Abstract
Chronic inflammation and fibrosis are the leading causes of chronic allograft failure. The nuclear receptor peroxisome proliferator-activated receptor (PPAR)γ is a transcription factor known to have antidiabetogenic and immune effects, and PPARγ forms obligate heterodimers with the retinoid X receptor (RXR). We have reported that a retinoic acid (RAR)/RXR-agonist can potently influence the course of renal chronic allograft dysfunction. In this study, in a Fischer to Lewis rat renal transplantation model, administration of the PPARγ-agonist, rosiglitazone, independent of dose (3 or 30 mg/kgBW/day), lowered serum creatinine, albuminuria, and chronic allograft damage with a chronic vascular damage score as follows: 35.0 ± 5.8 (controls) vs. 8.1 ± 2.4 (low dose-Rosi; P < 0.05); chronic tubulointerstitial damage score: 13.6 ± 1.8 (controls) vs. 2.6 ± 0.4 (low dose-Rosi; P < 0.01). The deposition of extracellular matrix proteins (collagen, fibronectin, decorin) was strikingly lower. The expression of transforming growth factor-β1 was inhibited, whereas that of bone morphogenic protein-7 (BMP-7) was increased. Intragraft mononuclear cells and activated fibroblast numbers were reduced by 50%. In addition, the migratory and proliferative activity of these cells was significantly inhibited in vitro. PPARγ activation diminished the number of cells expressing the proinflammatory and fibrogenic proteoglycan biglycan. In macrophages its secretion was blocked by rosiglitazone in a predominantly PPARγ-dependent manner. The combination of PPARγ- and RAR/RXR-agonists resulted in additive effects in the inhibition of fibrosis. In summary, PPARγ activation was potently immunosuppressive and antifibrotic in kidney allografts, and these effects were enhanced by a RAR/RXR-agonist.
Present immunosuppressive strategies are unable to prevent graft loss by atrophy and fibrosis that occur during chronic renal allograft dysfunction; the cellular and molecular mechanisms involved are complex.1 A persistent activity of T cells and monocytes/macrophages seems to be relevant for the subsequent activation and proliferation of (myo)fibroblasts and endothelial cells.2,3 Proinflammatory and fibrogenic mediators such as transforming growth factor (TGF)-β, platelet-derived growth factor, endothelin and angiotensin II are synthesized and secreted by both tissue-infiltrating inflammatory mononuclear cells and tubular epithelia.1,3 In addition, authors have recently reported that the small leucine-rich proteoglycans (SLRPs), biglycan (BGN), and decorin are not only structural components of the extracellular matrix; they may be involved in the synthesis of cytokines/chemokines and growth factors including basic fibroblast growth factor and TGF-β.4,5 We have reported that BGN is an endogenous ligand of the innate immunity receptors toll-like receptor (TLR) 2 and 4 and an activator of the Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, thereby increasing the synthesis and secretion of chemokines and collagen in monocytes/macrophages and fibroblasts.6,7 Adenovirus-mediated gene transfer of BGN has been shown to induce a fibroblastic response in the lung, indicating a role of BGN in fibrogenesis.8
Ligand activated transcription factors of the nuclear receptor superfamily might regulate these complex inflammatory/fibrotic networks.9 Thiazolidinediones, such as rosiglitazone, are high-affinity ligands for peroxisome proliferator-activated receptor (PPAR)γ, a member of the nuclear receptor family. They are used as insulin-sensitizing drugs in type 2 diabetes. In addition, it has been demonstrated that PPARγ-agonists suppress the synthesis and release of immunomodulatory cytokines/chemokines (eg, interleukin-1β, tumor necrosis factor-α, and interferon-γ) from various cell types and modulate cell cycle, differentiation, and apoptosis.10,11 After ligand-induced activation, PPARγ heterodimerizes with the retinoid X receptor (RXR). PPARγ/RXR heterodimers bind to specific DNA sequences in promoter regions of target genes, regulating genes of lipid and glucose homeostasis.10 Anti-inflammatory effects of PPARγ probably result by transrepression, impeding the activity of transcription factors such as activator protein-1 (AP-1), signal transducer and activator of transcription (STAT), and nuclear factor κB.11,12 PPARγ-independent anti-inflammatory effects of thiazolidinediones have been also reported.13,14
We have demonstrated that retinoids, which act through retinoic acid receptors (RAR) and RXRs, potently block inflammatory and fibrosing processes in renal allograft rejection.15,16 We have also found that rosiglitazone could ameliorate rejection phenomena in acute models of heart and renal transplantation (manuscript in preparation). On the basis of these results, we postulated that PPARγ-agonists could prevent chronic dysfunction of kidney allografts by inhibiting proinflammatory and fibrogenic mediators.
The effects of rosiglitazone in a chronic Fisher344 donor→Lewis recipient renal transplantation model have been investigated. Rosiglitazone led to a significant preservation of renal function and morphology. Mononuclear cell infiltration and secretion of cytokines/chemokines as well as proliferation were reduced in kidney allografts. In vitro, rosiglitazone inhibited the proliferation of monocytes/macrophages, endothelial cells, and fibroblasts as well as their migration in co-culture experiments. In addition, the PPARγ ligand, rosiglitazone, suppressed the expression of the SLRPs decorin and BGN in the treated allografts and inhibited the secretion of BGN by interleukin-6 stimulated macrophages in a mainly PPARγ-dependent fashion. The effectiveness of rosiglitazone in reducing chronic allograft damage was significantly increased by addition of an RXR agonist.
Materials and Methods
Animals
Male inbred Lewis (LEW, RT11) and Fisher (F344, RT11v1) rats were purchased from Charles River GmbH (Sulzfeld, Germany). Fisher or Lewis rats were used as recipients of Fisher kidney grafts. Donors and recipients weighed about 200 to 220 g at the time of renal transplantation.
Conditional PPARγ-Deficient Mice
Homozygous floxed PPARγ (B6.129-Ppargtm2Rev/J; PPARγfl/fl) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred in our animal facility. PPARγfl/fl mice were crossed with a transgenic mouse containing the Cre recombinase gene under the control of the murine M lysozyme promoter (LysCre) to achieve a selective deficiency of PPARγ in macrophages (PPARγfl/fl/LysCre; PPARγ−/−). C57Bl/6 wild-type mice were used as controls, as macrophages of PPARγfl/fl, and LysCre mice had shown similar characteristics of cytokine secretion.17 Animal experiments were performed according to German laws on animal protection.
Kidney Transplantation
Transplantation was performed under ether drop anesthesia.15,16 The left kidney of the donor F344 rat was isolated, perfused with ice-cold isotonic sodium chloride solution, excised, and transplanted orthotopically into a weight-matched F344 (isograft) or LEW recipient (allograft). In the recipient the left renal vein and artery were mobilized and clamped, the ureter was cut, and the left kidney was excised. End-to-end anastomosis of renal vessels and of ureter, without ureteral stenting, were performed with 10–0 nonabsorbable nylon sutures. Total ischemic time of the donor kidney varied between 30 and 45 minutes. The right kidney was left in place to enhance rejection and damage to the transplant by avoidance of potential endogenous immunosuppressive effects of renal insufficiency and by a reduction of the work load of the transplanted kidney;15,18 to obtain functional parameters of the graft at the end of the experiments, the right kidney was removed 48 hours before sacrifice. All transplant kidneys with hydronephrosis, which was evaluated both macroscopically and by light microscopy, were excluded from the experimental groups.
Experimental Protocol
Animals were randomly allocated to five experimental groups: (1) control group, 56 days (n = 20), F344 to LEW allografts fed with standard rat chow; (2) Rosiglitazone (Rosi) groups: (a) high dose (HD)-Rosi, 56 days (n = 9), and (b) low dose (LD)-Rosi, 56 days (n = 8), in which animals were treated with HD (30 mg/kgBW/day) or LD (3 mg/kgBW/day) to study the effects of rosiglitazone on renal allograft rejection.
To evaluate the effects of a combination of two ligands to RXR-heterodimers on chronic allograft dysfunction, two other groups were studied: (3) Isotretinoin (Iso), 56 days (n = 8), and (4) Rosi + Iso, 56 days (n = 8), in which animals were treated with isotretinoin (0.2 mg/kgBW/day) or a combination of rosiglitazone (3 mg/kgBW/day) and isotretinoin (0.2 mg/kgBW/day). The dose of isotretinoin used here corresponded to a 10-fold lower dose as that shown to be immunosuppressive in our previous experiments.16
Rosiglitazone (Avandia; GlaxoSmithKline, Münich, Germany) and isotretinoin (Roaccutan; Ratiopharm, Ulm, Germany) were administered orally for 8 weeks starting at the day of renal transplantation. None of the recipients were treated with any other immunosuppressant.
In addition, F344 to F344 isograft transplantation was performed and evaluated after 56 days (n = 5).
