Abstract
Overexpression of hypoxia inducible factor-1 (HIF-1)α, which is common in most solid tumors, correlates with poor prognosis and high metastatic risk in breast cancer patients. Because HIF-1α protein stability is tightly controlled by the tumor suppressor von Hippel-Lindau (VHL), deletion of VHL results in constitutive HIF-1α expression. To determine whether VHL plays a role in normal mammary gland development, and if HIF-1α overexpression is sufficient to initiate breast cancer, Vhl was conditionally deleted in the mammary epithelium using the Cre/loxP system. During first pregnancy, loss of Vhl resulted in decreased mammary epithelial cell proliferation and impaired alveolar differentiation; despite these phenotypes, lactation was sufficient to support pup growth. In contrast, in multiparous dams, Vhl−/− mammary glands exhibited a progressive loss of alveolar epithelium, culminating in lactation failure. Deletion of Vhl in the epithelium also impacted the mammary stroma, as there was increased microvessel density accompanied by hemorrhage and increased immune cell infiltration. However, deletion of Vhl was not sufficient to induce mammary tumorigenesis in dams bred continuously for up to 24 months of age. Moreover, co-deletion of Hif1a could not rescue the Vhl−/−-dependent phenotype as dams were unable to successfully lactate during the first lactation. These results suggest that additional VHL-regulated genes besides HIF1A function to maintain the proliferative and regenerative potential of the breast epithelium.
The tumor suppressor protein von Hippel-Lindau (VHL) provides substrate specificity to a large Elongin B/C, RING-box 1 (RBX1), Cullin2 (CUL2) complex that functions as an E3 ubiquitin ligase to target proteins for polyubiquitinylation and degradation by the 26S proteasome.1 Under normoxic conditions, VHL mediates the rapid, oxygen-dependent destruction of the hypoxia-inducible factor (HIF)α subunits, HIF-1α and HIF-2α.1 In contrast, in response to decreasing oxygen, or as a result of inactivating VHL mutations, HIF-1α and HIF-2α are stabilized, facilitating interaction with the constitutively expressed aryl hydrocarbon nuclear receptor translocator (ARNT; HIF-1ß) to form the HIF-1 or HIF-2 transcription factor complexes. Microarray profiling has demonstrated that the HIFs stimulate or repress transcription of over 100 genes in breast, renal, endothelial, or embryonic fibroblast cells as part of the hypoxic response.2,3,4 These targets are implicated in control of a variety of cellular processes, including glucose transport, glycolysis, angiogenesis, cell cycle, stress response, and apoptosis.
The biological function of the VHL/HIF axis is highly relevant to tumorigenesis. VHL disease is an autosomal dominant syndrome characterized by development of benign hemangioblastomas in the central nervous system and retina; a subset of patients develop pheochromocytomas, cysts in the pancreas or kidneys, multifocal, bilateral clear cell-type renal cell carcinomas or pancreatic islet cell tumors.5,6 The reason for the tissue-specificity of VHL disease remains unclear since VHL is ubiquitously expressed and expression is enriched in all epithelial cell types including breast epithelium.7,8 Although mutations in VHL leading to constitutive expression of HIF-1α and/or HIF-2α have been detected in the majority of sporadic renal cell carcinomas9,10 and in some sporadic pheochromocytomas,11 to date, no mutations in VHL have been reported in sporadic breast cancer.12,13 In a recent study of breast cancer specimens, both VHL protein and mRNA levels were found to be decreased in tumors of increasing grade, particularly for those patients that relapsed compared with the patients that were disease-free post-therapy.14
Overexpression of HIF-1α is observed in a variety of tumor types and their corresponding metastases,15 including poorly differentiated breast tumors.16 Several studies have demonstrated a correlation between HIF-1α overexpression in breast tumors, poor prognosis, and increased risk of metastasis.17,18,19 Moreover, a “hypoxic signature” identified in breast cancers was found to be more predictive of patient relapse than the previously identified wound signature.2
Using genetically modified mouse models, we have previously demonstrated that HIF-1α function is essential in the mammary epithelium for full alveolar differentiation and for lactation.20 Moreover, we have also demonstrated in a transgenic mouse model of breast cancer that HIF-1α enhances primary mammary tumor progression and strongly promotes lung metastasis.21 Yet, as overexpression, rather than loss of function of HIF1A, occurs in breast cancer patients, it remains unclear if increased HIF-1α activity is sufficient to promote breast cancer or is a consequence of tumor progression.
Several studies in mouse models have clearly demonstrated that deletion of Vhl is deleterious to development. For example, Tang et al22 showed that loss of Vhl specifically in the endothelium via Tie2-Cre conditional deletion results in placental defects, as was observed in the global knockout.23 Deletion of Vhl in chondrocytes results in drawfism, increased extracellular matrix and decreased proliferation,24 whereas deletion of Vhl in the renal proximal tubule and hepatocytes using phosphoenolpyruvate carboxykinase-Cre produces liver hemangiomas, polycythemia, and renal cysts.25 Therefore, Vhl is essential to the normal development of a variety of embryonic and adult tissues, as reviewed by Haase.26 Likewise, deletion of Hif1a or Hif2a in mice is deleterious since global knockout of either gene results in embryonic lethality and conditional deletion impairs function of multiple tissues, including the liver, kidney, endothelium, and skin.27,28 Therefore, the expression levels of the HIFα subunits must be tightly regulated, since either deletion or overexpression is detrimental to normal development and tissue physiology.
To clarify the impact of HIF-1α overexpression during normal mammary gland development and to determine whether overexpression is sufficient for breast cancer initiation, we conditionally deleted Vhl in the mammary epithelium. Deletion of Vhl was accomplished using previously characterized mouse mammary tumor virus (MMTV)-Cre29 or whey acidic protein (Wap)-Cre transgenic mice.30 To determine whether the phenotypes resulting from Vhl deletion were dependent on HIF-1α and, therefore, could be rescued by loss of HIF-1α function, Hif1a was co-deleted with Vhl via MMTV-Cre. Our results demonstrate a novel role for Vhl in controlling mammary alveologenesis and secretory differentiation and suggest that VHL acts through both HIF-1-dependent and HIF-1-independent pathways to control normal mammary gland development. We also demonstrate that overexpression of HIF-1α through loss of Vhl is not sufficient to initiate mammary tumors in constitutively bred, multiparous female mice. Finally, we propose that one key function of VHL function in the breast epithelium is to regulate self-renewal of a pregnancy-responsive, progenitor cell population.
Materials and Methods
Animals
Generation of the Vhl and Hif1a conditional (flanked by loxP sites, or “floxed”) mouse models has been previously described.31,32 Vhl mice were obtained directly from Dr. Volker Haase. Mice expressing the MMTV-Cre (line D) or Wap-Cre transgene were obtained from Dr. Kay-Uwe Wagner (University of Nebraska, Eppley Cancer Center).29,33 Female mice used in experiments were generated by mating floxed/floxed; MMTV- or Wap-Cre-positive male mice with floxed/floxed; Cre-negative females, producing littermate control (Cre-negative) and test (Cre-positive) progeny. To co-delete Hif1a and Vhl, Vhl/Hif1a compound floxed/floxed females were bred to Vhl/Hif1a floxed/floxed males positive for MMTV-Cre. Therefore, in all cases, expression of Cre would induce homozygous deletion of the floxed gene. Animals were housed in microisolator cages and provided food and water ad libitum in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility under protocols approved by the University of California San Diego or the University of Tennessee Health Science Center Laboratory Animal Care and Use Committee.
Protein Extraction and Western Blotting
Whole inguinal mammary glands were harvested from C57BL/6 female mice (JAX, Bar Harbor, ME) at the following developmental stages: mature virgin (12 weeks of age), day 6 (6-P), 10 (10-P), 15 (15-P), and 18 (18-P) of pregnancy, based on the date of plug observed as day 0, day 10 of lactation and at involution (following 4 days of forced weaning from day 10 of lactation); n = 3 mice per time point. Using procedures previously described,34 tissue extracts were prepared from whole mammary glands for each time point from frozen, pooled tissue that was ground to a fine powder under liquid nitrogen and immediately homogenized in a modified radioimmunopreciptiation assay buffer to produce whole cell extracts (WCE) comprised of cytoplasmic and nuclear proteins. WCE were resolved on 10% Bis-Tris NuPAGE gels (Invitrogen, Carlsbad, CA) and blotted to polyvinylidene difluoride membrane (Millipore, Bellirica, MA). The membrane was blocked in 5% nonfat dry milk before addition of anti-VHL antibody (clone Ig32, BD Biosciences, San Jose, CA) at 1:200 for 3 hours at room temperature, followed by incubation in a 1;10,000 dilution of anti-mouse IgG-HRP (GE Life Sciences, Piscataway, NJ) for 30 minutes, and development in ECLPlus substrate (GE Life Sciences). Membranes were stripped and re-probed with an anti-guinea pig cytokeratin 8/18 primary antibody (Progen GmbH; Heidelberg, Germany) at 1:5000 for 1 hour at room temperature, followed by incubation in a 1:5000 dilution of donkey anti-guinea pig IgG-HRP (Jackson Immunologicals, West Grove, PA), before exposure to ECLPlus substrate.
