Abstract
In this study we observed that mice pretreated with tumor exosomes had a significant acceleration of tumor metastasis in the lung. Tumor metastasis correlated significantly with an increase in recruitment of more Myeloid-derived suppressor cells (MDSCs) in the lung of C57BL/6j (B6) mice pretreated with tumor exosomes. These effects were blunted when MyD88 knockout (KO) mice were pretreated with tumor exosomes. MDSCs induced by tumor exosomes and isolated from wild-type B6 mice also more potently inhibited T cell activation and induction of interleukin-6 and tumor necrosis factor-α than MDSCs isolated from the lung of MyD88 KO mice. In vitro, addition of tumor exosomes to bone marrow-derived CD11b+ Gr-1+ cells isolated from wild-type B6 mice resulted in more cytokine production, including tumor necrosis factor-α, interleukin-6, and the chemokine CCL2, than CD11b+Gr-1+ cells isolated from MyD88 KO mice. Moreover, lower levels of CCL2 were observed in the lungs in MyD88 KO mice pretreated with tumor exosomes than that in wild-type mice. Together these data demonstrate a pivotal role for MyD88 in tumor exosome-mediated expansion of MDSCs and tumor metastasis.
Numerous studies have provided convincing evidence supporting the notion that inflammatory processes play a role in tumorigenesis.1,2,3,4 Besides extraneous agents like bacteria, recent studies have shown that tumor cells releasing membrane microvesicles, also known as exosomes, can stimulate inflammation through induction of Myeloid-derived suppressor cells (MDSC) derived interleukin-6 and tumor necrosis factor-α (TNF-α).5,6 Tumor exosome-mediated induction of inflammatory cytokines, such as interleukin-6 and TNF-α, further promotes expansion of MDSCs, which adversely effects antitumor immunity and promotes tumor growth by activating immunosuppressive networks.7,8,9,10,11,12,13,14 However, the signaling pathway(s) that leads to tumor exosome-mediated induction of proinflammatory cytokines and subsequent expansion of myeloid derived suppressor cells has not been identified.
MyD88 is a cytoplasmic adaptor molecule essential for integrating and transducing the signals generated by the Toll-like receptor (TLR) family. Ultimately, the activation of transcription factors like nuclear factor κB occurs and permits the transactivation of proinflammatory cytokine genes.15 Although a few endogenous ligands for the TLR pathway have been proposed,16 their role in tumor progression has not been addressed. Very little is known about whether tumor exosome-mediated induction of interleukin-6 and TNF-α in MDSCs is regulated through MyD88. Such knowledge is important because MyD88 mediated innate immune responses play a role in tumorigenesis.17,18 Therefore, we investigated the involvement of MyD88 in the immune response to tumor exosomes by using a B16 melanoma tumor metastasis model. Based on our results MyD88 is essential for tumor exosome-mediated induction of interleukin-6, TNF-α, induction of MDSCs, and tumor metastasis.
Materials and Methods
Animals
C57BL/6j (B6) male mice were purchased from the Jackson Laboratories (Bar Harbor, ME). MyD88, TRIF, TLR2, and TLR4 male mice on a B6 background were kindly provided by Dr. Shizuo Akira (University of Osaka, Osaka, Japan). All of the animal experiments were performed humanely under protocols approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Purification of Exosomes from B16-luc Cells, 4T-1-luc, and 16-Day Embryonic Fibroblasts of B6 and BALB/c Mice
Exosomes from 36-hour culture supernatants of B16-luc tumor cells19 were purified by using gradient centrifugation followed by a sucrose gradient method described previously.5 After a final wash with PBS, exosomes were resuspended in PBS and centrifuged at 10,000 × g for 5 minutes to remove aggregated exosomes. Nonexosomal fractions (fractions 6 and 7)5 from the sucrose gradient served as a control (E-control) for experiments. To test if tumor exosome-mediated promotion of tumor metastasis is also applicable to a breast cancer model, exosomes from 36-hour culture supernatants of 4T-1 breast tumor cells were also purified by using an identical method described for B16-luc exosomes. In addition, exosomes from cultured fibroblasts isolated from 16-day embryos of B6 mice or BALB/c mice were also used as controls. The protein content of the exosomes and E-control was determined by using a bicinchoninic acid protein assay kit (Bio-Rad Laboratories, Hercules, CA). The samples were aliquoted and stored at −80°C until used.
In addition to the precautions described previously20 and to further minimize potential contamination in the process of exosomal purification, exosomal endotoxin levels were quantified by using the limulus amebocyte lysate assay (Associates of Cape Cod, Inc., Falmouth, MA) according to manufacturer’s protocol. Endotoxin was undetectable in all samples used in this study, including exosomes and E-control.
Exosome Effects on the Differentiation of Bone Marrow Precursor Cells
Bone marrow (BM)-derived precursor cell cultures were prepared and differentiated from wild-type or MyD88, TRIF, TLR2, and TLR4 knockout (KO) mice in the presence of Granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/ml) plus B16 exosomes, EF-exosomes, or E-control (10 μg/ml) as described previously.5 After 7 days, cell cultures were stained with anti-CD11b, Gr-1, CD11c, and MHC-II antibodies and analyzed by using Fluorescence activated cell sorting (FACS). Cell culture supernatants were collected and assayed by enzyme-linked immunosorbent assay (ELISA) for cytokine induction.
