Abstract
Background
In spite of many similarities in the psychopathology of anorexia nervosa (AN) and bulimia nervosa (BN), the 2 groups seem to differ in terms of body image disturbances. Therefore, the aim of the present study was to compare neuronal correlates of viewing photographs of one’s own body and another woman’s body in patients with these forms of eating disorders as well as controls.
Methods
We performed functional magnetic resonance imaging while women with AN (n = 13), BN (n = 15) and healthy controls (n = 27) viewed 16 standardized pictures of their own body and another woman’s body, taken while the participants were wearing a bikini.
Results
When viewing their own body, participants with AN and BN showed reduced activity in the inferior parietal lobule compared with healthy women. In response to looking at another woman’s body, participants with AN had higher amygdala activity than did those in the BN and control groups.
Limitations
The generalizability of the results is limited by the small sample size.
Conclusion
Our data suggest decreased attentional processes in AN and BN toward one’s own body, possibly reflecting body-related avoidance behaviour. Enhanced limbic activity elicited by looking at another woman’s body in participants with AN might be a neural correlate of stronger emotional activation and enhanced vigilance, possibly resulting from social comparison processes. Our study reveals hints about body image–associated alterations in brain activity, which seem to be more pronounced among women with AN than among those with BN.
Introduction
Body image disturbances are a central feature of the various forms of eating disorders. For anorexia nervosa (AN) and bulimia nervosa (BN), one criterion according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR)1 is the undue influence of weight and shape on selfevaluation. Previous research has demonstrated that body image disturbances are a factor preceding the onset of eating disorders2 and are also a predictor of the maintenance3 and relapse process of these disorders.4
Disturbances of body image can be separated into perceptual, cognitive–affective and behavioural components. The perceptual component includes the mental representation of one’s own body. Previous research has demonstrated that women with AN and BN seem to overestimate their own body dimensions to a similar degree, whereas healthy women show a tendency to underestimate them.5,6 The cognitive–affective component of a disturbed body image comprises negative body-related attitudes and emotions. Although in Western cultures, discontent with one’s shape and weight seems to be widespread in the general female population,7 women with eating disorders exceed healthy women in terms of body dissatisfaction. This body disparagement seems to be higher among women with BN compared with those with AN, possibly owing to the fact that women with AN come closer to their ideal of extreme slimness than do women with BN, who are generally of normal weight (for a meta-analysis, see Cash and Deagle5). Laboratory studies in which participants were confronted with their own bodies demonstrated that, in patients with eating disorders, a higher degree of negative body-related emotions and cognitions was elicited compared with healthy controls. However, these enhanced emotional reactions did not seem to be accompanied by a higher peripheral physiologic and endocrine response.8 A negative evaluation of one’s own body is often associated with body-related avoidance (e.g., not looking in the mirror or hiding one’s body under baggy clothes9), which represents the behavioural component of a disturbed body image.
Recently, functional magnetic resonance imaging (fMRI) studies analyzing neurophysiologic responses to the visual processing of one’s own or another person’s body have been performed to gain an understanding of the neuronal correlates of body image in both healthy people and those with eating disorders. Kurosaki and colleagues10 compared the brain activation patterns of men and women when looking at photographs of their own body that were either nondistorted or that had been distorted as fat or thin. When looking at the fat images, women displayed higher activation bilaterally in the prefrontal cortex and left parahippocampal area including the amygdala, whereas men showed higher activation in the right occipital lobe, including the primary and secondary visual cortices. These results were interpreted as indicating that women tend to perceive their distorted body through a complex processing of emotion, possibly because they evaluated the fat shape of their body as fearful information, whereas men tend to perceive their own distorted body predominantly through object and spatial visual processing.
In another fMRI study with healthy women in which a social comparison paradigm was used, Friederich and colleagues11 compared brain responses to images of slim-idealized bodies with responses to images depicting interior designs. The authors found that when the women compared themselves to the photographs of models, higher activation was found in the lateral fusiform gyrus, right inferior parietal lobule, right lateral prefrontal cortex and left anterior cingulate compared with the control condition. It is noteworthy that the extent of self-reported anxiety during the exposure was positively correlated with brain responses in the basal ganglia of both sides, left amygdala, bilateral dorsal anterior cingulate and left inferior lateral prefrontal cortex. These results suggest that social comparison processes are associated with activation in brain areas reflecting negative emotional arousal.
Beyond body image research in healthy women, some studies have examined the neuronal underpinnings of body image disturbances in eating disorders. In their pilot study involving 3 adolescents with AN, Seeger and colleagues12 used digital pictures of the participants, which were individually distorted by the participants before the scanning session to depict the subjective maximum of unacceptability. It emerged that the participants with AN displayed a stronger activation in the right amygdala, right fusiform gyrus and brainstem region. However, these results could not be replicated in a subsequent study using the same paradigm in a larger sample.13 Instead, participants with AN displayed a higher activation in distinct brain areas such as the inferior parietal lobule, including the anterior part of the intraparietal sulcus, areas specifically involved in visuospatial processing and attentional processes.14,15 The authors concluded that patients with AN are extremely preoccupied with their body appearance and thus process their body image in a different way than controls.
Uher and colleagues,16 in an fMRI study, presented adult AN and BN participants with line drawings of female bodies. Participants with eating disorders were found to display lower activation in the inferior parietal lobule compared with healthy controls. This finding was even stronger in participants with AN compared with those with BN, contradicting the finding by Wagner and colleagues.13 Furthermore, the authors found underactivation in the lateral fusiform gyrus, covering the extrastriate body area, a region specialized in the visual perception of human bodies.17 This relative under-activation was smaller in AN compared with BN and was discussed as underlying the perceptual and cognitive–affective component of body image disturbance.
Whereas in the studies described above, distorted self-images or line-drawings of female bodies were used as stimulus material, Sachdev and colleagues18 presented AN and healthy women with nondistorted self-images and images of another woman. Their results indicate a similar pattern in the 2 groups when looking at the pictures of the other female, with activation in the inferior and middle frontal gyri, superior parietal lobule and thalamus. However, participants with AN displayed stronger activation in the medial frontal gyrus. When looking at their own body, participants with AN did not display significant activation, which was interpreted by the authors as being the result of a lack of activation in the attentional system, probably underlying body image distortion.
Although these studies about body processing in healthy controls and individuals with eating disorders provide valuable information about the neurobiologic underpinnings of body image disturbances, several questions still remain unanswered. First, Seeger and colleagues,12 Wagner and colleagues13 and Sachdev and colleagues18 restricted their studies to women with AN, meaning that individuals with BN have not yet been examined regarding their brain responses to viewing their own body and have consequently not been compared with those with AN and healthy controls. However, this aspect is highly relevant, because previous research has demonstrated that body dissatisfaction seems to be more pronounced in BN compared with AN.5 Furthermore, in these studies, state-like emotional responses to looking at one’s own and another female’s body as well as more trait-like indicators of body image and eating disturbances were not assessed in detail by use of validated questionnaires and were not related to brain activation patterns.
Therefore, in the present fMRI study, the neuronal correlates of looking at pictures of one’s own and another woman’s body as well as self-reported emotional responses to these stimuli were compared between participants with AN, BN and healthy controls. Based on previous findings indicating higher negative affective reactions, including fear, to looking at one’s own body in eating disorders,8 we hypothesized that, in relation to healthy controls, participants with AN and BN would display a higher activity in limbic areas including the amygdala, which is generally known to be involved in emotion processing, especially regarding fear.19 Owing to the higher degree of body dissatisfaction in BN than in AN,5 we assumed that participants with BN would show higher activation in this area compared with those with AN. Furthermore, we hypothesized that participants with AN and BN would display higher activity in the limbic regions, including the amygdala, when looking at another woman’s body, because people with eating disorders generally show more unfavourable social comparisons,20 resulting in a higher fear level, which has, in turn, been shown to be correlated with amygdala activity in healthy women.11 Additionally, we assumed that participants with eating disorders would have reduced activity in the extrastriate body area when looking at the photographs compared with controls, because previous research provided evidence for relative underactivation of the extrastriate body area in eating disorders, especially AN.16 Additionally, owing to the high comorbidity rates between eating disorders and depression21 and between amygdala hyper-activity and depression,22 we expected that participants with eating disorders and depression would have stronger brain responses to viewing their own or another woman’s body compared with participants without depression.
