Abstract
Duchenne muscular dystrophy (DMD) is characterized by progressive skeletal muscle wasting and weakness, leading to premature death from respiratory and/or cardiac failure. A clinically relevant question is whether myostatin inhibition can improve function of the diaphragm, which exhibits a severe and progressive pathology comparable with that in DMD. We hypothesized that antibody-directed myostatin inhibition would improve the pathophysiology of diaphragm muscle strips from young mdx mice (when the pathology is mild) and adult mdx mice (when the pathology is quite marked). Five weeks treatment with a mouse chimera of anti-human myostatin antibody (PF-354, 10 mg/kg/week) increased muscle mass (P < 0.05) and increased diaphragm median fiber cross-sectional area (CSA, P < 0.05) in young C57BL/10 and mdx mice, compared with saline-treated controls. PF-354 had no effect on specific force (sPo, maximum force normalized to muscle CSA) of diaphragm muscle strips from young C57BL/10 mice, but increased sPo by 84% (P < 0.05) in young mdx mice. In contrast, 8 weeks of PF-354 treatment did not improve muscle mass, median fiber CSA, collagen infiltration, or sPo of diaphragm muscle strips from adult mdx mice. PF-354 antibody-directed myostatin inhibition completely restored the functional capacity of diaphragm strips to control levels when treatment was initiated early, but not in the later stages of disease progression, suggesting that such therapies may only have a limited window of efficacy for DMD and related conditions.
Duchenne muscular dystrophy (DMD) is the most severe of the muscular dystrophies and affects approximately 1 in 3500 live male births. It is characterized by progressive skeletal muscle weakness and wasting that leads to premature death caused by respiratory or cardiac failure.1 DMD is caused by the absence of dystrophin, a membrane stabilizing cytoskeletal protein that confers protection from contraction-mediated trauma.2 The fragility of dystrophic muscle fibers renders them susceptible to injury and ongoing cycles of damage, degeneration, and incomplete regeneration. Currently, there is no cure for DMD, and despite their potential, widely lauded gene therapies have yet to be perfected nor will they be optimized in time to treat current patients.3 Therefore, it is crucial to develop therapeutic strategies that can increase muscle strength, enhance muscle fiber regeneration and/or reduce degeneration, and protect muscles from contraction-mediated injury.4,5
Myostatin, originally termed growth and differentiation factor-8, is a member of the transforming growth factor-β superfamily. Myostatin negatively regulates skeletal muscle growth,6 an effect attributed to inhibition of both myoblast proliferation and differentiation.7 Livestock and humans with a loss-of-function mutation in the myostatin gene exhibit hypermuscularity.6,7,8,9 Numerous studies have demonstrated that myostatin inhibition, via genetic deletion or pharmacological inactivation, can increase skeletal muscle size and strength.10,11,12,13,14 Not surprisingly, there is considerable interest in developing strategies to modulate myostatin activity in clinical situations where enhancing muscle growth and strength may have beneficial effects for age-related muscle wasting, cancer cachexia, denervation, sepsis, and the muscular dystrophies.15,16,17,18,19,20
Several strategies have been used to inhibit myostatin in dystrophic mdx mice. Transgenic deletion of myostatin18 or overexpression of follistatin, an endogenous antagonist of myostatin,21 in 5-week-old to 9-month-old mdx mice increased muscle mass and fiber cross-sectional area (CSA), improved diaphragm pathology, and reduced infiltration of connective tissue in the diaphragm. Similar improvements in limb muscle mass and fiber CSA as well as in diaphragm pathology were also found after 3 months administration of a myostatin inhibitory antibody (JA16)19 or myostatin propeptide20 to 4-week-old mdx mice. However, the limb muscles of mdx mice undergo the first profound bout of muscle degeneration at 19 to 21 days after birth, and this is when the pathology in the limb muscles of mdx mice most closely resembles that in DMD.22 Early treatment in mdx mice is sometimes difficult to translate to humans, because DMD is usually detected only when the condition has progressed to a stage when functional impairments are evident. Therefore, to comprehensively assess the therapeutic potential of such interventions, it is recommended that studies in mdx mice should examine effects in young (2- to 3-week-old) mdx mice before or during the initial bout of severe muscle fiber degeneration and in older mice after several cycles of degeneration and less than successful regeneration, at the time when clinical treatments for DMD are usually first implemented.23
Although the mdx mouse is a commonly used model of DMD, the limb muscles have only a relatively mild myopathy.24 In contrast, the diaphragm exhibits a more severe and progressive dystrophic pathology.24 To assess the therapeutic potential of myostatin inhibition for improving the dystrophic pathology in the mdx mouse, the effects on diaphragm muscle function are important clinically because respiratory insufficiency is a predictor of mortality in DMD.
The aim of this study was to investigate the therapeutic potential of myostatin inhibition, administered via a novel myostatin blocking antibody (PF-354), on the pathology and function of the diaphragm muscle of young (16- to 17-day-old) and adult (12-week-old) mdx mice. The efficacy of PF-354 for inhibiting myostatin activity has been shown previously in muscles from aged mice with 4 weeks treatment with PF-354 reducing Smad3 phosphorylation (a downstream event involved in myostatin signaling) and increasing skeletal muscle mass.15 We tested the hypothesis that PF-354 antibody mediated myostatin inhibition would improve the pathology and function of the diaphragm muscle of young and adult mdx mice.
Materials and Methods
Efficacy of PF-354 for Inhibiting Myostatin Activity in Vitro and in Wild-Type Mice
The generation of PF-354 has been described in detail previously.15 C3H10T1/2 cells stably carrying pcDNA3.1 (CAGA)15-luc (which carries 15 copies of the myostatin-responsive AGCCAGACA “CAGA” SMAD binding element, SBE, upstream of the Photinus pyralis firefly luciferase gene derived from the pGL3 vector) were generated by transfecting cells with the plasmid and selecting on 700 μg/ml G418. Stable clones were cultured in basal medium eagle, supplemented with 10% fetal bovine serum, 2 mmol/L glutamine, and 700 μg/ml G418. Assays were performed by treating cells for 6 hours at 37°C in serum-free basal medium eagle with 20 ng/ml myostatin (which typically activates the reporter in these cells approximately two- to threefold) preincubated with varying concentrations (0.8 nmol/L to 0.5 μmol/L) of a mouse chimera of anti-human myostatin antibody (PF-354, Pfizer Global Research and Development, Groton, CT) for 30 minutes before addition to the cells. Luciferase activity was measured on a Wallac 1450 Microbeta Trilux luminescence reader using the Dual-light® luciferase assay system (Applied Biosystems, Inc., Foster City, CA; Cat. No. T1004). Myostatin was purchased from R&D Systems (Minneapolis, MN). The pGL3 vector was purchased from Promega (Madison, WI). Fetal bovine serum, basal medium eagle, G418, glutamine, and pcDNA3.1 were purchased from Invitrogen (Grand Island, NY).