Continuous Breeding and Lactation Studies
To examine the effects of Vhl deletion in multiparous females, homozygous floxed, Cre-negative or Cre-positive females were housed singly and continuously bred to FVB males. During constitutive breeding, all pups were weaned at 21 days or age. For glands harvested during the lactation period, litter size was normalized on the date of birth to eight pups per dam, using either the endogenous litters (born to the dam) or by cross-fostering pups that were born to lactating FVB dams. To permit accumulation of milk in the lactating gland, all litters were weaned from nursing dams for 2 to 3 hours before gland harvest, and all pups then returned to the mother once she recovered from anesthesia.
Tissue Harvest, Histology, and Whole-Slide Scanning
Individual inguinal (#4) or thoracic (#3) mammary glands were biopsied from anesthetized mice at specified time points during the first pregnancy or lactation and/or during sequential survival surgeries performed on the same animal over the course of multiple gestations. Each gland was fixed in phosphate-buffered formalin for 6 hours at room temperature and/or flash frozen in liquid nitrogen for preparation of RNA or protein. Fixed tissue was embedded in paraffin and 5 μm sections prepared. Sections were stained with H&E or used for immunohistochemistry. Whole-slide, digitized images of representative H&E-stained mammary glands were captured using the Aperio ScanScope XT automated scanning system (Vista, CA). Images may be viewed from a publicly available database maintained by the Department of Pathology at the University of Tennessee Health Science Center, see Supplemental Database at http://ajp.amjpathol.org.
Immunohistochemistry
Antigen retrieval, and glucose transporter (GLUT)-1 and CD34 immunostaining and the calculation of microvessel density using Chalkley analysis were performed as previously described.20,21 To detect HIF-1α, sections were incubated with a 1:100 dilution of a rabbit polyclonal anti-human HIF-1α antibody (generated to amino acids 600 to 800) generously provided by Dr. Robert Abraham, Burnham Institute, La Jolla, CA. Leukocytes were detected with the pan-leukocyte marker CD45 (BD Biosciences) at a 1:100 dilution. Vascular endothelieal growth factor (VEGF) was detected with a 1:40 dilution of a goat polyclonal antibody to murine VEGF (R&D Biosystems, Minneapolis, MN). The Vector ABC Elite staining kit (Vector Laboratories, Burlingame, CA) was used in conjunction with 3,3′-diaminobenzidine or DAB Impact peroxidase reagent (Vector Laboratories) to visualize all immunoreactive complexes. All slides were counterstained with Harris hematoxylin. To determine the percentage of proliferating mammary epithelial cells (MECs) during pregnancy, 15-P females were injected 2 hours before tissue harvest with 0.1 ml/10g body weight of cell proliferation reagent (bromodeoxyuridine, BrdU, GE LifeSciences). BrdU incorporation was detected by anti-BrdU-FITC antibody (BD Biosciences, San Jose, CA) and the average percentage of BrdU+ cells/total MEC (n = 5 animals/genotype) was determined as previously described.35 Similar procedures were used to calculate the percentage of CD45+ cells/total MEC in glands harvested from multiparous dams, except that five random fields at ×40 magnification were observed (n = 4 animals/genotype). To visualize the gross vasculature of the mammary gland, thrice parous lactating dams were anesthetized, the thoracic mammary gland was bathed in PBS and the glands were digitally imaged under a Leica dissecting stereozoom microscope. Dams were then returned to their respective weaned litters following recovery from surgery.
Automated Analysis of Epithelial Cell Content
The Aperio Pixel Density algorithm was used to quantitate the number of MEC contained within inguinal, H&E-stained glands harvested from mice at 15-P (n = 4 mice/genotype) or 18-P (n = 3 mice/genotype). Default settings for this algorithm were adjusted until pixels from the stroma and the vasculature were not detected. The following settings were used: Image Zoom, 1; Mark-up Compression, 0; Compression Quality, 30; Classifier Neighborhood, 30; Classifier, 30; Hue Value, 0.75; Hue Width, 0.35; Color Saturation Threshold, 0.25; Iwp (high), 200; Iwp (low), 175; Ip (low), 100, Isp (low); Inp (high), −1. The total number of positive pixels was calculated by adding the weak, median, and strongly positive pixel values (Nweak, Np, and Npositive, respectively) and dividing this total by the area analyzed (in mm2). The average number of positive pixels/mm2 was then compared between genotypes. In all cases, the area occupied by the inguinal gland lymph node was excluded.
Gene Expression Analysis by Real-Time Reverse Transcription-PCR
Total RNA was prepared from flash-frozen, whole, inguinal mammary glands harvested at 18-P or on the date of birth during the first lactation. Tissue was ground to a fine powder under liquid nitrogen and homogenized immediately in RNABee reagent (Isotex Diagnostics, Friendswood, TX). Total RNA was prepared according to manufacturer instructions and RNA quality was analyzed by the Agilent 2100 Bioanalyzer (Santa Clara, CA). Only samples with RNA Integrity Number values higher than 9.0 were chosen for preparation of cDNA using the High-Capacity cDNA Reverse Transcription kit (ABI, Foster City, CA). Real-time PCR was performed using optimized primer and FAM-labeled probe sets designed by the Roche Universal Probe Library Assay Design Center software available on the Roche website. The primer sequences and Universal Probes used for each assay are provided as Table 1. Real-time PCR was performed using the Roche LightCycler 480 machine for 40 cycles. The crossing point (Cp) for each sample was determined using the Roche absolute quantitation algorithm. The average sample Cp value was then calculated for all independent replicates/genotype/time point. To control for cDNA input, the average sample Cp values were first normalized based on expression of the Ints3 gene. To compensate for changes in MEC content among genotypes, Ints3-normalized, average Cp values were then normalized for expression of cytokeratin 18. The average fold-change in gene expression was determined by comparing the dual-normalized Cp values between control (floxed/floxed) and gene-deleted samples using the delta/delta Cp method.
Table 1.
qRT-PCR Primers and Roche Universal Library Probes
| Gene | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| Integrator complex subunit 3 (Ints3) | 5′-GTGGCTGTTATTGACTCTGCAC-3′ | 5′-CAGGTTCCCCATCATCACAT-3′ | 17 |
| Cytokeratin 18 | 5′-AGATGACACCAACATCACAAGG-3′ | 5′-CTTCCAGACCTTGGACTTCCT-3′ | 78 |
| Whey acidic protein (Wap) | 5′-ACATGTACACCCCCAGTGC-3′ | 5′-CTGGTCACTCCCGACAGG-3′ | 81 |
| β-casein (Csn2) | 5′-TCCGTTTCTGTCTAAGAGGATTTC-3′ | 5′-CATTTCCAGTTTCAGTCAGTTCA-3′ | 63 |
| Glucose transporter 1 (Glut1) | 5′-ATGGATCCCAGCAGCAAG-3′ | 5′-CCAGTGTTATAGCCGAACTGC-3′ | 52 |
| Vascular endothelial growth factor (Vegf) | 5′-AACGATGAAGCCCTGGAGT-3′ | 5′-AGGTTTGATCCGCATGATCT-3′ | 9 |
| Adipose differentiation-related protein (Adrp) | 5′-TGAGTCCCACTGTGTTGAGC-3′ | 5′-AGCTGCTGGGTCAGGTTG-3′ | 106 |
Statistics
Statistical significance was determined using the unpaired student’s t-test (Prism 4.0, GraphPad, La Jolla, CA). All P values less than 0.05 were considered to reflect statistically significant changes between genotypes.
Results
VHL Expression Increases During Pregnancy and Decreases During Involution
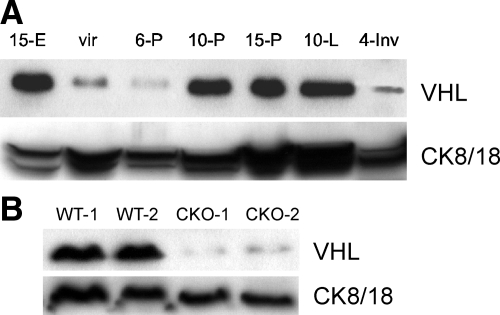
To determine the expression levels of VHL over the course of development of the normal mammary gland, WCE were prepared from whole mammary glands harvested from C57BL/6 nulliparous, pregnant, lactating, or involuting females, and VHL was detected by Western blotting. As a positive control, extract from an E15 embryo was included. A single isoform of VHL was detectable at 24 kDa at all stages of development. Expression of VHL increased dramatically from early (6-P) to mid-pregnancy (10-P), a period of rapid alveolar expansion, and levels remained high until involution, a time of extensive epithelial cell death and tissue remodeling (Figure 1A). Based on the increased expression of cytokeratins 8/18 over the course of mammary gland development, known markers of the luminal mammary epithelium, VHL expression appears to increase as the number of epithelial cells increases. These data suggest that murine VHL is preferentially expressed by MECs relative to the mammary stroma, in agreement with prior reports for the human breast.7
Figure 1.
