Abstract
Heterologous immunity associated with cross-reactive T-cell responses is proposed to contribute to variations among individuals in the pathogenesis of human viral infections. In genetically identical mice with similar infection histories, marked variations in the magnitude and specificities of T-cell responses under conditions of heterologous immunity occur and have been linked to the private specificity of T-cell repertoires in individual immune mice. Variations in immunopathology in the form of panniculitis are observed in lymphocytic choriomeningitis virus-immune mice after vaccinia virus infection. By adoptively transferring splenocytes from individual lymphocytic choriomeningitis virus-immune donors into paired recipients, we show here that, on vaccinia virus infection, similar levels of panniculitis were generated in recipients from a single donor, but the severity of panniculitis varied among recipients receiving cells from different donors. This indicates that virus-induced immunopathology under conditions of heterologous immunity is a function of the private specificity of the immune repertoire.
A number of studies have shown that T cell responses to viral infections may be influenced by memory T cells generated in response to unrelated pathogens.1,2,3,4,5 This alteration in responses is displayed by changes in T cell immunodominance hierarchies,1 by alterations in viral loads and protective immunity, and by marked changes in immunopathology.2,4 This “heterologous immunity” can be a consequence of unanticipated T cell cross-reactivity between different pathogens. Indeed, T cell specificity can be quite degenerate, and cross-reactivity between different viruses is common. For example, human studies have revealed strong T cell cross-reactivity between influenza A virus (IAV) and Epstein-Barr virus (EBV) epitopes and between IAV and hepatitis C virus (HCV) epitopes. Acute infectious mononucleosis and fulminant hepatitis have been suggested as pathological manifestations of such CD8 T cell cross-reactivity.3,5
Heterologous immunity can best be studied and manipulated in mouse model systems, and C57BL/6 mice immune to lymphocytic choriomeningitis virus (LCMV) and subsequently challenged with vaccinia virus (VV) show enhanced protective immunity and altered immunopathology, in contrast to naïve mice infected with VV.2,4 These are manifestations of heterologous immunity that can be duplicated by transfer of LCMV-immune T cell populations into naïve mice, followed by challenge with VV. VV challenge of LCMV-immune mice by the i.p. route results in a severe panniculitis, or inflammation of visceral fat, with pathology similar to that of human erythema nodosum. The fat tissue is infiltrated with T cells cross-reactive between LCMV and VV, and the pathology is dependent on interferon-γ (IFN-γ). VV challenge of LCMV-immune mice by the intranasal route results in abnormally pronounced T cell infiltrates in the lungs, sometimes with the accompanying pathology known as bronchiolitis obliterans.
A property of heterologous immunity noted both in the human and murine systems is that there can be dramatic variation in pathogenesis and in the cross-reactive specificities of the T cell responses between individuals. This is caused in part by the “private specificities” of unique T cell repertoires generated by random DNA recombination events even in genetically identical hosts.6,7 Mouse studies have shown that such variation can even occur in genetically identical individuals subjected to similar infection histories. In syngeneic LCMV-immune mice challenged with VV, T cell immunodominance, and cross-reactivity patterns vary, such that, for example, in some mice VV selectively expands T cells cross-reactive with the LCMV epitope NP205-212, whereas in other mice there is selective expansion of T cells specific to GP34-41 or to GP118-125.8 T cell adoptive transfer studies showed that VV-challenged recipient mice receiving T cells from a single LCMV-immune donor responded with similar T cell responses, but differed from recipients of immune cells from a different donor.8 This indicated that individual LCMV-immune mice had unique patterns of T cell cross-reactivity to VV, and that the private specificities of the T cell repertoires in individuals regulated the magnitude and the specificities of T cell responses under conditions of heterologous immunity.8
Human diseases involving suspected heterologous immunity include EBV-induced infectious mononucleosis,3 HCV-induced hepatitis,5 and dengue virus-induced hemorrhagic fever and shock syndrome.9,10 All present with marked variations in immunopathology. Here we tested the hypothesis that variations in immunopathology under conditions of heterologous immunity are, like the specificity of the T cell response, also regulated by the private specificity of the immune repertoire. We show this to be true in the panniculitis model described above.