Animal experimentation procedures performed at Pfizer Inc. (Groton, CT) were approved by the Pfizer Institutional Animal Care and Use Committee and conducted in accord with accepted standards of humane animal care. Mice were housed under a 12-hour light/dark cycle, with drinking water and standard chow provided ad libitum. To assess the efficacy of PF-354 for increasing muscle mass, and to determine appropriate dose for studies on mdx mice, 8-week-old male C57BL/6 mice (Charles River, Wilmington, MA) were randomly assigned to one of three experimental groups. Groups received an equivalent volume of either Saline (Control, n = 5), PF-354 at 3 mg/kg/week (s.c., n = 5), or PF-354 at 10 mg/kg/week (s.c., n = 5) for 2 weeks. Body mass and quadriceps muscle mass were determined at the conclusion of the treatment period.
Experimental Animals
All experiments were approved by the Animal Experimental Ethics Committee of The University of Melbourne and conducted in accordance with the Australian code of practice for the care and use of animals for scientific purposes as stipulated by the National Health and Medical Research Council (Australia).
Study 1
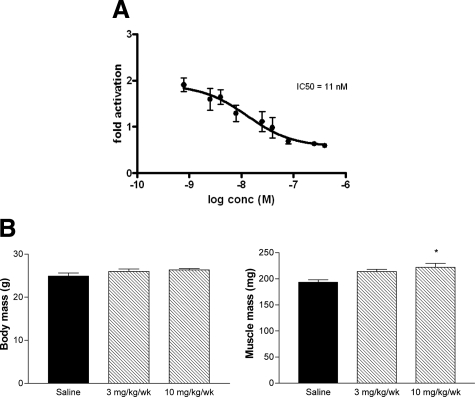
To assess the efficacy of PF-354 antibody-mediated myostatin blockade for improving the dystrophic pathology in young mdx mice, we used 16- to 17-day-old male C57BL/10ScSn (C57BL/10; n = 20) and C57BL/10ScSn-mdx/J (mdx, n = 20) dystrophic mice. Mice were obtained from the Animal Resource Centre (Canning Vale, Western Australia), and housed in the Biological Research Facility at The University of Melbourne under a 12-hour light/dark cycle, with drinking water and standard chow provided ad libitum. Animals were allocated randomly into one of two experimental groups; one received PF-354 (10 mg/kg/week, s.c., n = 20), and another received an equivalent volume of saline (Control, 0.1 ml/10 g body mass, s.c., n = 20) for 5 weeks. The dose of PF-354 was based on in vivo dose–response studies to maximize skeletal muscle hypertrophy (Figure 1).
Figure 1.
A: C3H10T1/2 cells, stably transfected with the SMAD reporter pcDNA3.1(CAG)15-luc, were treated with 20 ng/ml myostatin that had been preincubated for 30 minutes with or without increasing amounts of PF-354. Preincubation with PF-354 neutralized myostatin activity dose-dependently with an IC50 of 11 nmol/L. Data are means ± SEM, n = 3, and representative of at least three separate experiments. Whole body and quadriceps muscle mass of eight-week-old C57BL/six mice after two weeks treatment with saline (Control), or 3 mg/Kg/wk or 10 mg/kg/wk of PF-354 (B). Data are means ± SEM; n = 5. *P < 0.05 versus Saline.
Study 2
To assess the efficacy of PF-354 antibody-mediated myostatin blockade for improving the dystrophic pathology in adult mdx mice, 12-week-old male mdx dystrophic mice were allocated randomly into one of the two experimental groups described for Study 1, using the same delivery method. Two treatment periods were used in Study 2; the first of 5 weeks to facilitate comparisons with Study 1. In this study, adult mdx mice (n = 13) received either saline (Control, n = 6) or PF-354 (n = 7) for 5 weeks and body mass, muscle mass (extensor digitorum longus [EDL], soleus, plantaris, tibialis anterior [TA], gastrocnemius, and quadriceps), and heart mass were determined at the end of the treatment. Functional properties of diaphragm muscle strips were not evaluated after 5 weeks of treatment because this study was conducted separately from Study 1 and therefore did not use the same experimental design. However, the second treatment period, for 8 weeks, was used to investigate the potential of PF-354 antibody-mediated myostatin inhibition to reverse the dystrophic pathology in adult mdx mice, whereas in our studies on young mdx mice, we investigated the potential of myostatin inhibition to attenuate the disease progression. Adult mdx dystrophic mice (n = 16) received either saline (Control, n = 8) or PF-354 (n = 8) for 8 weeks, after which body mass, muscle and heart mass, and diaphragm muscle function and histology were assessed.
Assessment of Contractile Properties
Mice were anesthetized with sodium pentobarbitone (Nembutal, 60 mg/kg, Sigma-Aldrich Co., Castle Hill, NSW, Australia) via i.p. injection. The TA, EDL, soleus, plantaris, gastrocnemius, and quadriceps muscles were carefully excised, blotted on filter paper, and weighed on an analytical balance. The entire diaphragm and ribcage were surgically excised and placed in a dish containing oxygenated Krebs-Ringer solution (composition in mM: NaCl, 1.37; NaHCO3, 24; D-glucose, 11; KCl, 5; CaCl2, 2; NaH2PO4 H2O, 1; MgSO4 7H2O, 0.487; D-tubocurarine chloride, 0.293; pH 7.4) as described previously.25,26,27 Costal diaphragm muscle strips composed of longitudinally arranged full-length muscle fibers were tied firmly with braided surgical silk (6/0, Pearsalls Suture, Somerset; UK) at the central tendon at one end and sutured through a portion of the rib attached to the distal end of the strip at the other end. Mice were euthanized as a consequence of diaphragm and heart excision while anesthetized deeply.
The procedure for assessing contractile properties of diaphragm muscle strips in vitro has been described in detail by us previously.25,26,27 Briefly, the diaphragm strips were transferred to a custom built Plexiglas bath filled with oxygenated Krebs-Ringer solution thermostatically maintained at 25°C for optimal oxygen diffusion. The muscles were aligned horizontally and tied directly between a fixed pin and a dual-mode force transducer-servomotor (300B-LR, Aurora Scientific, Aurora, Ontario, Canada). Two platinum plate electrodes were positioned in the organ bath to flank the length of the muscle preparation. Muscles were field-stimulated by supramaximal square wave pulses (0.2 ms duration) that were amplified (EP500B power amplifier, Audio Assemblers, Campbellfield, Victoria; Australia) to increase and sustain current intensity to a sufficient level to produce a maximum isometric tetanic contraction.