Cell Migration Assays
Twenty-four hours cultured B16-luc cells were added to the upper chambers of 24-well transwell plates having a 5-μm pore membrane (Corning, Corning, NY). The lower chambers contained 7-day cultured BM precursor cells treated with exosomes purified from the supernatants of cells, including B16-luc melanoma cells, 4T-1-luc, TS/A-luc murine breast tumor cells, and B6 derived EF primary fibroblasts (10 μg/ml) in the presence or absence of hamster anti-mouse CCL2 antibody (1 μg/ml; the dose used is based on the manufacturer’s instructions, e-Bioscience, San Diego, CA). The culture medium was RPMI 1640. Seven-day cultured BM precursor cells treated with the exosome diluent (PBS) served as a control. The transwell cultures were incubated for 24 hours at 37°C in 5% CO2 and then the BM precursor cells in the bottom chamber were lysed, and luciferase activity was determined by using a chemiluminescence assay according to the manufacturer’s instructions (Promega, Madison, WI). The cell lysates harvested at 0 hours were also used for quantification of luciferase activity as a control for initial nonspecific activity. Percent specific luciferase activity in the bottom chambers was calculated as follows: percent luciferase activity = (counts per 5 seconds [bottom chamber of BM precursor cells treated with exosomes or E-control]/counts per 5 seconds of cells treated with PBS) × 100.
Cytokine Measurements
Quantification of mouse serum interleukin-6, TNF-α, and CCL2 was done by using a commercially available ELISA kit (e-Bioscience). All sera samples were analyzed in triplicate. Cell culture supernatants were analyzed for cytokines levels by using a Bio-Plex mouse cytokine 39-plex array (Millipore, Bedford, MA) according to the manufacturer’s instructions. The data were analyzed by using Bio-Plex Manager software (Bio-Rad Laboratories).
Lungs of mice were harvested. The right lungs were harvested and homogenized in 1.0 ml of lysis buffer containing 0.5% Triton X-100, 150 mmol/L NaCl, 15 mmol/L Tris, 1 mmol/L CaCl2, and 1 mmol/L MgCl2, pH 7.4. The supernatant was collected and kept at −80°C. Cytokines were measured by using commercial ELISA kits according to the manufacturer’s protocols (e-Bioscience). The left lungs were fixed, subjected to H&E staining, and the lung metastases were quantified by using a method described below.
H&E Stained Lung Section
Mouse lungs were fixed in 10% buffered formalin, subjected to H&E staining, and the lung metastases per H&E stained slide were quantified by using a microscope. Five slides per lung tissue blot were randomly examined for counting total numbers of tumor metastases. The data are presented as mean ± SEM of tumor metastases per slide.
Western Blotting Analysis of Stat3
Western blot analysis of total Stat3 and phosphorylated Stat3 in B16 exosomes, EF-exosomes, or E-control-treated BM cells has been described in detail previously.5 In brief, 2-day cultured BM cells treated with B16 exosomes, EF-exosomes, or E-control were lysed in radioimmunoprecipitation assay lysis buffer and total cell lysate (50 μg protein) was used in Western blot analysis for total Stat3 and phosphorylated Stat3 (BD Biosciences, San Jose, CA). Blotted proteins were detected by using an Odyssey infrared imaging system (LI-COR, Lincoln, NE).
In Vivo Induction of CD11bGr-1 Myeloid Cells
B6 wild-type mice or MyD88 knockout mice were intravenously injected with exosomes, EF-exosomes, or E-control (100 μg/mouse) isolated from the 36-hour culture supernatants. Mice received two injections of the exosomes or E-control per week for 3 weeks. One day after the last injection, the following analyses were done: B16-luc tumor cells migrating and remaining in the lung (in vivo luciferase imaging); ability of induced CD11bGr-1 myeloid cells to inhibit CD8 T cell tumor cytotoxicity (luciferase releasing assay); induction of CD11bGr-1 myeloid cells in the lung and liver (FACS based assay); and induction of serum interleukin-6 and TNF-α (ELISA).
In Vivo Luciferase Imaging
B6 mice or MyD88 KO mice treated as described above were injected with B16-luc cells (1 × 105/mouse) via the tail vein. Using identical protocols, BALB/c mice were intravenously injected twice per week for 3 weeks with 4T-1-luc exosomes or EF-exosomes (100 μg/mouse) and 1 day after the last exosome treatment, mice were injected subcutaneously with 1 × 105 4T-1-luc cells in the right flank. Twenty-one days after tumor cell injection, mice were injected intraperitoneally for each imaging session with 2.5 mg D-luciferin dissolved in 100 μl PBS. Whole body images were taken 10 minutes after the D-luciferin injection. The animals were repeatedly imaged in the same position for 2 minutes by using an IVIS-100 imaging system (Xenogen, Alameda, CA) to acquire photons of light emitted from the mice. Regions of luciferase signal interest were analyzed by using Living Image 2.50 software (Xenogen), and the results were expressed as units of relative photon counts per second. To determine the intensity of the signal for the area analyzed, the signal-to-noise ratios were calculated as follows: the area-weighted light signal of selected areas for exosome treated mice was compared with the E-control treated groups and the ratio was calculated. This experiment was repeated once with eight additional mice.
CD8 T Cell Proliferation Assay
Mice were injected intravenously with B16 exosomes, EF-exosomes, or E-control (100 μg in 100 μl of PBS) twice a week for 3 weeks. One day after the last injection, leukocytes were isolated from the lungs of mice by using a previously described method.21 CD11b+Gr1+ cells from isolated leukocytes were then FACS sorted to >95% purity and seeded in triplicate at 5 × 105 cells per well. CD8 cells were prepared from naïve spleens by negative selection by using a magnetic bead sorting (MACS) CD8-negative selection kit (Miltenyi, Auburn, CA) to achieve at least 90% purity. Sorted CD8 T cells were co-cultured at 37°C in a CO2 incubator with FACS sorted CD11bGr-1 cells (1:5 ratio) in the presence of anti-CD3 antibody (clone 145-2C11; 1 μg/ml) and pulsed at days 4 and 6 after the culture for 18 hours with 4 μCi/ml [3H]thymidine to assess their suppression of T cell proliferation.
Isolation of Leukocytes from Liver and Lung
The methods used for isolation of liver and lung leukocytes have been described in detail elsewhere,21 and isolated leukocytes were stained with anti-CD11bGr-1 antibody.
Blocking CCL2 with Neutralizing Antibody
Mice were injected intraperitoneally every 4 days from the date of tumor cell inoculation to the end of the experimental period with hamster anti-mouse CCL2 (eBioscience) neutralizing antibody or control hamster IgG.
