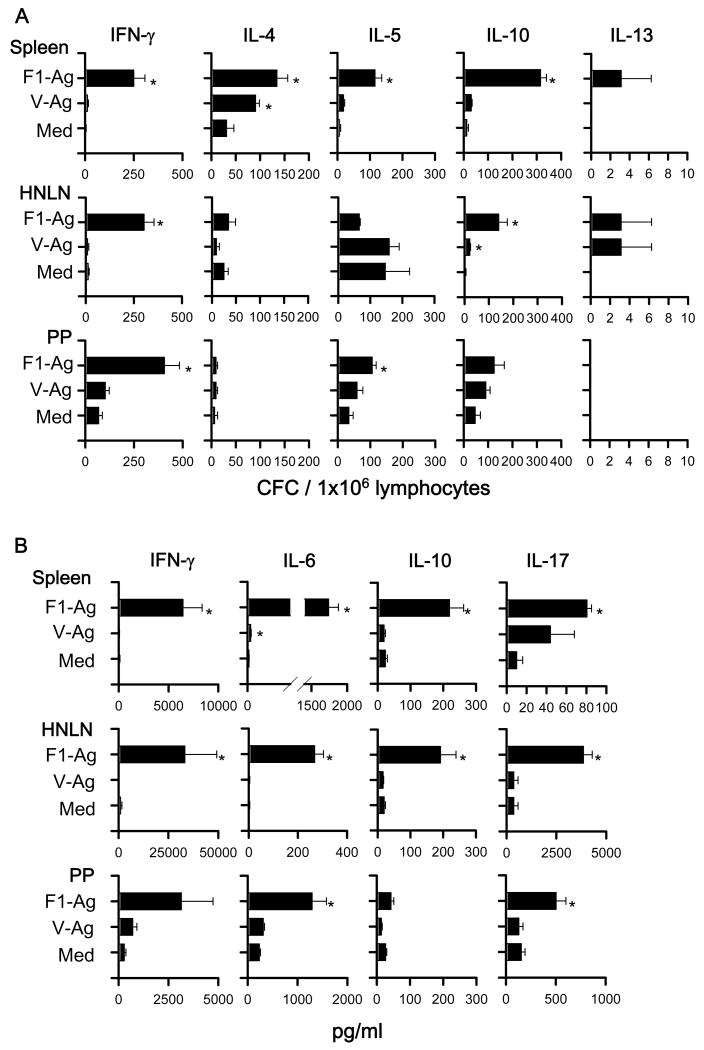
To assess the types of Th cell responses elicited by the DNA priming, cytokine-forming cell (CFC) responses were measured at 7 or 14 wks post-primary immunization by cytokine-specific ELISPOT. To evaluate the precise effects of LTN DNA vaccine priming when vaccines are given nasally and not affected by nasal F1-Ag protein boosts, the nasal immunization regimen was slightly modified, eliminating the nasal protein boosts, as previously done [25, 31]. For Th cell evaluations for i.m.-immunized mice, the vaccination regimen was left unchanged, as in the Th cell analyses [25, 31]. Lymphocytes from spleens, HNLNs, and PPs, which were obtained from LTN/F1-V DNA vaccinated mice at 7 wks, were restimulated with F1-Ag, V-Ag, or media for 2 days (Fig. 8A). Production of IFN-γ in spleens and LNs was significantly enhanced by LTN/F1-V vaccine, as were IL-4, IL-5, and IL-10. In addition, to assess Ag-specific Th cell responses, IL-6, IL-17, and TGF-β were measured in cell supernatants from lymphocytes restimulated with F1- and V-Ag by sandwich ELISA, as were IFN-γ and IL-10 (Fig. 8B). Although TGF-β was not detected (data not shown), Ag-specific IL-6 and IL-17 production was enhanced significantly, as well as IFN-γ and IL-10.
Fig. 8.
Cytokine-forming cell (CFC) responses by nasally LTN/F1-V DNA primed-mice show Ag-specific Th1/Th2/Th17 cell responses. BALB/c mice were nasally dosed with the DNA vaccines on wks 0, 1, and 2, and on wk 7, total lymphocytes were isolated from spleens, HNLNs, and PPs, and Ag-pulsed for 2 days; and (A) CFC responses and (B) cytokine production were measured by cytokine-specific ELISPOT and sandwich ELISA, respectively. Depicted is the mean ± SEM of two experiments (16 mice/group). In some instances, significant differences in the different tissues were detected comparing with media (Med) (*P < 0.05).