Methods
Participants
A total of 55 women participated in the study. We included 13 patients with AN (including 8 with binge eating episodes and 6 with purging behaviour) and 15 with BN (12 with purging behaviour) according to the criteria of the DSM-IV-TR.1 We recruited participants from the Center for Psychotherapy of the Ruhr-University Bochum. The diagnoses were assessed by experienced clinical psychologists using the Structured Clinical Interview for DSM-IV.23 We included 27 healthy women aged 18 to 50 years as controls; these women had no present or past eating disorder (verified with the Eating Disorder Examination Questionnaire24). We included only right-handed women in the study. We excluded participants if they had a personality disorder, suffered from claustrophobia or had metal parts in their body.
After the study had been described to each participant, written consent was obtained. The protocol was approved by the local ethics committee of the Ruhr-University of Bochum.
Stimulus material
One set of 16 photographs of each participant’s own body and one set of 16 photographs of a standardized unknown woman’s body (body mass index [BMI] 19, age 28 years; Fig. 1) were presented to each participant. All photographs were taken in the same room under identical conditions, with participants wearing a uniform pink bikini in their size in front of a white wall. Each participant was photographed from 16 standardized perspectives (e.g., front, back, right, left and between perspectives) without the head, because previous research demonstrated that including the face decreases activation of the extrastriate body area.25 Furthermore, research revealed that healthy women and those with eating disorder symptoms show different gaze patterns concerning the face; women with eating disorders neglect the face when looking at body pictures and healthy women scan the whole body.26,27
Fig. 1.
Standardized photographs of the comparison woman’s body from 16 different perspectives.
Each of the 16 photographs was presented for 3 seconds in a random order (total duration of each stimuli set 48 s). The photographs were presented in a block design in which each set of stimuli was presented 3 times without direct repetition of the same block. Before each block, a slide with the text “own body” or “other female’s body” was presented to the participants, indicating which slides would be shown next. Between each of the sets of stimuli, a fixation cross was presented for 48 seconds. The sequence of the presentation of the stimuli was the same across all participants to keep the conditions for each participant as homogeneous as possible. The participants were instructed to look at the pictures and not to close their eyes. For ethical reasons, each participant was informed in advance that she would be photographed in a standardized bikini and that she would be presented with these pictures as well as pictures of an unknown woman in the scanner.
Image acquisition and processing
We acquired images using a 1.5-T Symphony scanner (Siemens). A total of 440 T2*-weighted whole brain volumes omitting parts of the cerebellum were acquired in an ascending order for each condition. Each volume consisted of 25 slices of 3 mm (interslice gap 1 mm). The repetition time was 80 ms per slice (total repetition time 2000 ms) with an echo time of 40 ms and a flip angle of 90°. Additionally, we acquired high-resolution T1-weighted images with a voxel size of 1 × 1 × 1 mm, a repetition time of 2110 ms, an echo time of 3.93 ms and a flip angle of 15° for localization and coregistration of the functional data.
Questionnaires
To assess the degree of body image and eating disturbance, we administered several questionnaires to the participants. All questionnaires used are well established in eating disorders research. We used the 4 subscales Restraint, Eating Concern, Weight Concern and Shape Concern of the Eating Disorder Examination Questionnaire28,29 to assess relevant characteristics of eating disorders that had occurred within the past 28 days. The Restraint scale consists of 5 items (e.g., “Over the past 4 weeks, have you wanted your stomach to be empty? Has this been to influence your shape and weight?”), and the Eating Concern scale comprises 5 items (e.g., “Over the past 4 weeks, have you eaten in secret?”). The Weight Concern subscale comprises 5 items (e.g., “Over the past 4 weeks, have you been dissatisfied with your weight? Have you been so dissatisfied that it has made you unhappy?”) and the Shape Concern scale comprises 8 items (e.g., “Over the past 4 weeks, have you been dissatisfied with your shape? Have you been so dissatisfied that it has made you unhappy?”). Each item was scored on a 7-point scale, ranging from 0 (attribute not present) to 6 (attribute present every day). Internal consistencies of the subscales were good, with Cronbach α ranging from 0.76 to 0.93. Test–retest reliability varied from rtt = 0.68 to rtt = 0.74.
Additionally, we administered the subscales Drive for Thinness, Bulimia and Body Dissatisfaction from the Eating Disorder Inventory-2.30,31 These subscales include fear of getting fat, thoughts about diet and weight, binge-eating behaviour and the evaluation of one’s own body as negative. The Drive for Thinness scale includes 7 items (e.g., “I eat sweets and carbohydrates without feeling nervous”), the Bulimia scale includes 7 items (e.g., “I eat when I am upset”) and the Body Dissatisfaction scale consists of 9 items (e.g., “I think that my stomach is too big”). Each item was scored on a 6-point scale, ranging from 1 (never) to 6 (always). Internal consistencies for these ranged from α = 0.73 to α = 0.94 for different samples. The test–retest reliability varied between rtt = 0.86 and rtt = 0.94 for an intervening period of 7 days.31
We used the Body Image Avoidance Questionnaire9,32 to assess the behavioural component of body image disturbances. This questionnaire consists of 19 items (e.g., “I wear baggy clothes”) covering several areas such as clothing and social activities. The general score has sufficient psychometric properties, with Cronbach α of 0.89 and a test–retest reliability of rtt = 0.87 for an intervening period of 2 weeks.9
We used the Beck Depression Inventory33,34 as a measure of depressiveness. This self-report questionnaire consists of 21 items asking about how the participant has been feeling in the last week. The items are scored on a scale ranging from 0 (“I do not feel sad”) to 3 (“I am so sad or unhappy that I can’t stand it”), which can be summarized into one sum score. The internal consistency of the sum score ranged from α = 0.74 to α = 0.92 in various samples. The test–retest reliability was rtt = 0.75 for an intervening period of one week.
To assess positive and negative affect when looking at the participant’s own body and the other woman’s body on a computer screen, we administered the Positive and Negative Affect Schedule35,36 after the scanning session. The questionnaire consists of 20 items (10 items for positive affect [e.g., “interested”] and 10 items for negative affect [e.g., “scared”]). Participants had to indicate on a scale ranging from 1 (not at all) to 5 (very much) the extent to which each of the listed emotions had occurred during viewing the pictures of each set. The internal consistency of the questionnaire ranged from α = 0.85 to α = 0.86.
Procedure
After agreeing to participate, participants were given the Structured Clinical Interview for DSM-IV23 to assess the diagnoses. Following this, the participants filled out questionnaires, including the Eating Disorder Examination Questionnaire, the Eating Disorder Inventory-2, the Body Image Avoidance Questionnaire and the Beck Depression Inventory. On a separate day, 16 photographs of each participant were taken, followed by the scanning session. Afterwards, each participant was asked whether she had looked at the pictures and did not close her eyes. Additionally, the sets of photographs of one’s own and another woman’s body were again presented on a laptop computer. Directly after the presentation of each set, participants were asked to fill in the Positive and Negative Affect Schedule to evaluate the degree of positive and negative affect that they had experienced during the scanning session when viewing the photographs. At the end of the experiment, participants discussed their experiences during data collection with a clinical psychologist.
