Abstract
Many vision-threatening diseases are characterized by intraocular neovascularization, (e.g., proliferative diabetic retinopathy and age-related macular degeneration). Although a new therapy with anti-VEGF antibodies is being used to treat these intraocular neovascular disorders, the visual recovery is limited, mainly because of the remnants of fibrovascular tissues. The ideal goal of the treatment is to prevent the invasion of new vessels into the avascular tissue through a matrix barrier. The purpose of this study was to determine the role played by cathepsin L, a matrix degrading enzyme, on intraocular angiogenesis. Used established animal models of retinal and choroidal neovascularization, we demonstrated that an inhibition of cathepsin L by specific inhibitors resulted in a significant decrease of intraocular neovascularization. A similar decrease of neovascularization was found in cathepsin L–deficient mice. Transplantation of bone marrow from cathepsin L–deficient mice into wild-type mice significantly reduced the degree of intraocular neovascularization. In addition, immunocytochemical analyses demonstrated that VE cadherin–positive endothelial progenitor cells, but not CD43-positive or Iba-1–positive cells, were the major cells contributing to the production of cathepsin L. These data indicate that cathepsin L expressed in endothelial progenitor cells plays a critical role in intraocular angiogenesis and suggest a potential therapeutic approach of targeting cathepsin L for neovascular ocular diseases.
The eye as an optical instrument must maintain a clear optical pathway. As such, it contains different transparent avascular tissues (e.g., cornea, crystalline lens, vitreous body, and outer retina), but an invasion of blood vessels into the avascular tissue can lead to hemorrhage and exudates, which significantly impairs their transparency and hence vision. In fact, the majority of diseases that lead to vision depression in industrialized countries are disorders that are characterized by intraocular neovascularization, (e.g., proliferative diabetic retinopathy, retinal vein occlusion, retinopathy of prematurity, and age-related macular degeneration). Among these diseases, the proliferative diabetic retinopathy, retinal vein occlusion, and retinopathy of prematurity are characterized by the development of new vessels in the retina that proliferate into the vitreal cavity. Age-related macular degeneration is characterized by the formation of new vessels from the choroidal vessels, which invade the outer layers of the neural retina. This formation of new blood vessels is called a choroidal neovascularization (CNV).
For the cells of the new vessels to invade the avascular tissue within the eye, the cells must penetrate a matrix barrier separating the vascular tissue from the avascular tissue. In retinal neovascularization, retinal vascular endothelial cells need to degrade their own basement membrane and also the basement membrane of the Mueller cells forming the internal limiting membrane to migrate and proliferate into the avascular vitreous. In a CNV, the choroidal neovessels need to breach the Bruch membrane, an extracellular matrix composed mainly of elastin and collagen laminae, and grow into the neural retina. Although the alterations of the matrix composing the Bruch membrane have been investigated in detail,1 the mechanism of the degradation and invasion through the matrix barrier within the eye has not been fully explored.
Until recently, it was assumed that the neovascularization develops from the activation, migration, and proliferation of resident endothelial cells. This idea was changed when Asahara et al2 reported that peripheral blood contains a population of bone marrow–derived endothelial progenitor cells (EPCs) that differentiate into endothelial cells at the sites of postnatal vasculogenesis and pathological neovascularization.
The results of studies on animal models of retinal neovascularization3 and CNV4,5,6,7,8 have provided evidence that EPCs may be major contributors to intraocular angiogenic disorders. For example, experiments on laser-induced CNV in chimeric mice (viz., C57BL/6 mice with bone marrow transplantation from green fluorescent protein [GFP]-transgenic mice) showed that 50% to 60% of the endothelial cells of a CNV were GFP-positive.6 In addition, cells expressing the EPC marker AC133 were identified in the specimens of surgically excised CNVs of human patients.9
An important property of EPCs is their ability to invade the extracellular matrix.10 Thus, Bagley and coworkers10 studied AC133+/CD34+ bone marrow progenitor cells in a coculture assay using human SKOV3 ovarian cancer cell clusters in collagen as a stimulus for the invasion of EPCs. They showed that EPCs were able to invade the malignant cell cluster through a matrigel barrier, whereas human microvascular endothelial cells were not able to invade the malignant cell cluster. These results suggested that the EPCs have a greater proliferative and invasive capacity than mature vascular endothelial cells.
It has recently been shown that the major factor responsible for the greater angiogenic activity of EPCs was their high expression of cathepsin L.11 Thus, Urbich and associates11 demonstrated that the protease cathepsin L was essential for the degradation and invasion of the matrix in vitro by EPCs using a mouse hind limb ischemia model. They concluded that cathepsin L plays a critical role in the EPC-mediated neovascularization. The cathepsins include the catalytic classes of serine, asaparate, and cysteine peptidases exhibiting endo- or exopeptidase activities.12
Anti-VEGF therapy is being used to treat intraocular neovascular disorders, and some improvement of vision has been obtained.13,14 Nevertheless, it is still difficult to regain a complete visual recovery because of the remnants of fibrovascular scar tissue and the concomitant damage of the neural retina. Therefore, a goal of an ideal treatment against intraocular neovascular disorders is to prevent the development and progression of new vessels into the avascular tissue. Although the critical roles of cathepsin L and EPCs have been demonstrated in the angiogenesis in other organs, a PubMed search did not identify any studies investigating the role of cathepsin L in ocular angiogenesis.
Thus, the purpose of this study was to investigate the role played by cathepsin L in ocular neovascularization. To accomplish this, we used established animal models of retinal and choroidal neovascularization. We shall show that an inhibition of cathepsin L by specific inhibitors resulted in a significant decrease in the size of the intraocular neovascularization. Similar findings were made in cathepsin L gene–deficient mice (cathepsin L−/− mice). Immunocytochemical analyses demonstrated that VE cadherin–positive cells, highly likely EPCs, were the major cells that express cathepsin L.