For the i.m. immunization study, lymphocytes from spleens, HNLNs, and PLNs, which were obtained from each two DNA vaccinated mice at 14 wks, were re-stimulated with F1-Ag, V-Ag, or media for 2 days (Fig. 9A). I.m. LTN DNA immunization also showed significantly Ag-specific enhancement of IFN-γ production, as well as IL-4, IL-5, and IL-10 in both spleens and LNs. In addition, IFN-γ, IL-6, IL-10, IL-17, and TGF-β were also measured in cell supernatants from lymphocytes restimulated with F1- and V-Ag by sandwich ELISA (Fig. 9B). Although TGF-β were not detected (data not shown), Ag-specific IL-6 and IL-17 production was enhanced significantly, as well as IFN-γ and IL-10. These results suggest that both LTN DNA vaccines primed for Ag-specific T cells, and Th1-, Th2-, and Th17-type cytokines in the i.n.- and i.m.-immunized mice.
Fig. 9.
Cytokine responses cell by i.m. LTN/V-Ag or LTN/F1-V DNA primed-mice show Ag-specific Th1/Th2/Th17 cell responses. BALB/c mice were dosed i.m. with the DNA vaccine with nasal F1-Ag boost, as described in Fig. 4. On wk 14, total lymphocytes were isolated from spleens, HNLNs, and peripheral lymph nodes (PLNs), containing LLNs and PopLNs, and Ag-pulsed for 2 days; and (A) CFC responses and (B) cytokine production were measured by cytokine-specific ELISPOT and sandwich ELISA, respectively. Depicted is the mean ± SEM of two experiments (16 mice/group). In some instances, significant differences in the different tissues were detected compared to media (Med) (*P < 0.05).
4. Discussion
In this study, to obtain an effective DNA vaccine against pneumonic plague, two DNA vaccines were constructed co-expressing the V-Ag or F1-V fusion protein in combination with LTN DNA as a molecular adjuvant. Since Y. pestis is a facultative intracellular pathogen, Parent M. A. and co-workers suggested that plague vaccines should be designed to maximally prime both cellular and humoral immunity for effective protection [13-15]. LTN was selected as a molecular adjuvant because past studies have shown that LTN exhibits both Th1- and Th2-type properties when applied mucosally and parenterally [18-24]. LTN is produced by CD8+ T cells, NK cells, and γδ TCR+ IEL, indicating induction of protection immunity against tumors through chemotaxis of T cells and natural killer (NK) cells [32,33]. LTN has also been adapted as a molecular adjuvant for development of vaccines against pathogens, including human immunodeficiency virus (HIV) [34] and avian coccidiosis [35]. For the development of an effective plague vaccine, we tested LTN as a molecular adjuvant against Y. pestis. In this study, the mucosal adjuvant effect by LTN to stimulate protective immunity was not as apparent when given nasally. Although nasal immunization with LTN/βgal DNA vaccine plus F1-Ag did appear to confer improved protection against pneumonic plague challenge, this was not significantly different from any of the vaccinated groups. Likewise, for i.m. DNA vaccinated mice, protection conferred by the LTN/βgal DNA vaccine was not significantly different from the LTN/V or LTN/F1-V immunized mice. However, these results show that i.m. immunization with LTN DNA vaccines showed significant improved protection against pneumonic plague challenge than nasally immunized mice. Examination of the supportive Th cells revealed a spectrum of Th1-, Th2-, and Th17-type cytokines. I.m. immunization influenced the production of Th17 cell responses, further supporting the notion that LTN can be used as a molecular adjuvant for vaccine to enhance protective immunity against plague.
In mice immunized with LTN DNA vaccine by either i.n. or i.m. route, Ab responses to F1- and V-Ag began to increase by wk 6. Although three DNA immunizations were insufficient to elevate the anti-F1- and -V-Ag Ab responses, robust Ag-specific responses were induced in mice nasally boosted with F1-Ag protein. These results were consistent with previous observations that DNA immunization effectively primes the host [25,36,37], and the combination of DNA and protein immunizations offers one means to effect optimal immunity to plague. Our results also showed that i.n. and i.m. LTN DNA vaccinations provide sufficient priming effect on induction of immunity to F1- and V-Ag in the peripheral immune compartment, resulting in improved efficacy when compared to nasal application of recombinant F1-Ag alone. Thus, LTN DNA vaccines provide effective priming that ultimately leads to protective immunity against plague.