Expression of murine VHL increases over the course of normal mammary gland development as epithelial cell content increases. A: Western blotting for VHL was performed using tissue whole cell extracts (WCE, 100 μg/lane) prepared from mid-gestation embryos (15-E) or whole mammary glands harvested from mice over the course of gestation and lactation. Extracts were prepared from mature nulliparous (vir) mice, at day 6, 10 or 15 of pregnancy (6-P, 10-P, 15-P), at mid-lactation (10-L) or 10-L followed by 4 days of forced involution (4-Inv). The same blot was stripped and reprobed with antibodies to cytokeratins 8/18 (CK8/18) to indicate the relative abundance of mammary epithelial cell (MEC) content at each stage of development. Note the increased expression of VHL from day 6 of pregnancy to day 10 of pregnancy that persists throughout lactation, and then dramatically decreases at 4-Inv, a peak period of MEC cell death. The virgin sample was overloaded as indicated by the increased expression of CK8/18 in this lane relative to sample prepared from 6-P mice. B: To confirm efficient deletion of Vhl in response to Wap-Cre-mediated recombination (conditional knockout, CKO), tissue WCE were prepared from whole glands isolated from two independent control (WT) or CKO lactating dams during the third round of lactation. VHL expression is reduced by >90% when normalized for MEC content based on CK8/18 expression.
To specifically delete Vhl in the mammary epithelium, a Cre/lox strategy was used. Previously characterized Vhl homozygous floxed mice were bred to either MMTV-Cre or WAP-Cre transgenic mice. Whereas in line D of MMTV-Cre mice the Cre transgene is activated on puberty and induces recombination throughout mammary gland development,29 the Wap-Cre transgene is only induced at high levels beginning at mid-pregnancy.30 Furthermore, there is promiscuous expression of Cre when its expression is driven by the MMTV promoter since recombination activity was observed in other tissues, including the skin, B and T cells and the pancreas. In contrast, expression of the Wap-Cre transgene is highly restricted to the mammary epithelium of pregnant and lactating mice.29
To confirm that we successfully generated Vhl conditional knockout mammary epithelium (Vhl −/−MEC), WCE were prepared from whole mammary glands of age-matched, littermate lactating dams that were either negative or positive for the Wap-Cre transgene and subjected to Western blotting for VHL. VHL was barely detectable in extracts prepared from Wap-Cre-positive mice, confirming efficient deletion of Vhl. The blots were stripped and re-probed with antibodies to cytokeratin 8/18 (CK8/18) (Figure 1B). The extent of deletion of VHL observed at lactation was similar between Wap-Cre and MMTV-Cre transgenic mice (data not shown).
Conditional Deletion of Vhl Impairs MEC Proliferation and Differentiation During Pregnancy
We next assessed the contribution of Vhl to mammary gland development by comparing the histology of wild-type and Vhl −/−MEC mammary glands in which deletion was achieved via expression of the MMTV-Cre (line D) transgene. In this particular line of MMTV-Cre transgenic mice, Cre is activated in epithelial cells at approximately 3 weeks of age, when ductal morphogenesis in is progress.29 However, no gross differences in ductal branching or extent of outgrowth into the mammary fat pad were observed in mature nulliparous mice that had completed ductal morphogenesis (Supplemental Database at http://ajp.amjpathol.org). Likewise, no gross changes were observed in alveolar number or appearance at day 6 of pregnancy (early pregnancy) or at day 10 of pregnancy (mid-pregnancy) (data not shown).
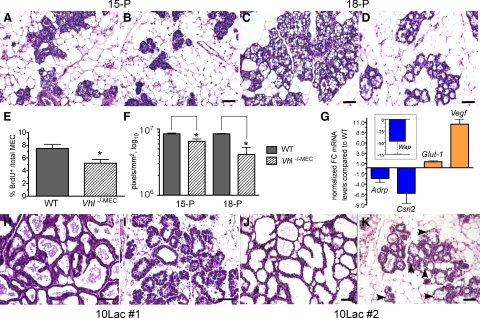
The transition from differentiation during pregnancy to successful milk secretion at lactation has been divided into two stages, termed secretory differentiation and secretory activation.36 The first phase, secretory differentiation, begins at mid-gestation with the production of significant quantities of milk protein and lipid. The second phase, secretory activation, depends on the completion of secretory differentiation, and is coordinated with the birth of pups to begin lactation. Therefore, we next analyzed tissue histology at day 15 of pregnancy (15-P), a period well within the secretory differentiation phase. At this stage, differentiation may be visualized by H&E staining by the presence of large cytoplasmic lipid droplets (CLD) within MEC, as well as the presence of proteinaceous material in the alveolar lumen, which is likely comprised of milk proteins that accumulate in preparation for lactation.37
At day 15 of pregnancy, alveoli formed by Vhl −/−MEC glands were not differentiated since the alveolar lumens remained closed (Figure 2B; Supplemental Database at http://ajp.amjpathol.org). This was in contrast to the control glands (Figure 2A; Supplemental Database at http://ajp.amjpathol.org), which contained alveoli with defined lumens and the presence of CLD. Moreover, there appeared to be fewer alveoli per field in Vhl −/−MEC glands compared with controls. To determine whether there were differences in MEC proliferation at this stage, the percentage of MEC incorporating BrdU was measured. The average percentage of cells positive for BrdU incorporation decreased by 30% in Vhl −/−MEC glands compared with control glands (5.2% vs. 7.5%) (Figure 2E). To confirm these results, we also compared the MEC content of glands at day 15 of pregnancy by automated quantitation of images of whole-slide digitized H&E-stained sections using a pixel density algorithm as described in the methods. The number of pixels corresponding to the mammary epithelium/mm2 of tissue was decreased by 22% (n = 4 mice/genotype, Figure 2F). By day 18 of pregnancy (18-P), just before parturition, analysis of H&E-stained slides showed that the glands containing Vhl −/−MEC appeared to maintain a reduced number of alveoli per field (Figure 2, C and D; Supplemental Database at http://ajp.amjpathol.org). Moreover, there were also lobuloalveolar units within the gland that did not appear to be well-differentiated. Automated pixel density analysis revealed that epithelial content was reduced by ∼50% at 18-P (n = 3 mice/genotype, Figure 2F).
Figure 2.
Reduced alveolar proliferation and differentiation in response to Vhl deletion via MMTV-Cre. Mammary glands were harvested from floxed control (A, C, H, J) or Vhl −/−MEC (B, D, I, K) glands at day 15 (A–B; 15-P, ×200 magnification) or day 18 of pregnancy (C–D; 18-P, ×200 magnification), day 10 of the first lactation (H–I; 10Lac#1) or day 10 of the second lactation (J–K; 10Lac#2), sectioned and stained with H&E. Scale bars = 10 μm. A–B: Note the decreased number of alveoli per field and the lack of differentiation in Vhl −/−MEC glands at 15-P. C–D: Fewer alveoli were also observed in Vhl −/−MEC glands at 18-P. E: The percentage of BrdU+ cells per total number of MEC was determined at day 15-P in individual mice (n = 5/genotype), and the grand mean compared between genotypes. *P < 0.043 F: The number of hematoxylin-positive pixels associated with MEC/mm2 gland scan area was compared using whole-slide scanned, digitized slides by the automated Aperio Pixel Density algorithm as described in the methods. The mean number of pixels plus the SEM is presented. *P < 0.02 G: Real-time PCR was performed to compare expression of Adrp, ß-casein (Csn2), Wap, Glut-1, and Vegf mRNAs in control and Vhl −/−MEC glands harvested at day 18 of pregnancy. The normalized fold-change (FC) in gene expression is expressed relative to expression levels observed in cDNA samples from control glands. Blue or orange bars indicate those genes for which relative expression decreased or increased, respectively. The average fold-change plus the SEM is presented. H–K: H&E-stained sections were prepared from mammary glands harvested from mid-lactation dams during the first (10Lac#1; H–I, ×400 magnification) or second (10Lac#2; J–K, ×200 magnification) cycles of lactation from the same cohort of test and control mice. As expected, the morphology of the wild-type gland remains relatively unchanged from the first to the second lactation (H versus J), whereas large lipid droplets are still present when Vhl is deleted (K; black arrowheads). Digitized images of representative H&E-stained slides from these experiments are available for viewing in the Supplemental Database at http://ajp.amjpathol.org.
To assay for molecular changes in differentiation, real-time PCR was also performed using cDNA prepared from 18-P glands (Figure 2G). Three markers of secretory differentiation were analyzed, adipose differentiation-related protein (ADRP, also known as adipophilin), ß-casein (Csn2), and WAP. Whereas ß-casein is one of the earliest markers of differentiation induced by pregnancy hormones, WAP and ADRP are expressed during later stages of differentiation.37,38 Antibodies to ADRP have also been used to immunostain the surface of CLD produced by differentiated MEC.37 In addition, we assayed for changes in expression of two direct HIF-1 target genes, Glut-1 and Vegf. Compared with control glands, expression of Adrp, Csn2, and Wap mRNA decreased by 2.6-fold, 6.2-fold, and 46.4-fold, respectively, in Vhl −/−MEC glands. As would be expected when Vhl is deleted, expression of Glut–1 mRNA increased by 40% and the expression of Vegf mRNA increased by 10.5-fold. Together, these results demonstrate that loss of Vhl activity delays both MEC proliferation and secretory differentiation during pregnancy and impacts gene expression.