Materials and Methods
Mice
Male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME; Ly5.2) and Ly5.1 mice (B6.SJL-Ptprca; Taconic Farms, Hudson, NY) were maintained under specific pathogen-free conditions in the University of Massachusetts Medical School Department of Animal Medicine.
Adoptive Transfer and Infections
LCMV-immune mice were inoculated i.p. with 5 × 104 plaque-forming unit (PFU) LCMV Armstrong and were used after 6 weeks.8 For some experiments, LCMV-immune and nonimmune mice were infected with 106 PFU of the WR strain of VV i.p. For T-cell enrichment, whole splenocytes were reacted with CD90.2 microbeads (130-049-101; Miltenyi Biotec, Auburn, CA) and sorted by Miltenyi Biotech MACS system. For paired cell transfers, cells from one Ly5.1 LCMV-immune mouse were transferred via the tail vein into two Ly5.2 mice. Alternatively, cells from five or more Ly5.1 LCMV-immune mice were pooled and transferred into four or five Ly5.2 mice. One day post-transfer, recipients were challenged with 106 PFU WR strain of VV i.p.
Virus Titration
At day 6 of infection, fat pads were harvested, and VV titers were determined by plaque assays by using tissue homogenate taken from individual mice, as described previously.4
Scoring Panniculitis
Levels 1 to 7 of panniculitis were scored visually based on the severity of disease as previously described4; levels 1 to 2, mild disease with a few necrotic white spots on abdominal fat-pads; levels 3 to 4, moderate disease with larger patches of necrosis that now also extends into the left upper quadrant splenic fat-pad; levels 5 to 6, severe disease with extensive large patches of necrosis throughout; level 7, very severe disease such that the abdominal organs adhere to each other; and level 8, mice moribund with panniculitis and unlikely to survive.
Isolation of Peritoneal Exudate Cells (PEC)
Leukocytes from the peritoneal cavity were collected by lavaging with 10 ml cold RPMI 1640 medium (GIBCO, Gaithersburg, MD).
Flow Cytometry and Intracellular IFN-γ Staining
Cells were stimulated and stained for cell surface markers and intracellular cytokines by using monoclonal antibodies specific for CD45.1 (A20; eBioscience, San Diego, CA), CD44 (IM7), CD8α (53-6.7), and IFN-γ (XMG1.2; BD Biosciences, San Jose, CA), as described previously.4 The samples were analyzed by using a BD Biosciences LSR II and FlowJo version software.
Synthetic Peptides
LCMV-specific epitopes NP396-404 (FQPQNGQFI), GP33-41 (KAVYNFATC), GP276-286 (SGVENPGGYCL), NP205-212 (YTVKYPNL), and GP118-125 (ISHNFCNL) were purchased from BioSource International Camarillo, CA and were purified with reverse phase-HPLC to 90% purity.
Statistical Analyses
Intraclass correlation was used to analyze panniculitis data from paired transfer experiments.11 Other data where designated were analyzed by the Mann-Whitney test or Student’s t-test and presented ± SE of the means.