Data analysis
We analyzed the data using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5/), starting with slice time correction followed by motion correction. Each scan was realigned to the first scan of the session. We normalized the acquired images to the standard brain of the Montreal Neurological Institute as provided by SPM5. Following this, the images were smoothed using a Gaussian kernel of 8 mm. We applied a general linear model to the data. In the first-level analysis, contrasts of one’s own body and the other woman’s body were calculated with the fixation condition serving as an implicit baseline. These contrast images were fed into a second-level analysis using a one-way analysis of variance (ANOVA) comparing the 3 groups regarding their brain responses to viewing their own and the other woman’s body, and posthoc 2-sample t tests were used to further specify group differences. All results were thresholded at p < 0.001 (uncorrected) with an extent threshold of 8 voxels. We transformed the resulting coordinates into the space defined by Talairach and Tournoux37 using the algorithm suggested by Brett (http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/mni2tal.m).
To compare the 3 groups in terms of age, height, BMI, duration of illness and the scores on the questionnaires, we used a one-way ANOVA (SPSS 16 software). If the overall group difference reached the statistical significance threshold of p < 0.05, we used the Dunnett T3 test as posthoc test for a pairwise comparison of the 3 groups. For the comparison of participants with AN and BN for the duration of illness, we used a 2-sample t test.
We extracted the percentages of signal change in selected brain areas using the MarsBaR software package (marsbar.sourceforge.net); these signal changes were correlated with the scores from the questionnaires separately for participants with AN, BN and healthy controls using the Pearson coefficient.
To check whether depressive symptoms affect brain activation, we categorized the AN and BN participants’ scores on the Beck Depression Inventory as depressive (scores ≥ 18) or nondepressive (scores ≤ 17). We compared the neuronal responses of these 2 groups while looking at pictures of their own and the other woman’s body using a 2-sample t test.
Results
Participant characteristics
The descriptive statistics for the 3 groups are presented in Table 1. A one-way ANOVA revealed that the 3 groups did not differ in age (F2,52 = 0.44, p = 0.65) or height (F2,52 = 0.06, p = 0.94). However, there were significant differences in BMI between the 3 groups (F2,52 = 47.23, p < 0.001). Posthoc tests (Dunnett T3) revealed that the AN participants had a significantly lower BMI than those with BN (p < 0.001) and healthy controls (p < 0.001), whereas participants with BN and healthy controls did not differ (p = 0.63). The mean duration of illness was not significantly different between participants with AN and BN (t24 = 1.69, p = 0.10).
Table 1.
Characteristics of participants with anorexia nervosa or bulimia nervosa and healthy controls
| Group; mean (standard deviation) [range] |
|||
|---|---|---|---|
| Characteristic | Anorexia nervosa, n = 13 | Bulimia nervosa, n = 15 | Healthy controls, n = 27 |
| Age, yr | 29.08 (9.79) [18–49] | 28.40 (7.07) [20–42] | 26.74 (7.60) [19–50] |
| Height, cm | 168.46 (7.26) [162–186] | 168.53 (5.11) [158–176] | 169.07 (5.83) [160–181] |
| Body mass index | 15.78 (1.28) [12.92–17.38] | 21.34 (2.26) [17.68–24.44] | 22.06 (2.06) [17.95–26.74] |
| Duration of illness, yr | 7.21 (6.20) [1–17] | 11.82 (7.51) [4–27] | — |
| Eating Disorder Examination Questionnaire24,28,29 score | |||
| Restraint | 4.11 (1.16) [1.80–6.00] | 3.57 (1.60) [0.40–6.00] | 0.67 (0.56) [0.00–2.00] |
| Eating Concern | 3.86 (0.86) [2.20–5.00] | 3.24 (1.40) [0.40–5.60] | 0.26 (0.31) [0.00–1.20] |
| Weight Concern | 3.63 (1.14) [1.40–4.80] | 3.84 (1.48) [0.80–5.80] | 0.82 (0.47) [0.20–2.20] |
| Shape Concern | 4.44 (1.14) [1.50–5.88] | 4.21 (1.59) [1.29–6.00] | 1.01 (0.64) [0.38–2.75] |
| Eating Disorder Inventory-230,31 score | |||
| Drive for Thinness | 4.44 (0.85) [3.14–5.57] | 4.93 (0.71) [3.86–5.86] | 1.96 (0.65) [1.00–3.86] |
| Bulimia | 3.05 (1.33) [1.29–5.43] | 3.94 (0.56) [3.00–4.86] | 1.25 (0.23) [1.00–1.86] |
| Body Dissatisfaction | 4.47 (0.94) [3.22–6.00] | 4.84 (1.04) [2.67–6.00] | 2.66 (0.72) [1.56–4.33] |
| Body Image Avoidance Questionnaire9,32 score | 1.83 (0.44) [1.16–2.42] | 1.80 (0.51) [0.84–2.68] | 0.95 (0.31) [0.53–1.95] |
| Beck Depression Inventory33,34 score | 26.07 (6.33) [17–36] | 16.67 (10.09) [4–34] | 3.41 (4.09) [0–14] |
In a one-way ANOVA, significant group differences were detected with respect to the subscales of the Eating Disorder Examination Questionnaire Restraint (F2,52 = 59.50, p < 0.001), Eating Concern (F2,52 = 100.11, p < 0.001), Weight Concern (F2,52 = 58.92, p < 0.001) and Shape Concern (F2,52 = 63.67, p < 0.001), as well as for the scales Drive for Thinness (F2,52 = 102.28, p < 0.001), Bulimia (F2,52 = 74.00, p < 0.001) and Body Dissatisfaction (F2,52 = 37.16, p < 0.001) from the Eating Disorder Inventory-2 (Table 1). Similarly, in the Body Image Avoidance Questionnaire, significant group differences were found (F2,52 = 31.43, p < 0.001). Posthoc tests revealed that participants with AN and BN differed significantly from healthy controls on each of the Eating Disorder Examination Questionnaire and Eating Disorder Inventory-2 scales as well as on the general score of the Body Image Avoidance Questionnaire (p < 0.001), whereas no significant differences emerged between women with AN and BN (p = 0.112 to 0.998). In the Beck Depression Inventory, significant group differences were also shown between the 3 groups (F 2,52 = 54.25, p < 0.001). Posthoc tests indicated that the participants with AN (p < 0.001) and BN (p < 0.001) had significantly higher depression scores compared with those in the control group. Participants with AN had significantly higher depression scores than those with BN (p = 0.019).
Self-reported affective responses
When looking at the photographs of one’s own body, the ANOVA revealed significant group differences in the extent of self-reported positive (F2,52 = 6.38, p = 0.003) and negative affect (F2,52 = 27.52, p < 0.001; Table 2). Posthoc analyses indicated that participants with AN (p = 0.001) and BN (p < 0.001) showed a higher degree of negative affect compared with healthy women. Participants with BN (p < 0.001) also displayed a lower degree of positive affect compared with healthy controls, whereas participants with AN (p = 0.29) did not. Participants with AN and BN did not differ significantly from each other in either positive (p = 0.41) or negative affective reactions (p = 0.99) to looking at their own body.
Table 2.
| Group; score, mean (standard deviation) [range] |
|||
|---|---|---|---|
| Images viewed | Anorexia nervosa | Bulimia nervosa | Healthy controls |
| Participant’s own body | |||
| Positive affect | 20.85 (6.72) [11–37] | 17.80 (3.88) [10–23] | 24.56 (6.53) [14–38] |
| Negative affect | 27.62 (8.82) [16–42] | 28.27 (5.85) [17–38] | 14.81 (5.67) [10–33] |
| Other woman’s body | |||
| Positive affect | 26.00 (6.60) [17–39] | 21.67 (6.77) [13–34] | 22.67 (6.37) [14–35] |
| Negative affect | 17.46 (4.84) [12–28] | 16.13 (5.33) [11–28] | 12.04 (4.43) [8–32] |
No significant group differences were found for positive affect when looking at photographs of another woman’s body (F2,52 = 1.70, p = 0.19), whereas negative affective reactions to viewing another woman’s body differed significantly between the 3 groups (F2,52 = 6.94, p = 0.002; Table 2). Posthoc tests indicated that participants with AN displayed a higher degree of negative affect compared with healthy controls (p = 0.007), whereas the difference between participants with BN and healthy controls was not significant (p = 0.052). Participants with either BN or AN did not differ from each other (p = 0.87).