Deletion of Vhl Results in Subtle Changes in Secretory Activity During First Lactation
In contrast to the strong lactation phenotype we observed previously on deletion of Hif1a in MEC during the first period of lactation,20 lactating mammary glands comprised of Vhl −/−MEC were able to successfully produce milk, as no striking changes in pup size were apparent at weaning of the first litter. However, on close histological examination of biopsied glands, subtle defects in MEC secretory function were observed in lactating glands at day 10 of lactation, a period of peak milk production (Figure 2, H–I; Supplemental Database at http://ajp.amjpathol.org). After a weaning period of 2 to 3 hours to permit milk accumulation in the gland, much less evidence of milk production was present in the Vhl −/−MEC alveoli compared with controls. Moreover, the majority of MEC within lobuloalveolar units of Vhl −/−MEC glands were not completely flattened with a basal nuclear location, as was observed in control glands. In addition, the Vhl −/− MEC alveoli exhibited reduced lumen size and areas of adipose tissue were still visible in each field.
Multiple lobuloalveolar units present within the Vhl −/− MEC glands also exhibited an abundance of cytoplasm that stained heavily basophilic. This feature is indicative of hypersecretory, apocrine metabolism, and is reminiscent of the Arias-Stella reaction. The Arias-Stella phenomenon refers to a pathology of the endometrium that is found in a subset of pregnant women characterized by nuclear hyperchromatism, cytoplasmic swelling, and vacuolization of apocrine epithelium due to a premature response to pregnancy hormones.39 Therefore, at first lactation, deletion of Vhl produces a mild secretory phenotype.
In contrast, during the second round of lactation, more severe defects were noted. Histological examination of biopsied glands revealed a more dramatic inhibition of alveolar differentiation and secretion at day 10 of lactation (Figure 2, J–K; Supplemental Database at http://ajp.amjpathol.org). As expected, the histology of the glands of wild-type mice between the first and second lactation periods was indistinguishable. Because the phenotypes observed in MMTV-Cre-positive transgenic females did not manifest until pregnancy and lactation, we then proceeded to confirm these observations using the Wap-Cre transgenic line. Furthermore, by using Wap-Cre to delete Vhl, we avoided any potential indirect effects on mammary gland development due to the promiscuous expression of MMTV-Cre in other cell types besides the mammary epithelium.29
Deletion of Vhl Contributes to Progressive Lactation Failure
The primary goal of using Wap-Cre transgenic mice to delete Vhl in continuously bred, multiparous females was to determine whether its deletion, and therefore, HIF-1α constitutive expression, was sufficient to result in mammary tumorigenesis. We also sought to determine whether use of Wap-Cre transgenic mice would phenocopy the Vhl deletion lactation phenotype observed in the MMTV-Cre transgenic mice. To perform continuous breeding, littermate Vhl control (wild-type; WT) or Vhl −/−MEC females were constitutively housed with FVB males since prior studies have shown that continuous breeding dramatically accelerates mammary tumorigenesis.40,41 Therefore, we reasoned that if loss of the tumor suppressor Vhl is sufficient for mammary tumor initiation, then mammary tumors would be observed in aged, multiparous females due to selection for stochastic mutations in Vhl −/−MEC. Furthermore, continual breeding would maintain constitutively high Cre transgene levels due to pregnancy hormone stimulation of the Wap promoter.38
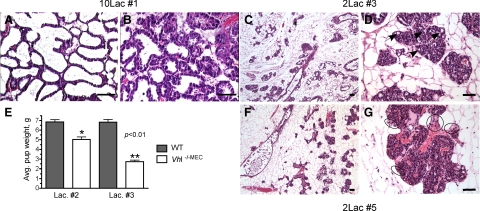
To observe changes in histology during successive rounds of lactation, individual thoracic or inguinal mammary glands were harvested from the same cohort of lactating dams during survival surgery at the first, third, and fifth period of lactation (Lac#1, Lac#3, or Lac#5) and sections prepared for H&E analysis. As observed following deletion of Vhl by the MMTV-Cre transgene, at day 10 of the first round of lactation, although there were mild defects in secretory activation in Vhl −/−MEC glands (Figure 3A vs. 3B), pups were able to thrive and appeared to be normal size at weaning of the first litters. In contrast, by the second round of lactation, there was a 27% decrease in the mean pup weight at day 10 of lactation (Figure 3E), although these litters survived and could be successfully weaned to independent cages at 21 days of age.
Figure 3.
Deletion of Vhl via Wap-Cre phenocopies results obtained with MMTV-Cre. Mammary glands were harvested from the same cohort of control or Wap-Cre+ multiparous dams during successive rounds of lactation and stained with H&E (A–D, F–G). Scale bars = 10 μm. A–B: During the first cycle of lactation (10Lac#1), the Vhl −/−MEC mammary gland accumulated less milk and there were subtle changes in MEC architecture, including the more frequent presence of basophilic cytoplasm in lobuloalveolar units (B) as compared with control glands (A), ×400 magnification. Progressive changes in MEC content were determined by H&E staining of glands biopsied from dams at day 2 of the third (C–D) or fifth (F–G) of lactation. As observed at low power magnification (×50), there are relatively few alveoli present in the Vhl −/−MEC mammary gland during the third (C, 2Lac#3) or the fifth (F, 2Lac#5) rounds of lactation. As indicated from higher power images obtained from the same Vhl −/−MEC mammary glands (D, G, ×200 magnification), in response to Vhl deletion there is a complete collapse of the alveolar lumen and there are multiple, large CLD still trapped in the MEC at the third lactation that should not be present (D, black arrows). By the fifth lactation, the lobuloalveolar structure is more disorganized, there are no distinct alveolar lumens and there appears to be increased microvessel density (D versus G) and immune cell infiltration (G, black circles). The mean pup weight per litter +/− SEM was calculated between control and Vhl −/−MEC dams (n >5 dams/genotype) on day 10 during the second (Lac. #2) and third (Lac. #3) cycles of lactation. Changes in pup weight between control and test dams were determined at Lac. #2 (single asterisk) and Lac. #3 (double asterisks) by an unpaired t-test. Digitized images of representative H&E-stained slides of mammary glands from multiparous animals are available for viewing in the Supplemental Database at http://ajp.amjpathol.org.
In contrast, by the third round of lactation, 100% of litters born to Wap-Cre positive dams were visibly severely runted throughout lactation and pup weight was decreased by 62% at day 10 of lactation (Figure 3E). As evident in H&E-stained sections of glands harvested during the third lactation period, there were dramatic reductions in the both the number of Vhl −/−MEC alveoli and the ability of the alveoli formed by Vhl −/−MEC to produce milk. Moreover, the majority of alveoli present were poorly organized, and in several areas, no distinct alveolar lumen could be observed. Whereas the entire lactating mammary gland of wild-type multiparous mice is compromised of fully differentiated alveoli, in the Vhl −/−MEC glands, the paucity of alveoli present was striking. In fact, the Vhl −/−MEC glands were only 20 to 30% filled with alveoli by the third round of lactation (Figure 3, C–D). Alveoli exhibiting hypersecretory, basophilic cytoplasmic secretions were also frequently observed. By H&E staining, there also appeared to be increased leukocyte infiltration and microvessel density around the remaining islands of alveoli, which became even more pronounced in the Vhl −/−MEC glands after subsequent rounds of lactation (for example, Figure 3G, fifth lactation). However, patches of well-differentiated lobuloalveolar units could also be detected in the thrice parous gland (Supplemental Database at http://ajp.amjpathol.org). The non-uniform appearance of the lobuloalveolar units in the Vhl −/−MEC glands likely results from the mosaic expression pattern of the Wap-Cre transgene during pregnancy and lactation (Supplemental Figure S1 at http://ajp.amjpathol.org).
To test whether these impaired alveologenesis and differentiation phenotypes were incremental in nature and progressively escalated on additional rounds of breeding, we then harvested glands from the same cohort of mice between days 2 to 10 lactation during the fifth lactation. No defects were noted in multiparous control glands (data not shown), however the phenotype of the multiparous Vhl −/−MEC gland was even more pronounced. Only 10 to 15% of the Vhl −/−MEC glands were filled with alveoli (Figure 3, F–G; Supplemental Database at http://ajp.amjpathol.org) and the alveoli exhibited little evidence of differentiation or milk production. By the fifth round of lactation, no endogenous litters born to Vhl −/−MEC dams survived past the date of birth, therefore, pups obtained from lactating FVB females were cross fostered to Cre-positive dams daily to ensure a continuous suckling stimulus. These glands were harvested at day 2 of lactation since lactation failure was evident due to lack of milk in the stomachs of nursing pups. None of the Vhl −/−MEC glands harvested from dams bred five times contained areas that appeared by H&E-staining to be differentiated enough to produce milk. In summary, extreme changes in gland structure were observed beginning at the third round of lactation, including loss of alveoli, evidence of decreased secretory differentiation, increased immune cell infiltration, and increased microvessel density, which all became more prominent during subsequent rounds of breeding. Because the proportion of affected MECs increased with each period of gestation and lactation, it is possible that VHL is necessary for maintaining the regenerative potential of the secretory mammary epithelium.