Results
Variation in the Levels of VV-Induced Pathology in LCMV-Immune Mice Occurs but Does Not Correlate with Viral Loads
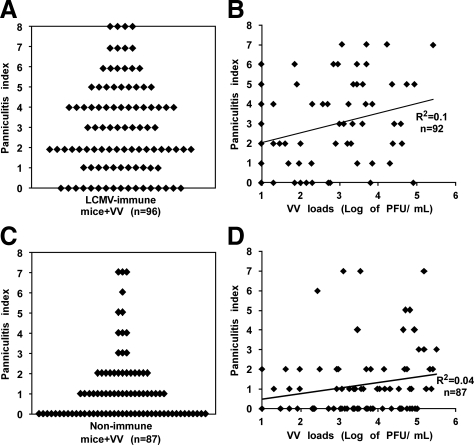
Naïve mice infected i.p. with 106 PFU of VV, strain WR, usually show little or no pathology in visceral fat pads at 6 days postinfection, whereas LCMV-immune male mice, on infection with VV, develop panniculitis in the form of acute fatty necrosis.4 The variation in levels of panniculitis in VV-infected LCMV-immune mice ranges from none to very severe levels, as quantified in Figure 1, A and B, and this is substantially greater than the low levels of panniculitis seen in nonimmune mice infected with VV as seen in Figure 1, C and D. There were variations in panniculitis in both groups of mice, but the VV-challenged LCMV-immune group averaged a panniculitis index of 2.9 ± 0.2 (n = 96), whereas the VV-challenged nonimmune groups averaged 1.2 ± 0.2 (n = 87) with P < 0.0001 when analyzed by the nonparametric Mann-Whitney test. The average values obscure the common appearance of extremes in pathology and the high variation in pathology in the VV-challenged LCMV-immune group. One possible explanation for this variation is that higher VV loads in the fat tissue may result in more cell death and more severe tissue damage. However, after titrating viral PFU in the fat pad, we found variations in panniculitis in mice with undetectable VV, and mice that had VV loads of more than 104 PFU/ml showed both low and high levels of panniculitis. The correlation coefficient (R2) between the levels of panniculitis and VV loads in VV-challenged LCMV-immune mice was 0.1, which means that the variation in levels of VV-induced panniculitis in LCMV-immune mice did not correlate with VV titers (Figure 1B). Similarly, viral loads did not correlate with the lower but varied levels of panniculitis in the VV-challenged nonimmune mice, where R2 = 0.04. These data suggest that the host response rather than the viral load was associated with variations in the pathology.
Figure 1.
Variable levels of panniculitis independent of VV load in genetically identical LCMV-immune mice during acute VV infection. LCMV-immune (A and B) and nonimmune (C and D) mice were challenged with VV i.p. At day 6 of infection, the levels of panniculitis were recorded, and VV loads in fat-pads were assayed. A and C: different levels of panniculitis developed after VV challenge: LCMV-immune = 2.9 ± 0.2 versus naïve = 1.2 ± 0.2; P < 0.0001 by Mann Whitney test. No correlation between VV loads and levels of panniculitis were found in either LCMV-immune (B) or nonimmune (D) mice.
Pooled LCMV-Immune CD8 T Cells from Multiple Donors Result in Similar Levels of Immunopathology
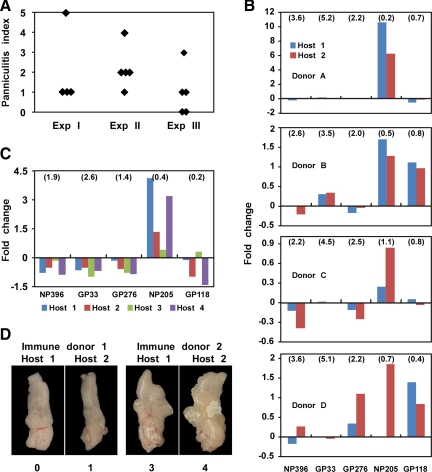
We hypothesized that because variations in immunopathology did not correlate well with viral loads, they might be a function of the immune responses within the fat. We had previously shown that LCMV-immune but not naïve splenocytes transferred into naïve mice protected them against VV infection while inducing panniculitis and that this protection and panniculitis were ablated in the absence of IFN-γ. Protective immunity was also eliminated by depletion of CD8 or CD4 T cells before transfer.4 Because naive cells transferred neither protective immunity nor panniculitis, we focused our subsequent studies herein on transferring immune cells only (Figure 2, A–D). To begin an assessment of the variations in pathology, we first transferred splenocytes from pooled groups of LCMV-immune Ly5.1+ mice into naïve Ly5.2+ recipients, challenged them with VV, and looked for induction of panniculitis (Figure 2A). Here, enriched populations of CD3+ T cells were transferred in experiments 1 and 2 and total spleen leukocytes in experiment 3. At day 6 of infection, the donor cell responses were examined, and the levels of panniculitis were recorded. All recipients receiving pooled LCMV-immune donor cells generated similar Ly5.1+ donor T cell responses, with preferential expansion of the LCMV-encoded NP205-specific T cell population (Figure 2C). For example, in experiment 1, before transfer, the NP205-specific response was 7% of the total LCMV-specific response to five epitopes. This proportion of the NP205-specific T cell population was increased in all four recipients to an average of 36 ± 8% of the total LCMV-specific response (ranged from 20% to 55%). Figure 2C depicts the data in another way, showing for experiment 1 the fold increase or decrease of epitope-specific T cells under these conditions of pooled T cell transfers and shows that the NP205-specific T cell response preferentially expanded. In all three experiments, most of the recipients developed panniculitis (Figure 2A), indicating that donor T cells can elicit this pathology. The levels of panniculitis were in general similar among recipients, but, in each experiment, there was one mouse that behaved quite differently from the rest of the recipients. These experiments suggest that immune T cells from pooled populations can induce similar levels of panniculitis, but that occasionally other uncontrolled factors may influence this process.