Optimal muscle length (Lo) was determined from the micromanipulation of muscle length to produce maximum isometric twitch force (Pt). Optimum fiber length (Lf) was equal to Lo based on the alignment of the fibers in the diaphragm preparation, as determined previously.25 Maximum isometric force (Po) was recorded from the plateau of the frequency-force relationship after successive stimulations at 10 to 150 Hz for 450 ms with 2 minutes rest between stimuli. In addition, we examined the susceptibility of the diaphragm muscle to contraction-mediated injury based on the protocol described by Schertzer and colleagues.28 Briefly, muscles were lengthened at a velocity of 2 Lf/s at progressively increasing magnitudes of stretch beyond Lf (5, 10, 20, 30, 40 and 50% for Study 1, and 2, 4, 6, 8, 10, 12.5, 15, 17.5, 20, 25, 30, 35, 40, 45, and 50% for Study 2), with Po determined after each lengthening contraction. The cumulated “force deficit” after contraction-mediated injury was determined by calculating the difference between Po measured 2 minutes after the lengthening contractions and Po determined before the lengthening contractions, expressed as a percentage of the Po determined before the protocol of successive lengthening contractions.29
At the conclusion of the contractile measurements, the diaphragm muscle strip was trimmed of tendon and any adhering non-muscle tissue, blotted once on filter paper and weighed on an analytical balance. The muscle strips were then frozen in thawing isopentane for later histological examination. Muscle mass, Lf and Po were used to calculate specific force (sPo), or the force normalized per total muscle fiber CSA (kN/m2) taking into account muscle density (1.06 mg/mm3) as described elsewhere.25,26
Assessment of Skeletal Muscle Pathology
Serial sections were cut transversely through the diaphragm muscle strip using a refrigerated cryostat (−20°C, CTI Cryostat, IEC, Needham Heights, MA) and stained with hematoxylin and eosin (H&E) and/or laminin and DAPI30 to determine general muscle architecture, median myofiber CSA, and the proportion of fibers with centrally located nuclei. Additional sections were stained with Van Gieson stain for assessment of collagen infiltration, as described previously.27
Digital images of stained sections were obtained using an upright microscope with camera (Axio Imager D1, Carl Zeiss, Wrek Göttingen, Germany), controlled by AxioVision AC software (AxioVision AC Rel. 4.7, Carl Zeiss Imaging Solutions, Wrek, Göttingen, Germany). Images were quantified using AxioVision 4.7 software.
Western Blotting Analyses
Muscle samples (≈20 mg) were homogenized (Polytron 2100, Kinematica, Lucerne, Switzerland) for 3 × 15 seconds on ice in a 1:10 dilution with homogenizing buffer (10 mmol/L Tris-HCl, pH 7.5, 100 mmol/L sodium chloride, 1 mmol/L ethylenediamine-tetraacetic acid [EDTA], 1 mmol/L ethylene glycol-bis[2-aminoethylether]-N,N,N′,N′-tetraacetic acid [EGTA], 10% glycerol, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 1 mmol/L sodium fluoride, 20 mmol/L sodium pyrophosphate, 2 mmol/L sodium orthovanadate, 0.5% sodium deoxycholate, 1 mmol/L phenylmethanesulphonylfluoride [PMSF], 0.1% protease inhibitor cocktail [P8340, Sigma-Aldrich], 0.1% phosphatase inhibitor cocktail [P2850, P5726, Sigma-Aldrich]). Samples were centrifuged at 10,000g for 10 minutes at 4°C, and the resulting supernatant analyzed for total protein content (Bio-Rad DC Protein Assay, Bio-Rad, Gladesville, NSW, Australia), with bovine serum albumin (BSA) as the standard. Protein loading buffer (40% glycerol, 6% SDS, 250 mmol/L Tris-HCl, pH 6.8, 0.04% bromophenol blue and 415 nmol/L DTT) was added to 50 μg of sample in a 1:4 dilution, and samples were heated at 95°C for 5 minutes before being stored at −20°C for immunoblotting.
SDS-PAGE (10 to 14% separating, 4% stacking gel) was performed and loaded with 50 μg of protein. After electrophoresis (40 minutes at 90 V, 200 minutes at 120 V), the protein was wet transferred overnight (200 mA) at 4°C (X Cell II Blot, Invitrogen, Carlsbad, CA) to polyvinylidene difluoride membrane (Immobilon-P; Millipore, North Ryde, NSW, Australia). The following day the membrane was incubated in Ponceau stain for 5 minutes to visually verify equal protein transfer. The membrane was destained with distilled water and washed with Tris-buffered saline-Tween 20 (TBST) before being blocked for 2 hours at room temperature in TBST containing 5% skim milk powder or BSA. Membranes were incubated for 2 hours at room temperature in primary antibodies, including: phospho-Smad3 (58 kDa, #1880–1, Epitomics, Burlingame, CA); Smad3 (52 kDa, #9513, Cell Signaling, Danvers, MA); and α-tubulin (50 kDa, #T6074, Sigma). Membranes were washed for 3 × 10 minutes in TBST and incubated for 1 hour at room temperature in horseradish peroxidise–conjugated secondary antibodies (donkey anti-goat or goat anti-mouse immunoglobulins) diluted in TBST containing 5% skim milk powder or BSA. After 3 × 10 minutes washes in TBST, membranes were treated with enhanced chemiluminescence (ECL plus; Amersham Biosciences, Buckinghamshire, UK). The signal was imaged using ChemiDoc XRS machine (Bio-Rad) and blots were quantified using Quantity One® software (Bio-Rad) and normalized against α-tubulin protein abundance.
Statistical Analysis
All values are expressed as mean ± SEM, unless otherwise stated. Groups were compared using an unpaired t test or a two-way analysis of variance (treatment, % stretch), where appropriate. Bonferonni post hoc test was used to determine significant differences between individual groups. The level of significance was set at P < 0.05 for all comparisons. Muscle fiber CSA was not normally distributed (Anderson Darling Normality test), and so data for diaphragm CSA were presented as 95% confidence intervals of the median. Differences were considered significant when no overlap existed between the 95% confidence interval of the median.31
Results
PF-354 Inhibits Myostatin Activity in Vitro
PF-354 is chimeric antibody designed to neutralize myostatin activity and be useful for long-term in vivo studies in mice.15 Preincubation of C3H10T1/2 cells with increasing concentrations of PF-354 reduced myostatin-induced p(CAGA)15 transcription activity, with an IC50 of 11 nmol/L (Figure 1A). These findings demonstrated the efficacy of PF-354 antibody mediated blockade of myostatin activity in vitro. Dose selection for studies in mdx mice was based on in vivo efficacy studies conducted in 8-week-old wild-type C57BL/6 mice treated with 3 or 10 mg/kg/week (s.c.) PF-354 for 2 weeks, with assessments of body mass and quadriceps muscle mass (Figure 1B). Consistent with circulating myostatin levels of 60 to 80 ng/ml for wild-type mice of this age, mice treated with 10 mg/kg/week PF-354 (but not 3 mg/kg/week) had an ≈15% higher quadriceps muscle mass than in saline-treated control mice (P < 0.05, Figure 1B). There was no significant difference in body mass between groups (P = 0.20, Figure 1B). Subsequent in vivo experiments therefore used PF-354 at a dose of 10 mg/kg/week.