Deletion of Vhl Does Not Promote Mammary Carcinoma in Aged Females
Although we had expected to observe that deletion of a tumor suppressor that causes HIF-1α overexpression would result in mammary tumorigenesis, we did not observe any hyperplastic alveolar nodules or carcinomas in any mammary glands biopsied from multiparous Wap-Cre-positive mice. No palpable masses or tumors were observed in any of the Vhl conditional knockout females subjected up to eight pregnancies and maintained up to 24 months of age (n = 9 mice/genotype). Instead, as described, we observed a progressive loss of the secretory epithelium with each round of pregnancy. Therefore, it is clear that deletion of Vhl in the MEC is not sufficient to promote mammary tumorigenesis. These data are consistent with the lack of report of mutations in VHL in sporadic breast cancer.
Although no overt tumors were detected, we did observe in H&E-stained sections from multiparous, lactating females (third−fifth lactation period) features suggestive of mild dysplasia (n = 5 mice). These areas were estimated to occur in less than 3% of the epithelium. The dysplastic lesions were noted only during the lactation period in Cre-positive dams that had been bred at least three times. For example, there were ducts or acini that contained multiple, fingerlike projections characterized by large MEC with dark, snout-like, amphophilic cytoplasms that projected into the ductal/alveolar lumens. The nuclei of these regions also were occasionally hyperchromatic. Within these regions, there were also pockets of cells that appeared to be syncitia with two or more nuclei observed per cell (Supplemental Figure S2 at http://ajp.amjpathol.org). However, these changes were not permanent since no dysplastic regions were observed in the same dams when contralateral glands were prepared for histology 14 days after removal of the litter (Supplemental Database at http://ajp.amjpathol.org). Therefore, we did not observe any direct evidence that loss of Vhl transforms the mammary epithelium.
Constitutive Expression of HIF-1α, GLUT-1, and VEGF and Enhanced Recruitment of Leukocytes in Response to Vhl Deletion
To further characterize markers downstream of VHL in the Vhl −/−MEC glands, including HIF-1α and its direct transcriptional targets, Vegf and Glut1, we performed immunostaining on glands harvested from multiparous Wap-Cre transgenic females during the third lactation period. Of note, we had previously demonstrated that GLUT-1 immunoreacitivity is strongly down-regulated in the normal mammary gland when Hif1a is deleted in MECs.20 Because high levels of VEGF can induce leukocyte infiltration and blockage of VEGF signaling can reduce immune cell infiltration into breast tumors,42 we also stained sections with a pan-leukocyte marker, CD45.
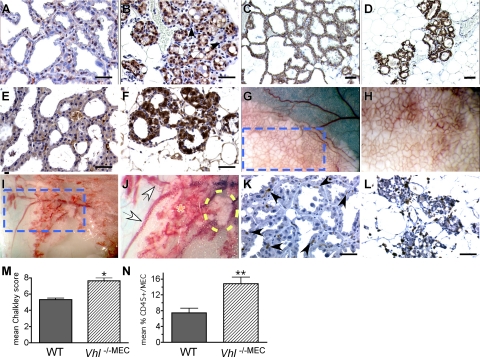
In wild-type glands, HIF-1α was detected primarily in the nucleus, as expected, and was expressed by approximately 60% of the acinar MEC (Figure 4A). In contrast, deletion of Vhl caused up-regulation of HIF-1α expression (Figure 4B). It should be noted that in response to Vhl deletion, HIF-1α immunoreactivity was observed in both the nucleus and the cytoplasm of MEC, but that no expression was detected in the more basally located myoepithelial cells or in the cells immediately adjacent to the alveoli, which by H&E-staining appeared to be infiltrating immune cells (Figure 3G, circled regions). The expression of GLUT-1 (basolateral localization) and cytoplasmic VEGF was also strongly up-regulated in the Vhl −/−MEC glands compared with wild-type glands (Figure 4, C–D and Figure 4, E–F), confirming that the HIF-1α protein detected by immunostaining was transcriptionally functional.
Figure 4.
Changes in HIF-1α, GLUT-1, and VEGF expression, microvessel density and immune cell infiltration in multiparous mice. A–F: Deletion of Vhl in the mammary epithelium results in uniform over-expression of HIF-1α (B), GLUT-1 (D), and VEGF (F) by MEC in thrice multiparous mice. Paraffin sections from formalin-fixed control (WT) or Vhl −/−MEC mammary glands generated from Wap-Cre transgenic stocks were prepared from glands isolated from lactating dams during the third round of lactation. Scale bars = 10 μm. Following antigen retrieval in citrate buffer, sections were stained with antibodies to HIF-1α, GLUT-1, VEGF, or the pan-leukocyte marker CD45. A–B: HIF-1α signal (brown nuclear stain) was detected in approximately 60% of MEC in WT lactating tissue (A), whereas intense nuclear staining as well as cytoplasmic staining of HIF-1α was observed in all MEC in which Vhl was deleted (B). Note the lack of HIF-1α staining in the stromal cells immediately adjacent to and surrounding the alveoli (B); the majority of these stromal cells have the morphology of myoepithelial cells (white arrowheads) or leukocytes (black arrowheads), ×400 magnification. C: As expected, GLUT-1 is expressed by all WT MEC at lactation, but there was an extreme up-regulation of GLUT-1 expression in the Vhl −/−MEC glands (D), ×200 magnification. E–F: Similarly, low levels of VEGF were expressed by WT glands (E), whereas expression of VEGF was strongly up-regulated in the Vhl −/−MEC glands (F), ×400 magnification. G–H: Deletion of Vhl results in hypervascularity and hemorrhage of the mammary gland. The gross appearance of thrice parous wild-type (G–H) or Vhl −/−MEC (I–J) thoracic mammary glands was compared by imaging live, anesthetized mice under a stereozoom dissecting microscope. The edge of the thoracic gland is shown in each low power field (G, I, ×12.5 magnification). The dashed blue box indicates the area highlighted in panels (G) and (I). In these panels individual alveoli and their supporting blood vessels can be visualized (H, J, ×25 magnification). In control dams, all alveoli are fully distended with milk (G, white opaque material), and the uniform basketlike network of vasculature that surrounds each alveolus can be identified (H). In contrast, in Vhl −/−MEC glands, the majority of vessels supporting the alveoli are hyperdilated, overall vessel density appears to be increased and there are areas of hemorrhage (J, orange asterisk). The large intramammary vessels are also dilated (J, white arrows). Moreover, the paucity of milk-containing alveoli in the Vhl null gland is striking, as there are only a few lobules capable of producing milk (yellow dashed oval). K–L: Sections were stained with anti-CD45 antibodies. Very few CD45+ cells were detected in the stroma of wild-type lactating glands (K, arrowheads), whereas there was an increase in the number of CD45+ cells present in the Vhl −/−MEC glands (L). To quantitate changes in microvessel density and leukocyte infiltration, Chalkley analysis was performed following immunostaining for CD34 (M) and the mean percentage of CD45+ cells/total number of MEC was determined (N). The mean Chalkley score and the percentage of CD45+/MEC plus SEM are presented; asterisks indicate statistically significant changes, as measured by an unpaired t-test (M–N). *P = 0.007, **P = 0.02.
Epithelial-Specific Deletion of Vhl Induces a Stromal Response Including Increased Microvessel Density
Although deletion of Vhl produces multiple MEC phenotypes, there were also severe defects in the mammary-associated stromal vasculature resulting from MEC-specific deletion of Vhl. This was first noticed during gross dissection of lactating glands. As shown in Figure 4, G–J, Vhl −/−MEC glands harvested from thrice lactating multiparous dams were hypervascular, and the blood vessels leading to the mammary glands were dilated, as compared with vessels in control glands. The Vhl −/−MEC glands in multiparous mice also bled profusely during surgical resection, requiring heavy cauterization. Closer observation under a stereozoom microscope revealed the presence of large areas of hemorrhage within the glands (Figure 4J). In addition, there was very little accumulated milk within the Vhl −/−MEC glands; the only milk present was restricted to few patches of presumably nonrecombined alveoli. Finally, during milk collection, blood could occasionally be extracted along with milk from dams harboring Vhl −/−MEC, but not from wild-type dams (data not shown). These data demonstrate VHL expression in the mammary epithelium is necessary for maintenance of the vasculature of the normal mammary gland and that VHL-regulated signals originating from the mammary epithelium control the proper branching and integrity of the mammary gland vasculature. To quantitate the changes in microvessel density between genotypes, Chalkley analysis was performed following immunostaining with anti-CD34 antibodies. As shown in Figure 4M, there was a 30% increase in the mean Chalkley score in multiparous Vhl −/−MEC glands.
Finally, whereas CD45+ cells were occasionally detected in the stroma surrounding lobuloalveolar units in the wild-type lactating gland (Figure 4K), we observed a dramatic increase in the number of CD45+ cells present in the stroma of the Vhl −/−MEC glands immediately surrounding abnormal acini (Figure 4L), possibly due to increased production of VEGF. The mean percentage of CD45+ cells/total MEC increased >2.0-fold in multiparous Vhl −/−MEC glands (Figure 4N).