Graft Functional Parameters and Systolic Arterial Blood Pressure
For measurements of serum creatinine and albuminuria, rats were kept in metabolic cages 24 hours before the end of the experiment. Serum creatinine (enzymatic determination), urea nitrogen, cholesterol, tiglycerides, Glutamix oxalacetic transaminase (GOT), Glutamic pyruvic transaminase (GPT), AP, Lactate dehydrogenase (LDH), and urine (albuminuria) samples were analyzed by using a Hitachi 9-17-E autoanalyzer (Hitachi, Frankfurt/M, Germany).19 Systolic blood pressure was determined before sacrifice by tail cuff plethysmography under light ether anesthesia.20
Histological Analysis
Renal allografts were removed in deep anesthesia, quickly blotted free of blood, weighed, and processed as required for histology, immunohistology, and molecular analysis. For histology, the kidneys were cut into 1-mm coronal slices and either immersion fixed in 4% formaldehyde in PBS (99 mmol/L NaH2PO4, 108 mmol/L, NaH2PO4, and 248 mmol/L NaCl) at ph 7.35 for 24 hours at 4°C or fixed in methacarn (60% methanol, 30% chloroform, and 10% acetic acid) for 8 hours and then embedded in paraffin. In addition, tissue slices were snap frozen in liquid nitrogen and stored at −80°C.
Light microscopy was performed on 3-μm sections stained by PAS. In brief, kidneys were evaluated for evidence of acute and chronic vascular (endothelialitis/vasculitis, fibrointimal thickening), glomerular (glomerulitis, transplant glomerulopathy, glomerulosclerosis), and tubulointerstitial damage (thinning/denudation/necrosis of the tubular epithelia and interstitial edema; tubular atrophy and interstitial fibrosis) on a scale ranging from 0 to 3 as previously described.15,16
Tubulointerstitial inflammation was judged as 0 (no mononuclear cells in the interstitium), 0.5 (focal mononuclear cell infiltration in the interstitium), 1 (focal mononuclear infiltration in the interstitium with tubulitis), 2 (diffuse mononuclear cell infiltration of the interstitium), and 3 (diffuse mononuclear cell infiltration of the interstitium with tubulitis). Tubulitis was defined as 1 or more mononuclear cells/tubular cross section. Tubulointerstitial inflammation index was defined as the percentage of fields with respective degree of the injury encountered in 10 fields (objective ×20) of cortex and outer stripe of outer medulla. The tubulointerstitial inflammation score was calculated as the sum of all specific indices, whereby the index of fields with degree 0.5 was multiplied by 0.5, that of degree 1 × 1, that of degree 2 × 2, and that of degree 3 × 3. Vascular and glomerular injuries were scored in an analogous pattern.
Immunohistochemistry
Immunohistochemical staining was performed on 30-μm sections of paraffin-embedded tissue except for CD4, which was labeled on frozen sections. Mouse anti-rat monoclonal antibodies against ED1 (Serotec, Oxford, UK) in methacarn-fixed tissue, CD4, CD8 (Serotec), and Ki-67 (clone MIP-5, Dianova, Germany) as well as anti-major histocompatibility complex (MHC)-class-II Ia antibody (OX6; Abcam, Cambridge, UK) in formaldehyde-fixed tissue were used. Formaldehyde-fixed tissues were microwave treated. To detect potential subpopulations of fibroblasts, antibodies to vimentin (from guinea pig; Progen Biotechnik, Heidelberg, Germany), desmin (Dako, Glastrup, Denmark), and α- smooth muscle actin (α-SMA; mouse ascites fluid; Sigma, St. Louis, MO) were used. Biglycan and decorin were stained by using LF-113, a rabbit anti-murine decorin antiserum, and MAY-01, a chicken anti-rat biglycan antiserum, as described previously.21
An alkaline phosphatase anti-alkaline phosphatase detection system was applied (Dako Cytomation A/S). For staining of osteopontin (mouse monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA), fibronectin (rabbit anti-human; Dako), collagen I (rabbit anti-rat; Biogenesis, Poole, UK), and collagen III (Chemicon, Temecula, CA) streptavidin-biotin enhanced horseradish peroxidase immunostaining was performed.
Positive glomerular cells were counted in at least 50 glomerular cross sections and given as the mean per glomerular section; interstitial positive cells were counted in 20 high-powered fields (HPFs; ×40) of cortex and outer medulla and recorded as mean per HPF. The intensity of the staining for collagen and fibronectin was evaluated as follows: not detectable (degree 0), faint (degree 1), moderate (degree 2), and intense staining (degree 3). A degree-specific staining index was defined as the percentage of the fields with the degree of staining in 20 HPFs (×400) of cortex and outer medulla. The staining score was calculated as the sum of the degree-specific indices by which degree 1 was multiplied by 1, that of degree 2 × 2, and that of degree 3 × 3.
Real-Time RT-PCR of Kidney Allografts
Total RNA was extracted from the kidney allografts by using the method of Chomczynski and Sacchi22 (n = 4 to 6 animals per group). RNA quality was characterized by using a RNA6000 Nanochip (Agilent Technologies, Waldbronn, Germany). Ten micrograms of total RNA was digested with DNase I according to standard protocol. Three micrograms of total RNA (DNA free) was used for the first-strand cDNA synthesis by using Superscript II reverse transcriptase and oligo d(T)12-18 as primer (LifeTechnologies, Karlsruhe, Germany). Real-time PCR was performed by LightCycler using LightCyler-FastStart DNA MasterSYBR Green I kit (Roche Diagnostics, Mannheim, Germany) as described.15 The primer sequences for target genes are shown in Table 1. Expression levels of the studied genes are given relative to the expression levels of tubulin.
Table 1.
Sequences of Primers Used for Real-Time RT-PCR Analysis of Kidney Allografts
| Gene | Gene ID | Sense | Antisense | Amplicon, bp |
|---|---|---|---|---|
| a-Tubulin | 64158 | 5′-TTTGATCTGATGTATGCCAAGC-3′ | 5′-TCCTTCTTCCTCACCCTCAC-3′ | 162 |
| CD36 | 29184 | 5′-ATTTGTTCTTCCAGCCAACG-3′ | 5′-ATGTCCAGCACACCATACGA-3′ | 114 |
| LXR-α | 58852 | 5′-TGATGCTGAATTTGCTCTGC-3′ | 5′-GGCTCACCAGCTTCATTAGC-3′ | 185 |
| TGF-β1 | 59086 | 5′-GCAACACGTAGAACTCTACC-3′ | 5′-CCCTGTATTCCGTCTCCTTG-3′ | 153 |
| PAI-1 | 24617 | 5′-TTTGTGTTCCAGTCACACTC-3′ | 5′-ATCTGTCTATCTGCTGCCC-3′ | 153 |
| BMP-7 | 85272 | 5′-GAAAACAGCAGCAGTGACCA-3′ | 5′-GGTGGCGTTCATGTAGGAGT-3′ | 165 |
Antigen Presentation by Bone Marrow Derived Dendritic Cells
Bone marrow derived dendritic cells (BMDCs) were cultivated as described in Lutz et al.23 Briefly, on day 0, 4 × 106 nucleated bone marrow cells were plated on uncoated Petri dishes (Greiner Bio-One, Frickenhausen, Germany) in Iscove’s Modified Dulbecco’s Medium with L-Glutamine (PAA, Cölbe, Germany), 10% fetal calf serum (FCS), 1% penicillin/streptomycin, and 50 mmol/L β-mercaptorthanol (all Invitrogen, Karlsruhe, Germany) supplemented with 25 ng/ml granulocyte macrophage colony-stimulating factor (R and D Systems, Wiesbaden, Germany). On day 4 all cells were collected and again 4 × 106 cells were seeded. On day 7 BMDCs were harvested and incubated for an additional 3 hours in pure media or in media supplemented with 100 μmol/L rosiglitazone (Alexis Biochemicals, Grünberg, Germany). Rosiglitazone was dissolved in ethanol. For antigen presentation, 0.5 × 106 BMDCs were first incubated at 37°C in 96-well plates (Greiner Bio-One) in 100-μl media supplemented with endotoxin-free ovalbumin (OVA; Profos, Regensburg, Germany) at 1 mg/ml. After 3 hours, the media was completely removed, and 0.1 × 106 BO17.10 (MHC-II restricted OVA-specific T-cell hybridomas)24 were added in pure media or media supplemented either with 10 μmol/L or 100 μmol/L rosiglitazone. In parallel, also BO17.10 cells pretreated with 100 μmol/L rosiglitazone for 3 hours were applied. After overnight incubation, interleukin-2 concentration was measured in the culture media by using interleukin-2 flex set, FACSCalibur (both BD Biosciences, Heildelberg, Germany), and FCAP array software (Soft Flow, Pécs, Hungary).
Isolation of Peritoneal Macrophages and Generation of Bone Marrow Derived Macrophages
Wild-type and PPARγ−/− (PPARγfl/fl/LysCre) mice were used for the experiments. Peritoneal macrophages were harvested 4 days after injection of thioglycolate and cultured in serum-free RPMI 1640 (Invitrogen) in 6-well plates at a concentration of 1 × 106/well. After 12 hours of incubation at 37C°/5%CO2, the macrophages were thoroughly washed and adherent cells were used for experiments. Purity was controlled by flow cytometry and Giemsa staining.