Study 1
Myostatin Inhibition via PF-354 Increases Muscle Mass in Young mdx Mice
Five weeks treatment with PF-354 antibody did not affect the percentage increase in body mass from initial in the young (16- to 17-day-old) C57BL/10 (P = 0.72) or mdx mice, but there was a trend for PF-354 to induce a greater percentage increase in body mass in the mdx mice (P = 0.18, Table 1). Absolute body mass was also not different between saline- and PF-354–treated young C57BL/10 or mdx mice (P = 0.14, Table 1).
Table 1.
Selected Morphometric Parameters of Young (16- to 17-Day-Old) C57BL/10 and mdx Mice after 5 Weeks Treatment with Either Saline (Control) or Myostatin Inhibitory Antibody (PF-354)
| Morphometric Parameter | C57BL/10
|
mdx | ||
|---|---|---|---|---|
| Control | PF-354 | Control | PF-354 | |
| Body mass, g | 23 ± 1 | 24 ± 1 | 23 ± 1 | 26 ± 1 |
| % increase body mass from initial | 208 ± 16 | 215 ± 1 | 197 ± 12 | 217 ± 9 |
| EDL/body mass, mg · g−1 | 0.23 ± 0.01 | 0.31 ± 0.01* | 0.31 ± 0.01 | 0.35 ± 0.01* |
| Soleus/body mass, mg · g−1 | 0.18 ± 0.02 | 0.24 ± 0.01* | 0.27 ± 0.01 | 0.30 ± 0.02 |
| Plantaris/body mass, mg · g−1 | 0.59 ± 0.03 | 0.68 ± 0.03 | 0.74 ± 0.03 | 0.78 ± 0.04 |
| TA/body mass, mg · g−1 | 1.63 ± 0.03 | 1.86 ± 0.05* | 2.27 ± 0.10 | 2.34 ± 0.09 |
| Gastrocnemius/body mass, mg · g−1 | 4.53 ± 0.10 | 4.89 ± 0.08 | 5.35 ± 0.12 | 5.92 ± 0.22* |
| Quadriceps/body mass, mg · g−1 | 6.89 ± 0.22 | 9.09 ± 0.34* | 8.87 ± 0.12 | 9.87 ± 0.29* |
| Heart mass, mg | 130.2 ± 3.7 | 126.1 ± 7.2 | 118.5 ± 6.3 | 125.4 ± 4.9 |
| Heart/body mass, mg · g−1 | 5.60 ± 0.15 | 5.38 ± 0.16 | 5.26 ± 0.23 | 4.84 ± 0.24 |
EDL indicates extensor digitorum longus; TA, tibialis anterior. Data are means ± SEM, n = 7 to 10.
P < 0.05 versus respective control strain.
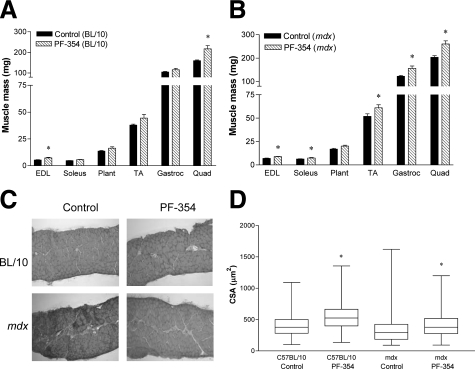
The mass of the EDL and quadriceps muscles was increased with PF-354 treatment in C57BL/10 mice (by 35% and 36%, respectively, P < 0.001, Figure 2A). EDL and quadriceps muscle mass corrected for body mass were also increased (by 32% for both muscles, P < 0.001), as were the soleus (by 32%, P < 0.05) and TA muscles (by 14%, P < 0.05, Table 1).
Figure 2.
Mass and histological analyses of muscles in young (16- to 17-day-old) C57BL/10 and mdx mice after five weeks treatment with either saline (Control) or myostatin inhibitory antibody (PF-354). Mass of selected hindlimb muscles in C57BL/10 (A) and mdx mice (B). Representative images of H&E stained tranverse sections (C) and box-and-whisker plots showing the median and range of the distribution of fiber cross-sectional area (CSA) measurements (D) of diaphragm muscle strips in C57BL/10 and mdx mice. EDL indicates extensor digitorum longus; Plant, plantaris; TA, tibialis anterior; Gastroc, gastrocnemius; Quad, quadriceps. Data are means ± SEM (A, B); n = 7 to 10 animals. *P < 0.05 versus respective Control.
In mdx mice, PF-354 treatment increased absolute mass of the EDL (by 27%, P < 0.01), soleus (by 19%, P < 0.05), TA (by 18%, P < 0.05), gastrocnemius (by 28%, P < 0.01) and quadriceps (by 28%, P < 0.01, Figure 2B). However, when expressed relative to body mass, increases with PF-354 treatment were observed only for the EDL (by 15%, P < 0.05), gastrocnemius (by 11%, P < 0.05) and quadriceps (by 11%, P < 0.05, Table 1).
PF-354 treatment did not affect absolute heart mass (P = 0.80) or heart mass relative to body mass (P = 0.13) in either C57BL/10 or mdx mice (Table 1).
Myostatin Inhibition via PF-354 Improves Diaphragm Pathology and Fiber CSA in Young mdx Mice
Representative H&E stained sections (Figure 2C) showed infiltration of connective tissue, smaller fiber sizes, and increased central nucleation; characteristic hallmarks of the dystrophic pathology in diaphragm muscle of mdx mice. However, 5 weeks treatment with PF-354 reduced infiltration of connective tissue and increased fiber CSA (Figure 2C). PF-354 treatment increased median fiber CSA in both young C57BL/10 and mdx mice, by 39% and 32%, respectively (both P < 0.05, Figure 2D).