Conditional Co-Deletion of Hif1a Does Not Rescue the VHL-Dependent Phenotype
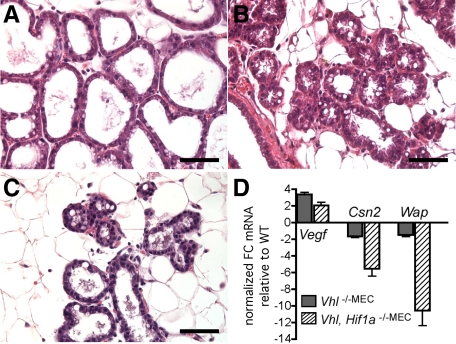
Because HIF-1α is a key substrate of VHL that at least partially confers the phenotypes observed in VHL disease, we then tested whether conditional co-deletion of Hif1a could rescue the VHL-dependent phenotypes. To achieve deletion as early as possible during mammary gland development, the MMTV-Cre line D transgene was used. As shown in Figure 5, deletion of Hif1a was not sufficient to rescue the phenotypes resulting from Vhl deletion. In fact, during the first lactation period, there were fewer alveoli present in the double knockout gland (Figure 5C) than were observed in glands in which either Vhl (Figure 3D) or Hif1a (Figure 5B) had been singly deleted. Moreover, the few alveoli present in the co-deleted gland were more collapsed and poorly differentiated compared with alveoli present in either the control glands (Figure 5A) or the glands in which Hif1a alone was deleted (Figure 5B). No pups survived that suckled the co-deleted gland, even during the first lactation period, therefore, exogenous pups were continuously cross-fostered to maintain the suckling stimulus.
Figure 5.
Co-deletion of Vhl and Hif1a does not rescue the Vhl lactation phenotype. Mammary glands were harvested from mice of each genotype at day 2 of lactation of the first gestation, sectioned and stained with H&E. Scale bars = 10 μm. In each case, deletion was achieved using the MMTV-Cre line D transgene. A: Lactation is normal in Cre-negative Vhl/Hif1a floxed control dams. B: Single deletion of Hif1a via MMTV-Cre line D produces mammary glands with poorly differentiated alveoli and trapped CLD, as previously reported,20 although dams were capable of nursing litters. C: Co-deleted Vhl/Hif1a −/−MEC glands contain relatively few alveoli, which are poorly differentiated and characterized by small lumens. D: Expression of Vegf, ß-casein (Csn2) and Wap mRNA was compared between Vhl −/−MEC and Vhl/Hif1a −/−MEC glands on the date of birth, when secretory activation begins. The normalized fold-change (FC) in gene expression is expressed relative to expression levels observed in cDNA samples from control glands. The average fold-change (FC) +/− SEM is presented per genotype per gene (dark gray bars, single Vhl deletion, hatched bars, Vhl/Hif1a co-deletion).
To measure changes in gene expression, cDNA was prepared from glands biopsied from co-deleted or Vhl-deleted glands on the date of birth, when secretory activation begins (n = 3 mice/genotype). Relative to control glands, expression of Vegf mRNA was up-regulated 3.4-fold in Vhl-deleted mice compared with 2.0-fold in co-deleted glands. In addition, expression of ß-casein mRNA was down-regulated 67% in Vhl-deleted glands and 5.5-fold in co-deleted glands and the expression of Wap mRNA was decreased 50% in Vhl-deleted glands and 10.6-fold in co-deleted glands (Figure 5D). Therefore, the decreased expression of differentiation markers in the co-deleted gland corresponded with changes observed by histology.
Discussion
We have generated the first conditional knockout mouse model to target deletion of Vhl and co-deletion of Vhl and Hif1a within the epithelium of the mammary gland. We have shown that in the normal murine mammary gland, VHL expression increases over the course of pregnancy as MEC content increases and then decreases sharply at involution when MEC regress. These data are supported by previous observations that expression of VHL is primarily localized to the epithelium rather than the stroma.7,8,14 Conditional deletion of Vhl in response to either MMTV-Cre or Wap-Cre transgene expression reduced alveolar proliferation and secretory differentiation during the first round of pregnancy.
Although there were subtle defects in MEC architecture during the first period of lactation, dams were able to complete lactation without impacting pup growth. In contrast, beginning during the second period of lactation, average pup weight was decreased. In addition, with each subsequent gestation, deletion of Vhl resulted in the continued loss of alveoli capable of producing milk, leading to complete lactation failure. The potential of Vhl −/−MEC to re-populate the parous mammary gland with functional alveoli was strikingly reduced by the third lactation, which resulted in a decrease in average pup weight of ∼60%. The progressive nature of this phenotype was revealed by the observations that the both number of Vhl −/−MEC alveoli and the differentiation potential of these MEC continued to decrease with each subsequent pregnancy until lactation completely failed by the fifth period of lactation. Changes in MEC architecture and secretory function were also accompanied by changes in the mammary stroma, including increased microvessel density, hemorrhage, and increased leukocyte infiltration.
Moreover, Vhl deletion in the mammary epithelium and the downstream up-regulation of HIF-1α was not sufficient to induce mammary hyperplasias or carcinomas. These results are consistent with observations that no VHL mutations have been detected in sporadic breast cancer and the lack of reports of increased lifetime incidence of breast cancer in VHL patients.8 Although mutations in VHL may not play a role in breast cancer, recent studies have shown that VHL mRNA levels are altered in breast cancer patients. For example, real-time PCR analysis of 124 breast cancer tissue samples indicated that levels of VHL mRNA are decreased in node-positive breast cancers, in patients with poor prognosis and in patients with metastases or high grade tumors.14 These results imply that decreases in VHL expression may occur as breast cancer cells become less-differentiated and more aggressive, which would parallel with the observed timing of overexpression of HIF-1α during tumor progression in breast cancer patients.16
A Role for VHL in Mammary Progenitor Cells?
The striking reduction in the number of alveoli observed in Vhl −/−MEC glands with each sequential lactation, combined with the previously described ability of the Wap-Cre transgene to target a population of self-renewing, progenitor cells known as parity-induced MECs (PI-MECs),30,40 suggests that loss of Vhl may inhibit the renewal capacity of the MEC. The PI-MECs are hormone-responsive progenitor cells that have the capacity to proliferate and to renew over several generations.30,43 It has been observed using the flox-STOP-ROSA26 reporter line of mice that the MEC exposed to Wap-Cre during the first gestation period constitute an increased percentage of the total regenerated mammary epithelium during subsequent pregnancy cycles, forming both ducts and alveoli comprised of luminal epithelial cells.30 Therefore, the inability of Vhl −/−MEC glands of multiparous mice to fully repopulate with alveoli and to achieve complete secretory activation on multiple, successive pregnancies is particularly intriguing since the Wap-Cre transgene targets Cre expression to differentiated cells as well as the PI-MECs.
The most dramatic decreases in alveolar number accompanied by the reduced ability to differentiate fully to produce milk were noted in Wap-Cre transgenic females bred at least 3 times. Because the MEC derived from the PI-MEC population expressing Wap-Cre will expand with successive rounds of pregnancy and lactation, it is possible that there would be a larger proportion of Vhl-deleted MEC present in the Wap-Cre+ glands with each successive round of lactation. This phenomenon could explain why the Vhl deletion phenotypes were enhanced with each gestation period and why dams were able to successfully nurse their litters at the first and second lactations, but could not maintain their litters during subsequent lactations.
There is increasing evidence that the VHL/HIF axis is involved in stem cell renewal, as reviewed by Keith et al.44 For example, in murine embryonic stem cells, HIF-1α and HIF-2α are expressed, but HIF-2α is not transcriptionally active.45 In human cancer cell lines, HIF-1α has been show to physically interact with the Notch intracellular domain and with ß-catenin, which modulates transcriptional target gene activation46,47; both the Notch and Wnt/ß-catenin pathways are key players in stem cell regulation. The relationship of this axis to cancer stem cell biology is particularly intriguing since hypoxic regions of solid tumors are known to be highly resistant to radiation and chemotherapy48 and since cancer stem cells are thought to represent the therapeutic-resistant fraction of tumors.49 Therefore, anti-HIF therapies may enhance the clinical response to chemotherapy or radiation.50,51
In the normal murine mammary gland and in various mouse models of breast cancer, the pluripotent population of primitive stem cells defined by enriched self-renewal in vitro and outgrowth potential in vivo has been defined as CD24+ (heat stable antigen) and CD29hi or CD49fhi (α6-integrin).52,53,54,55,56 A subpopulation of the PI-MECs have been shown to co-express CD24/CD49f.57 This population is thought to exhibit characteristics of a more committed progenitor MEC with limited pluripotent potential rather than a more primitive stem cell since most PI-MECs did not belong to the long-term label-retaining population.58 Together, these observations are consistent with our data that that the predominant defect in Vhl −/−MEC glands from multiparous females is a failure of the alveoli to regenerate and to differentiate during pregnancy. We propose that the luminal progenitor cell population that has the potential to form ductal and alveolar luminal MECs decreases in response to loss of Vhl.