Brain responses to looking at one’s own body
In the one-way ANOVA, the 3 groups differed significantly in activation in the left inferior temporal gyrus (Brodmann Area [BA] 20) when looking at their own bodies. Pairwise posthoc t tests indicated that participants with BN showed lower activation in this brain area compared with healthy controls. The ANOVA revealed a significant group difference in the left inferior parietal lobule (BA 40), with weaker activation in participants with AN compared with controls. Additionally, group differences in the left middle temporal gyrus (BA 21, 38) were found in the ANOVA, with participants with AN and BN displaying a weaker activation in comparison to controls.
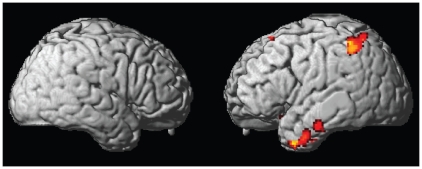
The t test comparing participants with AN and healthy controls revealed lower activation when looking at the photographs of one’s own body for participants with AN in the left uncus (BA 20), left superior parietal lobule (BA 7), left medial frontal gyrus (BA 10), left fusiform gyrus (BA 20), bilaterally in the inferior frontal gyrus (BA 47), left superior frontal gyrus (BA 6) and bilateral parahippocampal gyrus (BA 27) including the right hippocampus. Additional weaker activation in participants with BN compared with healthy controls were observed in the right inferior parietal lobule (BA 40) and right middle frontal gyrus (BA 11) when looking at the photographs of one’s own body. In the opposite contrast, the t test did not reveal stronger activation in AN and BN participants compared with healthy controls in any brain region when looking at pictures of their own bodies. When directly comparing participants with AN and BN, those with BN displayed stronger activation in the right medial frontal gyrus (BA 11), whereas in the opposite contrast, no significant difference was found (Table 3, Fig. 2).
Table 3.
Group comparisons of activation maps in response to viewing photographs of one’s own body
| Comparison; brain structure | Brodmann area | Talairach coordinates |
Cluster level | F or t value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| One-way analysis of variance: main effect of group | ||||||
| Inferior temporal gyrus | 20 | −32 | −10 | −36 | 49 | 3.99 |
| Inferior parietal lobule | 40 | −40 | −48 | 50 | 100 | 3.97 |
| Middle temporal gyrus | 21 | −42 | −4 | −34 | 40 | 3.75 |
| Middle temporal gyrus | 38 | −40 | 10 | −40 | 8 | 3.28 |
| Post-hoc t test: anorexia nervosa > healthy females | — | — | — | — | — | — |
| Post-hoc t test: healthy females > anorexia nervosa | ||||||
| Uncus | 20 | −30 | −10 | −36 | 261 | 4.57 |
| Middle temporal gyrus | 21 | −42 | −2 | −36 | 4.54 | |
| Middle temporal gyrus | 21 | −42 | 10 | −40 | 4.16 | |
| Inferior parietal lobule | 40 | −40 | −48 | 50 | 247 | 4.52 |
| Superior parietal lobule | 7 | −36 | −58 | 64 | 4.42 | |
| Inferior parietal lobule | 40 | −48 | −46 | 60 | 3.96 | |
| Medial frontal gyrus | 10 | −14 | 36 | −4 | 15 | 4.10 |
| Fusiform gyrus | 20 | −58 | −18 | −26 | 57 | 4.05 |
| Inferior frontal gyrus | 47 | 24 | 16 | −18 | 26 | 3.82 |
| Superior frontal gyrus | 6 | −6 | 28 | 60 | 15 | 3.79 |
| Parahippocampal gyrus | 27 | −22 | −38 | −2 | 19 | 3.66 |
| Inferior frontal gyrus | 47 | −24 | 18 | −16 | 9 | 3.57 |
| Parahippocampal gyrus–hippocampus | — | 26 | −10 | −26 | 8 | 3.56 |
| Post-hoc t test: bulimia nervosa > healthy females | — | — | — | — | — | — |
| Post-hoc t test: healthy females > bulimia nervosa | ||||||
| Inferior parietal lobule | 40 | 42 | −58 | 40 | 58 | 4.26 |
| Middle frontal gyrus | 11 | 24 | 26 | −20 | 30 | 4.06 |
| Middle temporal gyrus | 38 | −38 | 10 | −46 | 22 | 3.97 |
| Inferior temporal gyrus | 20 | −44 | −8 | −36 | 38 | 3.84 |
| Inferior temporal gyrus | 20 | −52 | −4 | −38 | 3.58 | |
| Post-hoc t test: anorexia nervosa > bulimia nervosa | — | — | — | — | — | — |
| Post-hoc t test: bulimia nervosa > anorexia nervosa | ||||||
| Medial frontal gyrus | 11 | 8 | 58 | −16 | 12 | 4.32 |
Fig. 2.
Activation differences in response to viewing photographs of one’s own body projected on a rendered brain showing areas where activation was greater in healthy controls than in participants with anorexia nervosa.
Brain responses to looking at another woman’s body
The ANOVA revealed a significant group difference in the bilateral middle temporal gyrus (BA 20, 21, 22, 38) when looking at photographs of the other woman’s body. The posthoc t test indicated that participants with AN displayed a higher bilateral activation in this brain area compared with controls. The t test indicated stronger bilateral activation in this brain region in participants with AN compared with those with BN. In the ANOVA, an additional group difference was found in the left inferior temporal gyrus (BA 20), with stronger activation in participants with AN compared to both controls and participants with BN. A further overall group difference emerged in the ANOVA in the right culmen, which was not significant in the t tests. Additionally, ANOVA indicated that there were group differences in the bilateral postcentral gyrus (BA 2, 5). Participants with AN and BN had stronger activation in this brain region compared with controls. Furthermore, the ANOVA revealed significant overall group differences in the bilateral parahippocampal gyrus (BA 36, 27), with participants with AN and BN displaying stronger activation than controls.
The ANOVA indicated significant activation differences in the bilateral superior temporal gyrus (BA 38, 39). Participants with AN displayed stronger activation in this brain region than controls and participants with BN. In the ANOVA, further activation differences emerged in the right amygdala, with participants with AN showing higher activation than controls and participants with BN. Furthermore, the ANOVA revealed group differences in the right inferior parietal lobule (BA 40). The t test indicated weaker activation in this area in participants with BN compared with controls. Finally, the ANOVA revealed a group difference in the right superior parietal lobule, with participants with BN displaying lower activation compared with controls and participants with AN.
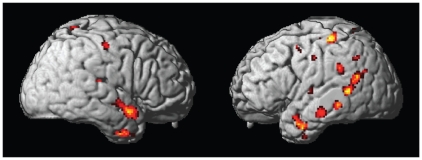
When looking at pictures of another woman’s body, participants with AN, relative to controls, showed additional activation in the left precentral gyrus (BA 4, 6), bilaterally in the thalamus including the bilateral ventral anterior nucleus and right pulvinar, medial paracentral lobule (BA 4), right posterior cingulate (BA 30), left hippocampus, left uncus (BA 28), bilaterally in the fusiform gyrus (BA 20), left insula (BA 13), bilaterally in the medial frontal gyrus (BA 6), left middle frontal gyrus (BA 6), right claustrum, left supramarginal gyrus (BA 40), left caudate body, left lingual gyrus (BA 18, 19), left inferior parietal lobule (BA 40) and left precuneus (BA 31). When comparing participants with BN and controls, additional activation was found in the right middle frontal gyrus (BA 11), left hippocampus and right inferior frontal gyrus (BA 45), with healthy controls showing higher activation. In the direct comparison of participants with AN and BN, participants with AN showed higher activation in the left inferior parietal lobule (BA 40), left supramarginal gyrus (BA 40), left precentral gyrus (BA 4, 6, 13), left uncus, right inferior temporal gyrus (BA 20), right cingulate gyrus (BA 31), left inferior frontal gyrus (BA 47), right precuneus (BA 7) and right insula (BA 13). In the opposite comparison, participants with BN did not show higher activation in any brain region compared with those with AN (Table 4, Fig. 3).