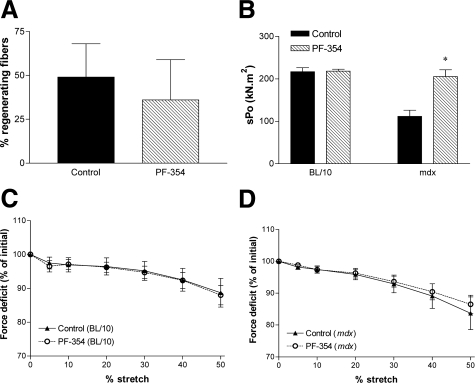
Diaphragm muscles from untreated or treated C57BL/10 mice did not exhibit any centrally nucleated fibers (Figure 2C), whereas diaphragm muscles from mdx mice comprised 49% centrally nucleated fibers (Figures 2C and 3A). There was no significant effect of 5 weeks PF-354 treatment on the percentage of centrally nucleated fibers in the diaphragm of mdx mice (Figure 3A).
Figure 3.
Percentage of regenerating fibers and functional properties of diaphragm muscle strips from young (16- to 17-day-old) C57BL/10 and mdx mice after five weeks treatment with either saline (Control) or myostatin inhibitory antibody (PF-354). Diaphragm strips from C57BL/10 mice did not exhibit any centrally nucleated fibers, and so the percentage of regenerating fibers is only shown for the mdx diaphragm (A). The specific force (sPo) was increased with PF-354 treatment in mdx mice (B). Progressive lengthening contractions (5%, 10%, 20%, 30%, and 40% stretches) leading to cumulative contraction-mediated damage in activated diaphragm muscle strips from C57BL/10 (C) and mdx mice (D). Data are means ± SEM, n = 6 to 8. *P < 0.001 versus Control.
Myostatin Inhibition via PF-354 Improves Diaphragm Muscle Function in Young mdx Mice
There was no effect of PF-354 treatment on the twitch characteristics of diaphragm strips from either young C57BL/10 or mdx mice (Table 2). Although PF-354 treatment had no effect on sPo of diaphragm muscle strips from C57BL/10 mice, sPo was increased by 84% in mdx mice (P < 0.05, Figure 3B).
Table 2.
Twitch Characteristics of Diaphragm Muscle Strips from Young (16- to 17-Day-Old) C57BL/10 and mdx Mice and from Adult (12-Week-Old) mdx Mice after Treatment with Either Saline (Control) or Myostatin Inhibitory Antibody (PF-354)
| Mice | Treatment | Pt (mN) | TPT (ms) | ½ RT (ms) | dPt/dt (mN · ms−1) |
|---|---|---|---|---|---|
| Young C57BL/10 | Control | 17.2 ± 2.5 | 38.9 ± 2.5 | 47.7 ± 4.8 | 3.4 ± 0.6 |
| PF-354 | 18.3 ± 1.2 | 40.8 ± 1.6 | 48.4 ± 3.9 | 3.3 ± 0.2 | |
| Young mdx | Control | 24.2 ± 6.8 | 46.2 ± 3.0 | 54.8 ± 6.5 | 3.9 ± 1.3 |
| PF-354 | 20.9 ± 2.2 | 43.7 ± 2.3 | 48.5 ± 4.2 | 3.7 ± 0.4 | |
| Adult mdx | Control | 35.3 ± 3.5 | 45.5 ± 2.6 | 56.5 ± 4.7 | 5.3 ± 0.5 |
| PF-354 | 34.1 ± 4.2 | 47.6 ± 1.5 | 62.7 ± 2.9 | 4.7 ± 0.4 |
Young mice were treated for 5 weeks, and adult mice were treated for 8 weeks with saline or PF-354. Pt indicates peak twitch tension; TPT, time to peak twitch tension; ½ RT, one-half relaxation time; dPt/dt, maximum rate of force development during a twitch contraction. Data are means ± SEM, n = 6 to 8. No significant differences were found between treatments.
Five weeks of PF-354 treatment had no effect on the susceptibility to contraction-mediated injury compared with controls in either C57BL/10 (Figure 3C) or mdx mice (Figure 3D).
Study 2
Myostatin Inhibition via PF-354 Increases Muscle Mass in Adult mdx Mice
Two experiments were performed using adult (12-week-old) mdx mice; one involving 5 weeks treatment (matching the treatment period for the young mdx mice) with analysis of body and muscle mass, and the other involving 8 weeks saline or PF-354 treatment with analysis of body and muscle mass and of diaphragm pathology and function.
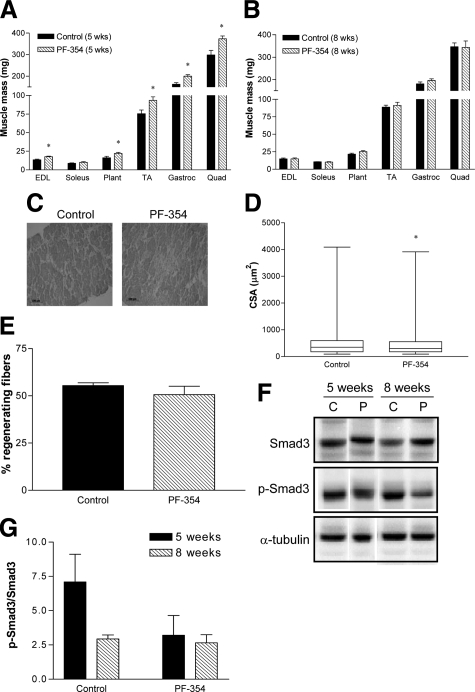
Five weeks PF-354 treatment tended to increase absolute body mass (Control, 32 ± 2 g; PF-354, 37 ± 2 g; n = 7, P = 0.07) and the percentage increase in body mass from initial, but this did not achieve statistical significance (Control, 14 ± 3%; PF-354, 22 ± 3%, n = 7, P = 0.06). However, PF-354 antibody-mediated myostatin inhibition increased the mass of the EDL (by 31%, P < 0.01), plantaris (by 35%, P < 0.05), TA (by 24%, P < 0.05), gastrocnemius (by 23%, P < 0.01), and quadriceps muscles (by 25%, P < 0.05, Figure 4A). When normalized to body mass, only the increase in EDL with PF-354 remained (by 14%, P < 0.01, data not shown). There was no effect of 5-week PF-354 treatment on absolute (P = 0.16) or relative heart mass (P = 0.52) in adult mdx mice (data not shown).
Figure 4.
