Abstract
Problem
Natural Killer (NK) cell numbers and cytotoxicity are suppressed during pregnancy. Little is known about postpartum NK number and function.
Method of study
Postpartum women (n = 39) were studied at one week and then monthly over the first six postpartum months. The standard natural killer cell cytotoxicity assay (NKCA) was performed. This is a Cr51 release assay from K562 cells cultured with peripheral blood mononuclear cells (PBMCs).
Results
Data indicate suppression of NK cytotoxicity in postpartum women. Cytotoxicity at each effector:target (E:T) ratio showed a drop from 1 week postpartum, reaching a nadir at around 2 months, and a trend towards recovery of cytotoxicity from 3 to 6 months. Lytic units (LUs) from pre-incubated cells from postpartum women were lower than age-matched, non-pregnant, non-postpartum controls through the fifth postpartum month.
Conclusion
These data suggest that the postpartum period, like pregnancy, is characterized by decreased NK cytotoxicity activity. This suppressed NK cytotoxic effect may result as a response to interaction with tolerized fetal microchimeric cells accumulated during pregnancy in maternal blood and tissues.
Keywords: Cytotoxicity, natural killer cells, postpartum, pregnancy
Introduction
Natural killer (NK) cells are essential components of the innate immune system due to their ability to produce cytokines and lyse foreign cells, cancer cells, rapidly dividing cells, and cells in distress such as virus infected cells. The killing of target cells does not require specificity and memory. NK killing is affected by binding to receptors and by recognition of peptides that are part of the MHC class I molecules expressed essentially on all cells in an individual. When the NK cell binds to a triggering receptor (signal 1), a second signal is activated if the cells express the normal components of self MHC class I molecules. Inhibitory receptors recognize MHC Class I, while activating receptors recognize stress-associated ligands and viral products.1 Co-stimulatory receptor engagement is also required, so that the functions and responses of NK cells are balanced by these three receptor-associated mechanisms.2 Cells which have low expression of MHC class I or have a decreased ability to trigger the negative inhibitory signal 2 are killed through release of proteases and perforating molecules (perforin) stored in the NK cell’s granules, which bore through and destroy the target cell membrane.3 This damage induces apoptosis of the target cell. Natural killer cells also have Fc receptors, which bind the Fc portion of immunoglobulin molecules. Thus, NK cells can cooperate with the humoral immune system and participate in cell lysis through antibody-dependent cytotoxicity (ADCC). In fact, the balancing and diversity of the repertoire of NK receptors place these cells as ‘conductors’ of the inflammatory response.4 There has been tremendous interest in NK cells, and hopes for clinical applications of the knowledge recently gained.
During normal human pregnancy, the peripheral CD56 dim population of NK cells decreases.5 The natural killer cell cytotoxicity assay (NKCA) of peripheral blood cells from pregnant women also shows diminished cytotoxicity compared with controls during pregnancy.6 The expression of a number of class I-specific KIR inhibitory receptors increased in the first weeks of pregnancy through the end of the first trimester in eight women studied through their pregnancies.7 The potential mechanism for inhibited NK function in pregnancy has been attributed in part to pregnancy hormones estrogen, progesterone, and prolactin.8 Progesterone may stimulate NK apoptosis, induce pregnancy protein TJ6, stimulate cytokines secreted by monocytes or trophoblasts, and other factors present in pregnant serum.9 Natural killer cells are key regulators of allograft rejection. Natural killer cells are believed to be involved in the absence of maternal rejection of the semi-allogeneic fetus, but many other mechanisms operate in concert. A complex system of interactions between NKs and many other cell types [T helper-1 (Th1), T helper 2 (Th2), T helper 3 (Th3) Tr1, CD4+ CD25+ (T regulatory) allows the fetus to be immunologically tolerated.9
As with pregnancy, the postpartum also seems to be characterized by low numbers and cytotoxicity of NK cells. We reported a significant decrease in the frequency of the CD56 dim population of NK cells at week’s 4 to 6 postpartum compared with controls.10 Baley and Schacter11 reported diminished NK cytotoxicity in pregnancy and the immediate postpartum. Webb, Bochan, Montel, Padilla, and Brahmi12 also observed low NKCA results in postpartum women. There has apparently been no investigation to date of the scientific basis for NK inhibition in the postpartum, and no exploration of the functional significance of such inhibition.