Statistical Analysis
Results were reported as mean ± SEM. A two-sided independent Student’s t-test without equal variance assumption or nonparametric Mann-Whitney test was performed to analyze the data with P < 0.05 considered as statistically significant.
Results
MyD88 Dependent and Tumor Exosomes-Mediated Inhibition of Myeloid Cell Differentiation
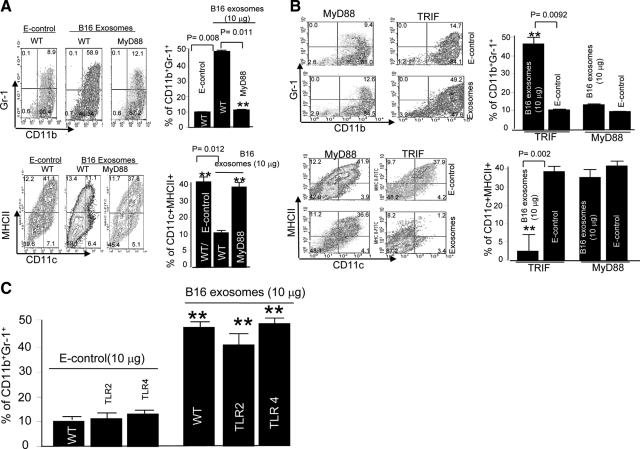
We previously showed that interleukin-6 plays a role in tumor exosome-mediated inhibition of myeloid cell differentiation.5 Given the importance of MyD88 in induction of interleukin-6,15 we wished to determine whether deficiency in this key adaptor protein impacted the ability of tumor exosomes to inhibit myeloid cell differentiation into dendritic cells. As shown in Figure 1A (top panel), using protocols identical to those described previously,5 we found that B16 melanoma exosomes do not have an inhibitory effect on the differentiation of BM precursor cells isolated from MyD88 KO mice. In contrast, an inhibitory effect is observed with BM precursor cells isolated from wild-type B6 mice.
Figure 1.
MyD88 dependent and tumor exosome-mediated blocking of the differentiation of GM-CSF-stimulated BM precursor cells. Erythrocyte-depleted BM cells of wild-type B6 mice or B6 mice with specific gene knockouts listed in A and B, were cultured in RPMI 1640 supplemented with 10% FBS and 20 ng/ml recombinant mouse GM-CSF. B16 exosomes or E-control (10 μg/ml) were added at day 0 after the addition of the GM-CSF to the cell cultures (A–C). After seven days in culture, the cells were analyzed by FACS for the expression of CD11b, Gr-1, CD11c, and MHCII. One representative of five independent experiments is shown (A–C). All data are given as the average values ± SEM obtained for five samples in three independent experiments (A–C). **P < 0.01.
This result was further supported by the evidence that B16 melanoma exosomes do not cause a significant reduction in the percentage of dendritic cells (CD11C+MHCII+; Figure 1A, bottom panel); ie, B16 exosome exposure to MyD88 knockout BM derived myeloid cells resulted in 39.4 ± 2.7% CD11C+MHCII+, whereas E-control exposure resulted in 43.1 ± 3.6% CD11C+MHCII+.
We further sought to determine whether KO of TRIF also affected the BM precursor cell differentiation because both MyD88 and TRIF molecules can be used as adaptors for the TLR signaling pathways. KO of MyD88, but not TRIF, had no significant effect on B16 exosome promotion of CD11b+Gr-1+ MDSC accumulation (Figure 1B, top panel) as well as blocking dendritic cells (DC) differentiation (Figure 1B, bottom panel), suggesting that this response is MyD88 specific.
The inhibition of myeloid cell differentiation is exosome specific because there was a significant difference in the percentages of CD11b+Gr-1+ cells; ie, exposure to B16 exosomes resulted in 47.4 ± 2.3% CD11b+Gr-1+ cells, whereas exposure to E-control resulted in 9.7 ± 1.1% CD11b+Gr-1+ cells (P < 0.008; Figure 1A, top panel). For CD11C+MHCII+ cells, B16 exosome exposure resulted in 11.4 ± 1.2% CD11C+MHCII+, whereas E-control exposure resulted in 42.0 ± 2.2% CD11C+MHCII+ (P < 0.012; Figure 1A, bottom panel).
Because MyD88 regulates both the TLR2 and TLR4 mediated pathways,22 we reasoned that a B16 exosome-mediated inhibition of myeloid differentiation into dendritic cells in TLR-deficient mice would be impaired. However, B16 exosome treatment of BM precursor cells isolated from TLR2 and TLR4 KO mice resulted in a significant inhibition of the precursor cell differentiation to dendritic cells (Figure 1C), suggesting that neither TLR2 nor TLR4 plays a role in B16 exosome-mediated induction of immature myeloid cells. Like the results from wild-type B6 mice, when BM precursor cells isolated from TLR4 and TLR2 KO mice were exposed to E-control, neither inhibition of myeloid cell differentiation nor dendritic cell maturation was observed (the results not shown). This suggests that the inhibition of myeloid cell differentiation is exosome specific.