Materials and Methods
Reagents
Cathepsin L inhibtor (Z-FF-FMK) and cathepsin S inhibitor (Z-Phe-Leu-COCHO) were obtained from Calbiochem (Darmstadt, Germany); polyclonal antiserum against mouse VE cadherin from R&D Systems (Minneapolis, MN) and from Abcam (Cambridge, MA); polyclonal antiserum against mouse CD43 from Becton Dickinson (Franklin Lakes, NJ); polyclonal antiserum against mouse Iba1 from Wako (Osaka, Japan); polyclonal antiserum against mouse cathepsin L from R&D Systems (Minneapolis, MN); biotinylated Griffonia simplicifolia lectin B4 (GSA) from Vector Laboratories (Burlingame, CA); streproavidin-phosphatase from Dako (Glostrup, Denmark); FITC-conjugated secondary antibody from Invitrogen (Carlsbad, CA); PE-conjugated secondary antibody from Invitrogen (Carlsbad, CA); and Topro 3 from Invitrogen (Carlsbad, CA).
Animals
All animals were treated in accordance with the principles described in the Guiding Principles in the Care and Use of Animals in Tokyo Medical and Dental University, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The research protocol was approved by the Ethics Committee for Animal Experimentation at Tokyo Medical and Dental University. C57BL/6 mice were obtained from CLEA Japan Inc. (Tokyo, Japan) and housed in our institutional animal care facilities. C57BL/6 mice with targeted disruption of the cathepsin L gene, cathepsin L−/− mice, were generated as described.15 Genotyping to verify the absence of the cathepsin L gene of the targeting vector was accomplished by polymerase chain reaction of the DNA from tail biopsy specimens.
Mouse Model of Oxygen-Induced Ischemic Retinopathy
Ischemic retinopathy was produced in C57BL/6 mice or cathepsin L−/− mice by the method described by Smith et al.16 On postnatal day (P) 7, pups and their mothers were placed in airtight incubators and exposed to an atmosphere of 75 ± 3% oxygen for 5 days. Oxygen was continuously monitored with a PROOX model 110 oxygen controller (Reming Bioinstruments, Redfield, NY). On P12, the mice were returned to room air.
On P17, the pups were sacrificed with an overdose of pentobarbital sodium, and their eyes were rapidly removed and frozen in optimal cutting temperature embedding compound (Miles, Elkhart, IN) and 10-μm-thick sections were cut.
To investigate the effect of specific pharmacological inhibition of cathepsin L, cathepsin L inhibitor (Z-FF-FMK) and cathepsin S inhibitor (Z-Phe-Leu-COCHO) were used. Periocular injections of 1 μl of the inhibitor were performed using a microinjector (CellTram, Eppendorf, Germany). Two independent experiments were performed to investigate the effect of periocular injections of cathepsin inhibitors. In the first experiment, mice received daily periocular injections of 40 μmol/L (8 mice) or 400 μmol/L (8 mice) of Z-FF-FMK and vehicle of the same concentration (DMSO) in the fellow eye from P12 to P17. In the second set of experiments, mice received daily periocular injections of 80 nmol/L (10 mice) or 800 nmol/L (10 mice) of Z-Phe-Leu-COCHO and vehicle in the fellow eye from P12 to P17.
Retinal flatmounts were prepared by a modification of a described technique.17 After the mice were anesthetized, the descending aorta was clamped, the right atrium was cut, and the animal was perfused through the right ventricle with 1 ml phosphate buffered saline (PBS) containing 50 mg/ml fluorescein-labeled dextran (2 × 106 average MW; Sigma, St. Louis, MO). The eyes were removed and fixed for 1 hour in 10% phosphate-buffered formalin. The cornea and lens were removed, and the entire retina was then carefully dissected from the eye cup. Then radial cuts were made from the edge of the retina to the equator in all four quadrants. The retinas were flatmounted on a microscope slide in an aqueous medium (Aquamount; BDH, Poole, UK) with the photoreceptors facing downward. Flatmounts were carefully examined by fluorescence microscopy (BX51; Olympus), and images were taken with a CCD camera and imported to a computer system.
Mouse Model of Laser-Induced CNV
The Bruch membrane was ruptured by laser photocoagulation to generate a CNV.18 Briefly, 4- to 5-week-old C57BL/6J mice or cathepsin L−/− mice were anesthetized with ketamine hydrochloride (100 mg/kg body weight), and the pupils were dilated with 1% tropicamide (Santen, Tokyo, Japan). Two diode laser photocoagulation spots (100-μm size, 0.1-second duration, 120 mW) were made on each retina with a slit-lamp delivery system (Ultima 2000SE, Coherent, Santa Clara, CA) and a handheld coverslip as a contact lens. Burns were made at the 11 to 1 and 5 to 7 o’clock positions around the optic nerve head. A production of a bubble at the time of laser irradiation, which indicated a rupture of the Bruch membrane, was an important end point that induced a CNV. Thus, only burns in which a bubble was produced were analyzed. Fourteen days after the laser irradiation, fluorescein angiography was performed using an intraperitoneal injection of 0.2 ml of 2.5% sodium fluorescein (Alcon Laboratories, Inc., Fort Worth, TX). Fourteen days after the laser irradiation, the mice were sacrificed by transcardiac perfusion of 5 ml 4% paraformaldehyde in PBS. The eyes were enucleated, fixed in paraformaldehyde, embedded in paraffin, and cut into 5-μm sections.
Three independent experiments were performed to investigate the effect of periocular injections of cathepsin inhibitors. In the first experiment, mice received periocular injections of 40 μmol/L (8 mice) or 400 μmol/L (8 mice) of Z-FF-FMK, and vehicle in the fellow every two days for 2 weeks after the laser irradiation. In the second experiment, mice received 3 μl periocular injections of 80 nmol/L (10 mice) or 800 nmol/L (10 mice) of Z-Phe-Leu-COCHO, and vehicle in the fellow eye every two days for 2 weeks after the laser irradiation. And in the third experiment, eight mice with laser-induced CNV and without injections were analyzed as non-treated controls.
The size of CNV lesions was measured in choroidal flatmounts. Mice were anesthetized and perfused with 1 ml PBS containing 50 mg/ml fluorescein-labeled dextran (Sigma, St. Louis, MO). After the eyes were enucleated and briefly fixed in 4% PFA, the anterior segment was removed and the retinas were carefully dissected from the eyecup. Four radial cuts were made from the edge to the equator, and the eyecup was flatmounted with the photoreceptors facing downward. The flatmounts were examined by fluorescence microscopy (BX51; Olympus) at ×40 magnification. Photographs of the retina were taken with a CCD camera and imported to a computer system.