The purpose of this study was to systematically examine the course of NK cytototoxicity, through the use of the NKCA, across the first six months of the postpartum. The rationale for this study was to follow up on the decrease in NK numbers we observed at 4–6 weeks postpartum and to evaluate whether the actual function of these important cells was also diminished in the postpartum period.
Method
Participants
This study was approved by the university institutional review board and all participants gave informed consent. The participants were women who were participating in a study of postpartum thyroiditis (PPT) and a control group of non-pregnant, non-postpartum women who provided blood samples for controls. Data from women (n = 39) who were negative for thyroid peroxidase (TPO) antibody titers during mid-pregnancy were used for the postpartum analyses reported here. Thyroid peroxidase negative women were selected to participate in the postpartum phase of the study from a screened group of over 300 TPO negative women through the use of a random number generator. Exclusion criteria for entry into the parent study included age less than 18 or older than 45 years old, immunological disease, immunosuppressive medications, low body mass index (BMI), twin pregnancies, in-vitro fertilization, and congenital anomalies. For the controls, the first four were considered exclusion criteria.
Natural Killer Cytotoxicity Assays were carried out at least three times over the course of 6 months of data collection on each postpartum participant’s blood samples, while the control group provided a single sample of blood for NK cytotoxicity analysis. It was not possible to collect data at all seven collection time points from all participants, due to various reasons. Blood was not always collected at a visit if the mother’s hematocrit was low. There were a few unsuccessful blood draws, and frequently there was not adequate blood for the NKCA, as many other study-related assays were being carried out with the available samples. Occasionally, viability of either the target cells or the effector cells was <80%, and so the assay was not carried out. Thus, each of the time points of data collection represents data from 12 to 25 individuals measured across the first 6 months of the postpartum.
Blood Sample Collection, Processing, and NKCA
Morning visits by a research nurse were made to the postpartum participants’ homes at one week, one month, and then through 6th month postpartum for the postpartum participants. Demographic and health data were collected and thyroid screening and stress and dysphoric mood instruments were administered. At that time, a venipuncture was performed if the hematocrit indicated that the participant was not anemic. Fifteen milliliters of venous blood were collected in heparinized vacutainers and brought immediately to the lab for processing. Four milliliters of heparinized blood were overlaid on 4 mL of Ficoll (Sigma-Aldrich, St. Louis, MO, USA), and centrifuged (Revco, Waltham, MA, USA) at room temperature at 300 g for 25 min with the brake off. The PBMC layer was removed and washed twice in 1640-RPMI supplemented with 10% fetal calf serum and 50 μg/mL of gentamicin. The PBMCs were counted in a Vi-Cell (Beckman Coulter, Fullerton, CA, USA) counter and typically had greater than 90% viability. Peripheral blood mononuclear cells were used at varying effector:target (E:T) ratios in the NKCA, as usual in this assay when peripheral blood is used. The NKCA was prepared according to the standard, recommended protocol.3 After initially determining that unstimulated cells had extremely low cytotoxicity in the NKCA, the PBMCs were incubated in a 37°C humidified incubator with 5% CO2 for 18–20 hr. One thousand units of recombinant [rIL-2 (Sigma-Aldrich)] were added to 2 × 106 cells in 2 mL of media. IL-2 acts to specifically amplify the cytotoxicity of NK cells. All NK cells express IL-2 receptors, which upon activation by IL-2 promotes cytotoxicity of the NK cell.6
After incubation, the cells were centrifuged, resuspended in media, and counted. Viability was always above 80%. K562 (ATCC) cells were maintained in culture at 37°C and 5% CO2. 1 × 106 K562 cells were labeled with 100 μCi of Cr51 for 1 hr and then added to wells in a 96-well plate at four different effector to target (E:T) ratios. K562 cells are the standard target cells in the NKCA, as they are highly sensitive to killing by NK cells and other cytotoxic effector cells in the NKCA. K562 cells were originally developed from a pleural effusion in a 53-year-old woman with chronic myelogenous leukemia. The four E:T ratios used in the experiments were 50:1, 25:1, 10:1, and 5:1. These ratios are, in fact, a ratio of PBMCs to target cells. The plates were incubated for 4 hr, and 100 μL of supernatant were removed from each well. Total radioactivity and spontaneous release wells were included. All measures were carried out in triplicate in a Packard Riastar gamma counter. Percent specific lysis was calculated for each E:T ratio, and the E:T ratio which produced 20% lysis was the LU for that experiment. However, LUs are normally expressed as the number of effector cells in 107 cells, so this calculation was used in the lytic units reported here. This method of using 4 or more E:T ratios is the preferred method for establishing LUs rather than a single percent cytotoxicity calculation, as it relies on the slope of the relationship between E:T ratios and radioactivity released into the supernatant at the end of the incubation.3
Twelve age-matched, non-pregnant and non-postpartum women were recruited to be a control group for the NKCA. The same procedures as above were utilized on single venous blood samples provided by these women, including rIL-2 pre-incubation.