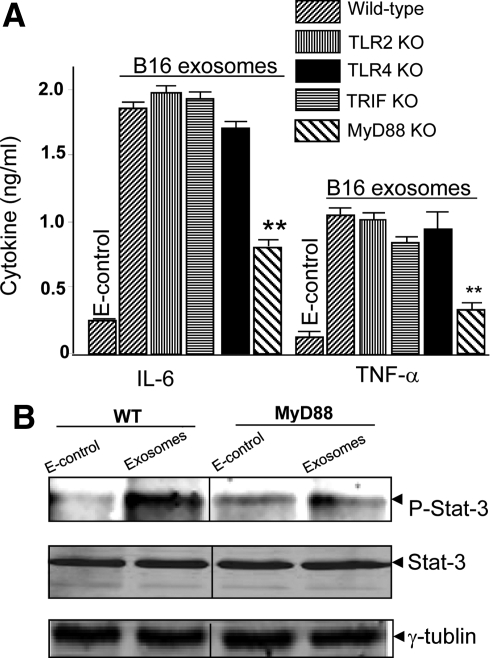
Our previous study indicated that tumor exosomes promote the induction of MDSCs in an interleukin-6 dependent manner via activation of the Stat3 pathway.5 Therefore, we analyzed whether MyD88 also regulates B16 exosome-mediated induction of interleukin-6 in BM precursor cells. The quantity of interleukin-6 (Figure 2A, left panel) and TNF-α (Figure 2A, right panel) was not significantly different in the cell culture supernatants of BM precursor cells isolated from MyD88 mice and cultured for 2 days after the addition of B16 exosomes or E-control (Figure 2A). In contrast, addition of tumor exosomes to BM precursor cells isolated from B6 wild-type and other TLR knockout mice, as noted in Figure 2A, resulted in a significant induction of interleukin-6 and TNF-α production (Figure 2A). Significant attenuation of tumor exosome-mediated induction of interleukin-6 is also associated with a reduction of phosphorylated Stat3 that is induced by B16 exosome inclusion in cultures of BM precursors derived from MyD88 KO mice (Figure 2B). These results were consistent with the data published previously with exosomes isolated from 4T-1 and TS/A murine breast cancer cell lines.5
Figure 2.
MyD88 dependent induction of proinflammatory cytokines and stat3 activation. Supernatants of two-day cultures treated as described in Figure 1, A–C, were collected, and the amount of interleukin-6 (A; left) and TNF-α (A; right) was measured by using a standard ELISA. **P < 0.01. To determine the effect of MyD88 on B16 exosome-mediated induction of phosphorylated Stat3, CD11b+ cells were treated with B16 exosomes or E-control (10 μg/ml), and phosphorylated Stat3 was detected in 48-hour cultured cells by Western blot analysis (B). Total cell lysates (100 μg/lane) were also used for determination of total Stat3 (B; middle) or γ-tubulin (B; bottom) by Western blot analysis.
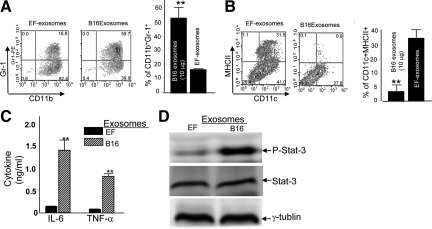
The tumor exosome specific in vitro induction of MDSCs, inhibition of DC differentiation, proinflammatory cytokines, including interleukin-6 and TNF-α, and activation of stat3 were further investigated by using identical approaches as described above. As expected, the addition of B16 exosomes to the BM cultures led to the induction of MDSCs (Figure 3A), the inhibition of DC differentiation (Figure 3B), and the induction of both interleukin-6 and TNF-α (Figure 3C). These phenotypes are associated with the activation of Stat3 (Figure 3D). In contrast, the addition of exosomes isolated from the cultured supernatants of embryonic fibroblasts lack the phenotypes induced by B16 exosomes (Figure 3). Collectively, these data suggest that the MyD88 mediated pathway plays a role in B16 tumor exosome-mediated inhibition of myeloid cell differentiation.
Figure 3.
Tumor exosome specific-mediated blocking of the differentiation of GM-CSF-stimulated BM precursor cells. B16 exosomes or EF-exosomes (10 μg/ml) were added at day 0 after the addition of the GM-CSF to the cell cultures (A–C). Exosomal effects on the production of CD11b+Gr-1+ cells (A), dendritic cells (B), interleukin-6 and TNF-α (C), and activation of Stat3 (D) were evaluated by using identical procedures as described in Figures 1 and 2. Data represent mean ± SEM of three samples in three independent experiments. **P < 0.01.
In Vivo MyD88 Dependent and Tumor Exosome-Mediated Expansion of MDSCs
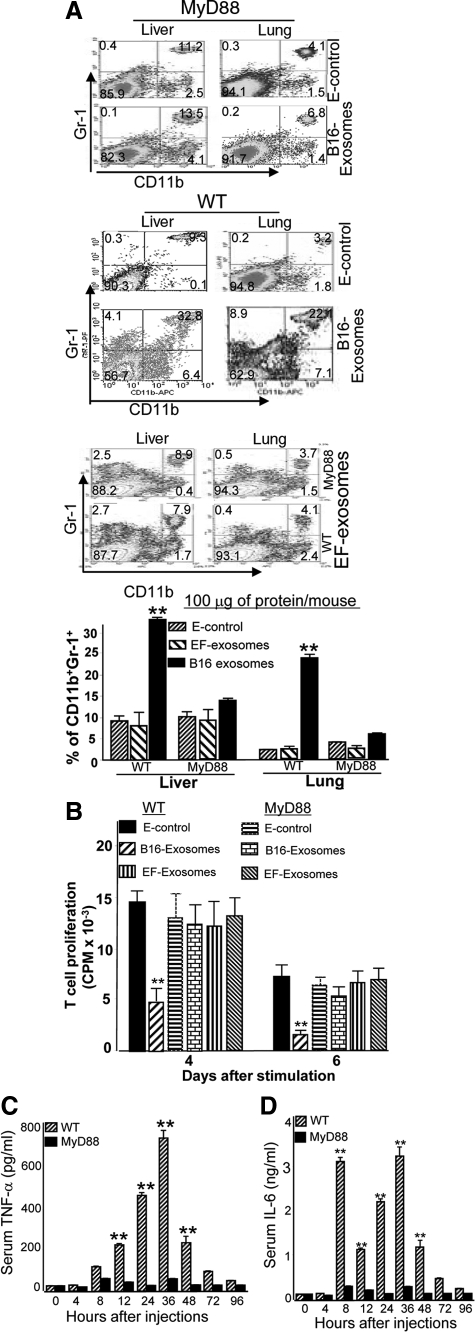
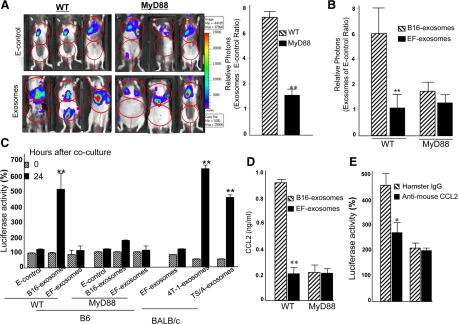
Experiments were conducted to determine whether in vivo B16 exosome-mediated accumulation of MDSCs and induction of interleukin-6 and TNF-α was MyD88 dependent. Figure 4A (upper and bottom panels) shows that there was no significant accumulation of CD11b+Gr-1+ cells in the liver and lung in MyD88 KO mice treated with B16 exosomes or E-control. MyD88 mediated attenuation of responses to exosomes is B16 exosome-specific as evidenced by the fact that wild-type B6 mice injected intravenously with B16 tumor exosomes (middle panel), but not EF-exosomes (bottle panel), results in an accumulation of CD11b+Gr-1+ cells in the liver and lung.