Analyses of Signal Intensity of Laser-Induced CNV by Fluorescein Angiography
Four to six minutes after injecting fluorescein into the intraperitoneal cavity, fluorescein angiograms were taken with a fundus camera (TRC-50IX; Topcon, Tokyo, Japan) with a built-in filter for fluorescein. The images were downloaded into a computer with a Windows operating system using a 3-color CCD color video camera (640 × 480 pixels; DXC-970MD; Sony, Tokyo, Japan). The intensity of the fluorescein staining of the CNV was determined as reported by Takehana and associates.19 Briefly, late-phase angiograms taken 100 to 140 seconds after the dye injection were graded by two examiners in a masked fashion. The angiograms were graded as: score 0, no staining; score 1, slightly stained; score 2, moderately stained; and score 3, strongly stained. The differences in the scores were evaluated by Wilcoxon signed rank tests.
Quantification of Neovascularization
Serial sections (10 μm) were cut through the entire eye, and the sections were histochemically stained with biotinylated GSA B4 as described.20 GSAB4 binds selectively to vascular cells. Slides were incubated in 4% paraformaldehyde for 30 minutes, washed with 0.05 M Tris buffer (TB; pH 7.4), incubated in methanol-H2O2 for 10 minutes at 4°C, washed with 0.05 M TB, and incubated for 30 minutes in 10% normal swine serum. Slides were rinsed with 0.05 M TB, and incubated 2 hours at 37°C with 1:20 GSA, rinsed again with 0.05 M TB, and incubated with undiluted streptoavidin-phosphatase for 30 minutes at room temperature. After a 10-minute wash in 0.05 M TB (pH 7.6), the slides were developed with diaminobenzidine (Dako).
For quantification, lectin-stained sections were examined at ×400 magnification with a model Q600 HR Leica microscope (Heidelberg, Germany). The images were digitized with a three-color CCD video camera and a frame grabber. The accompanying software (Quantimet; Leica) was used to delineate lectin-stained cells, and the area of neovascularization was measured. The area of the neovascularization was set to the area covered by the proliferated retinal pigmented epithelium, and the volume of CNV was determined with volume-calculating software (TRY/3D-SUFII, RATOC System Engineering Co Ltd, Tokyo, Japan). Mann–Whitney U tests were used to determine whether significant differences existed between experimental mean values. A P < 0.05 was considered significant. All statistical analyses were done using StatView software (SAS Institute, Cary, NC).
The area of CNV in the choroidal flatmounts was outlined with a cursor moved by the computer mouse, and the area was determined with the public domain NIH image program (developed at the NIH and available on the Internet at http://rsb.info.nih.gov/nih-image/). Image analysis was performed with the observer masked as to treatment or the types of mice used for the experiments.
Bone Marrow Transplantation
To determine the contribution of cathepsin L to the development of a CNV, bone marrow cells that contain EPCs that synthesize cathepsin L were transplanted into wild-type mice. The bone marrow cells were obtained from three cathepsin L −/− mice. To isolate the bone marrow cells, the femur and tibia were dissected and placed in RPMI 1640 culture medium (Invitrogen-Gibco, Grand Island, NY) containing 2.5% HEPES (1 M) and 1% gentamicin at 4°C. Bone marrow was obtained by slowly flushing the medium inside a diaphyseal channel with a syringe through a 27-gauge needle. The bone marrow was homogenized by passing the fluid through an 18-gauge needle and filtered through a nylon filter (70 μm; Spectrum, Houston, TX). The fluid was centrifuged and the pellets were resuspended in a medium described earlier.
Recipient mice (C57BL/6J) were lethally irradiated (950 cGy) and given 107 nonpurified bone marrow cells intravenously (200 μl). The blood components were allowed to reconstitute for 1 month. The survival rate of mice transplanted with exogenous bone marrow was 100%. In contrast, irradiated mice without exogenous bone marrow died approximately 10 to 14 days after the irradiation. One month after the bone marrow transplantation, CNVs were induced in recipient mice by making four separate choroidal burns in each eye with a red diode laser. Two weeks later, the animals were sacrificed and the eyes removed for histological examinations. Preliminary experiments using GFP transgenic mice, transgenic for the chicken β-actin promoter-GFP and cytomegalovirus enhancer (stock no. 003291; The Jackson Laboratory, Bar Harbor, ME), revealed that the chimerism of this bone marrow with this transplantation protocol was more than 90% (data not shown).
Immunostaining of Cathepsin L and Different Cell-Specific Marker during CNV Development
To determine the contribution of cathepsin L to the development of a CNV and to identify the cells that expressed cathepsin L in the process of CNV development, mice were sacrificed at 2 and 6 days after laser irradiation of the retina with an overdose of pentobarbital sodium. The eyes were enucleated, fixed in 4% paraformaldehyde, embedded in optimal cutting temperature embedding compound, and cut into 10-μm-thick frozen sections. The sections were incubated overnight in a humidified chamber at room temperature with rabbit polyclonal antibody against mouse cathepsin L (dilution 1:10; 10 μg/ml, R&D Systems, MN), or with VE-cadherin, which selectively binds to endothelial cells and mononuclear cells including monocytes and macrophages (dilution 1:50, BD Biosciences, NJ), or with CD43, which selectively binds to monocytes, granulocytes, and lymphocytes, (dilution 1:50, BD Biosciences, NJ), or with Iba1, which selectively binds to microglia/macrophages (dilution 1:50; 2 μg/ml, Wako, Osaka, Japan). Immunoreactive cells were identified by incubating the sections in FITC-conjugated secondary antibody or PE-conjugated secondary antibody. The nuclei were counterstained with Topro3 for fluorescence staining, and the sections were observed with a confocal optical microscopy (Carl Zeiss Inc., Germany).