Figure 2.
Variations in panniculitis in VV-infected LCMV-immune mice determined by the private nature of the immune T-cell repertoire. A and C: Pooled LCMV-immune cells derived from multiple Ly5.1 LCMV-immune donors were transferred into four or five naïve Ly5.2 mice, which were examined at day 6 of VV infection. A shows the levels of panniculitis from three independent experiments, where enriched CD3+ spleen T cells were transferred in Experiments (Exp) 1 and 2 and unfractionated spleen leukocytes were transferred in Exp 3, as described in Materials and Methods. C: the fold changes of LCMV-specific T cell responses, as detected by peptide-induced intracellular IFN-γ assay after subtracting background. The percentages of donor epitope-specific responses per CD8 cell before adoptive transfer in B and C are indicated in parentheses. B and D: LCMV-immune cells derived from a single donor were transferred equally into two congenic hosts. At day 6 of VV infection, LCMV-specific responses of donor cells isolated from PEC were examined by intracellular cytokine assay, and the levels of panniculitis were evaluated. The fold changes of LCMV-specific responses are shown in B, which presents data from Exp 4 of Table 1. Pictures of fat-pads from two representative pairs of mice are shown in D.
The Private Specificity of the LCMV-Immune Donor Determines the Levels of Immunopathology Induced by VV Infection
We next questioned whether variations in pathology could be a function of the private specificity of the immune repertoire. We did adoptive transfer studies as above in Figure 2A, but this time pooled immune samples were not used. Instead, pairs of recipient mice received lymphocytes from an individual LCMV-immune donor before infection with VV. At day 6 of VV infection, the levels of panniculitis were recorded, and the donor cell responses against LCMV epitopes were examined.
Figure 2D shows panniculitis in visceral fat pads from two sets of donor-recipient pairs. The two recipients receiving memory cells from immune donor 1 both showed very little panniculitis, whereas the fat pads isolated from recipients of cells from donor 2 both showed considerably higher levels of panniculitis. This type of experiment was done four times with four to five donors per experiment, and the results are displayed in Table 1, which shows, statistically, that the two recipients from a given donor had similar levels of panniculitis on VV challenge. With three exceptions, all paired recipients had either identical or very similar (±1) pathology scores. Two of the exceptions were just two units different. To compare whether the variation within paired recipients was significantly less than the variation between unpaired recipients within these same experiments, we used intraclass correlation to analyze our data.11 This analysis showed that the pathology in the paired samples had a moderate correlation with high significance (coefficient = 0.6; P = 0.002), indicating that the severity of VV-induced panniculitis in LCMV-immune mice was determined by the private specificity of the donor T-cell repertoire. However, because the correlation coefficient was not 1, additional factors could be involved, as was also suggested by the data in Figure 2A.
Table 1.
Two Hosts Receiving Cells from the Same Donor Develop Similar Levels of Panniculitis
| Experiment (Exp) | Host 1* | Host 2* |
|---|---|---|
| Exp 1 | 2 | 3 |
| 1 | 3 | |
| 4 | 4 | |
| 4 | 3 | |
| 1 | 2 | |
| Exp 2† | 2 | 1 |
| 3 | 6 | |
| 3 | 2 | |
| 3 | 3 | |
| Exp 3 | 1 | 0 |
| 3 | 2 | |
| 1 | 3 | |
| 2 | 1 | |
| 1 | 2 | |
| Exp 4 | 0 | 1 |
| 0 | 0 | |
| 3 | 4 | |
| 1 | 0 | |
| 2 | 2 | |
| ICC‡ | 0.6 | |
| P | 0.002 | |
ICC, intra-class correlation.