Generation of murine bone marrow derived macrophages was performed according to standard protocols.25,26 In brief, mouse femurs were dissected and each bone was flushed with 10 ml PBS. A bone marrow cell suspension was collected and centrifuged. Pellets were resuspended in RPMI 1640 medium supplemented by 20% Macrophage colony stimulating factor (MCSF)-containing L929 medium. The cells were plated on non-TC (tissue culture) treated 10-cm Petri dishes and incubated at 37°C/5% CO2. Fresh medium was provided at days 3 and 5, and experiments were performed at day 7. After preincubation with 10 or 100 μmol/L rosiglitazone for 1 hour, macrophages were stimulated with human interleukin-6 (10 ng/ml; R and D Systems) for 6 hours.
In Vitro Conditional PPARγ Deficiency in Peritoneal Macrophages
Conditional deficiency of PPARγ in vitro (PPARγfl/fl/HTNCre) in peritoneal and bone marrow derived macrophages of PPARγfl/fl mice was achieved as described previously.27,28 Briefly, thioglycolate-induced peritoneal macrophages from wild-type and PPARγfl/fl mice were treated immediately after isolation with 1 μmol/L of a membrane-permeable His-TAT-NLS-Cre (HTNCre) recombinase (provided by F. Edenhofer, Bonn) in a low serum (1% FCS) medium and washed extensively after 6 hours. The macrophages were then incubated with 10 μmol/L rosiglitazone 1 hour before the interleukin-6 stimulation.
Northern Blot and RT-PCR Analysis of Macrophages
Total RNA was extracted from peritoneal and bone marrow derived macrophages by using TRIzol (Invitrogen). The effectiveness of Cre-mediated recombination was determined by RT-PCR of PPARγ-transcripts (200 bp; sense: 5′-TGTAATGGAAGGGCAAAAGG-3′; antisense: 3′-TGGCTTCCAGTGCATAAGTT-5′). For northern blots, membranes were hybridized with 32P-labeled cDNA probes for BGN and S18r (GO (gene ontology) number X00686; Applied Biosystems/Ambion, Darmstadt, Germany).21 Northern blots were performed in triplicates and were quantified as described previously.29 Real-time RT-PCR for BGN was performed by LightCycler using LightCyler-FastStart DNA MasterSYBR Green I kit (Roche Diagnostics) as described by kidney allografts.15 The primer sequences for target genes were as follows: BGN: sense: 5′-AACATGAACTGCATTGAGATGG-3′; antisense: 5′- GATCTGATTGTGTCCTAAGCCC-3′. Expression levels of BGN are given relative to the expression levels of tubulin: sense: 5′-TCTCTCACCCTCGCCTTCTA-3′; antisense: 5′-GGGTTCCAGGTCTACGAACA-3′.
Cell Culture of Human Dermal Fibroblasts and Human Dermal Microvascular Endothelial Cells and THP-1 Cell Line
Primary isolated human dermal fibroblasts (HDF) and human dermal microvascular endothelial cells (HDMEC) were maintained in culture as described previously.18,30 The human monocytic cell line THP-1 was cultured in RPMI 1640 medium supplemented with 10% FCS, 1% penicillin/streptomycin, 1% glutamine, and 1% HEPES (Sigma). For macrophage differentiation of THP-1 cells, 160 nmol/L phorbol-12-myristate-13-acetate (Sigma) was added for 72 hours. Rosiglitazone maleate was purchased from Alexis Biochemicals.
Proliferation Assay
HDF, HDMEC, and THP-1 cells were harvested by trypsinization at 37°C and neutralized with trypsin-neutralizing solution. A suspension of 50,000 cells was added to 25-cm2 culture flasks (Becton Dickinson, Heidelberg, Germany) in triplicate. Cells were incubated for 2 hours with different concentrations of rosiglitazone (0.1, −1, and 10 μmol/L) in cytokine-free medium under standard conditions. After another 72 hours of incubation, the cells were trypsinized, resuspended, and counted.
Co-Culture Model for Cell Migration/Invasion
In vitro co-culture transmigration assay using a transwell model (Becton Dickinson) was performed as described previously with minor modifications.18,30 Briefly, matrigel-coated (0.78 mg/ml) transwells with 8-μm pore size (BD Biosciences) were used. HDF or HDMEC were seeded in 24-well plates (bottom wells). Phorbol-12-myristate-13-acetate-induced macrophages (THP-1) or HDF were added to the transwells (upper compartment) and preincubated with or without 1 μmol/L rosiglitazone for 2 hours. The transwells were transferred to 24-well plates. After 18 hours of incubation, THP-1 cells or fibroblasts that had invaded the matrigel and migrated through the 8-μm pores to the bottom of the transwell-membrane were fixed and stained with Diff-Quick II solution (Dade Behring, Marburg, Germany), sealed on slides, and counted by microscopy (number of migrated cells per 10 optical fields at 40× objective and 10× oculars). Experiments were performed in triplicates to quadruplets.
Statistical Analysis
All data were presented as mean ± SEM. Data were analyzed by the nonparametric Mann-Whitney U test or unpaired t-test as appropriate. A P value of less than 0.05 was considered to show a significant difference between two groups.
Results
The in vivo renal activity of rosiglitazone was corroborated by increased expression of specific target genes of PPARγ: CD36, Liver X receptor (LXRα) in kidney allografts31,32 (Table 2).
Table 2.
Effects of Rosiglitazone (Low Dose) on Intragraft Gene 56 Days After Transplantation
| Gene | Controls, 56 days | LD-Rosi, 56 days |
|---|---|---|
| CD36 | 0.42 ± 0.64 × 10−3 | 1.97 ± 3.29 × 10−3* |
| LXRα | 0.38 ± 0.64 × 10−3 | 2.63 ± 1.98 × 10−3* |
| TGF-β1 | 1.75 ± 0.002 × 10−2 | 1.00 ± 0.001 × 10−2† |
| PAI-1 | 0.17 ± 0.07 | 0.08 ± 0.02 |
| BMP-7 | 0.07 ± 0.01 | 0.14 ± 0.01† |
n = 5 to 6 animals per group. Expression levels of genes are given relative to the expression levels of tubulin.
P < 0.01 versus controls.
P < 0.05 versus controls.
As indirect parameters of kidney allograft function, allograft weights of animals with PPARγ activation were higher in comparison with untreated controls. The higher liver weights associated with ascites in the control group reflected the uraemic state of these animals. Treatment for 8 weeks with HD-Rosi led to a significant increase of the relative heart weights corresponding to a reported myocardial effect of thiazolidinediones.33 In addition, an increase in serum triglyceride levels and GPT activity was seen in some rats with HD-Rosi administration, but these phenomena were not consistent (triglyceride: 53.6 ± 2.9 vs. 58.11 ± 3.0 mg/ml; GPT: 12.20 ± 2.2 vs. 16.9 ± 2.5 U/L in controls versus HD-Rosi). No effects on serum GOT and AP activity were observed (data not shown). In all experimental groups, systolic blood pressure remained in the normal range (Table 3).
Table 3.
Body Weight, Relative Organ Weights, and Systolic Blood Pressure in the Experimental Groups 56 Days After Transplantation
| Groups | n | Body weight, g | Relative organ weights, g/100 g body weight
|
Systolic arterial pressure, mmHg | ||
|---|---|---|---|---|---|---|
| Allograft kidney | Heart | Liver | ||||
| Controls | 20 | 321.2 ± 5.2 | 0.23 ± 0.02 | 0.33 ± 0.01 | 2.65 ± 0.05 | 95 ± 2.8 |
| HD-Rosi | 9 | 305.8 ± 3.3 | 0.33 ± 0.02† | 0.38 ± 0.01† | 2.47 ± 0.05* | 93 ± 2.7 |
| LD-Rosi | 8 | 317.9 ± 6.5 | 0.26 ± 0.02 | 0.34 ± 0.01 | 2.26 ± 0.10† | 92 ± 2.6 |
| Iso | 8 | 298.2 ± 7.6* | 0.26 ± 0.02 | 0.33 ± 0.01 | 2.35 ± 0.06† | 87 ± 2.1 |
| LD-Rosi + Iso | 8 | 297.6 ± 7.3* | 0.27 ± 0.01 | 0.32 ± 0.01 | 2.33 ± 0.10* | 89 ± 3.0 |
n = number of animals per group.
P < 0.05 versus controls.
P < 0.01 versus controls.