Results
Effect of Inhibiting Cathepsin L on Extraretinal Neovascularization
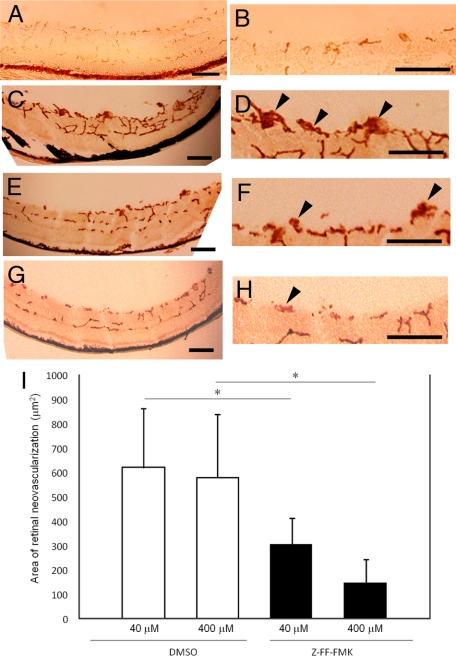
Daily periocular injection of 40 μmol/L or 400 μmol/L of Z-FF-FMK, a specific cathepsin L inhibitor, from P12 to P17 was well-tolerated and caused a statistically significant reduction in the area of the retinal neovascularization from 619.5 ± 48.0 μm2 with 40 μmol/L DMSO to 307.1 ± 108.0 μm2 with 40 μmol/L Z-FF-FMK, and from 578.4 ± 263.7 μm2 with 400 μmol/L DMSO to 150.4 ± 93.7 μm2 with 400 μmol/L Z-FF-FMK (Figure 1, A–I).
Figure 1.
Effects of periocular injections of Z-FF-FMK, a cathepsin L–specific inhibitor, on a mouse model of retinal neovascularizaion. A–H: Photomicrographs of retinal sections histochemically stained with the endothelial cell–selective lectin from Griffonia simplicifolia. Retinal blood vessels within the retina and neovascularization on the surface of the retinas are stained with the reaction product. Scale bars; 100 μm. A and B: The retina of P17 mice raised in room air (no oxygen exposure). No extraretinal neovascularization is observed. B: Higher magnification image of A. C and D: The retina of P17 mice that were exposed to 75 ± 3% oxygen from P5 to P12 and received daily intraperitoneal injection of DMSO (40 μmol/L) from P12 to P17. Extraretinal neovascularization is observed as brown clumps on the surface of the retina. D: Higher magnification image of C. Arrowheads point to the extraretinal neovascularization. E and F: The retina of P17 mice that were exposed to 75 ± 3% oxygen from P5 to P12 and received daily intraperitoneal injection of Z-FF-FMK (40 μmol/L) from P12 to P17. Extraretinal neovascularization is observed as brown clumps on the surface of the retina. F: Higher magnification image of E. Arrowheads point to the extraretinal neovascularization. G and H: The retina of P17 mice that were exposed to 75 ± 3% oxygen from P5 to P12 and received daily intraperitoneal injection of Z-FF-FMK (400 μmol/L) from P12 to P17. Extraretinal neovascularization appears to be reduced. H: Higher magnification image of G. Arrowheads point to the extraretinal neovascularization. I: Quantification of the total area of endothelial cell staining in retinal sections of mouse with oxygen-induced ischemic retinopathy. Only preretinal vascular cells were counted. Neovascularization in mice that had a periocular injection of Z-FF-FMK (n = 8 both at 40 μmol/L and 400 μmol/L) was significantly reduced compared with mice that had vehicle (DMSO) injection only (n = 8 both at 40 μmol/L and 400 μmol/L). *P < 0.01.
Effect of Inhibiting Cathepsin L on Development of CNV
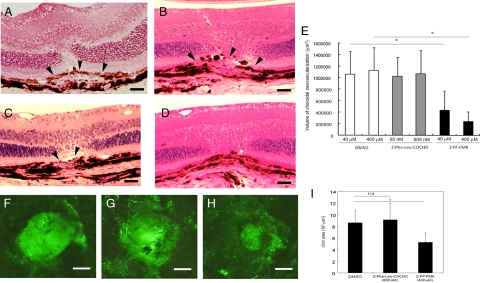
The volume of the CNV in eyes that received periocular injections of 40 μmol/L or 400 μmol/L of Z-FF-FMK were 4.34 × 105 ± 3.37 × 105 μm3 and 2.38 × 105 ± 1.70 × 105 μm3, respectively, which were significantly smaller than the 10.65 × 105 ± 3.90 × 105 μm3 with 40 μmol/L DMSO and 11.29 × 105 ± 3.90 × 105 μm3 with 400 μmol/L DMSO in eyes that received periocular injections of vehicle. The volumes of the CNV in eyes that received daily periocular injections of Z-Phe-Leu-COCHO, a specific inhibitor of cathepsin S, were 10.23 × 105 ± 3.33 × 105 μm3 with 80 nmol/L and 10.66 × 105 ± 4.06 × 105 μm3 with 800 nmol/L on day 14 after the laser irradiation. These volumes were not significantly different from that in the vehicle injected eyes (Figure 2, A–E).
Figure 2.
Effects of periocular injections of Z-FF-FMK, a cathepsin L–specific inhibitor, on laser-induced choroidal neovascularization (CNV) in a mouse. A–D: Representative photomicrographs of retinal sections stained with hematoxylin and eosin (HE). Arrowheads show the site of the CNV. Scale bars = 20 μm. A: The retinal section of the mice that received daily periocular injections of DMSO for 14 days after laser irradiation. B: The retinal section of the mice that received daily periocular injections of Z-Phe-Leu-COCHO (800 nmol/L) for 14 days after laser irradiation. C: The retinal section of the mice that received daily periocular injections of Z-FF-FMK (40 μmol/L) for 14 days after laser irradiation. D: The retinal section of the mice that received daily periocular injections of Z-FF-FMK (400 μmol/L) for 14 days after laser irradiation. The CNV is barely detectable at the site of laser injury. E: Quantification of the total volume of CNV at 14 days after laser irradiation of mouse retina. The neovascularizations in mice that had received periocular injections of Z-FF-FMK (n = 9 at 40 μmol/L and n = 8 at 400 μmol/L) are significantly smaller than that of mice that received vehicle (DMSO) injection alone (n = 9 at 40 μmol/L and n = 8 at 400 μmol/L). Injection of cathepsin S–specific inhibitor (Z-Phe-Leu-COCHO; n = 8 both at 80 nmol/L and 800 nmol/L) does not lead to an inhibition of the CNV compared with the mice that received vehicle injection alone. *P < 0.001. F–H: Representative figures of CNV as choroidal flatmounts. Scale bar = 200 μm. F: The vehicle (DMSO)-injected eye. G: The cathepsin S–specific inhibitor (Z-Phe-Leu-COCHO; 800 nmol/L)-injected eye. H: The cathepsin L–specific inhibitor (Z-FF-FMK; 400 μmol/L)-injected eye. I: CNV areas in the choroidal flatmounts were measured in the vehicle (DMSO)-injected eyes (n = 8), cathepsin S–specific inhibitor (Z-Phe-Leu-COCHO; 800 nmol/L)-injected eyes (n = 8), and cathepsin L–specific inhibitor (Z-FF-FMK; 400 μmol/L)-injected eyes (n = 8), and the results of the quantitative analyses are shown in the graph. CNV area is significantly smaller in cathepsin L inhibitor-injected eyes than cathepsin S inhibitor–injected eyes or vehicle-treated eyes. *P < 0.01.