The data were analyzed for normality and were found to have normal distributions. Control LUs were compared with postpartum women’s LUs by t-tests. Lytic units over the seven data collection intervals were also compared by t-tests.
Results
The sample consisted of 39 postpartum TPO women, followed at week 1 postpartum and then monthly for 6 months. The mean age of these women was 29 years (range of 19–44). There were 20 White, 13 Hispanic, four Black, and two Asian participants. The range of parity was from 1 to 6 with a mean of 2.25.
The control group consisted of 12 women (ten White, one Black, two Asian). The mean age of this group was 28.2 years.
We chose to pre-incubate with rIL-2 after initial assays with unstimulated PBMCs from the postpartum women showed very low levels of lysis. At 10:1 and 5:1 E:T ratios, there was no lysis in the unstimulated cell assays, compared with 9.2 ± 1.1 and 4.7 ± 1.03% cytototoxicity in rIL-2 pre-incubated cells. At 50:1, the percent cytotoxicity was 10.7 ± 2.9 in unstimulated cells, compared with 28.8 ± 2.5 in rIL-2 pre-incubated cells, and at 25:1 unstimulated cells showed a 4.1 ± 3.7% cytotoxicity, while the pre-incubated cells showed 21.5 ± 2.5% cytotoxicity. The mean LU of unstimulated cells (n = 18) was 31.26, compared with LU of 95.3 for the same cells in rIL-2 pre-incubation (P < 0.005).
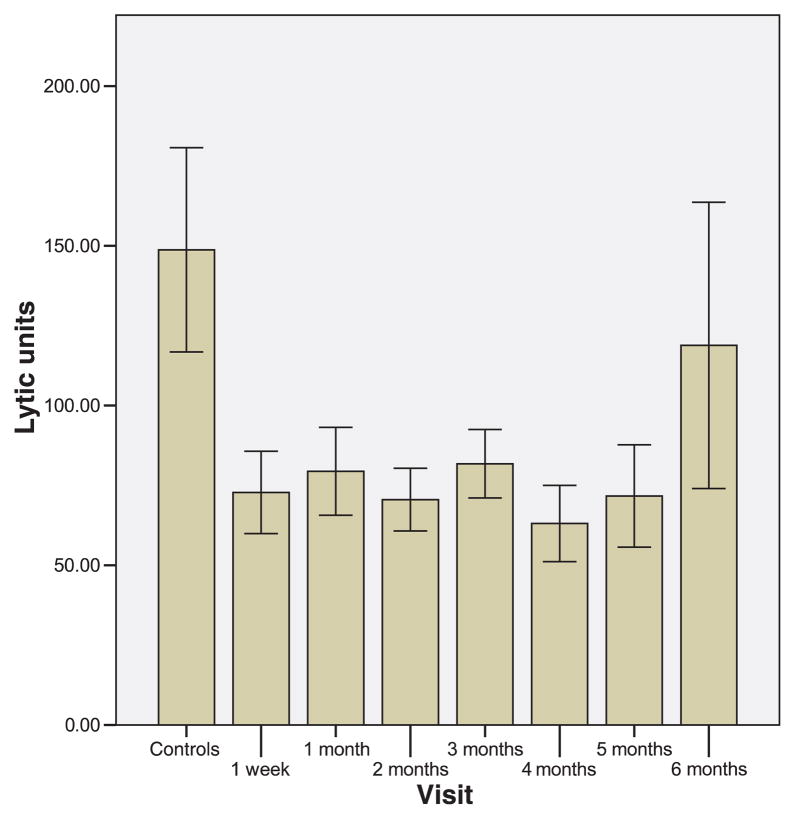
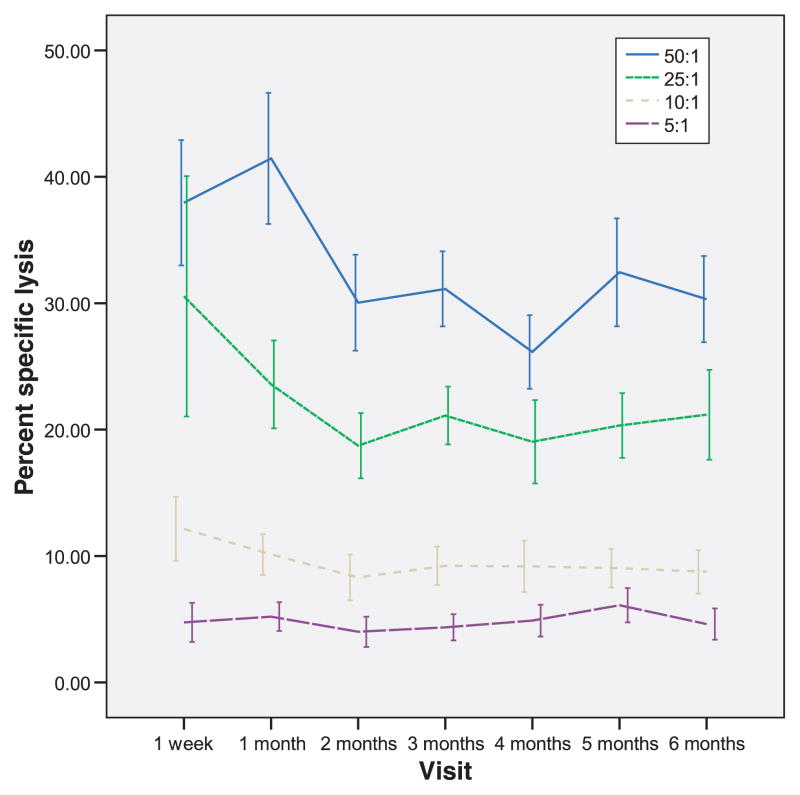
The mean LUs per time of measurement in the postpartum compared with controls are depicted in Fig. 1. Each interval represents a minimum of 12 and a maximum of 25 individual NKCAs. The median LU for the entire data set, when the top and bottom deciles are removed, which is the recommended approach, is 63.7 (range of 14.3 to 128.8).3 According to Friberg, Bryant & Whiteside3, the adult median value for unstimulated cells is in the order of 141 LUs (range of 66 to 341). The median for rIL-2 pre-incubated