Table 4.
Group comparisons of activation maps in response to viewing photographs of another woman’s body
| Comparison; brain structure | Brodmann area | Talairach coordinates |
Cluster level | F or t value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| One-way analysis of variance: main effect of group | ||||||
| Middle temporal gyrus | 22 | −54 | −50 | 6 | 122 | 4.50 |
| Middle temporal gyrus | 21 | −50 | 4 | −26 | 195 | 4.16 |
| Middle temporal gyrus | 38 | −40 | 8 | −42 | 3.96 | |
| Inferior temporal gyrus | 20 | −44 | −4 | −40 | 3.61 | |
| Culmen | — | 2 | −54 | 2 | 37 | 3.93 |
| Postcentral gyrus | 2 | −40 | −34 | 62 | 26 | 3.83 |
| Middle temporal gyrus | 20 | −52 | −38 | −10 | 34 | 3.81 |
| Parahippocampal gyrus | 36 | −24 | −42 | −4 | 15 | 3.78 |
| Superior temporal gyrus | 38 | 52 | 6 | −14 | 55 | 3.77 |
| Amygdala | — | 28 | −2 | −20 | 35 | 3.75 |
| Postcentral gyrus | 5 | 2 | −48 | 68 | 60 | 3.70 |
| Inferior parietal lobule | 40 | 42 | −44 | 50 | 12 | 3.57 |
| Middle temporal gyrus | 38 | 38 | 4 | −36 | 32 | 3.51 |
| Inferior temporal gyrus | 20 | −60 | −20 | −16 | 27 | 3.48 |
| Superior parietal lobule | 7 | 26 | −66 | 58 | 13 | 3.46 |
| Parahippocampal gyrus | 27 | 10 | −34 | 2 | 8 | 3.32 |
| Superior temporal gyrus | 39 | −46 | −56 | 20 | 14 | 3.29 |
| Post-hoc t test: anorexia nervosa > healthy females | — | — | — | — | — | — |
| Precentral gyrus | 4 | −36 | −30 | 60 | 105 | 6.12 |
| Superior temporal gyrus | 38 | 52 | 6 | −16 | 207 | 5.00 |
| Superior temporal gyrus | 22 | 46 | −4 | −8 | 4.11 | |
| Thalamus | — | 18 | −6 | 14 | 61 | 4.97 |
| Thalamus–ventral anterior nucleus | — | 10 | −6 | 6 | 3.74 | |
| Postcentral gyrus | 5 | 2 | −46 | 68 | 171 | 4.80 |
| Paracentral lobule | 4 | 0 | −38 | 68 | 3.90 | |
| Posterior cingulate | 30 | 2 | −54 | 2 | 181 | 4.66 |
| Thalamus | — | 10 | −34 | 2 | 4.02 | |
| Parahippocampal gyrus | 30 | 6 | −44 | −2 | 3.63 | |
| Subgyral–hippocampus | — | −24 | −40 | −4 | 40 | 4.45 |
| Amygdala | — | 28 | −2 | −20 | 98 | 4.45 |
| Uncus | 28 | −20 | −10 | −34 | 3.49 | |
| Superior temporal gyrus | 22 | −46 | −46 | 8 | 223 | 4.31 |
| Superior temporal gyrus | 39 | −48 | −54 | 18 | 4.15 | |
| Superior temporal gyrus | 22 | −54 | −50 | 6 | 4.13 | |
| Middle temporal gyrus | 21 | −50 | 4 | −26 | 130 | 4.29 |
| Fusiform gyrus | 20 | −44 | −4 | −30 | 4.14 | |
| Middle temporal gyrus | 21 | −52 | −38 | −10 | 57 | 4.27 |
| Insula | 13 | −40 | −14 | 24 | 28 | 4.24 |
| Medial frontal gyrus | 6 | 4 | −12 | 60 | 71 | 4.21 |
| Medial frontal gyrus | 6 | −2 | −24 | 70 | 3.77 | |
| Superior temporal gyrus | 22 | −40 | −26 | 4 | 28 | 4.19 |
| Uncus | 28 | −22 | −8 | −38 | 8 | 4.19 |
| Insula | 13 | −32 | 2 | 18 | 12 | 4.13 |
| Precentral gyrus | 4 | −26 | −22 | 56 | 31 | 4.12 |
| Middle frontal gyrus | 6 | −20 | −18 | 62 | 3.64 | |
| Middle temporal gyrus | 21 | −60 | −22 | −18 | 76 | 4.12 |
| Middle temporal gyrus | 21 | 42 | 2 | −36 | 89 | 4.10 |
| Inferior temporal gyrus | 20 | 52 | −4 | −38 | 3.67 | |
| Middle temporal gyrus | 21 | 44 | 12 | −40 | 3.44 | |
| Claustrum | — | 32 | 0 | 12 | 26 | 4.10 |
| Fusiform gyrus | 20 | 38 | −20 | −28 | 17 | 4.07 |
| Postcentral gyrus | 3 | 48 | −18 | 50 | 27 | 4.05 |
| Thalamus–pulvinar | — | 20 | −24 | 12 | 22 | 4.03 |
| Parahippocampal gyrus | 35 | −28 | −24 | −22 | 11 | 4.02 |
| Supramarginal gyrus | 40 | −56 | −40 | 30 | 48 | 4.01 |
| Thalamus–ventral anterior nucleus | — | −16 | −8 | 16 | 21 | 3.91 |
| Caudate–caudate body | — | −12 | 4 | 18 | 16 | 3.87 |
| Inferior temporal gyrus | 20 | −42 | −4 | −42 | 10 | 3.87 |
| Lingual gyrus | 18 | −8 | −78 | −8 | 25 | 3.83 |
| Lingual gyrus | 18 | −12 | −80 | −18 | 3.44 | |
| Parahippocampal gyrus | 35 | −30 | −2 | −26 | 9 | 3.82 |
| Postcentral gyrus | 5 | 18 | −46 | 70 | 11 | 3.81 |
| Parahippocampal gyrus | 36 | 26 | −20 | −30 | 3.45 | |
| Lingual gyrus | 19 | −28 | −60 | 0 | 22 | 3.75 |
| Parahippocampal gyrus | 36 | −20 | −28 | −30 | 3.41 | |
| Inferior parietal lobule | 40 | −54 | −58 | 40 | 16 | 3.69 |
| Precentral gyrus | 6 | −50 | −10 | 6 | 10 | 3.68 |
| Precentral gyrus | 4 | −52 | −14 | 40 | 10 | 3.67 |
| Precuneus | 31 | −14 | −72 | 22 | 11 | 3.62 |
| Middle frontal gyrus | 6 | −46 | 4 | 50 | 9 | 3.60 |
| Middle temporal gyrus | 39 | −52 | −64 | 26 | 13 | 3.60 |
| Postcentral gyrus | 40 | 64 | −24 | 16 | 8 | 3.45 |
| Post-hoc t test: healthy females > anorexia nervosa | ||||||
| Inferior parietal lobule | 40 | −42 | −44 | 48 | 10 | 3.58 |
| Post-hoc t test: bulimia nervosa > healthy females | ||||||
| Postcentral gyrus | 3 | 22 | −34 | 60 | 19 | 4.26 |
| Parahippocampal gyrus | 36 | 34 | −24 | −28 | 15 | 3.73 |
| Post-hoc t test: healthy females > bulimia nervosa | ||||||
| Middle frontal gyrus | 11 | 22 | 28 | −18 | 44 | 4.41 |
| Superior parietal lobule | 7 | 26 | −66 | 58 | 25 | 4.16 |
| Inferior parietal lobule | 40 | 42 | −44 | 50 | 73 | 3.87 |
| Inferior parietal lobule | 40 | 42 | −48 | 58 | 3.85 | |
| Parahippocampal gyrus–hippocampus | – | −34 | −12 | −22 | 8 | 3.54 |
| Inferior frontal gyrus | 45 | 54 | 32 | 4 | 9 | 3.47 |
| Post-hoc t test: anorexia nervosa > bulimia nervosa | ||||||
| Middle temporal gyrus | 21 | −58 | −50 | 6 | 199 | 6.12 |
| Middle temporal gyrus | 21 | −50 | 4 | −24 | 277 | 5.29 |
| Middle temporal gyrus | 38 | −40 | 8 | −42 | 5.11 | |
| Inferior temporal gyrus | 20 | −46 | −2 | −36 | 4.30 | |
| Middle temporal gyrus | 21 | −54 | −38 | −10 | 142 | 4.81 |
| Middle temporal gyrus | 21 | −58 | −22 | −10 | 4.28 | |
| Middle temporal gyrus | 21 | −66 | −30 | −6 | 4.27 | |
| Middle temporal gyrus | 38 | 34 | 6 | −38 | 43 | 4.77 |
| Inferior parietal lobule | 40 | −52 | −54 | 38 | 54 | 4.76 |
| Supramarginal gyrus | 40 | −44 | −54 | 34 | 3.49 | |
| Precentral gyrus | 13 | −50 | −10 | 10 | 33 | 4.52 |
| Superior temporal gyrus | 13 | −54 | −42 | 22 | 33 | 4.48 |
| Uncus | 28 | −20 | −12 | −36 | 3.90 | |
| Amygdala | — | 26 | −2 | −18 | 24 | 4.30 |
| Inferior temporal gyrus | 20 | 56 | −28 | −18 | 20 | 4.25 |