Effects of Rosiglitazone on Graft Morphology/Function, Intragraft Immune Cell Infiltration and Proliferation
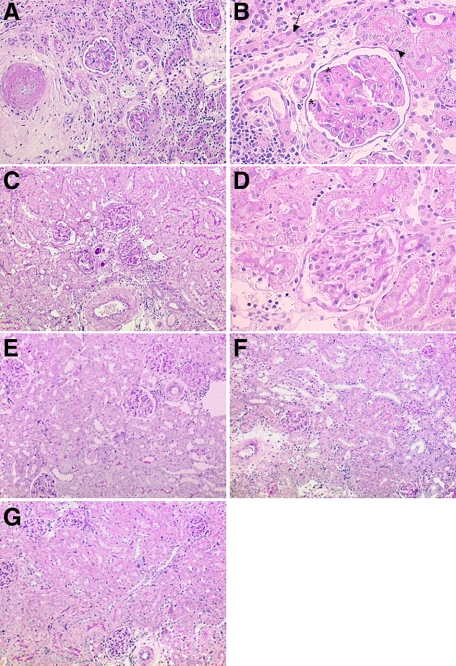
As shown in Supplemental Table 1 (http://ajp.amjpathol.org) at day 56 after transplantation, F344 to F344 kidney isografts did not show relevant histopathological lesions. By contrast, at day 56, post-transplant preglomerular arteries of untreated kidney allografts showed a severe obliteration with subendothelial fibrosis and a transmural infiltration of mononuclear cells. In glomeruli mesangial matrix increase and segmental thickening of basement membranes and/or segmental glomerulosclerosis were present (Figure 1, A and B). Vascular and glomerular changes as well as interstitial inflammation, fibrosis, and tubular atrophy were strikingly lower in rats treated with either low or high doses of rosiglitazone (Table 4; Figure 1, C–G). The inner stripe of the outer medulla and inner medulla of kidney allografts presented a slight to moderate cellular infiltrate without differences between the groups (Supplemental Figure 1, see http://ajp.amjpathol.org).
Figure 1.
Light microscopy of renal allografts 56 days after transplantation. A and B: An untreated renal allograft showing a preglomerular artery with significant obliteration by subendothelial matrix increase, glomeruli with mesangial matrix increase, and segmental extensive broadening of peripheral basement membrane (asterisk in B), surrounding tubulointerstitium with increased interstitial mononuclear cell infiltrate and matrix, surrounding collapsed atrophic tubules (arrow), which are focally infiltrated by mononuclear cells (arrowhead). C–G: Representative micrographs of treated allografts: low dose rosiglitazone (C and D), high dose rosiglitazone (E), isotretinoin (F), and low dose rosiglitazone and isotretinoin (G). The micrographs present preglomerular arteries with only few mononuclear cells sticking to endothelium without significant narrowing of lumen; glomeruli with slightly increased mesangial matrix and few mononuclear cells in the capillary lumen, surrounding tubulointerstitium with focal sparse mononuclear cell infiltrate; the majority of tubules are differentiated. Almost regular parenchyma after a combination of PPARγ and RXR ligand at low concentration (G; A–G: PAS). Original magnification: ×100 (A, C, E, F, G); ×400 (B and D).
Table 4.
Graft Morphology in Experimental Groups 56 Days After Transplantation
| Groups | Transplant glomerulopathy score | Glomerulosclerosis score | Vascular rejection score | Chronic vascular damage score | Tubulointerstitial inflammation score | Chronic tubulointerstitial damage score |
|---|---|---|---|---|---|---|
| Controls | 99.7 ± 18.4 | 91.5 ± 17.8 | 90.0 ± 10.10 | 35.0 ± 5.8 | 211.9 ± 25.40 | 13.6 ± 1.8 |
| HD-Rosi | 87.9 ± 24.3 | 7.6 ± 2.3* | 45.9 ± 6.4* | 2.8 ± 1.5* | 68.3 ± 15.2* | 2.2 ± 1.0* |
| LD-Rosi | 25.2 ± 5.6*† | 19.4 ± 4.6‡ | 34.1 ± 5.5* | 8.1 ± 2.4‡ | 72.8 ± 16.1* | 2.6 ± 0.4*† |
| Iso | 44.3 ± 7.4§ | 27.1 ± 5.7†‡ | 37.0 ± 2.9*† | 5.1 ± 1.6* | 83.9 ± 11.5‡§ | 2.7 ± 0.3†‡ |
| LD-Rosi + Iso | 7.8 ± 3.1¶ | 10.9 ± 4.1* | 23.8 ± 5.5¶ | 5.5 ± 2.5* | 34.4 ± 8.7¶ | 1.3 ± 0.3¶ |
Number of animals correspond to those in Table 3. Scoring system is described in Materials and Methods.
P < 0.01 versus controls.
P < 0.05 versus combination therapy.
P < 0.05 versus controls.
P < 0.01 versus combination therapy.
P < 0.001 versus controls.
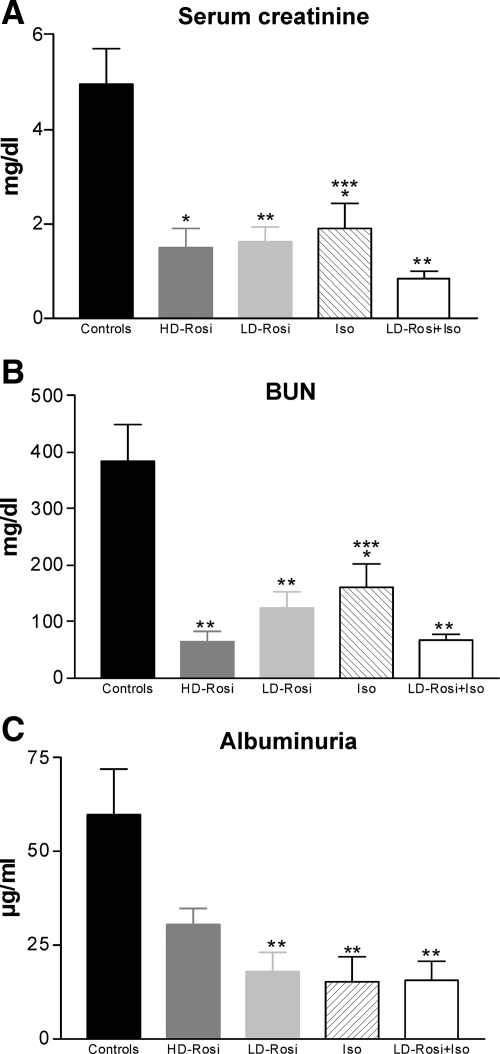
Corresponding to the improvement of morphological changes, excretory renal function increased in grafts with PPARγ activation or simultaneous activity of PPARγ and RXR: serum creatinine, blood urea nitrogen (BUN), and albuminuria were significantly lower in rosiglitazone animals than in untreated transplanted rats (approximately threefold lower in LD-Rosi and almost fourfold to fivefold lower in LD-Rosi + Iso groups in comparison with controls; Figure 2, A–C).
Figure 2.
Excretory function of kidney allografts in experimental groups 56 days after transplantation (bilaterally nephrectomized rats). Serum creatinine (A), BUN (B), and albuminuria (C) were significantly lower in treated groups in comparison with controls; combination therapy of LD-Rosi with Iso showed additive effects in improving graft function (A and B). n = 7 to 9 animals per group. Values are given as mean ± SEM. *P < 0.05; **P < 0.01 vs. control; ***P < 0.05 vs. combination therapy.
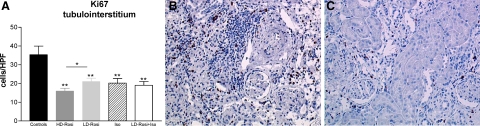
Glomerular and tubulointerstitial mononuclear cell infiltration consisting of ED1 positive macrophages, CD8, and CD4 T lymphocytes was significantly inhibited by PPARγ activation (Δ60% for ED1 and CD4 cells and Δ50% for CD8 T cells in the LD-Rosi group in comparison with controls; Table 5). In addition, immunohistochemistry depicted a reduced number of interstitial MHC-II+ cells, supposedly of recipient origin, as the antibody recognizes Ia antigens of Lewis rats (Table 5). Cell proliferation of interstitial cells, as determined by Ki-67 immunohistochemistry, was reduced by 35% to 50% in the different treatment groups; the antiproliferative effect was more evident in the HD-Rosi group (Figure 3, A–C).
Table 5.
Mononuclear Cell Infiltration in the Interstitial Area of Kidney Allografts in Experimental Groups 56 Days After Transplantation
| Groups | ED1+ | CD8+ | CD4+ | MHC-II+ |
|---|---|---|---|---|
| Controls | 85.1 ± 10.1 | 39.6 ± 9.4 | 65.2 ± 10.3 | 15.9 ± 1.1 |
| HD-Rosi | 47.8 ± 5.8*† | 23.3 ± 3.8‡ | 23.7 ± 1.9‡ | 7.5 ± 1.5* |
| LD-Rosi | 32.8 ± 2.1†§ | 19.9 ± 2.4‡ | 26.5 ± 0.4* | 9.2 ± 1.2* |
| Iso | 27.9 ± 2.1§ | 16.9 ± 1.4* | 24.6 ± 2.7* | 7.3 ± 1.1* |
| LD-Rosi + Iso | 24.4 ± 2.4§ | 15.5 ± 1.0* | 18.1 ± 2.1‡ | 6.5 ± 0.9* |
Number of animals correspond to those in Table 3. Interstitial positive cells were counted in 20 HPFs (×40) of cortex and outer medulla and recorded as mean ± SEM per HPF.
P < 0.01 versus controls.
P < 0.05 versus combination therapy.
P < 0.05 versus controls.
P < 0.001 versus controls.
Figure 3.
Effects of rosiglitazone on cell proliferation in vivo (A–C). The graph presents the number of Ki-67+ cells in the tubulointerstitium in all experimental groups. B: Micrograph presenting high number of proliferating cells in the tubulointerstitium, in glomeruli, and in neointima of a preglomerular artery in a control kidney allograft. Significantly fewer proliferating cells in a LD-Rosi treated transplanted kidney (C; n = 7 to 9 animals per group). A: Values presented as mean ± SEM. **P < 0.01 vs. control; *P < 0.05 vs. HD-Rosi. B and C: Alkaline phosphatase and anti-alkaline phosphatase (APAAP); original magnification, ×200.