The stimulation of neutralizing Abs when using LTN adjuvant was less apparent when applied nasally. Nasal LTN DNA vaccinations conferred less protection than the same vaccines given by the i.m. route. These results were unexpected, since we previously showed that Salmonella-based [27] and IL-12-based DNA vaccines [25] were effective against pneumonic plague challenge. Our results also showed, although serum Ab responses to F1- and V-Ag between i.n. and i.m. LTN DNA-vaccinated mice were similar after boosting with F1-Ag protein, Ab responses induced during the priming phase by the nasal LTN DNA vaccines were slightly lower than those Abs obtained by i.m.-vaccinated mice. Moreover, nasal immunization with LTN/F1-V produced less robust nasal Ab responses when compared to mice similarly immunized via the i.m. route. Although there did appear to be some tissue specificity, the cytokine analysis revealed the Th cell responses to V-Ag in the nasally DNA immunized mice were dampened, particularly the Th1 cell responses, when the same Th cell responses were compared to i.m. immunized mice. Such differences could account for the dampened efficacy by the nasally immunized mice. Thus, the molecular adjuvant, LTN, when given as a DNA vaccine, seems to perform better when given parenterally and provides better protection against pneumonic plague than the same vaccines given nasally. These data differ from that previously shown for the LTN protein when applied nasally with Ag [24].
No differences in IgG subclass responses were observed in mice nasally vaccinated with LTN DNA vaccines. However, IgG1 and IgG2a anti-F1-Ag responses were significantly greater than IgG2b responses in i.m.-immunized mice with LTN/V-Ag and LTN/F1-V DNA vaccines. In fact, the LTN/F1-V vaccine stimulated greater IgG2a than IgG1 anti-F1-Ag Abs from those mice primed with the LTN/V vaccine, although both groups were subsequently boosted with F1-Ag protein plus CT. CT is a well-known mucosal adjuvant that stimulates Th2-type responses [38,39]. Elevated IgG1 Abs to F1- and V-Ag were induced, which has been previously deemed important since enhanced IgG1 subclass titers to F1- and V-Ag correlated with protection against plague [40]. Thus, using the described vaccination regimens, mixed Th cell responses were induced supporting the varied IgG subclass responses.
Our results show that immunity to both V- and F1-Ags are required for protection against pneumonic plague evident by the similar levels of protection conferred by mice vaccinated i.m. with LTN/V or LTN/F1-V DNA vaccines plus F1-Ag boosts. These results are consistent with previous observations that a combination or fusion of these Ags has an additive protective effect when used to immunize mice against plague [9-12]. In addition, others have also reported that the F1- and V-Ag are considered the most effective candidates for vaccines against plague, although vaccination with each protein alone is sufficient for protecting mice against plague challenges [7,8]. Indeed, our Ab results in mice immunized with LTN DNA vaccine expressing V-Ag only or F1-V were consistent with Ab responses obtained in these other studies. Therefore, DNA vaccine expressing a combination of F1- and V-Ag, or as a fusion F1-V-Ag protein, is able to effectively prime for protection against plague.
In summary, this is the first description of LTN as a molecular adjuvant that tests DNA vaccines mucosally and parenterally for plague. Using a bicistronic plasmid encoding LTN plus the vaccine encoding V-Ag or F1-V-Ag, we showed effective priming by i.m. delivery of LTN DNA vaccine followed by booster immunizations with recombinant F1-Ag protein, resulting in protection against pneumonic plague. Th1, Th2, and Th17 cell responses were induced either by mucosal or parenteral vaccination; however, i.m. immunization with the LTN DNA vaccine markedly enhanced Th17 cell immunity when compared to the same vaccines administered nasally. These results suggest LTN can be used as a molecular adjuvant to allow inclusion of a cell-mediated component to enhance protective immunity against plague.