Mass and histological analyses of muscles in adult (12-week-old) mdx mice after treatment with saline (Control) or myostatin inhibitory antibody (PF-354). Mass of selected hindlimb muscles in mdx mice after 5 weeks (A) or 8 weeks treatment (B). Representative images of H&E-stained tranverse sections (C), box-and-whisker plots showing the median and range of the distribution of fiber cross-sectional area (CSA) measurements (D), and the percentage of regenerating fibers (E) of diaphragm muscle strips from mdx mice after 8 weeks treatment. Representative blots (F) and group data (G) of p-Smad3/Smad3 protein abundance normalized to α-tubulin expression in adult mdx mice treated for five weeks or eight weeks with either Saline (C) or PF-354 (P). EDL indicates extensor digitorum longus; Plant, plantaris; TA, tibialis anterior; Gastroc, gastrocnemius; Quad, quadriceps. Data are means ± SEM (A, B, E, G); n = 5 to 7 animals. *P < 0.05 versus Control.
Eight weeks treatment with PF-354 had no effect on body mass (P = 0.42) or on the percentage increase in body mass from initial in adult (12-week-old) mdx mice compared with saline-treated controls (P = 0.09, Table 3). After the 8-week treatment protocol, there was no difference in either absolute mass or muscle mass relative to body mass for the TA, EDL, soleus, plantaris, gastrocnemius, and quadriceps muscles from mdx dystrophic mice (Figure 4B). The only exception was a greater relative mass of the plantaris muscle (by 13%, P = 0.04, Table 3). There was also no effect of 8 weeks PF-354 treatment on either absolute heart mass or the heart mass relative to body mass in adult mdx mice (Table 3).
Table 3.
Selected Morphometric Parameters of Adult (12-Week-Old) mdx Mice after 8 Weeks Treatment with Either Saline (Control) or Myostatin Inhibitory Antibody (PF-354)
| Morphometric Parameter | Control | PF-354 |
|---|---|---|
| Body mass, g | 37 ± 2 | 39 ± 1 |
| % increase body mass from initial | 17 ± 3 | 24 ± 3 |
| EDL/body mass, mg · g−1 | 0.40 ± 0.01 | 0.39 ± 0.04 |
| Soleus/body mass, mg · g−1 | 0.29 ± 0.01 | 0.27 ± 0.02 |
| Plantaris/body mass, mg · g−1 | 0.58 ± 0.03 | 0.66 ± 0.02* |
| TA/body mass, mg · g−1 | 2.41 ± 0.04 | 2.36 ± 0.07 |
| Gastrocnemius/body mass, mg · g−1 | 4.91 ± 0.19 | 5.08 ± 0.12 |
| Quadriceps/body mass, mg · g−1 | 9.42 ± 0.46 | 8.82 ± 0.57 |
| Heart mass, mg | 129.4 ± 3.7 | 131.6 ± 2.5 |
| Heart/body mass, mg · g−1 | 3.51 ± 0.05 | 3.42 ± 0.10 |
EDL indicates extensor digitorum longus; TA, tibialis anterior. Data are means ± SEM, n = 7.
P < 0.05 versus control.
There was a tendency for lower phosphorylation of Smad3 in TA muscles of mice treated with PF-354 for 5 weeks (P = 0.14), but this did not reach statistical significance, probably because of the low statistical power (0.23, Figure 4, F and G). There was also no effect of 8 weeks treatment with PF-354 on the phosphorylation of Smad3 (P = 0.71, Figure 4, F and G).
Myostatin Inhibition Does Not Improve Diaphragm Pathology in Adult mdx Mice
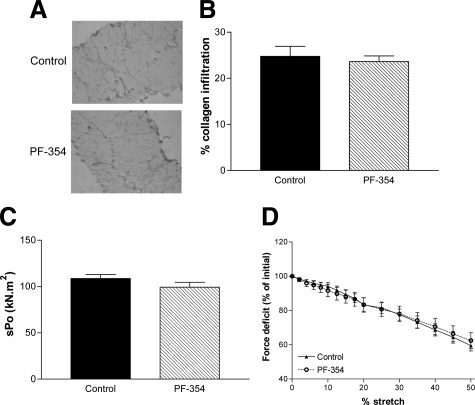
Eight weeks PF-354 treatment did not improve the pathology of diaphragm muscle strips from adult mdx dystrophic mice, compared with saline-treated controls (Figure 4C), as evident from measures of median fiber CSA (Figure 4D) and the proportion of centrally nucleated fibers (Figure 4E), with no improvements observed after PF-354 treatment. Fiber CSA was actually reduced by ≈13% (P < 0.05) after PF-354 treatment (Figure 4D).
Although diaphragm muscles from mdx dystrophic mice showed significant infiltration of collagen, there was no change after PF-354 treatment (Figure 5, A and B).
Figure 5.
Histological and functional analyses of diaphragm muscle strips in adult (12-week-old) mdx mice after eight weeks treatment with either saline (Control) or myostatin inhibitory antibody (PF-354). Representative images of Van Gieson–stained tranverse sections (A) and the percentage infiltration of collagen (B). Specific force (sPo, C) and progressive lengthening contractions (2% to 50%) leading to cumulative contraction-mediated damage (D). Data are means ± SEM, n = 6 to 7. No significant differences were found between treatment.
Myostatin Inhibition Does Not Improve Diaphragm Muscle Function or the Susceptibility to Contraction-Mediated Injury in Adult mdx Mice
Eight weeks of PF-354 treatment had no effect on the contractile characteristics of diaphragm strips from adult mdx mice including specific force (Table 2, Figure 5C). There was also no effect of PF-354 treatment on the susceptibility of the adult mdx diaphragm muscle to contraction-mediated injury (Figure 5D).
Discussion
This is the first study to our knowledge investigating whether myostatin inhibition can improve the functional characteristics of diaphragm muscle strips from mdx mice. Remarkably, PF-354 antibody mediated myostatin inhibition attenuated the normal loss of force producing capacity of the young (16- to 17-day-old) mdx diaphragm such that levels were comparable with those of healthy C57BL/10 mice. This finding has significant clinical relevance because the diaphragm of mdx mice undergoes severe muscle degeneration that most closely resembles the dystrophic pathology in DMD. Conversely, PF-354 antibody-directed myostatin inhibition did not improve the pathophysiology of the diaphragm in adult mdx mice. Thus, PF-354 antibody-directed myostatin inhibition is only beneficial for improving dystrophic diaphragm structure and function when administered during the early bouts of degeneration and regeneration.