Figure 4.
Intravenous injection of tumor exosomes leads to the induction of MDSCs via a MyD88-mediated pathway. A total of 100 μg of B16 exosomes, EF-exosomes, or E-control in 100 μl of PBS was injected into MyD88 knockout and B6 wild-type mice via the tail vein twice a week for three weeks. One day after the last injection, the mice were sacrificed, and the percentages of CD11b+Gr-1+ cells in lung and liver tissues were determined by FACS analysis (A). CD11b+Gr-1+ cells (5 × 105) were isolated from the lung by FACS sorting as described in the Materials and Methods, and the isolated cells were co-cultured with splenic T cells at a ratio of 5:1 (B). Cell proliferation was measured by [3H]thymidine incorporation after four and six days in culture. Data are presented as the mean ± SEM of triplicate wells representing three independent experiments. B6 wild-type or MyD88 mice were intravenously injected with B16 exosomes (100 μg/mouse in 100 μl of PBS). Sera were collected up to 96 hours after injection (C and D) and analyzed for TNF-α (C) and interleukin-6 (D) by using an ELISA. Data represent mean ± SEM of five mouse sera in two independent experiments. **P < 0.01.
MDSCs are well known to suppress T cell proliferation14,23,24; therefore, we analyzed the ability of B16 exosome induced MDSCs to affect the proliferation of CD8 T cells. T cells isolated from the spleens of naïve B6 mice were stimulated up to 6 days with anti-CD3 mAb in the presence or absence of CD11b+Gr-1+ cells (T cells : MDSCs = 1:5) isolated from the lung of B6 wild-type mice or MyD88 KO mice pretreated with B16 exosomes, EF-exosomes, or E-control. Proliferation of T cells was evaluated by using a [3H]thymidine incorporation assay. Apparent from these experiments was that the presence of MDSCs isolated from spleen of B6 mice treated with B16 exsomes inhibits normal T cell proliferation to anti-CD3 stimulation; however, this is not the case when EF-exosomes or E-control are used (Figure 4B). In contrast, no significant suppression of T cell proliferation was observed when CD11b+Gr-1+ cells isolated from MyD88 KO mice pretreated with B16 exosomes were tested (Figure 4B). These data suggest that the in vivo induction of CD11b+Gr-1+ cells by B16 exosomes in a MyD88 dependent fashion could inhibit T cell proliferation. Intravenous injection of B16 melanoma exosomes led to a rapid induction of TNF-α (Figure 4C) and interleukin-6 (Figure 4D) in B6 wild-type mice but not in MyD88 KO mice. Interestingly, induction of TNF-α peaks at 36 hours after injection (Figure 4C), whereas induction of interleukin-6 has two peaks, one at 8 hours and one at 36 hours after injection (Figure 4D).
The Effect of Tumor Exosomes on Lung Metastasis Is Attenuated in the Absence of MyD88
Our previous work21 and that of others suggest6,25 that mice pretreated with tumor exosomes have a more rapid tumor metastasis to the lung, and that accumulation of CD11b+Gr-1+ cells in tumor bearing mice plays a role in tumor lung metastasis.6,21 To determine whether MyD88 plays a role in B16 exosome-mediated tumor metastasis in the lung, the metastasis of B16-luc cells to the lung after intravenous injection was determined by quantifying in vivo luciferase activity. In vivo quantification of luciferase accumulation in the lungs of mice at day 21 after tumor cell injection showed an increased tumor burden in the B6 animals pretreated with B16 exosomes when compared with mice pretreated with E-control (Figure 5A). The aforementioned results were different from the results obtained for MyD88 mice pretreated with B16 exosomes or E-control; in the latter case there was no significant difference in the tumor burden when comparing B16 exosomes and E-controls between the sources of the exosomes (Figure 5A). Although we observed that only one out of nine MyD88 KO mice receiving tumor exosomes had obvious ascites develop (Figure 5A), no tumor was identified in the peritoneum. Because we do not know what causes large amounts of ascites to be produced, the imaging data generated from this mouse was treated as an outlier. To further investigate the specificity of tumor exosome-mediated enhancement of tumor metastasis to the lung, in vivo luciferase activity of injected B16-luc cells was determined after wild-type B6 or MyD88 KO mice were treated with either B16 exosomes or EF-exosomes by using identical protocols as described in Figure 5A. Unlike B16 exosomes, the results indicate that neither EF-exosomes nor E-control promote B16 tumor metastasis (Figure 5B). The depletion of Gr-1+ cells in B6 mice led to a reduction in the promotion of tumor growth in the lung of wild-type B6 mice pretreated with B16 exosomes (data not shown). Gr-1+ myeloid suppressor cells have been shown to promote tumor metastasis to the lung by releasing a number of chemotactic proteins.26,27 These results raise the possibility that B16 melanoma exosome-induced MDSCs may release an array of chemotactic proteins that attract tumor cells to the vicinity where MDSCs have accumulated or are accumulating. To test this concept, B16-luc tumor cells were co-cultured in transwell chambers with BM precursor cells pretreated with exosomes released from B16 melanoma tumor cells and B6 derived EF primary cells. We found that BM myeloid cells pretreated with B16 exosomes produce chemoattractants for B16-luc cells; however, this was not the case for the EF-exosomes or E-control (Figure 5C). Chemotaxis was mediated by a MyD88 pathway because migration was significantly reduced in co-cultures of B16-luc tumor cells with BM precursor cells isolated from MyD88 KO mice. Using Bioplex cytokine array technology, we further identified that CCL2 in 7-day cultured BM myeloid cells pretreated with B16 exosomes was up-regulated more than fivefold in comparison with MyD88 KO BM myeloid cells pretreated with B16 exosomes. This preliminary data were further confirmed by a standard ELISA (Figure 5D). To confirm the contribution of CCL2 to B16 exosome-mediated myeloid cell chemotaxis, CCL2-neutralizing Abs or control IgG were added to co-cultures and cultured as described above. Neutralization of CCL2 significantly decreased the migration of B16-luc cells to the myeloid cells (Figure 5E). To determine whether CCL2 is reduced in the lung of B16 tumor exosome-mediated lung metastasis, lung extracts from MyD88 KO mice, as well as wild-type B6 mice, were used for ELISA quantification of CCL2. The results of quantifying CCL2 (n = 9) at day 21 after tumor cells were injected indicated that KO of MyD88 significantly reduces CCL2 amounts in comparison with wild-type B6 mice (100 ± 8.2 pg/ml of lung tissue versus 1249 ± 27.1 pg/ml of lung tissue).