Hosts 1 and 2 on the same row represent the two mice reconstituted with immune cells derived from a single LCMV-immune donor, prior to challenge with VV.
Purified T-cells from individual LCMV-immune mice were used for adoptive transfer.
Here, the similarities of panniculitis scores of paired recipients from a single donor were compared with scores from random unpaired recipients within the same data set.
Our previous studies had shown that recipients receiving donor cells from the same individual LCMV-immune donor selectively expanded LCMV-specific memory cells with similar specificities, whereas recipients of different LCMV-immune donors expanded LCMV memory cells with different specificities.6,8 We found similar results in this study, as shown in Figure 2, B and C. Figure 2B first shows using the data in experiment 4 from Table 1 that recipient pairs from the same donor usually expanded the same epitope-specific T cell population, whether it be an expansion of one, two, or three specificities. Second, it shows variations in the epitope-specific T cell populations expanded, depending on the donor. In this experiment, NP205-specific T cells were preferentially expanded from host recipients of Donor A and C, GP33/34, NP205, and GP118 from Donor B, and GP276, NP205, and GP118 from Donor D. This analysis was also done with the paired hosts in experiment 1 and 2 from Table 1, and here five out of nine mouse pairs showed preferential expansion of GP118-specific T cell population, two showed skewing toward NP205, and the other two had no dramatic change in the proportion of LCMV-specific responses.
Discussion
Our results for the first time demonstrate that the private specificity of an individual’s immune repertoire can be a determinant of the highly variable disease course observed in genetically identical LCMV-immune mice during VV infection. These data may provide an explanation for some of the variations in human disease during viral infections, in addition to the effects of genetics, physiological state, and infection history. Humans are not immunologically naïve, and we suggest that the results of a viral infection may be greatly affected by the private specificities of T cell repertoires induced in response to previously acquired infections. There are several examples of human viral infections associated with both cross-reactive T cell responses and major differences in immunopathology. Dengue virus infections can range from asymptomatic to febrile (dengue fever), to dengue hemorrhagic fever, and dengue shock syndrome. The majority of the cases of dengue hemorrhagic fever/dengue shock syndrome is associated with secondary infection by a different dengue serotype,12,13 and cross-reactive CD8 T cells are found between the different serotypes.10,14 Although other models for pathogenesis in this system have been proposed, including antibody-dependent immune enhancement, the selective expansion of cross-reactive T cells with low binding affinity for the current serotype from the unique memory T cell pool of an individual may result in an inefficient immune response,10 leading to the progression to severe forms of disease.9 Because only a small number of patients with secondary infection experience dengue hemorrhagic fever/dengue shock syndrome, the private specificities of the immune repertoires could be regulating these differences in severity.15,16,17
HCV infections can also induce extremely different outcomes among individuals, varying from asymptomatic to severe illness. A study of eight HCV-infected patients showed that fulminant hepatitis in a subset of patients was associated with a robust and narrowly focused CD8 T cell response cross-reactive against an HLA-A2.1-restricted HCV immunodominant epitope NS31073–1081 and an IAV epitope NA231–239. This focused response was absent in other HLA-A2.1 patients, which had undoubtedly been exposed to IAV but instead developed a very diverse T cell response and had only mild disease.5 These data are consistent with our finding that the immunopathology and cross-reactivity are controlled by the private specificities of the memory T cell repertoire of the host. Similar examples are also seen in EBV-infected patients. The pathogenesis of EBV-associated mononucleosis is also highly variable and associated with T-cells cross-reactive between EBV and IAV.3