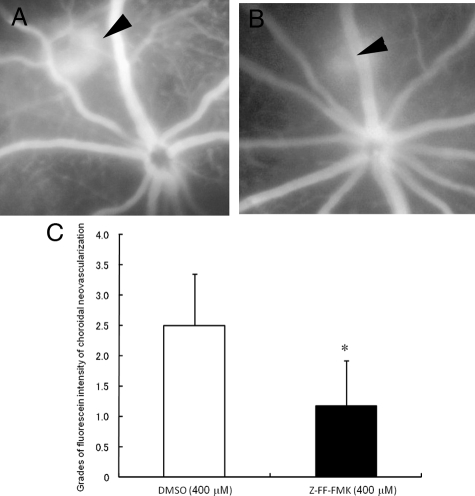
Choroidal flatmount analyses was used to quantify the CNV area, and the results demonstrated that the CNV area was smaller in the mice treated with 400 μmol/L Z-FF FMK (5.28 × 103 ± 1.63 × 103 μm2) than in the mice treated with 800 nmol/L Z-Phe-Leu-COCHO (9.12 × 103 ± 2.82 × 103 μm2) or DMSO-treated controls (8.64 × 103 ± 2.18 × 103 μm2; Figure 2, F–I). The intensity of the CNV in the fluorescein angiograms was significantly weaker in eyes that received Z-FF-FMK (1.17 ± 0.75) than in eyes that received vehicle injections (2.50 ± 0.84) (Figure 3, A–C).
Figure 3.
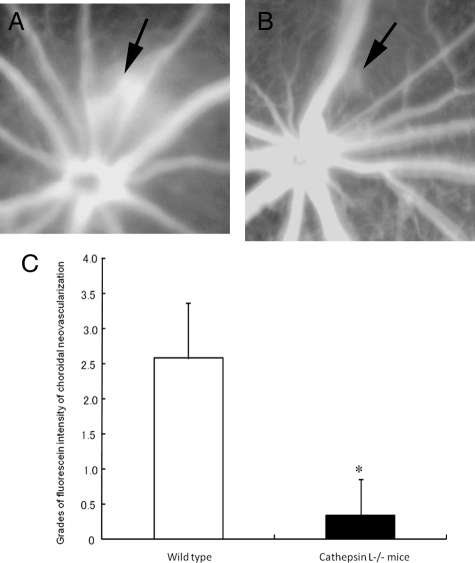
Effects of periocular injections of Z-FF-FMK on the fluorescein dye intensity of laser-induced choroidal neovascularization (CNV). A and B: Representative fundus angiograms at four minutes after fluorescein dye injection. Arrowheads show the site of CNV. A: The vehicle (DMSO)-injected eye. B: The eye that received cathepsin L–specific inhibitor (Z-FF-FMK; 400 μmol/L). C: Quantification of the grades of fluorescein dye intensity of the CNV in mice at 14 days after laser irradiation. The fluorescein intensity of CNV in mice that received periocular injection of Z-FF-FMK (n = 6) was significantly lower than that of mice that received vehicle (DMSO) injection alone (n = 6). *P = 0.03.
Inhibition of Retinal and Choroidal Neovascularization in Cathepsin L−/− Mice
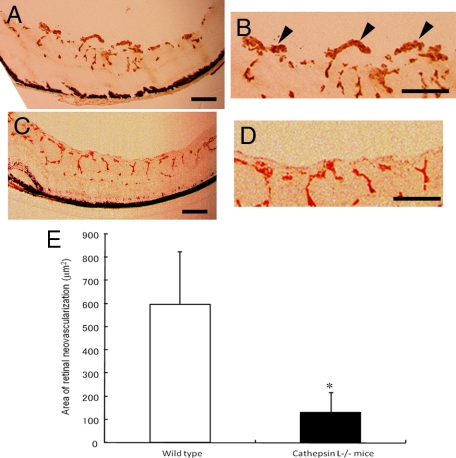
To determine the contribution of cathepsin L to the development of retinal neovascularization and CNV, the degree of ocular neovascularization in cathepsin L−/− mice was compared with that in wild-type mice. The results demonstrated that cathepsin L−/− mice had significantly smaller areas of retinal neovascularization: 130.9 ± 88.3 μm2 in cathepsin L−/− and 599.2 ± 226.1 μm2 in wild-type mice (Figure 4, A–E). No differences were detected in the development of retinal vasculature in P12 wild-type mice without any treatment, in P12 wild-type that had received periocular injections of 400 μmol/L of Z-FF-FMK, and in P12 cathepsin L−/− mice (see supplemental Figure S1 at http://ajp.amjpathol.org). In addition, the intensity of the CNV in the fluorescein angiograms was significantly weaker in eyes of cathepsin L−/− mice (0.33 ± 0.52) than in the eyes of wild-type mice (2.57 ± 0.79; Figure 5, A–C). The volume of the CNV in cathepsin L−/− mice (1.86 × 105 ± 1.68 × 105 μm3) was significantly smaller than that in wild-type mice (10.14 × 105 ± 4.14 × 105 μm3; Figure 6, A, B, and E).
Figure 4.