| Cingulate gyrus | 31 | 16 | −32 | 42 | 42 | 4.21 |
| Superior parietal lobule | 7 | 22 | −66 | 58 | 32 | 4.15 |
| Precentral gyrus | 6 | −32 | −2 | 40 | 12 | 4.14 |
| Inferior frontal gyrus | 47 | −44 | 32 | −18 | 9 | 4.08 |
| Precentral gyrus | 4 | −48 | −16 | 42 | 22 | 4.07 |
| Precuneus | 7 | 8 | −52 | 46 | 9 | 3.89 |
| Insula | 13 | 40 | −24 | −2 | 9 | 3.65 |
| Middle temporal gyrus | 21 | 50 | 8 | −20 | 12 | 3.64 |
| Post-hoc t test: bulimia nervosa > anorexia nervosa | — | — | — | — | — | — |
Fig. 3.
Activation differences in response to viewing photographs of the other woman’s body projected on a rendered brain indicating areas where activation was greater in participants with anorexia nervosa than in healthy controls.
Correlations between brain activation and behavioural measures
Because we found that the left inferior parietal lobule was underactivated in AN and BN in response to looking at one’s own body, we examined the correlation between activation in this cluster and behavioural data. However, within each of the 3 groups, no significant correlation was found between activation in this brain area and behavioural data from the Eating Disorder Examination Questionnaire, Eating Disorder Inventory-2, Body Image Avoidance Questionnaire, Beck Depression Inventory or the Positive and Negative Affect Schedule. Within each of the 3 groups, none of these questionnaire scales was correlated significantly with activation in the right amygdala, which was more strongly activated in participants with AN compared with those with BN and healthy controls when looking at the pictures of the other woman’s body.
Brain responses in participants with eating disorders and depression
When we compared AN and BN participants with a score of 18 or above on the Beck Depression Inventory (n = 19) to those with lower values (n = 9), a 2-sample t test showed no significant group difference when looking at the pictures of one’s own body. However, when looking at the other woman’s body, the participants with depression had higher activation in the left postcentral gyrus (BA 2, 40), left precentral gyrus (BA 4) and left middle temporal gyrus (BA 21). In the opposite contrast, participants with depression showed lower activation in the right cingulate gyrus (BA 24, Table 5).
Table 5.
Group comparisons of activation maps in response to viewing photographs of one’s own or another woman’s body between anorexia nervosa and bulimia nervosa participants with and without depression*
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Images viewed; comparison | Brodmann area | x | y | z | Cluster level | t value |
| Participant’s own body | ||||||
| Depressive > nondepressive | — | — | — | — | — | — |
| Nondepressive > depressive | — | — | — | — | — | — |
| Other woman’s body | ||||||
| Depressive > nondepressive | ||||||
| Postcentral gyrus | 40 | −38 | −32 | 54 | 52 | 4.32 |
| Precentral gyrus | 4 | −32 | −28 | 50 | 4.12 | |
| Postcentral gyrus | 2 | −42 | −32 | 62 | 3.92 | |
| Middle temporal gyrus | 21 | −56 | −22 | −12 | 9 | 3.89 |
| Nondepressive > depressive | ||||||
| Cingulate gyrus | 24 | 12 | 10 | 30 | 16 | 4.02 |
Discussion
The primary aim of our study was to compare women with AN and BN and healthy controls in the visual processing of their own bodies and that of another woman to gain insight into the neurophysiologic aspects of body image disturbance in eating disorders. Results from self-reports for the Positive and Negative Affect Schedule indicate that participants with AN and BN have a higher negative affective reaction to looking at pictures of their own body compared with healthy controls. However, in contrast to our hypothesis and the preliminary findings by Seeger and colleagues,12 this stronger emotional reaction in AN and BN participants in response to looking at one’s own body was not reflected in higher activation of the amygdala, which is generally involved in emotion processing.19 An explanation for this unexpected finding might be that participants with AN and BN were showing a form of body-related avoidance behaviour9 when presented with pictures of themselves, because these stimuli were experienced as threatening. Accordingly, previous research demonstrated that amygdala responses are modulated by attention toward a threatening stimulus such as electric shocks38 or toward presentation of emotional faces,39 indicating that sufficient attentional resources are necessary to elicit amygdala activity. A further indication of the postulated body-related avoidance behaviour might be the relative underactivation in the inferior parietal lobule in participants with AN and BN and additional lower activation in the superior parietal lobule in participants with AN, because these brain areas seem to be responsible for visuospatial processing and attentional processes.14,15,40
The assumed decreased attention toward the self-images, as well as the finding that participants with AN did not display higher activation than healthy controls in any brain region when looking at pictures of their own body, can be regarded in the context of the findings of Sachdev and colleagues,18 who reported that patients with AN had no significant activation in response to viewing images of their own bodies. They interpreted this as an indication that patients with AN show a lack of activation of the attentional system. However, it has to be considered that, in the present study, the correlation between the activation of the inferior parietal lobule with the general score on the Body Image Avoidance Questionnaire was not statistically significant. Because this questionnaire measures avoidance behaviour in certain everyday situations, such as choice of clothing or body-related behaviour in social contexts, the questionnaire might not properly assess the assumed more cognitive form of avoidance, which participants with AN may have displayed in the specific situation in the scanner when shown pictures of their own body.