Effects of Rosiglitazone on Fibroblasts and Extracellular Matrix Proteins in Allografts
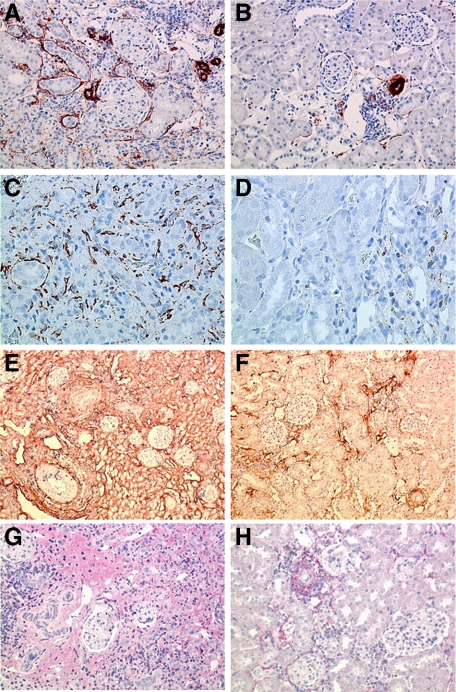
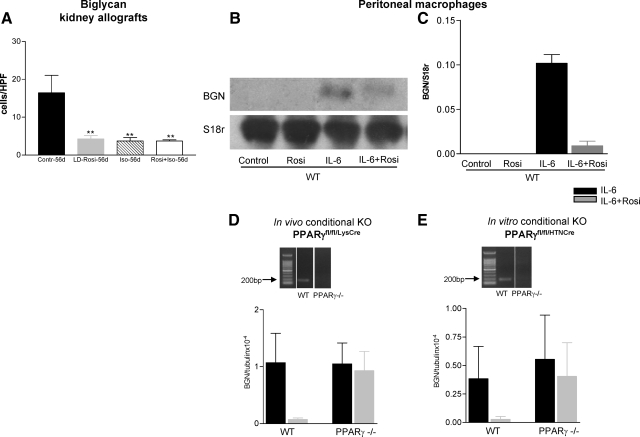
Stainings for desmin and α-SMA were performed to study fibroblasts/myofibroblasts in kidney allografts 56 after transplantation. Considerable numbers of desmin+ and α-SMA+ cells were found in the untreated kidney allografts. All these fibroblast populations were significantly decreased in the rosiglitazone treated groups (α-SMA: Δ50% by HD-Rosi, Δ70% by LD-Rosi and Iso, and Δ85% by combined administration; Table 6; Figure 4, A–D). The extracellular matrix components collagen I, III, and fibronectin were diffusely positive in the interstitium of untreated kidney allografts; they were reduced twofold to threefold by rosiglitazone (Table 6; Figure 4, E and F). In addition to collagens and fibronectin, proteoglycans are major components of the extracellular matrix. Reports have shown that changes in their expression may play a crucial role in the pathogenesis of renal diseases.34 Immunostainings for the SLRP BGN and decorin were performed. The extent of decorin deposition correlated to the distribution pattern of collagen I and III and was significantly decreased in rosiglitazone treated grafts (Table 6; Figure 4, G and H). In untreated kidney allografts, a high number of BGN positive cells were present in the interstitium as well as in the neointima of preglomerular arteries with transplant vasculpathy. BGN could also be detected on endothelial cells, distal tubular cells, and some collecting ducts. Rosiglitazone led to a significant reduction of BGN expression in treated allografts (Figure 5A).
Table 6.
Fibroblast Markers and Extracellular Matrix Proteins in the Tubulointerstitium of Kidney Grafts 56 Days After Transplantation
| Groups | Vimentin, cells/HPF | α-SMA, cells/HPF | Collagen I, score | Collagen III, score | Fibronectin, score | Decorin, score |
|---|---|---|---|---|---|---|
| Controls | 64.2 ± 7.8 | 36.4 ± 5.4 | 214.1 ± 7.2 | 217.5 ± 19.9 | 221.1 ± 16.6 | 193.0 ± 9.3 |
| HD-Rosi | 38.0 ± 4.6* | 18.9 ± 4.1* | 68.6 ± 5.9† | 77.2 ± 17.5† | 78.1 ± 12.8† | ND |
| LD-Rosi | 29.4 ± 2.7† | 9.3 ± 1.8† | 78.1 ± 10.7† | 70.0 ± 11.3†‡ | 79.4 ± 11.4†§ | 82.0 ± 15.9¶ |
| Iso | 26.5 ± 0.9† | 9.6 ± 1.6† | 60.0 ± 8.2† | 62.1 ± 6.2†∥ | 68.1 ± 11.1†§ | 75.0 ± 8.5¶ |
| LD-Rosi + Iso | ND | 5.5 ± 0.7† | 34.4 ± 5.3† | 35.0 ± 2.8† | 14.3 ± 2.5† | 65.0 ± 2.5¶ |
Number of animals correspond to those in Table 3. ND, not determined.
P < 0.05 versus controls.
P < 0.001 versus controls.
P < 0.01 versus combination therapy.
P < 0.001 versus combination therapy.
P < 0.01 versus controls.
P < 0.05 versus combination therapy.
Figure 4.
Effects of rosiglitazone on fibroblasts (A–D) and extracellular matrix proteins (E–H) in kidney allografts 56 days after transplantation. The micrographs show the high number of α-SMA+ (A) and desmin+ (C) activated fibroblasts in an untreated kidney allograft; considerably fewer α-SMA+ and desmin+ fibroblasts can be seen in the LD-Rosi treated graft (B and D). Diffuse deposition of collagen I (E) and decorin (G) in the tubulointerstitium of control renal allografts 56 days after transplantation is shown. By LD-Rosi treatment, a significant reduction of tubulointerstitial collagen I (F) and decorin (H) occurred. A–G: avidin-biotin complex. Original magnification: ×200 (A and B; G and H); ×400 (C and D); ×100 (E and F).
Figure 5.
Effects of rosiglitazone on BGN expression. A: The graph presents the number of BGN+ cells in the interstitium of kidney allografts, which are significantly fewer by PPARγ and RAR/RXR activation (mean ± SEM; **P < 0.01 vs. control). B: Northern blot for BGN expression in mouse peritoneal macrophages stimulated for 6 hours with interleukin-6 (10 ng/ml) with and without preincubation with rosiglitazone (100 μmol/L) for 1 hour (one representative figure from three experiments). C: Quantification of the Northern blots. Values represent mean ± SEM for three different experiments. Interleukin-6 stimulation for 6 hours resulted in a high expression of BGN mRNA in activated macrophages. Preincubation of the cells with rosiglitazone (100 μmol/L) for 1 hour led to an almost total inhibition of BGN expression. D and E: Graphs presenting the effects of rosiglitazone on interleukin-6 (10 ng/ml) induced BGN expression in peritoneal in vivo (D) and in vitro (E) generated PPARγ-deficient macrophages in comparison with wild-type macrophages as determined by real time RT-PCR. Rosiglitazone (10 μmol/L) mediated suppression of interleukin-6-induced BGN mRNA expression was mainly abrogated in peritoneal macrophages deficient in PPARγ. Values represent mean ± SEM for two different experiments in duplicates.
Effects of Rosiglitazone on Cytokines/Growth Factors Related to Inflammation and Fibrosis in Kidney Allografts and Macrophages
Reduced expression of osteopontin, a cytokine with potent migratory and proliferative effects,35 could be seen in kidney allografts with PPARγ activation (Supplemental Figure 2, see http://ajp.amjpathol.org). Renal cortical mRNA expression of the fibrogenic cytokine TGF-β1 and plasmonigen-activator inhibitor (PAI)-1 was suppressed by LD-rosiglitazone treatment (Table 2). By contrast, the expression of BMP-7 mRNA, an endogenous growth factor, which may inhibit TGF-β1-driven fibrogenesis, primarily by preventing the TGF-β1-dependent down-regulation of matrix degradation and up-regulation of PAI-1,36 was significantly higher in LD-Rosi treated kidney grafts (Table 2).
BGN constitutes a molecule with both inflammatory and fibrotic features. It was taken as an exemplary molecule to show the intricate relation of inflammation and fibrosis. To examine a direct role of rosiglitazone in BGN synthesis, mouse peritoneal macrophages were stimulated with interleukin-6 (10 nmol/L) for 6 hours. This resulted in a high expression of BGN mRNA (Northern blot) in activated macrophages. Preincubation of the cells with rosiglitazone (100 μmol/L) for 1 hour led to an almost total inhibition of BGN expression as shown in Figure 5, B and C. Similar results were obtained by using bone marrow derived macrophages (data not shown). To analyze whether the inhibitory effects of rosiglitazone on BGN in macrophages are dependent on the nuclear receptor PPARγ, in vivo and in vitro generated PPARγ deficient peritoneal macrophages (see methods) were used. Rosiglitazone (10 μmol/L) mediated suppression of interleukin-6-induced BGN mRNA expression was mainly abrogated in macrophages deficient in PPARγ (Figure 5, D and E).