cells would be expected to be somewhat higher. A total of 168 separate assays across the 6 postpartum months have been analyzed. Lytic units from postpartum women were lower than controls from 1 week to 5 months (P-values from <0.009 to <0.03). At 6 months, the postpartum LUs were not significantly different than the control LUs (P < 0.59). Fig. 2 depicts the percent cytotoxicity across time at the various E:T ratio in postpartum women. The trend appears a decline over time to the lowest cytotoxicity at month 4. However, none of the percents specific lysis over the six months is statistically significantly different from the cytotoxicity observed at week one.
Fig. 1.
Lytic Units per 107 peripheral blood mononuclear cells (PBMCs) in controls and postpartum women. Lytic Units were calculated from co-cultures of PBMCs, pre-incubated with rIL-2 for 18 hr, with Cr51- labeled K562 cells at four effector:target ratios. The cells were cultured together in RPMI for 4 hr, and the radioactivity in supernatants was counted and used in the calculation of lytic units.
Fig. 2.
Graphs depicting percent specific lysis at four different effector: target ratios over time in postpartum women. As anticipated, the greatest lysis of Cr51 labeled K562 cells was achieved at the E:T ratio of 50:1, with minimal lysis observed at the lowest ratio of 5:1. These graphs are significantly different from each other, but over time the percent specific lysis does not differ significantly at any of the ratios.
Discussion
These data suggest that the NK suppression observed in pregnancy continues through the 5th postpartum month. Lytic units remain low through month 5, and then appear to be recovering, as they are no longer significantly different from the control LUs at month 6. The fact that NK suppression is observed uniformly in the early months of the postpartum suggests a profound but essentially normal inhibition of cytotoxicity.