Further investigation is warranted to determine whether HIF-1α also plays a direct role in regulation of mammary progenitor cell expansion or lineage commitment. Our hypothesis is not without precedent as HIF-1α has been shown to act through the Notch3 pathway to promote breast cancer stem cell renewal when cells are cultured under hypoxic conditions59 and since the Rich laboratory has found that the HIFs directly regulate cancer stem cell activity in glioblastomas.60 In fact, in this study, there was HIF-dependent, preferential expression of VEGF and GLUT-1 in the cancer stem cell population compared with the non-stem population.60
A Key Role for Local VEGF in Regulating the Mammary-Associated Vasculature
The increased microvessel density, presence of hemorrhage and increased immune cell infiltration observed in multiparous glands may be due to the up-regulation of Vegf mRNA levels, which was initially observed in Vhl −/−MEC glands during the first round of pregnancy and lactation. By the third period of lactation, we also observed via immunostaining extremely high levels of VEGF protein localized to the mammary epithelium. These phenotypes contrast with our previous observations of the effects of conditional Hif1a deletion in the mammary gland, in which we observed profound defects in alveolar differentiation without any impact on alveolar cell number/proliferation, changes in microvessel density or changes in Vegf mRNA expression.20
However, it is unlikely that VEGF acts alone to cause the Vhl-dependent phenotype. This conclusion is derived from our observations that co-deletion of Hif1a in the Vhl null background only slightly reduced Vegf mRNA levels, but enhanced the block in lactation when compared with deletion of either Hif1a or Vhl alone. It is known that VEGF and VEGF receptor expression increases during pregnancy and lactation and that VEGF immunostaining switches from the stroma to the epithelium during late gestation and lactation.61,62 Therefore, local VEGF produced by MECs is critical for lactation as demonstrated by conditional deletion of Vegf in the mammary gland via expression of the cytokeratin-5 (K5)-Cre transgene. In these experiments, deletion of Vegf in MECs and myoepithelium resulted in decreased angiogenesis during pregnancy and blocked milk production at lactation.63 Therefore, it would be of interest to determine whether co-deletion of Vegf could reverse the multiple vascular phenotypes observed in the Vhl −/−MEC mammary gland. In this scenario, Vegf deletion would not be expected to rescue MEC secretory defects or the production of milk.
Hif1a Deletion Cannot Rescue the Vhl Phenotype, a Role for Other VHL-Regulated Targets?
Based on the strong correlation between loss of VHL function and constitutive HIF activity in VHL patients,64 we predicted that co-deletion of Hif1a would at least partially rescue the Vhl phenotype. We focused on deletion of Hif1a in these studies rather than Hif2a since up to 80% of genes in breast epithelial cells are transcriptionally regulated by HIF-1α, rather than HIF-2α.3,65 Moreover, in a panel of breast cancer cell lines, HIF-2α expression was low to undetectable, also pointing to HIF-1α as the primary regulator of the hypoxic response in the breast.66 The dependence of breast cancer cells on HIF-1α for mediating the hypoxic response was later confirmed using specific small interfering RNAs to either HIF-1α or HIF-2α and then analyzing expression of key HIF target genes.3
Instead of ameliorating the Vhl-dependent phenotypes, co-deletion of Hif1a resulted in a further reduction in the number of alveoli at lactation. In addition, the few alveoli that formed exhibited even less evidence of secretory activity by histology than was observed for single deletion of either Hif1a or Vhl. Furthermore, dams harboring co-deleted mammary glands were incapable of producing enough milk to support their litters even at the first round of lactation. These results suggest that in the context of the Vhl -deleted gland, HIF-1α overexpression acts in part to maintain pathways required for proper secretory differentiation and milk production. However, the phenotypes arising from deletion of Vhl cannot solely reflect enhanced HIF-1α activity as co-deletion of Hif1a could not rescue lactation and instead amplified the impaired lactation phenotype. Therefore, additional VHL-regulated substrates or interacting proteins besides the HIFs must contribute to the pathology observed in the Vhl −/−MEC glands.
There is increasing evidence that VHL regulates multiple proteins through HIF-independent pathways,67,68 including via protein-interaction activities that are independent of its ability to target proteins for proteasome-dependent degradation. For example, VHL has been implicated in regulation of the physical integrity of cells and the extracellular matrix network, through modulation of microtubule stability69 and the processing of the fibronectin extracellular matrix.22,70 VHL also physically interacts with several other proteins.68 For example, VHL interactions stabilize the putative tumor suppressor Jade-1 (gene for apoptosis and differentiation in epithelia), which then promotes ubiquitinylation of ß-catenin.71 Finally, it is possible that deletion of Vhl in the mammary epithelium may also lead to premature senescence independent of HIFα function via up-regulation of the cyclin-dependent kinase inhibitor p27, as recently described in mouse embryonic fibroblasts.72
A Role for VHL in the Prolactin Response?
The mammary gland is resilient and often compensates for lack of gene function in subsequent pregnancies by ameliorating the phenotype observed at first lactation. Our data suggest that VHL function is critical to regulate either the ability of progenitor cells to develop into differentiated epithelium. An alternate explanation is that the Vhl −/−MEC are unable to respond to pregnancy hormones. Interestingly, the incremental loss of alveolar differentiation phenotype observed on deletion of Vhl is similar to that observed previously on Wap-Cre mediated deletion of Jak2 in the murine gland, the kinase that activates STAT5 in response to prolactin. Conditional deletion of Jak2 completely blocks alveolar proliferation and differentiation, which is compounded by a failure to maintain differentiated cells during lactation.73 Similarly, deletion of Stat5 via Wap-Cre, in which Stat5 is deleted after alveolar differentiation has initiated, results in a loss of differentiated cells, suggesting that STAT5 is also needed for maintaining alveolar differentiation.74 Previous studies have shown that hypoxia can activate STAT5 through JAK2 in normal murine HC11 MECs and in MCF7 breast cancer cells,75 however, it is unclear whether STAT5 activation would also occur in response to deletion of Vhl since HIF-1α is constitutively expressed.
Summary
In conclusion, although VHL does not function as a classic tumor suppressor in the breast, we have shown for the first time that it is required to regulate proper development and expansion of the mammary epithelium during repetitive cycles of pregnancy and lactation. Since the mammary gland is not essential for survival of the individual animal, the conditional deletion model system we have developed will be useful to continue to dissect the general role of VHL in regulation of proliferation, differentiation, and/or self-renewal of secretory epithelium.
Acknowledgments
We thank Ms. Susanna Didrickson, Ms. Lauren Davie, and Ms. Molly Jumper for excellent animal care and technical support. Dr. Robert Cardiff (University of California, Davis, Mutant Mouse Pathology Laboratory) generously consulted on the histopathology. Dr. Robert Abraham provided the anti-HIF-1α antibody to R.S.J. We thank Crystal Stanton and Dr. Anand Kulkarni (Department of Pathology) for creating and maintaining the Aperio database.
Footnotes
Address reprint requests to Tiffany N. Seagroves, Ph.D., Center for Cancer Research, Department of Pathology and Laboratory Medicine, University of Tennessee Health Science Center, Rm 262 Cancer Research Bldg., 19 S. Manassas St., Memphis, TN 38163. E-mail: tseagro1@uthsc.edu.
Supported by NIH, R01CA082515 (R.S.J.), California Breast Cancer Program, 7PB-0108 (R.S.J.), Susan G. Komen Foundation PDF-499 (T.N.S.), Department of Defense, DAMD-17–01-1–0186 (T.N.S.) and W81XWH-04–1-0417 (D.L.), and the University of Tennessee Health Science Center Maston Callison Bowld Cancer Research Fund (T.N.S.). We also acknowledge support of the Molecular Resource Center of Excellence at the University of Tennessee Health Science Center for use of shared equipment. The Aperio ScanScope XT system is maintained with support from NCRR 1S10RR025665–01.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
Current address of D. L.: Department of Immunology, Scripps Research Institute, La Jolla, CA.