In this context, it should also be kept in mind that for ethical reasons, participants had to be informed that they would be photographed wearing a bikini and would be presented with these pictures. Because all patients agreed to participate, we speculate that women with very severe body-related avoidance would not have been willing to take part in the study and, thus, are not included in the present sample. In addition to decreased attention toward one’s own body, the relative underactivation in the inferior parietal lobule in participants with AN and BN might be interpreted in the context of previous research on the participation of this brain region in somatosensory body representation (e.g., in the relocation of one’s own limb41 and in the building of differential representations of one’s own and other’s bodies).42 In general, the relative underactivation in AN participants when looking at their bodies in this study was located on the left side. This finding is in agreement with previous research demonstrating that body image disturbance in AN seems to located predominantly in the left hemisphere.43
Our study indicates that, when looking at photographs of another woman’s body, participants with AN showed stronger self-reported negative affective reactions compared with healthy controls in their scores on the Positive and Negative Affect Schedule, whereas participants with BN did not. Accordingly, as we had hypothesized, brain activation data indicate that when looking at another women’s body, participants with AN displayed a more pronounced activation in parts of the limbic system (i.e., in the right amygdala and bilateral parahippocampal gyrus, also covering the left hippocampus and left uncus) compared with controls. A comparison of participants with BN and healthy controls did not reveal differences in amygdala activation when looking at another woman’s body. Additionally, when we directly compared brain responses to looking at another woman’s body between participants with the 2 types of eating disorders, we found higher amygdala activation in participants with AN compared with those with BN. The stronger amygdala activation in participants with AN might be explained by an enhanced emotional activation (e.g., regarding fear19) and a higher degree of vigilance44 in this group. We speculate that the assumed enhanced emotional activity and vigilance that occurred in AN participants when looking at the other woman’s body might result from unfavourable social comparison processes. Accordingly, findings from previous research indicate that, in general, body image and eating disturbances are associated with elevated social comparison levels,45,46 and people with AN have been shown to display more unfavourable social comparisons than those without eating disorders.20 Based on these findings and in line with Festinger’s social comparison theory,47 participants with AN may have judged themselves as being inferior to the other woman in terms of appearance, leading to an enhanced degree of body dissatisfaction.47 According to information processing theories,48 in general, body dissatisfaction leads to negative affect (e.g., fear) probably reflected by the stronger amygdala activation in the AN group than in the BN or control group in this study. This enhanced amygdala activation was accompanied by stronger responses in the inferior and superior lobule, providing a further hint for a higher degree of directing attention toward the pictures of the other woman’s body.14,15
Additionally, the finding of higher activation in the right posterior cingulate in participants with AN compared with controls also points to negative emotional activation, because this brain area is not only involved in attentional functions,39,49 but it also seems to play a role in the processing of negative emotions.50 Goethals and colleagues51 observed a significant correlation between regional brain perfusion in the right posterior cingulate with the degree of body dissatisfaction and ineffectiveness in patients with eating disorders. Furthermore, participants with AN displayed stronger activation of the bilateral fusiform gyrus, middle temporal gyrus, superior temporal gyrus and left lingual gyrus compared with controls when looking at the other woman’s body. In previous research, these and vicinal brain regions have been shown to be activated when participants reflect on the physical appearance or personality traits of another person compared with when they reflect on their own attributes.52 However, because previous research has indicated that patients with BN are even more body dissatisfied than those with AN,5 it is unclear why we found that participants with BN did not display a stronger negative emotional response and no altered brain activation patterns in the brain regions assumed to reflect unfavourable social comparison processes, as was found in AN.
Despite the various indications that participants with AN most likely performed social comparison in our study, the assumption of the social comparison processes has to be regarded with caution, because the correlation of the activation of the amygdala and questionnaire data indicating body image disturbance and negative affect following looking at one’s own body was not statistically significant. Although we had expected such a correlation based on the findings described above, we did not use a questionnaire that directly assessed the extent to which participants had compared themselves with the unknown woman. Furthermore, no explicit instructions were given to compare oneself with the other woman shown in the photographs, as was the case in a recent study involving healthy women.11 Therefore, to confirm the hypothesis of more unfavourable social comparison processes in people with AN compared with those with BN and controls, it would be useful to vary the instructions to encourage or discourage social comparison processes and to vary the characteristics of the person with whom the participants should compare themselves (e.g., a slim or overweight woman). Such research designs would allow distinctions to be drawn between the neural correlates of social comparison processes and those of the outcome of this process (i.e., the feeling of superiority or inferiority relative to the presented person and its impact on the associated affective reaction in patients with eating disorders).
We found that participants with AN compared with controls displayed altered activation in the frontal and temporal cortex when looking at their own body as well as that of the other woman. This observation corresponds with previous research indicating a general alteration in frontotemporal circuits in AN and BN.53,54 Interestingly, previous research showed that frontotemporal dementia is associated with changes in eating behaviour including alterations in appetite, food preferences and eating habits (e.g., overeating).55, 56
In spite of the generally higher reactivity of the amygdala in individuals with depression22 and enhanced amygdala activity in participants with AN when looking at the other woman’s body in the present study, we found no differences in amygdala activity between participants with and without depression in the eating-disordered group when either viewing one’s own or the other woman’s body. Furthermore, in contrast to our hypotheses, we did not find any group difference in the extrastriate body area,17 either when looking at photographs of one’s own body or the other woman’s body. This finding is in contrast to previous research, which highlighted a possible role of the extrastriate body area in body image disturbance in AN.16 These diverging results might be because of differences in stimulus material, because Uher and colleagues16 presented line drawings of female bodies.
Limitations
Several limitations have to be considered when interpreting our data. The generalizability of the results is limited by the small sample size. Because the main purpose of the present study was the between-groups comparison of participants with AN, BN and healthy controls, we used a standardized set of pictures of a female body to present each participant from the 3 groups with exactly the same stimulus material for the condition of the unknown woman’s body because previous research showed that the attractiveness of the individual with whom people compare themselves affects self-evaluation.57 However, these standardized photographs were taken of a woman with a BMI of 19; therefore, this woman was heavier than the participants with AN, but her BMI was comparable to or lower than that of the participants with BN and healthy controls. This stimulus may, thus, have caused differential effects in the participants of the 3 groups. Because of this and the fact that evaluation of attractiveness can be regarded as a subjective issue, it would have been informative to assess the participants’ individual evaluation of the standardized photographs of the other woman’s body. Accordingly, in future research, it might be of interest not only to present standardized pictures of an unknown woman’s body but also to provide stimulus material that is matched for BMI18 to check whether these different kinds of stimuli lead to divergent results.
Although we assessed the emotions that arose when participants were looking at their own and the other woman’s body, we did not consider mood and the amount of food eaten before the scanning session. We cannot exclude the possibility that these state-like variables might have influenced the results because previous research demonstrated that mood58 and recent food consumption59 influence state body image. Future research should include these variables to test their impact on the neurobiologic underpinnings of body-image processing in eating disorders.
Furthermore, despite the frequent transitions that occur between the various forms of eating disorders,60 we did not systematically assess the history of any other eating disorder or obesity. However, it might have been of relevance to consider minimum and maximum lifetime body weight, because previous research demonstrated that weight changes in the past influence current body image.3 Additionally, we did not include a behavioural component in the task to ensure compliance with the main task in the scanner and to prevent participants from looking away or distracting themselves. Instead, the participants were systematically interviewed after the scanning session, and each of them confirmed that she had looked at the photographs and did not close her eyes during image acquisition. However, because this answer might be affected by social desirability, it might be useful in future studies to use an fMRI-compatible eye-tracker to reliably record gaze patterns.
Conclusion
Our data indicate that body image processing brain circuits are altered in AN and also, to some extent, in BN. Whereas reduced activation in the brain areas belonging to the attentional network (e.g., the inferior and superior parietal lobule) in response to looking at one’s own body provide indications of body-related avoidance behaviour in AN, enhanced limbic activation in the amygdala when looking at another woman’s body might indicate negative affective reactions, possibly resulting from unfavourable social comparison processes. A challenge for further research is to analyze whether these abnormalities in body processing can be altered through targeted interventions (e.g., exposure tasks to overcome body avoidance8) and cognitive techniques to modify self-defeating body-related thoughts61 to introduce a more realistic view of one’s own and other women’s bodies in patients with AN and BN.
Footnotes
Competing interests: None declared.