Reduced retinal neovascularization in cathepsin L−/− mice with oxygen-induced ischemic retinopathy. A–D: Representative photomicrographs of retinal section histochemically stained with the endothelial cell–selective lectin from Griffonia simplicifolia. Retinal blood vessels within the retina and neovascularization on the surface of the retina are stained positively. Scale bars = 100 μm. A: The retina of P17 wild-type mice that were exposed to 75 ± 3% oxygen from P5 to P12. Extraretinal neovascularization is observed as brown clumps on the surface of the retina. B: Higher magnification image of A. Arrowheads point to the extraretinal neovascularization. C: The retina of P17 cathepsin L−/− mice that were exposed to 75 ± 3% oxygen from P5 to P12. Extraretinal neovascularization is barely observed. D: Higher magnification image of C. E: Quantification of the total area of endothelial cell staining in retinal sections of mice with oxygen-induced ischemic retinopathy. Neovascularization after hyperoxia is significantly reduced in cathepsin L−/− mice (n = 8) compared with that in wild-type mice (n = 8). *P < 0.001.
Figure 5.
Reduction of the fluorescein dye intensity of laser-induced choroidal neovascularization (CNV) in cathepsin L−/− mice. A and B: Representative fundus angiogram at four minutes after fluorescein dye injection. A: The fluorescein angiogram of wild-type mouse at 14 days after laser irradiation. Intense dye leakage from the CNV is observed (arrow). B: The fluorescein angiogram of cathepsin L−/− mouse at 14 days after laser irradiation. Dye leakage at the site of laser irradiation is reduced remarkably (arrow). C: Quantification of the grades of fluorescein dye intensity of the CNV in a mouse retina 14 days after laser irradiation. The fluorescein intensity of the CNV in the cathepsin L−/− mice (n = 7) is significantly weaker than that of wild-type mice (n = 7). *P < 0.01.
Figure 6.
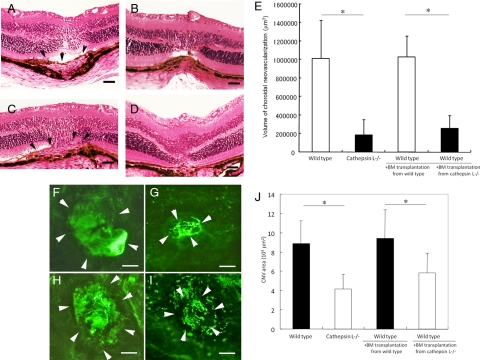
Inhibition of laser-induced choroidal neovascularization (CNV) by the transplantation of bone marrow cells derived from a cathepsin L−/− mice into a wild-type mouse. A–D: Representative photomicrographs of a retinal section stained with hematoxylin and eosin (H&E). Laser treatment was performed 30 days after bone marrow transplantation. Scale bars = 20 μm. A: The retina of wild-type mouse without bone marrow transplantation at 14 days after laser irradiation. Arrowheads point to the CNV. B: The retina of cathepsin L−/− mouse without bone marrow transplantation at 14 days after laser irradiation. CNV is barely detectable at the laser site. C: The retina of wild-type mouse that received bone marrow transplantation from a wild-type mouse and at 14 days after laser irradiation. Arrowheads point to the CNV. D: The retina of wild-type mouse that received bone marrow transplantation from a cathepsin L−/− mouse and at 14 days after laser irradiation. Neovascularization is barely detectable. E: Quantification of the total volume of CNV in a mouse retina at 14 days after laser irradiation. Neovascularization in wild-type mice that received transplantation of bone marrow cells from cathepsin L−/− mice (n = 7) is significantly reduced compared with the wild-type mice that received a transplantation of bone marrow derived from wild-type mice (n = 8). Neovascularization in wild-type mice that received transplantation of bone marrow derived from cathepsin L−/− mice is comparable with that of cathepsin L−/− mice (n = 8). *P < 0.01. F–I: Representative photographs of CNV in choroidal flatmounts at 14 days after laser irradiation. Arrowheads show the site of the CNV. Scale bars = 100 μm. F: Wild-type mouse without bone marrow transplantation. G: Cathepsin L−/− mouse without bone marrow transplantation. H: Wild-type mouse that received bone marrow transplantation from wild-type mouse. I: Wild-type mouse that received bone marrow transplantation from a cathepsin L−/− mouse. Neovascularization appears smaller than that of the wild-type mouse that received bone marrow transplantation from wild-type mouse. J: CNV areas in the choroidal flatmounts were measured in wild-type mice (n = 7), cathepsin L−/− mice (n = 8), wild-type mice that received transplantation of bone marrow cells from wild-type mice (n = 8), and wild-type mice that received transplantation of bone marrow cells from cathepsin L−/− mice (n = 8). CNV area in wild-type mice that received transplantation of bone marrow cells from cathepsin L−/− mice is significantly reduced compared with the wild-type mice that received a transplantation of bone marrow derived from wild-type mice. *P < 0.01.
Transplantation of Cathepsin L−/− Mice–Derived Bone Marrow Cells Decreases Size of CNV
To determine the contribution of bone marrow–derived cells to the development of a CNV through the expression of cathepsin L, we transplanted bone marrow cells from cathepsin L−/− mice into wild-type mice. One month after the transplantation, a laser-induced CNV was produced. Fourteen days after laser irradiation, the CNV volume in wild-type mice which received bone marrow transplantation from cathepsin L−/− mice (2.57 × 105 ± 1.38 × 105 μm3) was significantly smaller than that in wild-type mice that received a bone marrow transplantation from wild-type mice (10.30 × 105 ±2.29 × 105 μm3; P < 0.001, Figure 6, C, D, and E). The CNV volume in wild-type mice that received a bone marrow transplantation from cathepsin L−/− mice was not significantly different from that of cathepsin L−/− mice without a bone marrow transplantation (Figure 6E). In addition, the difference in the CNV volume between wild-type mice with and without transplantation of bone marrow from wild-type mice was not significant.
Choroidal flatmount analyses demonstrated that the CNV area in wild-type mice that received bone marrow transplantation from cathepsin L−/− mice (4.152 × 103 ± 1.54 × 103 μm2) was significantly smaller than that in wild-type mice that received a bone marrow transplantation from wild-type mice (8.88 × 103 ± 2.32 × 103 μm2; Figure 6, F through J).