Myostatin Inhibition Increases Muscle Mass in Young but not Adult mdx Mice
The inhibition of myostatin using the antibody, JA-16, has been shown previously to increase muscle mass by 23% to 35% in 4- to 8-week-old wild-type13 and dystrophic mdx mice.19 In the present study, we demonstrated the efficacy of the myostatin inhibitory antibody, PF-354, for inhibiting myostatin induced transcriptional activity in vitro (Figure 1A). More importantly, the efficacy of the antibody was also demonstrated in vivo because 5 weeks of myostatin inhibition with PF-354 administration increased the mass of EDL, soleus, TA, gastrocnemius, and quadriceps muscles by 18% to 28% in young mdx mice, and in young C57BL/10 mice, increased the mass of the EDL and quadriceps muscles by 35% and 36%, respectively. Because most of these increases remained when muscle mass was normalized to body mass, there was a greater effect of PF-354 in muscles of dystrophic compared with wild-type mice. PF-354 was administered for a longer period (8 weeks) in adult mdx mice to ensure that the treatment period of myostatin inhibition was sufficient to potentially reverse the dystrophic pathology. However, to make comparisons with Study 1, we also investigated the effects of 5 weeks PF-354 treatment on muscle mass in adult mdx mice. Surprisingly, 8 weeks of treatment had no effect on either absolute or relative mass of any of the hindlimb muscles tested, and 5 weeks of PF-354 treatment only increased the relative EDL mass. This finding is in contrast with those reported in 7- to 8-week-old BALB/c mice where 8 weeks of JA-16 treatment induced greater increases in muscle mass (by 7% to 9%) compared with 5 weeks of treatment.13 The lack of effect of 8 weeks PF-354 treatment for enhancing muscle mass may reflect the older age of the animals and therefore a more severe dystrophic pathology at the cessation of treatment compared with 5 weeks treatment. It may also reflect a reduced responsiveness to PF-354, with 5 weeks of PF-354 treatment inducing a trend toward lower phosphorylation of Smad3, whereas a similar trend was not seen after 8 weeks of PF-354 treatment. Interestingly, a previous study reported higher grip strength in male Mstn−/−/mdx mice compared with mdx mice at 3 months and 6 months of age, but not at 9 months of age, supporting an age-specific effect of myostatin inhibition for ameliorating the dystrophic phenotype.18 The overall finding, however, is that PF-354 treatment enhances dystrophic muscle mass when initiated before the onset of severe muscle degeneration, but not when initiated later in the disease progression. In mdx mice, the first major bout of muscle degeneration occurs at 3 to 4 weeks of age.22 Our data show that treatment was most successful when initiated at or before this period but not after.
PF-354 antibody-mediated myostatin inhibition did not cause cardiac hypertrophy in either young or adult mdx mice, or in young C57BL/10 mice. These findings are consistent with those of Cohn and colleagues32 who found no difference in heart mass, fiber CSA, or fibrosis in hearts of Mstn−/− mice, but contrast with those of Artaza and colleagues who found increased heart mass in Mstn−/− mice.33 However, the latter study found no impairment in cardiac function in Mstn−/− mice33 and more recently, aged Mstn−/− mice were reported to have improved cardiac function and reduced cardiac fibrosis compared with age-matched control mice.34 Myostatin blockade therefore does not impair cardiac function and may actually have beneficial cardiac outcomes. However, this remains to be investigated futher.7
Myostatin Inhibition Improves Diaphragm Muscle Pathology and Function in Young but not Adult mdx Mice
PF-354 antibody-mediated myostatin inhibition restored the pathology and function of the diaphragm of young mdx mice to levels comparable with those in healthy C57BL/10 mice (Figure 3B). These findings have significant implications for DMD, because respiratory failure is one of the main causes of death in affected patients and because the pathology of the diaphragm in mdx mice closely resembles that in DMD. Thus, PF-354 antibody-mediated myostatin inhibition initiated before the major episode of muscle fiber degeneration helped preserve diaphragm function and attenuate the disease progression. When PF-354 antibody mediated myostatin inhibition was initiated later in the disease progression, at a stage comparable with when DMD is diagnosed and treatments initiated, there was no improvement in the pathophysiology of the diaphragm in adult mdx mice (Figures 4 and 5). A limitation of this study is that Studies 1 and 2 were undertaken separately and did not use an identical experimental design. As such, function of diaphragm muscle strips from adult mdx mice was not assessed after the same treatment duration (5 weeks) as in young mdx mice. However, the finding that 8 weeks of treatment had little beneficial effect for diaphragm pathology and function in adult mdx mice has important clinical implications because not all DMD patients have access to early interventions and so it is imperative that the efficacy of a treatment be determined when initiated well after the disease onset. These findings expand on those observed in a different model of muscular dystrophy, the δ-sarcoglycan null mouse (scgd−/−), where 3 months of myostatin inhibition induced via an inhibitory antibody (JA16) enhanced hindlimb muscle mass and improved hindlimb and diaphragm muscle pathology in young (4-week-old) but not adult (20-week-old) scgd−/− mice. Diaphragm muscle function was not investigated in that study.17
Similar to previous studies conducted on 4-week-old mdx mice, PF-354 antibody mediated myostatin inhibition improved the pathology of the diaphragm of young mdx mice, increasing fiber CSA and reducing the infiltration of connective tissue.19,20 There was no effect of PF-354 on the proportion of diaphragm fibers with centrally located nuclei, suggesting that regeneration was not improved with PF-354 antibody mediated myostatin inhibition. This is consistent with the finding that hypertrophy associated with myostatin blockade does not involve altered satellite cell proliferation.35 The finding that PF-354 antibody-mediated myostatin inhibition induced muscle fiber hypertrophy in young but not adult mdx mice, without increasing the percentage of centrally located nuclei in either group, suggests that in contrast to young mdx mice, fiber hypertrophy in adult mdx mice may require an increase in the percentage of centrally located nuclei. This notion is consistent with the finding that satellite cell proliferation in mdx mice is maximal at 4 to 8 weeks but declines thereafter.36 Therefore, there is the capacity for treatments to enhance satellite cell proliferation and therefore induce muscle fiber hypertrophy in adult but not young mdx mice.
Taken together, our findings indicate that PF-354 antibody-directed myostatin inhibition would need to be introduced at the earliest stages of the disease progression for it to improve the pathophysiology of the diaphragm muscle.