Effects of Rosiglitazone on Antigen Presentation
The ability of rosiglitazone to interfere with antigen presentation and cross presentation was tested by using OVA loaded BMDCs and OVA-specific MHC-II restricted T-cell hybridomas. Rosiglitazone present during the co-culture of OVA-loaded BMDCs and OVA-specific T-cell hybridomas dampened (10 μmol/L) or abolished (100 μmol/L) the process of antigen presentation (Supplemental Figure 3, see http://ajp.amjpathol.org). A 3-hour pretreatment of BMDCs with 100 μmol/L rosiglitazone was also able to abolish the antigen presentation. Similarly, T-cell hybridomas pretreated for 3 hours with 100 μmol/L rosiglitazone showed significantly diminished response to antigen (Supplemental Figure 3, see http://ajp.amjpathol.org).
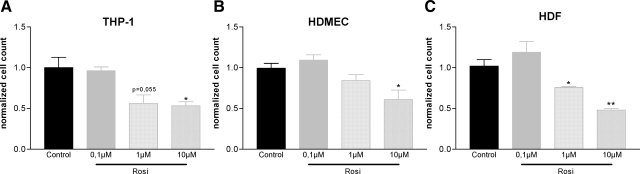
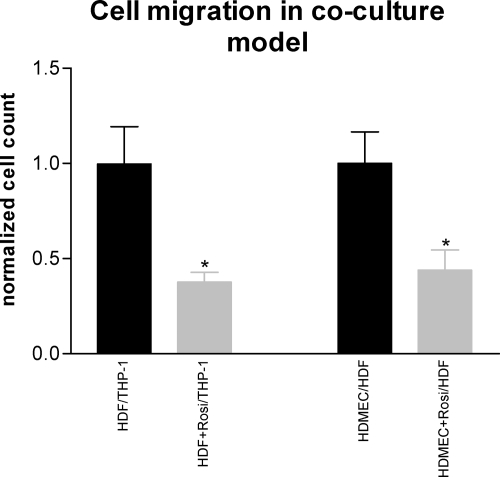
Effects of Rosiglitazone on Cell Proliferation and Transmigration of Endothelial Cells, Macrophages and Fibroblasts in Co-Culture Experiments
Cell migration and proliferation are important processes that contribute to the amplification of the inflammatory response and destruction of the transplanted organs. In in vitro proliferation assays, rosiglitazone reduced the number of proliferating fibroblasts, microvascular endothelial cells and THP-1-cells in a concentration dependent manner (Figure 6, A–C). Due to the close intercellular interaction of monocytes/macrophages, fibroblasts, and peritubular capillary endothelial cells in the interstitium of kidney grafts,37 we performed co-culture experiments to study the effects of rosiglitazone (1 μmol/L) on migration of these cells. Endothelial cell-induced migration of fibroblasts and fibroblast-induced migration of macrophages were investigated. Rosiglitazone markedly reduced the transmigration of both cell types through Matrigel—a model of extracellular matrix—by 50% (Figure 7).
Figure 6.
Effects of rosiglitazone on cell proliferation in vitro. In proliferation assays treatment with rosiglitazone reduced the number of proliferating THP-1 cells (A), microvascular endothelial cells (HDMEC; B), and fibroblasts (HDF; C) in a concentration dependent manner. Values represent mean ± SEM for one experiment in triplicates. *P < 0.05; **P < 0.01 vs. control.
Figure 7.
Transmigration in co-culture transwell assay. Microvascular endothelial cell (HDMEC)-induced migration of fibroblasts (HDF) and fibroblast-induced migration of macrophages (THP-1) through Matrigel (taken as model of extracellular matrix) was markedly inhibited by rosiglitazone (1 μmol/L). Values represent mean ± SEM for one experiment in triplicates or quadruplets. *P < 0.05 vs. control.
Combination of the PPARγ Ligand Rosiglitazone with the RAR/RXR-Ligand Isotretinoin Led to Additive Antifibrotic Effects in Kidney Allografts
As RXR forms a permissive heterodimeric complex with PPARγ and RAR, and as the activation of PPARγ as well as RAR/RXR exerts anti-inflammatory and antifibrotic effects, it seemed reasonable to expect additive/synergistic effects of a combination of PPARγ and RAR/RXR agonists in reducing allograft rejection. The combination of low dose rosiglitazone with isotretinoin (0.2 mg/kgBW/day, corresponding to doses 10 times lower as used in earlier kidney transplantation experiments)15 led to a strong inhibition of inflammatory and fibrotic changes in the kidney allografts when compared with sole administration of rosiglitazone at 3 mg/kgBW/day or isotretinoin at 0.2 mg/kgBW/day (Tables 4–6; Figure 2).
Discussion
The prevention of chronic vascular and tubulointerstitial damage associated with chronic rejection is a central issue in kidney transplantation because approximately 6% of renal allografts are lost to these processes each year after the first year of transplantation.38,39 Chronic allograft dysfunction is characterized by hyalinosis and fibrosis of preglomerular vessels, transplant glomerulopathy/glomerulosclerosis, interstitial fibrosis with a variable degree of mononuclear cell infiltrate, tubulitis, and tubular atrophy.
In this study we could show that in a rat model of chronic allograft dysfunction administration of the PPARγ-agonist, rosiglitazone, in the absence of additional immunosuppression, inhibited the development of renal graft atrophy and fibrosis; graft function was preserved.
Rosiglitazone was applied at two different doses; low dose (3 mg/kgBW/day) was as efficient as the high dose (30 mg/kgBW/day) in reducing chronic rejection phenomena, and at this dosage no adverse effects such as cardiac hypertrophy were observed. Because all animals had systolic arterial blood pressure values in the normal range, the renoprotective effects of rosiglitazone were probably not due to systemic hemodynamic changes.
Reduced renal injury and neutrophil infiltration in a model of bilateral clamping of the renal pedicles was found with PPARγ-agonists.40 However, the lack of evident chronic changes in isografts 56 days after transplantation suggest that the antifibrotic effects of rosiglitazone in our transplantation model were not mediated by a reduction of ischemia-reperfusion injury.
The dominant action of rosiglitazone appeared to be its immunosuppressive and antifibrotic effects. Antigen presentation is one of the primary and critical processes in rejection. By immunohistochemistry we detected significantly fewer MHC-II+ cells in the interstitium of rosiglitazone treated kidney allografts; rosiglitazone may have blocked MHC-II-mediated T-lymphocyte activation and proliferation. We tested the ability of rosiglitazone to interfere with antigen presentation by using OVA loaded BMDCs and OVA-specific MHC-II restricted T-cell hybridomas. Rosiglitazone inhibited antigen presentation in a concentration dependent manner. This is in concordance with earlier studies showing that PPARγ is a negative regulator of dendritic cell maturation and function, resulting in inhibition of naïve T cells to undergo differentiation and subsequent clonal expansion.41,42 PPARγ has been shown to specifically inhibit the promoter IV of the class II transactivator gene—an obligatory mediator of interferon-γ induced MHC-II expression.43 The effect though could also be seen with singular PPARγ activation in dendritic cells or T cells indicating a direct effect of PPARγ on T cell activation. CD4+ and CD8+ T cells were significantly reduced in rosiglitazone treated kidney allografts. It has also been reported that treatment of endothelial cells with PPARγ-activators significantly inhibited interferon-γ-induced expression of CXCL10/IP-10, CXCL9/Mig, and CXCL11/I-TAC chemokines known to regulate lymphocyte trafficking.44
As prominent monocyte and macrophage infiltration of transplanted kidneys typically precedes and accompanies fibrosis, these innate immunity cells seemed to constitute an important factor in the pathophysiology of chronic graft deterioration. A 50% to 60% reduction in the number of infiltrating monocytes/macrophages (ED1+) in the tubulointerstitium was seen after rosiglitazone treatment.
The reduced inflammatory cell recruitment in the allografts may explain in part the reduced kidney fibrosis. However, a significant level of infiltrating mononuclear cells was still present in the treated allografts; therefore, the inflammatory or fibrogenic activity of these remaining mononuclear cells might also be influenced by rosiglitazone. Osteopontin is a secreted phosphoprotein that is involved in diverse biological functions, including mononuclear cell adhesion, migration, and signaling, and has been shown to be directly regulated by PPARγ.35,45 Its expression has been shown to be increased during renal allograft rejection as well as neointima formation in arteries.46,47 By rosiglitazone administration, a significant decrease of osteopontin-expressing cells was found in kidney allografts. Other inflammatory mediators involved in the development of chronic allograft dysfunction (eg, interferon-γ and tumor necrosis factor-α) have been demonstrated to be inhibited by PPARγ in different cell types including monocytes/macrophages and T cells.48,49,50
Monocyte/macrophages and activated fibroblasts are thought to be key mediators of fibrosis by providing profibrotic factors and extracellular matrix proteins.51 Fibroblasts are activated by a variety of mechanisms, including paracrine signals derived from lymphocytes and macrophages and autocrine factors secreted by myofibroblasts, particularly TGF-β1. The close proximity of monocytes/macrophages and fibroblasts to peritubular capillary endothelial cells in the interstitium of kidney grafts37 prompted us to perform co-culture experiments, to mimic the in vivo situation, and to study potential intercellular interaction induced by PPARγ-activation. We could show that fibroblast induced migration of macrophages and migration of fibroblasts induced by endothelial cells were efficiently inhibited by rosiglitazone.