Bone Marrow–Derived EPCs Contribute to CNV Development
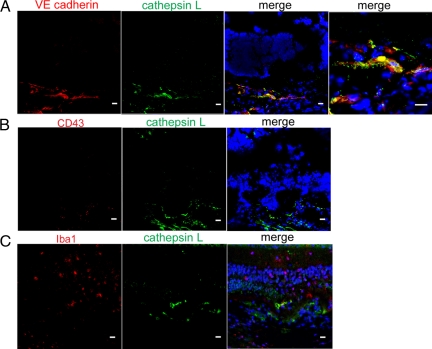
To identify the cells that express cathepsin L during the CNV development, eyes were immunohistochemically analyzed using different cell markers after laser irradiation. Two days after laser irradiation, VE cadherin–positive cells were observed at the laser site as individual cells, and cathepsin L staining was detected at the corresponding site of the VE cadherin–positive cells (Figure 7, A–C). There were only a few CD43-positive cells and Iba1-positive cells at the laser site at two days after laser irradiation, and these cells did not express cathepsin L.
Figure 7.
Immunohistochemical examination of the expression of cathepsin L and various cell markers at the site of the choroidal neovascularization (CNV) in the mouse retina with laser-induced CNV at 2 days after laser irradiation. Cell nuclei are counterstained with Topro 3. Scale bars = 10 μm A: Immunostaining of cathepsin L (green) and VE cadherin (red). At 2 days after laser irradiation, staining of cathepsin L (green) can be seen at the laser site. The staining of cathepsin L is observed at the corresponding area of VE cadherin-positive cells (red). A higher magnification image confirms the colocalization of cathepsin L–positive cells and VE cadherin–positive cells. B: Immunostaining of cathepsin L (green) and CD43 (red). Only a few CD43-positive cells are observed at the laser irradiation site, and these cells do not show cathepsin L expression. C: Immunostaining of cathepsin L (green) and Iba1 (red). Only a few Iba1-positive cells are observed at the laser irradiation site, and these cells do not show cathepsin L expression.
Discussion
Our results demonstrated that the size and fluorescein intensity of the intraocular neovascularization was significantly reduced in cathepsin L−/− mice compared with that in wild-type mice. In addition, wild-type mice that had received daily injections of specific inhibitors of cathepsin L had reduced neovascularization size. These findings indicate that cathespin L must play a significant role in the development of neovascularization in mice eyes. This was supported by the transplantation of bone marrow cells of cathepsin L−/− mice into wild-type mice, which significantly reduced the size of the CNV, almost to a level comparable with that in cathepsin L−/− mice. To the best of our knowledge, these results reveal for the first time the critical role of cathepsin L in the development of an intraocular neovascularization.
The process of matrix degradation is important in the development of intraocular neovascularizations. The basement membranes of vascular endothelial cells, Mueller cells, and the Bruch membrane serve as physical barriers to cell movement. They restrict the movement of cells between the vascular and avascular tissues within the eye and prevent the development of retinal angiogenesis and CNVs. The Bruch membrane is a thick complex matrix that lies between the neural retina and the vascular choroid. The Bruch membrane is made up of the basement membrane of the choriocapillaris and the basement membrane of the RPE cells. It is composed of two collagen-rich layers that flank a central domain of elastin and elastin-associated proteins.20,21
Proteases are necessary for the formation of new blood vessels. Proteolytic enzymes, such as the matrix metalloproteinases (MMPs) and the cathepsin cysteine proteases, can degrade the extracellular matrix and thereby contribute to various pathological conditions. These broad-spectrum proteases are potent contributors to the degradation of several extracellular matrix proteins such as laminin, fibronectin, collagens I and IV, elastin, and other structural proteins of basement membranes.
Mature endothelial cells are equipped with a set of proteases including the MMPs and the urokinase-type plasminogen activator. There is little evidence on the different roles played by the MMPs and cathepsins in pathological conditions. Urbich and associates11 performed gene expression profiling of EPCs and mature endothelial cells and identified genes that might be important for the neovascularization capacity of EPCs. Cathepsin L was found to be highly expressed in EPCs as opposed to endothelial cells, and it was essential for matrix degradation and invasion by EPCs in vitro and in an ischemic hind limb model in mice. In addition, pharmacological inhibition of cathepsin L, but not cathepsin S, MMP-3, MMP-9, and elastase, significantly decreased the angiogenic activity of EPCs. Reddy et al22 also showed that human monocyte-derived macrophages synthesize both elastinolytic MMPs and cathepsins B, L, and S. However, only the cathepsins were detected extracellularly as processed active enzymes. The inhibition of cathepsins L and S, but not MMPs, almost completely blocked macrophage-mediated elastinolytic activity, indicating that cathepsins are the most potent elastases secreted by human macrophages.
Finally, we tried to identify the cells in the intraocular angiogenesis that expressed cathepsin L. The results of the immunohistochemical analyses revealed that the VE cadherin-positive, but not CD43-positive or Iba1-positive cells, were the major cells that expressed cathepsin L at the site of the laser irradiation, which induced the development of the CNV. Earlier studies have reported an infiltration of different types of cells including inflammatory cells, e.g., macrophages,23 leukocytes,24 and lymphocytes,24 retinal pigmented epithelial cells, fibroblasts, vascular endothelial cells, and EPCs6,7,8,9 that migrated into the site of a CNV. VE cadherin is mainly expressed on endothelial cells as well as mononuclear cells including monocytes and macrophages, and CD43 is expressed on lymphocytes, neutrophils, monocytes, and platelets. Iba1 is known to bind selectively to microglia/macrophages. The findings that the cells that expressed cathepsin L were VE cadherin-positive, but not CD43-positive and Iba1-postive, suggest that these cells are vascular endothelial cells. In addition, the VE cadherin–positive cells that expressed cathepsin L did not show tube formation at 2 days after laser irradiation, and they were observed as separate single cells, which suggests that the VE cadherin–positive cells expressing cathepsin L may not be mature endothelial cells. This supports our previous study,25 where it was demonstrated that the tube forming activity of injected EPCs was much weaker than that of injected late EPCs after 12 to 21 days of cultivation of human cord blood cells in the ear occlusion model.
Combining all of these reports, our results suggest that EPCs can regulate the intraocular neovascularization through the expression of cathepsin L. Our results and those by Urbich et al11 and by Bagley et al10 suggest that an increased expression of cathepsin L by EPCs might be involved in the development of intraocular neovascularization as seen in neovascularization of cancerous tissue as well as ischemia-induced neovascularization of a hind limb model. This suggests that the molecular mechanism that allows cells to invade into the extracellular matrix and facilitate the neovascularization might be common regardless of the angiogenic stimuli and the type of organ.