Figure 5.
MyD88 adaptive molecule plays a role in tumor exosome-mediated promotion of B16 tumor cell growth in the lung and in tumor chemotaxis. Seven-week-old wild-type B6 or MyD88 knockout mice were injected intravenously with B16 exosomes (100 μg/mouse in 100 μl of PBS) or E-control twice a week for three weeks. One day after the last injection, the mice were injected intravenously with B16–luc cells (1 × 105). Twenty-one days after B16-luc injection, the mice were imaged at 0 and 4 hours (A) after injection of D-luciferin, and the total photon count per minute (photons per minute) was calculated (nine animals/group, three representative mice are shown) by using Living Image software (A; left). The growth potential of injected B16–luc cells was determined by dividing photon emissions at 4 hours by the photon emissions at 0 hours (A; right) for mice treated with exosomes or the E-control. To determine the specificity of B16 exosome-mediated promotion of B16-luc growth in the lung, mice were treated with EF-exosomes or B16 exosomes by using an identical protocol as described in A. In vivo luciferase activity was measured as described in A. All results in B are based on two independent experiments with data pooled for mice in each experiment. Results in both A and B are presented as the mean ± SEM; **P < 0.01. Seven-day cultured BM precursor cells isolated from B6 wild-type mice or BALB/c mice and treated as described in Figure 2A were co-cultured with B16-luc or 4T-1-luc in a 24-well transwell plate. Twenty-four hours later the cells in the bottom chamber were harvested, lysed, and luciferase activity was determined. Results are presented as percent of luciferase activity. Data presented are mean ± SEM of triplicates wells for three independent experiments (C). Supernatants of seven-day cultures treated as described in Figure 1, A–C, were collected, and the amount of CCL2 (D) was measured by using a standard ELISA. Data represent mean ± SEM of three samples in three independent experiments. **P < 0.01. Seven-day cultured BM precursor cells treated as described in Figure 2A were co-cultured with B16-luc in the presence of either anti-mouse CCL2 (1.0 μg/ml) or a control hamster IgG in a 24-well transwell plate. Twenty-four hours later the cells in the bottom chamber were harvested, lysed, and luciferase activity was determined. Results are presented as percent of luciferase activity. Data presented are mean ± SEM of triplicates wells for three independent experiments (E).
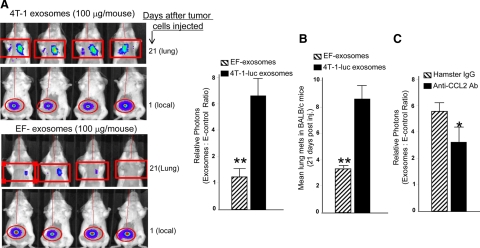
We then further assessed the influence of murine breast tumor exosomes on lung metastases by using the metastasizing cell line 4T-1-luc that stably expresses luciferase protein. Mice were intravenously injected with 4T-1 exosomes or BALB/c derived EF-exosomes by using the same protocols as described for B16 exosome treatment in Figure 5A. 4T-1-luc tumor cells were then injected into the right flank of BALB/c mice. The next day, mice were imaged to verify that approximately the same numbers of tumor cells were injected (Figure 6A, top panels). On day 10 after injection of 4T-1-luc tumor cells, the tumor was surgically removed. Tumor metastasis to the lung was quantified by measuring in vivo luciferase activity at day 21 after tumor cells were injected. In vivo quantification of luciferase accumulation in the lungs showed an increased tumor burden in the BALB/c animals pretreated with 4T-1 exosomes when compared with mice pretreated with EF-exosomes (Figure 6A, bottom panels). In agreement with the luciferase activity in the lung, the numbers of tumors in the lung were significantly (P < 0.01) increased in the mice administered 4T-luc exosomes (Figure 6B). Furthermore, mice treated with neutralizing anti-CCL2 antibody also demonstrated a reduction in luciferase activity in the lung (Figure 6C), suggesting that CCL2 plays a role in tumor exosome-mediated enhancement of 4T-1 tumor metastasis to the lung.
Figure 6.