Gene expression analysis revealed a reduced mRNA expression of TGF-β1 and PAI-1 in rosiglitazone kidney allografts, which partly corresponded to the lower number of fibroblasts/myofibroblast partly to a PPARγ-dependent silencing of these cells. Both natural and synthetic agonists of PPARγ have been shown to inhibit the stimulation of collagen and fibronectin synthesis and myofibroblast differentiation induced by TGF-β1 in vitro.52,53,54 The proposed mechanisms are antagonistic cross talk of PPARγ with the intracellular TGF-β/Smad signal transduction pathway and inhibition of TGF-β1 induced AP-1 binding activity.53,55 We have now detected an increased expression of BMP-7 in rosiglitazone treated allografts. BMP-7 is a member of the TGF-β superfamily, which can inhibit TGF-β1-driven fibrogenesis, primarily by preventing the TGF-β1-dependent down-regulation of matrix degradation and up-regulation of PAI-1.36 BMP-7 is expressed primarily in tubular epithelial cells and its expression has been shown to be diminished in experimental models of kidney injury.56 Its increased tubular expression in rosiglitazone allografts when compared with controls may have contributed to the well-preserved renal morphology in these grafts.
Extracellular matrix proteins including collagen I and III, fibronectin, and the SLRP decorin were impressively reduced by PPARγ activation, underlining the antifibrotic activity of the PPARγ-ligand rosiglitazone in chronically rejecting allografts. Reports have shown that changes in the SLRPs may play an important role in the pathogenesis of renal diseases. Decorin staining was found to be significantly enhanced in patients with chronic renal disease and was found to be a robust predictor both of the development of renal failure.34 In unilateral ureteral obstruction, the development of tubulointerstitial fibrosis and increased expression of TGF-β1 were associated with the infiltration of BGN-expressing macrophages.57 BGN was increased also in transplant coronary arteriopathy.58 These SLRPs are not only structural components of the extracellular matrix, but have multiple functions, including the control of extracellular matrix assembly through interactions with collagen proteins, the activation and inactivation of growth factors as well as direct effects on innate immunity cell surface receptors and adhesion molecules.4,5 Recently we have shown that BGN can signal through the innate immunity receptors TLR4 and 2 and activate the NLRP3 inflammasome and interleukin-β1 secretion. BGN is thus thought to amplify and propagate both inflammation and fibrosis in the context of chronic inflammation.6,7
A high number of BGN-expressing mononuclear cells could be seen in the interstitium of control kidney allografts 56 days after transplantation. In addition, BGN was expressed at the apical surface of distal tubular epithelial cells and in the neointima of preglomerular arteries with obliterative changes. Because T cells do not synthesize BGN, macrophages, which can be activated by cytokines such as interleukin-1β and interleukin-6 to produce BGN, are the likely source of BGN in addition to myofibroblasts in the interstitium.57 These results clearly hint at the intimate relation, at the cellular and molecular level, of immunological and fibrogenic processes. To study if these effects are a result of direct PPARγ-activation and not only secondary to the reduced number of interstitial cell infiltrate, interleukin-6 stimulated peritoneal macrophages were preincubated with rosiglitazone, leading to a strong inhibition of BGN mRNA expression. Using PPARγ-deficient peritoneal macrophages achieved in vivo by crossing homozygous floxed PPARγ mice with transgenic mice containing the Cre recombinase gene under the control of the murine M lysozyme promoter or generated in vitro by Cre recombinase (HTNCre) mediated ablation of the PPARγ gene, we have now demonstrated that the effects of rosiglitazone on BGN expression were mainly dependent on PPARγ. Interleukin-6 induced BGN mRNA expression was only minimally reduced in PPARγ-deficient macrophages in comparison with wild-type mice by rosiglitazone.
The development of chronic allograft dysfunction involves an increased proliferative activity of infiltrated mononuclear cells and resident interstitial cells. Thiazolidinediones have been shown to block proliferation in some tumor models, but also to inhibit the growth of vascular smooth muscle cells and mesangial cells.33,59 Several mechanisms have been proposed for the antiproliferative actions of PPARγ ligands, including decreased cyclin D1 expression (by inhibiting cyclin D1 transcription as well as enhancing cyclin D1 protein degradation) and up-regulation of CDK inhibitors p21 and p27.60 In congruence with those data, rosiglitazone significantly inhibited in vitro the proliferation of monocytes, microvascular endothelial cells, and fibroblasts and lowered the number of Ki-67+ cells in the allografts.
In vitro and in vivo findings suggest that different nuclear receptors can function in a combinatorial manner to coordinately regulate immune responses, simultaneously targeting inflammatory genes.61 In former experiments we found that isotretinoin (13-cis-retinoic acid), which acts through RAR/RXR heterodimers, had strong and complex immunosuppressive effects in renal allograft rejection.15,16 Beneficial effects for the combined treatment with PPAR ligands plus retinoids have been reported in preclinical studies of hematological malignancies.62 Here we have now found that the combination of rosiglitazone at low dose and isotretinoin at a dosage 10 times lower as used in our previous experiments was associated with increased anti-inflammatory and antifibrotic effects. These data may have clinical relevance, as in many cases, the ability to achieve desirable therapeutic effects with a natural or synthetic nuclear receptor agonist is limited by dose related adverse effects.
In summary, our data have shown that PPARγ activation successfully prevents the development of chronic dysfunction in experimental kidney allografts. The antiproliferative, antimigratory, and anti-inflammatory/antifibrotic actions resulted in improved graft function. In this context a direct effect of rosiglitazone on matrix synthesis and secretion including inflammatory/fibrogenic SLRPs might be important. The current clinical application of thiazolidinediones to treat type 2 diabetes and their ability to counteract the effects of steroids on glucose homeostasis63 together with the beneficial actions on experimental allograft rejection may lead to an assessment of PPARγ ligands as add-on compounds in solid organ transplantation.
Acknowledgments
We gratefully acknowledge the advice of Stefan Herzig (DKFZ) for the macrophage experiments.
Footnotes
Address reprint requests to Hermann-Josef Gröne, M.D., Department of Cellular and Molecular Pathology, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany. E-mail: h.-j.groene@dkfz.de.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 405, B10; GR 880/3; SCHA 1082/2-1), European Union grant INNOCHEM to H.-J.G., National Research Program Tumor–Vessel Interface grant SPP1190, and National Aeronautics and Space Administration Specialized Center of Research grant NNJ04HJ12G to A.A.