Acknowledgments
This work was supported by NIH-NIAID R01 AI-56286, NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185 and, in part, by Montana Agricultural Station and USDA Formula Funds. The challenge studies were partly supported by the Rocky Mountain Regional Center of Excellence for Biodefense and Emerging Infectious Diseases, NIH U54 AI-06537. We thank Ms. Nancy Kommers for her assistance in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anisimov AP, Lindler LE, Pier GB. Intraspecific diversity of Yersinia pestis. Clin Microbiol Rev. 2004;17:434–64. doi: 10.1128/CMR.17.2.434-464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry RD, Fetherston JD. Yersinia pestis-Etiologic agent. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koirala J. Plague: disease, management, and recognition of act of terrorism. Infect Dis Clin North Am. 2006;20:273–87. doi: 10.1016/j.idc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Baker E, Sommer H, Foster LE, Meyer KF. Studies on immunisation against plague. J Immunol. 1952;68:131–45. [PubMed] [Google Scholar]
- 5.Winter CC, Cherry WB, Moody MD. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull WHO. 1960;23:408–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Sing A, Rost D, Tvardovskaia N, Roggenkamp A, Wiedemann A, Kirschning CJ, et al. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J Exp Med. 2002;196:1017–24. doi: 10.1084/jem.20020908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leary SEC, Williamson ED, Griffin KF, Russell P, Eley SM, Titball RW. Active immunisation with V-antigen from Yersinia pestis protects against plague. Infect Immun. 1995;63:2854–58. doi: 10.1128/iai.63.8.2854-2858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller J, Williamson ED, Lakey J, Pearce MJ, Jones SM, Titball RW. Macromolecular organisation of recombinant Yersinia pestis F1 antigen and effect of structure on immunogenicity. FEMS Immunol Med Microbiol. 1998;21:213–21. doi: 10.1111/j.1574-695X.1998.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 9.Heath DC, Anderson GW, Mauro M, Welkos SL, Andrews GP, Adamovicz J, Friedlander AM. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine. 1998;16:1131–37. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- 10.Williamson ED, Eley SM, Griffin KF, Green M, Russell P, Leary SEC, et al. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol Med Microbiol. 1995;12:223–30. doi: 10.1111/j.1574-695X.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 11.Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washing and protects immunized animals against pneumonic plague. Vaccine. 1997;15:1079–84. doi: 10.1016/s0264-410x(96)00303-9. [DOI] [PubMed] [Google Scholar]
- 12.Williamson ED, Sharp GJE, Eley SM, Vesey PM, Pepper TC, Titball RW, et al. Local and systemic immune response to a microencapsulated sub-unit vaccine for plague. Vaccine. 1996;14:1613–19. doi: 10.1016/s0264-410x(96)00151-x. [DOI] [PubMed] [Google Scholar]
- 13.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005;73:7304–10. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006;74:3381–86. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philipovskiy AV, Smiley ST. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect Immun. 2007;75:878–885. doi: 10.1128/IAI.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelner GS, Kennedy J, Bacon KB, Kleyensteuber S, Largaespada DA, Jenkins NA, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–99. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy J, Kelne GS, Kleyensteuber S, Schall TJ, Weiss MC, Yssel H, et al. Molecular cloning and functional characterization of human lymphotactin. J Immunol. 1995;155:203–9. [PubMed] [Google Scholar]
- 18.Hedrick JA, Saylor V, Figueroa D, Mizoue L, Xun Y, Menon S, et al. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol. 1997;158:1533–540. [PubMed] [Google Scholar]
- 19.Hedrick JA, Zlotnik A. Lymphotactin. Clin Immunol Immunopathol. 1998;87:218–222. doi: 10.1006/clin.1998.4546. [DOI] [PubMed] [Google Scholar]