Myostatin Inhibition Does Not Increase the Susceptibility of Diaphragm Muscle Strips to Contraction-Mediated Damage
One of the major factors contributing to the dystrophic phenotype is the cumulative muscle damage associated with repeated contractile activity, where the absence of dystrophin renders skeletal muscle fibers highly susceptible to contraction-mediated injury.2,37 The mdx diaphragm is susceptible to both eccentric and isometric contractions, and also to passive lengthenings.37 In the present study, PF-354 antibody-mediated myostatin inhibition improved the specific force of diaphragm muscle strips from young mdx mice and did not increase the susceptibility to contraction-mediated injury. There was also no effect of PF-354 antibody mediated myostatin inhibition on the susceptibility to contraction-mediated injury of diaphragm muscle strips from young C57BL/10 or adult mdx mice. Previous studies have reported no protective effect of treatment with myostatin inhibitory antibodies or propeptides on contraction-mediated injury in EDL muscles from mdx mice.19,20 It is important to emphasize that the increase in myofiber CSA after myostatin inhibition19,20 did not increase the susceptibility to contraction-mediated injury because it contradicts the supposition that larger muscle fibers are more susceptible to contraction-mediated injury and that smaller caliber muscle fibers are protected from the dystrophic pathology.2,38,39
In conclusion, myostatin inhibition with the novel antibody PF-354 attenuated the progression of the pathophysiology of the diaphragm of mdx mice when initiated early and before the onset of major muscle fiber degeneration. The findings highlight the therapeutic potential of pharmacological inhibition of myostatin in muscular dystrophy. Because in many cases DMD is not diagnosed until well into the disease progression, other strategies are required for interventions that can improve functional capacity.
Acknowledgments
We thank Tim Naim and Jennifer Trieu for expert technical assistance.
Footnotes
Address reprint requests to Gordon S. Lynch, Ph.D., Basic and Clinical Myology Laboratory, Department of Physiology, The University of Melbourne, Victoria 3010 Australia. E-mail: gsl@unimelb.edu.au.
Supported by research grants from Pfizer Global Research and Development. K.T.M. is supported by a Biomedical Australian Fellowship from the National Health and Medical Research Council (NHMRC). J.G.R. is supported by a Biomedical Overseas Research Fellowship from the National Health and Medical Research Council of Australia (520034). R.K. was supported by a Rubicon Research Fellowship from the Netherlands Organization for Scientific Research (NWO).
K.T.M. and J.G.R. contributed equally to this study.
Current address for J.G.R.: The Laboratory of Muscle Stem Cells and Gene Regulation, National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health (NIH), Bethesda, MD.
References
- Emery AE. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- Lynch GS. Role of contraction-induced injury in the mechanisms of muscle damage in muscular dystrophy. Clin Exp Pharmacol Physiol. 2004;31:557–561. doi: 10.1111/j.1440-1681.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Davies KE. The allure of stem cell therapy for muscular dystrophy. Neuromuscul Disord. 2007;17:206–208. doi: 10.1016/j.nmd.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Radley HG, De Luca A, Lynch GS, Grounds MD. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. Int J Biochem Cell Biol. 2007;39:469–477. doi: 10.1016/j.biocel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008;29:513–534. doi: 10.1210/er.2008-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Quadrupling muscle mass in mice by targeting TGF-β signaling pathways. PLoS ONE. 2007;2:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol. 2007;292:E985–E991. doi: 10.1152/ajpendo.00531.2006. [DOI] [PubMed] [Google Scholar]
- Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, Maylor R, O'Hara D, Pearson A, Quazi A, Ryerson S, Tan XY, Tomkinson KN, Veldman GM, Widom A, Wright JF, Wudyka S, Zhao L, Wolfman NM. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hadhazy M, Wehling M, Tidball JG, McNally EM. Dominant negative myostatin produces hypertrophy without hyperplasia in muscle. FEBS Lett. 2000;474:71–75. doi: 10.1016/s0014-5793(00)01570-2. [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64:940–948. doi: 10.1093/gerona/glp068. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118:3531–3541. doi: 10.1242/jcs.02482. [DOI] [PubMed] [Google Scholar]
- Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol. 2006;168:1975–1985. doi: 10.2353/ajpath.2006.051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J. 2005;19:543–549. doi: 10.1096/fj.04-2796com. [DOI] [PubMed] [Google Scholar]
- Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, Murakami T, Uezumi A, Takeda S, Noji S, Sunada Y, Tsuchida K. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008;22:477–487. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- Shavlakadze T, White J, Hoh JF, Rosenthal N, Grounds MD. Targeted expression of insulin-like growth factor-I reduces early myofiber necrosis in dystrophic mdx mice. Mol Ther. 2004;10:829–843. doi: 10.1016/j.ymthe.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Rafael JA, Hinkle RT, Cole NM, Chamberlain JS, Faulkner JA. Contractile properties of diaphragm muscle segments from old mdx and old transgenic mdx mice. Am J Physiol. 1997;272:C2063–C2068. doi: 10.1152/ajpcell.1997.272.6.C2063. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol. 2002;161:2263–2272. doi: 10.1016/S0002-9440(10)64502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt LJ, Holmes AG, Gregorevic P, Schertzer JD, Stupka N, Plant DR, Lynch GS. Interleukin-15 administration improves diaphragm muscle pathology and function in dystrophic mdx mice. Am J Pathol. 2005;166:1131–1141. doi: 10.1016/S0002-9440(10)62333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertzer JD, Gehrig SM, Ryall JG, Lynch GS. Modulation of insulin-like growth factor (IGF)-I and IGF-binding protein interactions enhances skeletal muscle regeneration and ameliorates the dystrophic pathology in mdx mice. Am J Pathol. 2007;171:1180–1188. doi: 10.2353/ajpath.2007.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22:467–475. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- Koopman R, Zorenc AHG, Gransier RJJ, Cameron-Smith D, van Loon LJC. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol. 2006;290:E1245–E1252. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- Sim J, Reid N. Statistical inference by confidence intervals: issues of interpretation and utilization. Phys Ther. 1999;79:186–195. [PubMed] [Google Scholar]
- Cohn RD, Liang HY, Shetty R, Abraham T, Wagner KR. Myostatin does not regulate cardiac hypertrophy or fibrosis. Neuromuscul Disord. 2007;17:290–296. doi: 10.1016/j.nmd.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artaza JN, Reisz-Porszasz S, Dow JS, Kloner RA, Tsao J, Bhasin S, Gonzalez-Cadavid NF. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol. 2007;194:63–76. doi: 10.1677/JOE-07-0072. [DOI] [PubMed] [Google Scholar]
- Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8:573–583. doi: 10.1111/j.1474-9726.2009.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci U S A. 2009;106:7479–7484. doi: 10.1073/pnas.0811129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD, Partridge TA, Morgan JE. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpati G, Carpenter S. Small-caliber skeletal muscle fibers do not suffer deleterious consequences of dystrophic gene expression. Am J Med Genet. 1986;25:653–658. doi: 10.1002/ajmg.1320250407. [DOI] [PubMed] [Google Scholar]
- Gehrig SM, Koopman R, Naim T, Tjoakarfa C, Lynch GS. Making fast-twitch dystrophic muscles bigger protects them from contraction injury and attenuates the dystrophic pathology. Am J Pathol. 2010;176:29–33. doi: 10.2353/ajpath.2010.090760. [DOI] [PMC free article] [PubMed] [Google Scholar]