E.K. and Z.V.P. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Yates PJ, Nicholson ML. The aetiology and pathogenesis of chronic allograft nephropathy. Transpl Immunol. 2006;16:148–157. doi: 10.1016/j.trim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Joosten SA, Sijpkens YW, van Kooten C, Paul LC. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 2005;68:1–13. doi: 10.1111/j.1523-1755.2005.00376.x. [DOI] [PubMed] [Google Scholar]
- Kresse H, Schönherr E. Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189:266–274. doi: 10.1002/jcp.10030. [DOI] [PubMed] [Google Scholar]
- Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte M, Malle E, Schaefer RM, Gröne HJ. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne HJ, Schaefer L. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284:24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Sarnstrand B, Xing Z, Graham F, Fisher L, Gauldie J. Adenovirus-mediated gene transfer of the proteoglycan biglycan induces fibroblastic responses in the lung. Chest. 1997;111(Suppl 6):137S. doi: 10.1378/chest.111.6_supplement.137s. [DOI] [PubMed] [Google Scholar]
- Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr Opin Genet Dev. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab. 2006;17:321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- Adams J, Kiss E, Arroyo AB, Bonrouhi M, Sun Q, Li Z, Gretz N, Schnitger A, Zouboulis CC, Wiesel M, Wagner J, Nelson PJ, Gröne HJ. 13-cis retinoic acid inhibits development and induces regression of chronic allograft nephropathy. Am J Pathol. 2005;167:285–298. doi: 10.1016/S0002-9440(10)62973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E, Adams J, Grone HJ, Wagner J. Isotretinoin ameliorates renal damage in experimental acute renal allograft rejection. Transplantation. 2003;76:480–489. doi: 10.1097/01.TP.0000066354.31050.5A. [DOI] [PubMed] [Google Scholar]
- Malur A, Mccoy AJ, Arce S, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J Immunol. 2009;182:5816–5822. doi: 10.4049/jimmunol.0803504. [DOI] [PubMed] [Google Scholar]
- Bedke J, Kiss E, Schaefer L, Behnes CL, Bonrouhi M, Gretz N, Horuk R, Diedrichs-Moehring M, Wildner G, Nelson PJ, Gröne HJ. Beneficial effects of CCR1 blockade on the progression of chronic renal allograft damage. Am J Transplant. 2007;7:527–537. doi: 10.1111/j.1600-6143.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gretz N, Schmidt R, Kloetzer HM, Groene HJ, Lelongt B, Meyer M, Sadick M, Pill J. Plasma creatinine determination in mice and rats: an enzymatic method compares favorably with a high-performance liquid chromatography assay. Kidney Int. 2007;71:74–78. doi: 10.1038/sj.ki.5001988. [DOI] [PubMed] [Google Scholar]
- Grone HJ, Helmchen U. Impairment and recovery of the clipped kidney in two kidney, one clip hypertensive rats during and after antihypertensive therapy. Lab Invest. 1986;54:645–655. [PubMed] [Google Scholar]
- Schaefer L, Hausser H, Altenburger M, Ugorcakova J, August C, Fisher LW, Schaefer RM, Kresse H. Decorin, biglycan and their endocytosis receptor in rat renal cortex. Kidney Int. 1998;54:1529–1541. doi: 10.1046/j.1523-1755.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R, Kappler J, Marrack P, Grey H. Antigen recognition by H-2-restricted T cells: cell-free antigen processing. J Exp Med. 1983;158:303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. Cold Spring Harb Protoc 2008, doi:10.1101/pdb.prot5080 [DOI] [PubMed] [Google Scholar]
- Gersuk GM, Razai LW, Marr KA. Methods of in vitro macrophage maturation confer variable inflammatory responses in association with altered expression of cell surface dectin-1. J Immunol Methods. 2008;329:157–166. doi: 10.1016/j.jim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Peitz M, Jäger R, Patsch C, Jäger A, Egert A, Schorle H, Edenhofer F. Enhanced purification of cell-permeant Cre and germline transmission after transduction into mouse embryonic stem cells. Genesis. 2007;45:508–517. doi: 10.1002/dvg.20321. [DOI] [PubMed] [Google Scholar]
- Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, Edenhofer F, Kurts C, Klockgether T, Limmer A, Knolle P, Burgdorf S. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor gamma is mediated by up-regulation of B7H1. J Immunol. 2009;183:129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Raslik I, Grone HJ, Schonherr E, Macakova K, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J. 2001;15:559–561. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- Domhan S, Muschal S, Schwager C, Morath C, Wirkner U, Ansorge W, Maercker C, Zeier M, Huber PE, Abdollahi A. Molecular mechanisms of the antiangiogenic and antitumor effects of mycophenolic acid. Mol Cancer Ther. 2008;7:1656–1668. doi: 10.1158/1535-7163.MCT-08-0193. [DOI] [PubMed] [Google Scholar]
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- Duan SZ, Ivashchenko CY, Usher MG, Mortensen RM. PPAR-gamma in the cardiovascular system. PPAR Res. 2008;2008:745–804. doi: 10.1155/2008/745804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MB, Holler S, Cui Y, Hudkins KL, Eitner F, Fogo A, Alpers CE. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000;57:487–498. doi: 10.1046/j.1523-1755.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitu G, Hirschberg R. Bone morphogenetic protein-7 (BMP7) in chronic kidney disease. Front Biosci. 2008;13:4726–4739. doi: 10.2741/3035. [DOI] [PubMed] [Google Scholar]
- Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol. 2008;130:247–262. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16:3015–3026. doi: 10.1681/ASN.2005050463. [DOI] [PubMed] [Google Scholar]
- Gourishankar S, Halloran PF. Late deterioration of organ transplants: a problem in injury and homeostasis. Curr Opin Immunol. 2002;14:576–583. doi: 10.1016/s0952-7915(02)00386-2. [DOI] [PubMed] [Google Scholar]
- Sivarajah A, Chatterjee PK, Patel NS, Todorovic Z, Hattori Y, Brown PA, Stewart KN, Mota-Filipe H, Cuzzocrea S, Thiemermann C. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol. 2003;23:267–276. doi: 10.1159/000072088. [DOI] [PubMed] [Google Scholar]
- Nencioni A, Grünebach F, Zobywlaski A, Denzlinger C, Brugger W, Brossart P. Dendritic cell immunogenicity is regulated by peroxisome proliferator-activated receptor gamma. J Immunol. 2002;169:1228–1235. doi: 10.4049/jimmunol.169.3.1228. [DOI] [PubMed] [Google Scholar]
- Klotz L, Dani I, Edenhofer F, Nolden L, Evert B, Paul B, Kolanus W, Klockgether T, Knolle P, Diehl L. Peroxisome proliferator-activated receptor gamma control of dendritic cell function contributes to development of CD4+ T cell energy. J Immunol. 2007;178:2122–2131. doi: 10.4049/jimmunol.178.4.2122. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Myit S, Mulhaupt F, Veillard N, Rufer N, Roosnek E, Mach F. PPARgamma but not PPARalpha ligands are potent repressors of major histocompatibility complex class II induction in atheroma-associated cells. Circ Res. 2002;90:356–362. doi: 10.1161/hh0302.104924. [DOI] [PubMed] [Google Scholar]
- Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby P, Plutzky J, Luster AD. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama Y, Akuzawa N, Nagai R, Kurabayashi M. PPARgamma ligand inhibits osteopontin gene expression through interference with binding of nuclear factors to A/T-rich sequence in THP-1 cells. Circ Res. 2002;90:348–355. doi: 10.1161/hh0302.105098. [DOI] [PubMed] [Google Scholar]
- Alchi B, Nishi S, Kondo D, Kaneko Y, Matsuki A, Imai N, Ueno M, Iguchi S, Sakatsume M, Narita I, Yamamoto T, Gejyo F. Osteopontin expression in acute renal allograft rejection. Kidney Int. 2005;67:886–896. doi: 10.1111/j.1523-1755.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Széles L, Töröcsik D, Nagy L. PPARgamma in immunity and inflammation: cell types and diseases. Biochim Biophys Acta. 2007;1771:1014–1030. doi: 10.1016/j.bbalip.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Cunard R, Eto Y, Muljadi JT, Glass CK, Kelly CJ, Ricote M. Repression of IFN-gamma expression by peroxisome proliferator-activated receptor gamma. J Immunol. 2004;172:7530–7536. doi: 10.4049/jimmunol.172.12.7530. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H, Daugherty L, Thatcher T, Lakatos H, Ray D, Redonnet M, Phipps R, Sime P. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- Guo B, Koya D, Isono M, Sugimoto T, Kashiwagi A, Haneda M. Peroxisome proliferator-activated receptor-gamma ligands inhibit TGF-beta 1-induced fibronectin expression in glomerular mesangial cells. Diabetes. 2004;53:200–208. doi: 10.2337/diabetes.53.1.200. [DOI] [PubMed] [Google Scholar]
- Milam J, Keshamouni V, Phan S, Hu B, Gangireddy S, Hogaboam C, Standiford T, Thannickal V, Reddy R. PPAR-gamma agonists inhibit profibrotic phenotypes in human lung fibroblasts and bleomycin-induced pulmonary fibrosis. Am J Physiol. 2008;294:L891–L901. doi: 10.1152/ajplung.00333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Bhattacharyya S, Lakos G, Chen SJ, Mori Y, Varga J. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis Rheum. 2004;50:1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Endo S, Okuda T, Economides AN, Valenzuela DM, Murphy AJ, Robertson E, Sakurai T, Fukatsu A, Yancopoulos GD, Kita T, Yanagita M. Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int. 2008;73:181–191. doi: 10.1038/sj.ki.5002626. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Macakova K, Raslik I, Micegova M, Gröne HJ, Schönherr E, Robenek H, Echtermeyer FG, Grässel S, Bruckner P, Schaefer RM, Iozzo RV, Kresse H. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Wilson JE, Roberts CR, Horley KJ, Winters GL, Costanzo MR, McManus BM. Biglycan, decorin, and versican protein expression patterns in coronary arteriopathy of human cardiac allograft: distinctness as compared to native atherosclerosis. J Heart Lung Transplant. 1996;15:1233–1247. [PubMed] [Google Scholar]
- Ghosh SS, Gehr TW, Ghosh S, Fakhry I, Sica DA, Lyall V, Schoolwerth AC. PPARgamma ligand attenuates PDGF-induced mesangial cell proliferation: role of MAP kinase. Kidney Int. 2003;64:52–62. doi: 10.1046/j.1523-1755.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- Chou FS, Wang PS, Kulp S, Pinzone JJ. Effects of thiazolidinediones on differentiation, proliferation, and apoptosis. Mol Cancer Res. 2007;5:523–530. doi: 10.1158/1541-7786.MCR-06-0278. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Yu HN, Noh EM, Kim JS, Song EK, Han MK, Kim BS, Lee SH, Park J. Peroxisome proliferator-activated receptor γ and retinoic acid receptor synergistically up-regulate the tumor suppressor PTEN in human promyeloid leukemia cells. Int J Hematol. 2007;85:231–237. doi: 10.1532/IJH97.A30615. [DOI] [PubMed] [Google Scholar]
- Willi SM, Kennedy A, Brant BP, Wallace P, Rogers NL, Garvey WT. Effective use of thiazolidinediones for the treatment of glucocorticoid-induced diabetes. Diabetes Res Clin Pract. 2002;58:87–96. doi: 10.1016/s0168-8227(02)00127-4. [DOI] [PubMed] [Google Scholar]