- 20.Muller S, Dorner B, Korthauer U, Mages HW, D'Apuzzo M, Senger G, et al. Cloning of ATAC, an activation-induced, chemokine-related molecule exclusively expressed in CD8+ T lymphocytes. Eur J Immunol. 1995;25:1744–48. doi: 10.1002/eji.1830250638. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida T, Imai T, Kakizaki M, Nishimura M, Takagi S, Yoshie O. Identification of single C motif-1/lymphotactin receptor XCR1. J Biol Chem. 1998;273:16551–4. doi: 10.1074/jbc.273.26.16551. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida T, Izawa D, Nakayama T, Nakahara K, Kakizaki M, Imai T, et al. Molecular cloning of mXCR1, the murine SCM-1/lymphotactin receptor. FEBS Lett. 1999;458:37–40. doi: 10.1016/s0014-5793(99)01114-x. [DOI] [PubMed] [Google Scholar]
- 23.Boismenu R, Feng L, Xia YY, Chang JC, Havran WL. Chemokine expression by intraepithelial γδ T cells. Implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–92. [PubMed] [Google Scholar]
- 24.Lillard JW, Jr, Boyaka PN, Hedrick JA, Zlotnik A, McGhee JR. Lymphotactin acts as an innate mucosal adjuvant. J Immunol. 1999;162:1959–65. [PubMed] [Google Scholar]
- 25.Yamanaka H, Hoyt T, Yang X, Golden S, Bosio CM, Crist K, et al. A nasal interleukin-12 DNA vaccine coexpressing Yersinia pestis F1-V fusion protein confers protection against pneumonic plague. Infect Immun. 2008;76:4564–73. doi: 10.1128/IAI.00581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maddaloni M, Staats HF, Mierzejewska D, Hoyt T, Robinson A, Callis G, et al. Mucosal vaccine targeting improves onset of mucosal and systemic immunity to botulinum neurotoxin A (BoNT/A) J Immunol. 2006;177:5524–32. doi: 10.4049/jimmunol.177.8.5524. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Hinnebusch BJ, Trunkle T, Bosio CM, Suo Z, Tighe M, et al. Oral vaccination with Salmonella simultaneously expressing Yersinia pestis F1 and V antigens protects against bubonic and pneumonic plague. J Immunol. 2007;178:1059–67. doi: 10.4049/jimmunol.178.2.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Hudson M, Walters N, Bargatze RF, Pascual DW. Selection of protective epitopes for Brucella melitensis using DNA vaccination. Infect Immun. 2005;73:7297–303. doi: 10.1128/IAI.73.11.7297-7303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochoa-Repáraz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–9. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual DW, Trunkle T, Sura J. Fimbriated Salmonella enterica serovar Typhimurium abates initial inflammatory responses by macrophages. Infect Immun. 2002;70:4273–81. doi: 10.1128/IAI.70.8.4273-4281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanaka H, Hoyt T, Bowen R, Yang X, Crist K, Golden S, et al. An IL-12 DNA vaccine co-expressing Yersinia pestis antigens protects against pneumonic plague. Vaccine. 2009;27:80–7. doi: 10.1016/j.vaccine.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao X, Zhang W, He L, Xie Z, Ma S, Tao Q, et al. Lymphotactin gene-modified bone marrow dendritic cells act as more potent adjuvants for peptide delivery to induce specific antitumor immunity. J Immunol. 1998;161:6238–44. [PubMed] [Google Scholar]
- 33.Dilloo D, Bacon K, Holeden W, Zhong W, Burdach S, Zlotnik A, et al. Combined chemokine and cytokine gene transfer enhances antitumor immunity. Nat Med. 1996;2:1090–95. doi: 10.1038/nm1096-1090. [DOI] [PubMed] [Google Scholar]
- 34.Waterman PM, Kitabwalla M, Hatfield GS, Evans PS, Lu Y, Tikhonov I, et al. Effects of virus burden and chemokine expression on immunity to SHIV in nonhuman primates. Viral Immunol. 2004;17:545–57. doi: 10.1089/vim.2004.17.545. [DOI] [PubMed] [Google Scholar]
- 35.Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ. Adjuvant effects of IL-1β, IL-2 IL-8, IL-15, IFN-α, IFN-γ, TGF-β4 and lymphotactin on DNA vaccination against Eimeria ecervulina. Vaccine. 2002;20:267–74. doi: 10.1016/s0264-410x(01)00270-5. [DOI] [PubMed] [Google Scholar]
- 36.Egan MA, Chong SY, Megati S, Montefiori DC, Rose NF, Boyer JD, et al. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res Hum Retroviruses. 2005;21:629–43. doi: 10.1089/aid.2005.21.629. [DOI] [PubMed] [Google Scholar]
- 37.Pal VS, Kalyanaraman B, Nair BC, Whitney S, Keen T, Hocker L, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348:341–53. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–29. [PubMed] [Google Scholar]
- 39.Vajdy M, Kosco-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4-targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 40.Williamson ED, Vesey PM, Gillhespy KJ, Eley SM, Green M, Titball RW. An IgG1 titre to the F1 and V antigens correlates with protection against plague in the mouse model. Clin Exp Immunol. 1999;116:107–14. doi: 10.1046/j.1365-2249.1999.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]