A highly diverse T cell response may be superior to a narrowly focused response in clearing an infection with limited immunopathology. Some narrowly focused responses, which vary from host to host, may do more harm than good by not being effective at combating the pathogen while preventing the generation of more effective and diverse T cell responses by immunodominance mechanisms. We show here that when mice were adoptively reconstituted with pooled LCMV memory T cells from multiple donors, most of the mice (11 out of 14) developed levels of panniculitis at the low end (Figure 2A). However, when mice were reconstituted with memory cells from single LCMV-immune donors, about 40% (15 out of 38) of the mice showed panniculitis at level 3 or higher (Table 1). Compared with a single memory T cell pool, the pooled LCMV memory T cell population would be more diverse, and the hosts receiving this broader T cell receptor (TCR) repertoire might have had a better chance at getting a high affinity protective cross-reactive response. Lower affinity cross-reactive responses may be poorer at rapidly clearing viruses and more likely to cause collateral damage to the fat by producing inflammatory cytokines such as IFN-γ. In a total of 106 VV-challenged LCMV-immune mice (without adoptive transfer), higher levels of pathology were seen when the dominant response was to GP118 (mean, 3.6 ± 0.4) than to GP33/34 (mean, 1.9 ± 0.5; P = 0.004) or NP205 (mean, 2.2 ± 0.4; P = 0.015). Future studies will investigate whether GP118-specific T cells are more capable of stimulating panniculitis. Thus, highly skewed or focused T cell responses may be dangerous, and, under conditions of heterologous immunity may commonly be produced with extreme variations among individuals, due to the private specificities of their immune repertoires. These issues should be carefully considered in the engineering of T cell vaccines.18
Acknowledgments
We thank Quan Yuan for helping us with photography and Stephen P. Baker for consultation about statistical analysis.
Footnotes
Address reprint requests to Liisa K. Selin, M.D., Ph.D., Department of Pathology, University of Massachusetts Medical School, 55 Lake Ave North, Worcester, MA 01655. E-mail: Liisa.Selin@umassmed.edu.
Supported by NIH grant AI-46578 (L.K.S.), AI-46629 (L.K.S.), and AR35506 (R.M.W.) and DR-32520.
Current address of S.N.: Biomedical Research Models, Inc., Worcester, MA; of S.-J.L.: Research Center for Emerging Viral Infection and Department of Medical Biotechnology and Laboratory Science, Chang Gung University, Taiwan, Republic of China.
References
- Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, Welsh RM, Selin LK. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005;115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, Ferrari C. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. 2005;201:675–680. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Kim SK, Cornberg M, Clute SC, Selin LK, Naumov YN. The privacy of T cell memory to viruses. Curr Top Microbiol Immunol. 2006;311:117–153. doi: 10.1007/3-540-32636-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM. Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005;201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J, Gu F, Vasudevan SG. Role of T cells, cytokines and antibody in dengue fever and dengue haemorrhagic fever. Rev Med Virol. 2006;16:263–275. doi: 10.1002/rmv.507. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- Cook C. Annual Meeting of the Southwest Educational Research Association; A review of intraclass correlation. 2000:35. [Google Scholar]
- Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Delgado I, Halstead SB. Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–799; discussion 804. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, Phanthumachinda B, Halstead SB. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand: the 1980 outbreak. Am J Epidemiol. 1984;120:653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Zivna I, Green S, Vaughn DW, Kalayanarooj S, Stephens HA, Chandanayingyong D, Nisalak A, Ennis FA, Rothman AL. T cell responses to an HLA-B*07-restricted epitope on the dengue NS3 protein correlate with disease severity. J Immunol. 2002;168:5959–5965. doi: 10.4049/jimmunol.168.11.5959. [DOI] [PubMed] [Google Scholar]
- Bravo JR, Guzman MG, Kouri GP. Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans R Soc Trop Med Hyg. 1987;81:816–820. doi: 10.1016/0035-9203(87)90041-1. [DOI] [PubMed] [Google Scholar]
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee S. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health. 2004;9:1022–1029. doi: 10.1111/j.1365-3156.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Fujinami RS. Pathogenic epitopes, heterologous immunity and vaccine design. Nat Rev Microbiol. 2007;5:555–563. doi: 10.1038/nrmicro1709. [DOI] [PMC free article] [PubMed] [Google Scholar]