Tumor exosome-mediated promotion of 4T-1 tumor metastasis in the lung. A: A total of 100 μg of 4T-1-luc exosomes or EF-exosomes in 100 μl of PBS was injected into BALB/c female mice via the tail vein twice a week for three weeks. One day after the last injection, 1 × 105 4T-1-luc cells were injected in the right flank of each mouse. The next day, to verify that approximately equal numbers of tumor cells were injected, mice were imaged by using a luciferase imaging assay as described in Figure 5A. A representative image from each group of mice is shown (A; top panels of each group). Ten days after 4T-1-luc injection, tumors were removed. Twenty-one days after tumor cells were injected, in vivo luciferase activity in the lung was determined by using an identical protocol as described in Figure 5A. Representative images of mice are shown (A; bottom panels of each group). The results are based on two independent experiments with data pooled for eight mice in each experiment. Results are presented as the mean ± SEM. **P < 0.01 (A; bar graph). Lung metastases on stained slides were counted manually by using a microscope, and the mean number per section was plotted (B). Statistical significance was analyzed by the nonparametric Mann Whitney t-test. Mice were treated with exosomes as described in A, and then identical protocols as described in Figure 5A were used for 4T-1-luc tumor cell injection and tumor removal. One day after tumor cells were injected, mice were intravenously injected at dose 10 mg/kg of body weight of mouse with hamster anti-CCL2 antibody or a control hamster IgG antibody every four days until the end of the mouse experiment. Mice were imaged at day 21 after the tumor cells were injected. The results are based on two independent experiments with data pooled for eight mice in each experiment. Results are presented as the mean ± SEM (C). *P < 0.05.
Discussion
The decreased cancer susceptibility associated with MyD88-deficiency has generally been attributed to its importance in regulation of inflammatory responses that promote tumor growth.17,28,29 Tumor exosomes have been shown to be potent inducers of proinflammatory cytokines such as TNF-α and interleukin-6.5,21 We report here that tumor exosomes markedly induce TNF-α and interleukin-6 production in a MyD88 dependent manner in MDSCs derived from BM precursor cells. Knockout of MyD88 significantly reduces tumor exosome-triggered production of interleukin-6, TNF-α, and CCL2 in BM precursor cell derived MDSCs; therefore, few MDSCs are induced. Intravenous injection of tumor exosomes significantly decreases production of serum proinflammatory cytokine interleukin-6, results in a decreased induction of MDSCs in the lung, and results in a slower growth of metastatic lung tumor in MyD88 KO mice. CCL2 released from MDSCs induced by tumor exosomes plays a role in lung metastasis. MyD88 regulates the expression of CCL2 in MDSCs. These results suggest that “cross talk” between the MyD88 mediated pathway and tumor exosomes is needed for promotion of tumor metastasis via a proinflammatory cytokine driven expansion of MDSCs.
Our results are in agreement with previous observations showing that tumor exosomes not only block BM derived DC differentiation,5,21 but also promote the induction of MDSCs.5,21 The induction of MDSCs is partially regulated by interleukin-6, and we further show that tumor exosomes can trigger induction of interleukin-6 via activation of the Stat3 pathway. The roles of interleukin-6 and Stat3 activation in tumorgenesis have been well documented. In a recent mouse study using diethylnitrosamine induced tumors, diethylnitrosamine exposure promoted production of interleukin-6 in Kupffer cells in a manner dependent on the TLR adaptor protein MyD88.17 Ablation of interleukin-6 protected male mice from diethylnitrosamine-induced hepatocarcinogenesis.17 A more recent study suggests that activation of Stat3 leads to the induction of tumor angiogenesis by MDSCs in vivo.24,30 These results support our belief that tumor exosomes promote tumor growth and metastasis through the induction of the inflammatory cytokine interleukin-6 in a MyD88 dependent manner. Stat3 has been reported to be recruited into endosomes along with MyD88 and is relocated with MyD88.31 The recruitment of MyD88 to the endosomes could lead to the modulation of cytokine signaling pathways. These results lead to us to further speculate that tumor exosomes may play a co-activator role in activation of both Stat3 and MyD88 mediated pathways. This hypothesis could be tested in mice with a double knockout for Stat3 and MyD88 genes.
TLR2 and TLR4 agonists can directly induce production of TNF-α and other inflammatory cytokines of in vitro activated myeloid cells and in a MyD88 dependent fashion.32,33,34,35 However, we found that KO of TLR2 and TLR4 in MDSCs did not lead to a significant reduction of interleukin-6 and TNF-α production. Recently, another group reported that versican released from tumor cells can activate macrophages through a TLR2-dependent pathway27 and can stimulate metastasis by induction of myeloid cell derived TNF-α. In our system, we could not detect versican in the tumor exosomes by Western blot analysis (data not shown). The importance of MyD88 in regulating the expansion of immature CD11b+Gr-1+ cells with immunosuppressive activity has also been reported in a bacterial induced sepsis mouse model.36 This group also showed that TLR4 signaling is not required for the expansion of these CD11b+Gr-1+ cells in sepsis. In the future study, it will be important to identify the ligand(s), which is required for triggering MyD88 mediated activation of MDSCs.
An alternative explanation could be that tumor exosomes facilitate cross talk between MyD88 and Stat3 in the endosomes apartment, leading to activation of Stat3 in a TLR/ligand independent manner and subsequently in a Stat3 dependent expansion of MDSCs. Therefore, once exosomes are taken up by recipient cells, they may be transferred along the endosomal pathway to modulate the function of proteins recruited into the endosomal compartment without ligand(s) binding to the TLR receptors.
Although we do not know the in vivo trafficking routes of exosomes from B16 or 4T-1 tumor cells, a previous study has shown that thymus and tumor exosomes, including 4T-1 and TS/A breast tumor cells, are predominantly seeded into the liver and lung after intravenous injection.5,21,37 Breast tumor exosomes are selectively taken up by CD11bGr-1 cells.5 Therefore, we further speculate that exosomes could be selectively taken up by newly arrived BM derived CD11bGr-1 cells in the lung and liver. Subsequently, the differentiation of BM derived CD11bGr-1 cells is blocked, which may signal to the BM to send more precursor cells to lung and liver. As a result, there is an increase in the number of MDSCs in lung and liver.