References
- Ohh M. Ubiquitin pathway in VHL cancer syndrome. Neoplasia. 2006;8:623–629. doi: 10.1593/neo.06442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Borresen-Dale AL, Giaccia A, Longaker MT, Hastie T, Yang GP, van de Vijver MJ, Brown PO. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL. Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res. 2003;63:6130–6134. [PubMed] [Google Scholar]
- Vengellur A, Woods BG, Ryan HE, Johnson RS, LaPres JJ. Gene expression profiling of the hypoxia signaling pathway in hypoxia-inducible factor 1alpha null mouse embryonic fibroblasts. Gene Expr. 2003;11:181–197. doi: 10.3727/000000003108749062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- Sano T, Horiguchi H. Von Hippel-Lindau disease. Microsc Res Tech. 2003;60:159–164. doi: 10.1002/jemt.10253. [DOI] [PubMed] [Google Scholar]
- Corless CL, Kibel AS, Iliopoulos O, Kaelin WG., Jr Immunostaining of the von Hippel-Lindau gene product in normal and neoplastic human tissues. Hum Pathol. 1997;28:459–464. doi: 10.1016/s0046-8177(97)90035-6. [DOI] [PubMed] [Google Scholar]
- Lantzsch T, Hefler L, Koebl H, Lampe D. Expression of the von Hippel-Lindau gene protein in breast cancer tissue. Gynecol Oncol. 2002;84:186–187. doi: 10.1006/gyno.2001.6419. [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, Lubensky I, Duan DR, Florence C, Pozzatti R, Walther MM, Bander NH, Grossman HB, Brauch H, Pomer S, Brooks JD, Isaacs WB, Lerman MI, Zbar B, Linehan WM. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- Shuin T, Kondo K, Torigoe S, Kishida T, Kubota Y, Hosaka M, Nagashima Y, Kitamura H, Latif F, Zbar B, Lerman MI, Yao M. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994;54:2852–2855. [PubMed] [Google Scholar]
- Dannenberg H, De Krijger RR, van der Harst E, Abbou M, Y IJ, Komminoth P, Dinjens WN. Von Hippel-Lindau gene alterations in sporadic benign and malignant pheochromocytomas. Int J Cancer. 2003;105:190–195. doi: 10.1002/ijc.11060. [DOI] [PubMed] [Google Scholar]
- Miyakis S, Sourvinos G, Liloglou TL, Stathopoulos GP, Field JK, Spandidos DA. The Von Hippel-Lindau (VHL) tumor-suppressor gene is not mutated in sporadic human colon adenocarcinomas. Int J Cancer. 2000;88:503–505. [PubMed] [Google Scholar]
- Sourvinos G, Miyakis S, Liloglou TL, Field JK, Spandidos DA. Von Hippel-Lindau tumour suppressor gene is not involved in sporadic human breast cancer. Tumour Biol. 2001;22:131–136. doi: 10.1159/000050607. [DOI] [PubMed] [Google Scholar]
- Zia MK, Rmali KA, Watkins G, Mansel RE, Jiang WG. The expression of the von Hippel-Lindau gene product and its impact on invasiveness of human breast cancer cells. Int J Mol Med. 2007;20:605–611. [PubMed] [Google Scholar]
- Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- Bos R, Zhong H, Hanrahan CF, Mommers E, Semenza GL, Pinedo HM, Abeloff MD, Simons JW, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1a during breast carcinogenesis. J Natl Cancer Instit. 2001;93:309–314. doi: 10.1093/jnci/93.4.309. [DOI] [PubMed] [Google Scholar]
- Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- Dales JP, Garcia S, Meunier-Carpentier S, Andrac-Meyer L, Haddad O, Lavaut MN, Allasia C, Bonnier P, Charpin C. Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer. 2005;116:734–739. doi: 10.1002/ijc.20984. [DOI] [PubMed] [Google Scholar]
- Generali D, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield S, Bersiga A, Allevi G, Milani M, Aguggini S, Gandolfi V, Dogliotti L, Bottini A, Harris AL, Fox SB. Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res. 2006;12:4562–4568. doi: 10.1158/1078-0432.CCR-05-2690. [DOI] [PubMed] [Google Scholar]
- Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W, Welm B, Wagner KU, Neville M, Johnson RS. HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development. 2003;130:1713–1724. doi: 10.1242/dev.00403. [DOI] [PubMed] [Google Scholar]
- Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- Tang N, Mack F, Haase VH, Simon MC, Johnson RS. pVHL function is essential for endothelial extracellular matrix deposition. Mol Cell Biol. 2006;26:2519–2530. doi: 10.1128/MCB.26.7.2519-2530.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander D, Kobayashi T, Knight MC, Zelzer E, Chan DA, Olsen BR, Giaccia AJ, Johnson RS, Haase VH, Schipani E. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development. 2004;131:2497–2508. doi: 10.1242/dev.01138. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin Cell Dev Biol. 2005;16:564–574. doi: 10.1016/j.semcdb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens A, Johnson RS. Transgenic models to understand hypoxia-inducible factor function. Methods Enzymol. 2007;435:87–105. doi: 10.1016/S0076-6879(07)35005-2. [DOI] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;V98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan H, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit J, Jonhson R. Hypoxia-inducible factor-1alpha is a positive regulator in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM. C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14:359–368. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- McManaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev. 2003;55:629–641. doi: 10.1016/s0169-409x(03)00033-4. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Rudolph MC, McManaman JL, Neville MC. Key stages in mammary gland development. Secretory activation in the mammary gland: it’s not just about milk protein synthesis! Breast Cancer Res. 2007;9:204. doi: 10.1186/bcr1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JM, Wyszomierski SL, Hadsell D. Regulation of milk protein gene expression. Annu Rev Nutr. 1999;19:407–436. doi: 10.1146/annurev.nutr.19.1.407. [DOI] [PubMed] [Google Scholar]
- Arias-Stella J. The Arias-Stella reaction: facts and fancies four decades after. Adv Anat Pathol. 2002;9:12–23. doi: 10.1097/00125480-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- Landis MD, Seachrist DD, Abdul-Karim FW, Keri RA. Sustained trophism of the mammary gland is sufficient to accelerate and synchronize development of ErbB2/Neu-induced tumors. Oncogene. 2006;25:3325–3334. doi: 10.1038/sj.onc.1209365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland CL, Lynn KD, Toombs JE, Dineen SP, Udugamasooriya DG, Brekken RA. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One. 2009;4:e7669. doi: 10.1371/journal.pone.0007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- Zhang M, Rosen JM. Stem cells in the etiology and treatment of cancer. Curr Opin Genet Dev. 2006;16:60–64. doi: 10.1016/j.gde.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- Rich JN, Bao S. Chemotherapy and cancer stem cells. Cell Stem Cell. 2007;1:353–355. doi: 10.1016/j.stem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Bechis SK, Slorach EM, Littlepage LE, Egeblad M, Ewald AJ, Pai SY, Ho IC, Werb Z. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D, Medina D, Tsimelzon A, Hilsenbeck S, Green JE, Michalowska AM, Rosen JM. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 2008;68:4674–4682. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Creamer BA, Triplett AA, Wagner KU. Longitudinal analysis of mammogenesis using a novel tetracycline-inducible mouse model and in vivo imaging. Genesis. 2009;47:234–245. doi: 10.1002/dvg.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C, Chieco P, Bonafe M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, Shi Q, Cao Y, Lathia J, McLendon RE, Hjelmeland AB, Rich JN. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey RC, Goldhar AS, Baffi J, Vonderhaar BK. Transcriptional regulation of vascular endothelial growth factor expression in epithelial and stromal cells during mouse mammary gland development. Mol Endocrinol. 2001;15:819–831. doi: 10.1210/mend.15.5.0635. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol. 1998;177:439–452. doi: 10.1002/(SICI)1097-4652(199812)177:3<439::AID-JCP7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rossiter H, Barresi C, Ghannadan M, Gruber F, Mildner M, Fodinger D, Tschachler E. Inactivation of VEGF in mammary gland epithelium severely compromises mammary gland development and function. FASEB J. 2007;21:3994–4004. doi: 10.1096/fj.07-8720com. [DOI] [PubMed] [Google Scholar]
- Maynard MA, Ohh M. Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer. Am J Nephrol. 2004;24:1–13. doi: 10.1159/000075346. [DOI] [PubMed] [Google Scholar]
- Aprelikova O, Wood M, Tackett S, Chandramouli GV, Barrett JC. Role of ETS transcription factors in the hypoxia-inducible factor-2 target gene selection. Cancer Res. 2006;66:5641–5647. doi: 10.1158/0008-5472.CAN-05-3345. [DOI] [PubMed] [Google Scholar]
- Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000;60:7106–7113. [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Meller J. von Hippel-Lindau tumor suppressor: not only HIF’s executioner. Trends Mol Med. 2004;10:146–149. doi: 10.1016/j.molmed.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Frew IJ, Krek W. Multitasking by pVHL in tumour suppression. Curr Opin Cell Biol. 2007;19:685–690. doi: 10.1016/j.ceb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Lolkema MP, Mans DA, Snijckers CM, van Noort M, van Beest M, Voest EE, Giles RH. The von Hippel-Lindau tumour suppressor interacts with microtubules through kinesin-2. FEBS Lett. 2007;581:4571–4576. doi: 10.1016/j.febslet.2007.08.050. [DOI] [PubMed] [Google Scholar]
- Stickle NH, Chung J, Klco JM, Hill RP, Kaelin WG, Jr, Ohh M. pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development. Mol Cell Biol. 2004;24:3251–3261. doi: 10.1128/MCB.24.8.3251-3261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, Bharti A, Seldin DC, Lecker SH, Dominguez I, Cohen HT. Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL. Nat Cell Biol. 2008;10:1208–1216. doi: 10.1038/ncb1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AP, Schlisio S, Minamishima YA, Zhang Q, Li L, Grisanzio C, Signoretti S, Kaelin WG., Jr VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Krempler A, Triplett AA, Qi Y, George NM, Zhu J, Rui H. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol. 2004;24:5510–5520. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung YH, Lim EJ, Lee MY, Park JH, Ye SK, Park EU, Kim SY, Zhang Z, Lee KJ, Park DK, Park T, Moon WK, Yang YM. Hypoxia activates the cyclin D1 promoter via the Jak2/STAT5b pathway in breast cancer cells. Exp Mol Med. 2005;37:353–364. doi: 10.1038/emm.2005.45. [DOI] [PubMed] [Google Scholar]