In conclusion, our results demonstrated that the cathepsin L expressed by VE cadherin–positive bone marrow–derived cells plays a critical role in the development of retinal and choroidal neovascularization. The use of the cathepsin L inhibitor should be considered as a preventive treatment for intraocular neovascular disorders.
Acknowledgments
We thank Prof. Duco Hamasaki for his critical discussion and revision of the final manuscript.
Footnotes
Address reprint requests to Kyoko Ohno-Matsui, M.D., Department of Ophthalmology and Visual Science, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyo-ku, Tokyo 113, Japan. E-mail: k.ohno.oph@tmd.ac.jp.
Supported in part by research grant 17659654, 19390441, 19659445, and 20659306 from the Japan Society for the Promotion of Science, Tokyo, Japan.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Chong NH, Keonin J, Luthert PJ, Frennesson CI, Weingeist DM, Wolf RL, Mullins RF, Hageman GS. Decreased thickness and integrity of the macular elastic layer of Bruch’s membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol. 2005;166:241–251. doi: 10.1016/S0002-9440(10)62248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative projenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Dorrell MI, Ritter MR, Marchetti V, Moreno SK, El-Kalay M, Bird AC, Banin E, Aguilar E. Progenitor cells and retinal angiogenesis. Angiogenesis. 2007;10:89–101. doi: 10.1007/s10456-007-9070-4. [DOI] [PubMed] [Google Scholar]
- Csaky KG, Baffi JZ, Byrnes GA, Wolfe JD, Hilmer SC, Flippin J, Cousins SW. Recruitment of bone marrow–derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res. 2004;78:1107–1116. doi: 10.1016/j.exer.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Caicedo A, Hernandez EP, Csaky KG, Cousins SW. Bone marrow-derived progenitor cells contribute to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4914–4919. doi: 10.1167/iovs.03-0371. [DOI] [PubMed] [Google Scholar]
- Espinosa-Heidmann DG, Reinoso MA, Pina Y, Csaky KG, Caicedo A, Cousins SW. Quantitative enumeration of vascular smooth muscle cells and endothelial cells derived from bone marrow precursors in experimental choroidal neovascularization. Exp Eye Res. 2005;80:369–378. doi: 10.1016/j.exer.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Sengupta N, Caballero S, Mames RN, Butler JM, Scott EW, Grant MB. The role of adult bone marrow–derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:4908–4913. doi: 10.1167/iovs.03-0342. [DOI] [PubMed] [Google Scholar]
- Tomita M, Yamada H, Adachi Y, Cui Y, Yamada E, Higuchi A, Minamino K, Suzuki Y, Matsumura M, Ikehara S. Choroidal neovascularization is provided by bone marrow cells. Stem Cells. 2004;22:21–26. doi: 10.1634/stemcells.22-1-21. [DOI] [PubMed] [Google Scholar]
- Sheridan CM, Rice D, Hiscott PS, Wong D, Kent DL. The presence of AC133-positive cells suggests a possible role of endothelial progenitor cells in the formation of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:1642–1645. doi: 10.1167/iovs.05-0779. [DOI] [PubMed] [Google Scholar]
- Bagley RG, Walter-Yohrling J, Cao X, Weber W, Simons B, Cook BP, Chartrand SD, Wang C, Madden SL, Teicher BA. Endothelial precursor cells as a model of tumor endothelium: characterization and comparison with mature endothelial cells. Cancer Res. 2003;63:5866–5873. [PubMed] [Google Scholar]
- Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, Vajkoczy P, Hofmann WK, Peters C, Pennacchio LA, Abolmaali ND, Chavakis E, Reinheckel T, Zeiher AM, Dimmeler S. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciulla TA, Rosenfels PJ. Antivascular endothelial growth factor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2009;20:158–163. doi: 10.1097/ICU.0b013e32832d25b3. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Rosenfeld PJ. Anti-vascular endothelial growth factor therapy for neovascular ocular diseases other than age-related macular degeneration. Curr Opin Ophthalmol. 2009;20:166–174. doi: 10.1097/ICU.0b013e328329d173. [DOI] [PubMed] [Google Scholar]
- Koike M, Shibata M, Waguri S, Yoshimura K, Tanida I, Kominami E, Gotow T, Peters C, von Figura K, Mizushima N, Saftig P, Uchiyama Y. Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease). Am J Pathol. 2005;167:1713–1728. doi: 10.1016/S0002-9440(10)61253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Ohno-Matsui K, Uetama T, Yoshida T, Hayano M, Itoh T, Morita I, Mochizuki M. Reduced retinal angiogenesis in MMP-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:5370–5375. doi: 10.1167/iovs.03-0249. [DOI] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana Y, Kurokawa T, Kitamura T, Tsukahara Y, Akahane S, Kitazawa M, Yoshimura N. Suppression of laser-induced choroidal neovascularization by oral tranilast in the rat. Invest Ophthalmol Vis Sci. 1999;40:459–466. [PubMed] [Google Scholar]
- Marshall J, Hussain A, Starita C, Moore D, Patmore A. Aging and Bruch’s membrane. Marmor M, Wolfensberger T, editors. New York: Oxford University Press.; The Retinal Pigment Epithelium. 1988:pp669–692. [Google Scholar]
- Guymer R, Bird A. Bruch’s membrane, drusen, and age-related macular degeneration. Marmor M, Wolfensberger T, editors. New York: Oxford University Press,; The Retinal Pigment Epithelium. 1988:pp693–705. [Google Scholar]
- Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B. L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K, Matsumura M, Ogura Y, Honda Y. The potential role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1891–1898. [PubMed] [Google Scholar]
- Tsutsumi-Miyahara C, Sonoda KH, Egashira K, Ishibashi M, Qiao H, Oshima T, Murata T, Miyazaki M, Charo IF, Hamano S, Ishibashi T. The relative contributions of each subset of ocular infiltrated cells in experimental choroidal neovascularization. Br J Ophthalmol. 2004;88:1217–1222. doi: 10.1136/bjo.2003.036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, Kobayashi A, Yamaguchi T, Abe M, Amagasa T, Morita I. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2007;314:430–440. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]