It is also well-known that MDSCs accumulate in the spleen of murine breast cancer models.38 Although our previous data indicate that tumor exosomes are initially taken up by CD11bGr-1 cells and predominantly accumulate in the liver and lung,5 we cannot exclude the possibility that tumor exosomes may also play a role in the expansion of MDSCs in the spleen. It would be very interesting to determine whether the trafficking routes of tumor exosomes is different in a tumor bearing host when compared with a naïve host (which our data were generated from). Tumor induces an array of chemokines that could alter the homing of tumor exosome induced MDSCs in spleen.
It is also conceivable that the biological effects of MDSCs induced by tumor exosomes on immune suppression, in particular MDSC mediated immune suppression of T cell tumor cytotoxicity, may also depend on microenvironmental factors. These factors could have effects on the production, composition, and trafficking routes of tumor exosomes. For an example, in the early stages of cancer, we assume that tumor exosomes are predominantly present at the tumor site and then released to the peripheral blood as the cancer progresses. We propose that exosome-mediated increases in MDSCs should take place in the tumor during the initial stage of tumor growth. As tumor cells continue to multiply, more exosomes are released into peripheral blood and travel to the lung and liver where breast metastasis takes place, and tumor exosome-mediated increases in MDSCs could then take place in these tissues. In addition, due to the differences in microenviroment factors, the properties of the MDSCs induced in the lung and liver may be different from those induced in the tumor, or spleen in terms of promotion of tumor growth, tumor metastasis, and immune suppression. Development of technology for tracking exosomes released from tumor cells in vivo over time will be fundamental to understanding how tumor exosomes communicate with peripheral organs, including the liver, lungs, and spleen, and lead to immunosuppression in a host. In addition, like what we have found previously, exosomes released from tumors may be more potent in terms of induction or expansion of MDSCs than exosomes released from the same type of tumor cells. As a result, it is conceivable that tumor exosomes may be more potent in enhancement of tumor metastasis than tumor cell derived exosomes.
Acknowledgments
We thank Dr. Sam Hwang for providing the B16-luc and 4T-1-luc tumor cell lines. We thank Dr. Jerald Ainsworth for editorial assistance.
Footnotes
Address reprint requests to Huang-Ge Zhang, Ph.D., Department of Microbiology and Immunology, James Graham Brown Cancer Center, University of Louisville, Louisville, KY 40202. E-mail: H0zhan17@louisville.edu.
Supported by grants from the NIH (RO1CA116092, RO1CA107181, R01AT004294, and R01CA137037), Louisville Veterans Administration Medical Center Merit Review Grants (H.-G.Z.), and a grant from the Susan G. Komen Breast Cancer Foundation.
References
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Lotze MT. Molecular basis of metastasis. N Engl J Med. 2009;360:1679. author reply 1679–1680. [PubMed] [Google Scholar]
- Melnikova VO, Bar-Eli M. Inflammation and melanoma metastasis. Pigment Cell Melanoma Res. 2009;22:257–267. doi: 10.1111/j.1755-148X.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- Mon NN, Kokuryo T, Hamaguchi M. Inflammation and tumor progression: a lesson from TNF-alpha-dependent FAK signaling in cholangiocarcinoma. Methods Mol Biol. 2009;512:279–293. doi: 10.1007/978-1-60327-530-9_15. [DOI] [PubMed] [Google Scholar]
- Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti L, Marigo I, Mantelli B, Peranzoni E, Zanovello P, Bronte V. Myeloid-derived suppressor cell role in tumor-related inflammation. Cancer Lett. 2008;267:216–225. doi: 10.1016/j.canlet.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LE, Trautwein C, Liedtke C. Is interleukin-6 a gender-specific risk factor for liver cancer? Hepatology. 2007;46:1304–1305. doi: 10.1002/hep.21982. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Silasi DA, Alvero AB, Illuzzi J, Kelly M, Chen R, Fu HH, Schwartz P, Rutherford T, Azodi M, Mor G. MyD88 predicts chemoresistance to paclitaxel in epithelial ovarian cancer. Yale J Biol Med. 2006;79:153–163. [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Kakinuma T, Wang J, Zhang H, Palmer DC, Restifo NP, Hwang ST. Sensitization of B16 tumor cells with a CXCR4 antagonist increases the efficacy of immunotherapy for established lung metastases. Mol Cancer Ther. 2006;5:2592–2599. doi: 10.1158/1535-7163.MCT-06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang HG. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J, Xiang J. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28:126–131. [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, Prendergast GC, Mellor AL. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Mukhopadhyay S, Sehgal PB. Live cell imaging of interleukin-6-induced targeting of “transcription factor” STAT3 to sequestering endosomes in the cytoplasm. Am J Physiol Cell Physiol. 2007;293:C1374–C1382. doi: 10.1152/ajpcell.00220.2007. [DOI] [PubMed] [Google Scholar]
- Covacu R, Arvidsson L, Andersson A, Khademi M, Erlandsson-Harris H, Harris RA, Svensson MA, Olsson T, Brundin L. TLR activation induces TNF-alpha production from adult neural stem/progenitor cells. J Immunol. 2009;182:6889–6895. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- Shoenfelt J, Mitkus RJ, Zeisler R, Spatz RO, Powell J, Fenton MJ, Squibb KA, Medvedev AE. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J Leukoc Biol. 2009;86:303–312. doi: 10.1189/jlb.1008587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoelen MA, Yang H, Florquin S, Meijers JC, Akira S, Arnold B, Nawroth PP, Bierhaus A, Tracey KJ, van der Poll T. Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock. 2009;31:280–284. doi: 10.1097/SHK.0b013e318186262d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JS, Riehle KJ, Brooling JT, Bauer RL, Mitchell C, Fausto N. Proinflammatory cytokine production in liver regeneration is Myd88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006;176:2522–2528. doi: 10.4049/jimmunol.176.4.2522. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Liu Y, Qin A, Shah SV, Deng ZB, Xiang X, Cheng Z, Liu C, Wang J, Zhang L, Grizzle WE, Zhang HG. Thymus exosomes-like particles induce regulatory T cells. J Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2001;Chapter 20:Unit 20.22. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]