Biological pathways for postpartum NK cytotoxicity suppression remain to be discovered. Soluble HLA-G, HLA-G on microchimeric cells, down regulation or blockade of activating receptors, activation of KIR receptors, or altered cytokine production are all possible mechanisms. General mechanisms by which NK cells are inhibited could include inhibition of the KIR receptors, inhibition or decreased numbers of activation markers such as CD69, inhibition of cytokine secretion or interruption of NK-target conjugate formation.13 Block of programming for lysis such as the MAPK and ERK activation is an additional possibility. 14 Understanding the mechanisms through which this suppression occurs is a goal that may have implications for many clinical scenarios. For example, understanding natural suppression pathways in normal NK cells may ultimately help in preventing graft rejection in transplant recipients, as NK cells play a major role in graft versus host and host versus graft disease, through both proinflammatory cytokine production and cytotoxic attack against allogeneic cells. Further studies of the postpartum NK phenomenon planned in our laboratory may help us to postulate how, for how long, and why this cytotoxicity suppression naturally occurs and how it impacts women’s health.
This study had several important limitations. There was no access to information about any illnesses or disruptions in pregnancy that could potentially influence NK function. We did have data about C-section versus vaginal birth, and this factor did not appear to exert any influence on the NKCA. It would have been preferable to have complete sets of longitudinal data on all participants, but logistics prevented this from being possible.
The study does not provide any insights into mechanisms or reasons for this NK cytotoxicity suppression. We can speculate that it allows for survival and tolerization of fetal microchimeric cells, but further work is necessary to investigate this possibility. It is possible that the pregnancy related suppression requires many months to return to normal levels. It seems unlikely that this suppression increases a women’ risk for postpartum illness, but this needs further investigation as well.
References
- 1.Achdout H, Manaster I, Mandelboim O. Influenza virus infection augments NK cell inhibition through reorganization of major histocompatibility complex class I proteins. J Virol. 2008;82:8030–8037. doi: 10.1128/JVI.00870-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Friberg DD, Bryant JL, Whiteside TL. Measurements of natural killer (NK) activity and NK-cell quantification. Methods A Companion to Methods in Enzymology. 1996;9:316–326. doi: 10.1006/meth.1996.0037. [DOI] [PubMed] [Google Scholar]
- 4.Di Santo JP. Natural killer cells: diversity in search of a niche. Nat Immunol. 2008;9:473–475. doi: 10.1038/ni.f.201. [DOI] [PubMed] [Google Scholar]
- 5.Gregory CD, Lee H, Rees GB, Scott IV, Shah LP, Golding PR. Natural killer cells in normal pregnancy: analysis using monoclonal antibodies and single-cell cytotoxicity assays. Clin Exp Immunol. 1985;62:121–127. [PMC free article] [PubMed] [Google Scholar]
- 6.Gregory CD, Lee H, Scott IV, Golding PR. Phenotypic heterogeneity and recycling capacity of natural killer cells in normal human pregnancy. J Reprod Immunol. 1987;11:135–145. doi: 10.1016/0165-0378(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 7.Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C, Bertone S, Moretta A, Moretta L, Mingari MC. Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci USA. 1999;96:5674–5679. doi: 10.1073/pnas.96.10.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev. 2005;26:44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- 9.Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. 2008;77:14–22. doi: 10.1016/j.jri.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Groer MW, Davis MW, Smith K, Casey K, Kramer V, Bukovsky E. Immunity, inflammation and infection in post-partum breast and formula feeders. Am J Reprod Immunol. 2005;54:222–231. doi: 10.1111/j.1600-0897.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Baley JE, Schacter BZ. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–3048. [PubMed] [Google Scholar]
- 12.Webb BJ, Bochan MR, Montel A, Padilla LM, Brahmi Z. The lack of NK cytotoxicity associated with fresh HUCB may be due to the presence of soluble HLA in the serum. Cell Immunol. 1994;159:246–261. doi: 10.1006/cimm.1994.1311. [DOI] [PubMed] [Google Scholar]
- 13.Gan X, Zhang L, Solomon GF, Bonavida B. Mechanism of norepinephrine-mediated inhibition of human NK cytotoxic functions: inhibition of cytokine secretion, target binding, and programming for cytotoxicity. Brain Behav Immun. 2002;16:227–246. doi: 10.1006/brbi.2000.0615. [DOI] [PubMed] [Google Scholar]
- 14.Jiang K, Zhing B, Gilvary D, Corliss B, Hong-Geller E, Wei S, Djeu J. Pivotal role of phosphoinoditide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]