Contributors: Drs. Vocks, Schulte, Herpertz and Suchan designed the study. Drs. Vocks, Busch and Grönemeyer acquired the data. Drs. Vocks and Suchan analyzed the data and wrote the article. Drs. Busch, Grönemeyer, Schulte, Herpertz and Suchan reviewed the article. All authors approved the final version submitted for publication.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revised. Washington (DC): The Association; 2000. [Google Scholar]
- 2.Jacobi C, Hayward C, de Zwaan M, et al. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Stice E. Risk and maintenance factors for eating pathology: a meta-analytic review. Psychol Bull. 2002;128:825–48. doi: 10.1037/0033-2909.128.5.825. [DOI] [PubMed] [Google Scholar]
- 4.Keel PK, Dorer DJ, Franko DL, et al. Postremission predictors of relapse in women with eating disorders. Am J Psychiatry. 2005;162:2263–8. doi: 10.1176/appi.ajp.162.12.2263. [DOI] [PubMed] [Google Scholar]
- 5.Cash TF, Deagle EA. The nature and extent of body-image disturbances in anorexia and bulimia nervosa: a meta-analysis. Int J Eat Disord. 1997;22:107–25. [PubMed] [Google Scholar]
- 6.Vocks S, Legenbauer T, Rüddel H, et al. Static and dynamic body image in bulimia nervosa: mental representation of body dimensions and biological motion patterns. Int J Eat Disord. 2007;40:59–66. doi: 10.1002/eat.20336. [DOI] [PubMed] [Google Scholar]
- 7.Garner DM. The 1997 Body Image Survey results. Psychol Today. 1997;30:30–44. [Google Scholar]
- 8.Vocks S, Legenbauer T, Wächter A, et al. What happens in the course of body exposure? Emotional, cognitive, and physiological reactions to mirror confrontation in eating disorders. J Psychosom Res. 2007;62:231–9. doi: 10.1016/j.jpsychores.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Rosen JC, Srebnik D, Saltzberg E, et al. Development of a body image avoidance questionnaire. Psychol Assess. 1991;3:32–7. [Google Scholar]
- 10.Kurosaki M, Shirao N, Yamashita H, et al. Distorted images of one’s own body activates the prefrontal cortex and limbic/paralimbic sys-tem in young women: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:380–6. doi: 10.1016/j.biopsych.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Friederich HC, Uher R, Brooks S, et al. I’m not as slim as that girl: neural bases of body shape self-comparison to media images. Neuroimage. 2007;37:674–81. doi: 10.1016/j.neuroimage.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Seeger G, Braus DF, Ruf M, et al. Body image distortion reveals amygdala activation in patients with anorexia nervosa — a functional magnetic resonance imaging study. Neurosci Lett. 2002;326:25–8. doi: 10.1016/s0304-3940(02)00312-9. [DOI] [PubMed] [Google Scholar]
- 13.Wagner A, Ruf M, Braus DF, et al. Neuronal activity changes and body image distortion in anorexia nervosa. Neuroreport. 2003;14:2193–7. doi: 10.1097/00001756-200312020-00012. [DOI] [PubMed] [Google Scholar]
- 14.Clower DM, West RA, Lynch JC, et al. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–91. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coull JT, Nobre AC. Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18:7426–35. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uher R, Murphy T, Friederich HC, et al. Functional neuroanatomy of body shape perception in healthy and eating-disordered women. Biol Psychiatry. 2005;58:990–7. doi: 10.1016/j.biopsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Peelen MV, Downing PE. The neural basis of visual body perception. Nat Rev Neurosci. 2007;8:636–48. doi: 10.1038/nrn2195. [DOI] [PubMed] [Google Scholar]
- 18.Sachdev P, Mondraty N, Wen W, et al. Brains of anorexia nervosa patients process self-images differently from non-self-images: an fMRI study. Neuropsychologia. 2008;46:2161–8. doi: 10.1016/j.neuropsychologia.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neu-roanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 20.Troop NA, Allan S, Treasure J, et al. Social comparison and submissive behaviour in eating disorder patients. Psychol Psychother. 2003;76:237–49. doi: 10.1348/147608303322362479. [DOI] [PubMed] [Google Scholar]
- 21.Hudson JI, Hiripi E, Pope HGJ, et al. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peluso MA, Glahn DC, Matuso K, et al. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res. 2009;173:158–61. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB, Gibbon M, Spitzer RL, et al. User’s guide for the Structured Clinical Interview for DSM-IV Axis 1 Disorders — research version (SCID-I; Version 20) Washington (DC): American Psychiatric Press; 1996. [Google Scholar]
- 24.Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? Int J Eat Disord. 1994;16:363–70. [PubMed] [Google Scholar]
- 25.Morris JP, Pelphrey KA, McCarthy G. Occipitotemporal activation evoked by the perception of human bodies is modulated by the presence or absence of the face. Neuropsychologia. 2006;44:1919–27. doi: 10.1016/j.neuropsychologia.2006.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewig J, Cooper S, Trippe RH, et al. Drive for thinness and attention toward specific body parts in a nonclinical sample. Psychosom Med. 2008;70:729–36. doi: 10.1097/PSY.0b013e31817e41d3. [DOI] [PubMed] [Google Scholar]
- 27.Freeman RJ, Touyz S, Sara G, et al. In the eye of the beholder: processing body shape information in anorexic and bulimic patients. Int J Eat Disord. 1991;10:709–14. [Google Scholar]
- 28.Fairburn CG, Cooper GT. The Eating Disorder Examination. In: Fairburn CF, Wilson GT, editors. Binge eating: nature, assessment and treatment. New York (NY): Guilford; 1993. pp. 317–32. [Google Scholar]
- 29.Hilbert A, Tuschen-Caffier B, Karwautz A, et al. Eating Disorder Examination Questionnaire: evaluation der deutschsprachigen Übersetzung. Diagnostica. 2007;53:144–54. [Google Scholar]
- 30.Garner DM. Eating Disorder Inventory-2: professional manual. Odessa (FL): Psychological Assessment Resources; 1991. [Google Scholar]
- 31.Paul T, Thiel A. Eating Disorder Inventory, Deutsche version. Göttingen (Germany): Hogrefe; 2004. [Google Scholar]
- 32.Legenbauer T, Vocks S, Schütt-Strömel S. Validierung einer deutschsprachigen Version des Body Image Avoidance Questionnaire BIAQ. Diagnostica. 2007;53:218–25. [Google Scholar]
- 33.Beck AT, Steer RA. Beck Depression Inventory — manual. San Antonio (TX): Psychological Corporation; 1987. [Google Scholar]
- 34.Hautzinger M, Bailer M, Worall H, et al. Beck-Depressions-Inventar (BDI) Testhandbuch. Bern (Switzerland): Huber; 1994. Bearbeitung der deutschen Ausgabe. [Google Scholar]
- 35.Krohne HW, Egloff B, Kohlmann C-W, et al. Untersuchungen mit einer deutschen Version der “Positive and Negative Affect Schedule” (PANAS) Diagnostica. 1996;42:139–56. [Google Scholar]
- 36.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 37.Tailarach J, Tournoux J. Coplanar stereotaxic atlas of the human brain: 3-dimensional proportional system — an approach to cerebral imaging. New York (NY): Thieme; 1988. [Google Scholar]
- 38.Straube T, Weiss T, Mentzel HJ, et al. Time course of amygdala activation during aversive conditioning depends on attention. Neuroimage. 2007;34:462–9. doi: 10.1016/j.neuroimage.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 40.Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Curr Opin Neurobiol. 2004;14:212–7. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Kammers MP, Verhagen L, Dijkerman H, et al. Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. J Cogn Neurosci. 2009;21:1311–20. doi: 10.1162/jocn.2009.21095. [DOI] [PubMed] [Google Scholar]
- 42.Felician O, Romaiguère P. Your body and mine: a neuropsycho-logical prespective. Clin Neurophysiol. 2008;38:183–7. doi: 10.1016/j.neucli.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Smeets MAM, Kosslyn SM. Hemispheric differences in body image in anorexia nervosa. Int J Eat Disord. 2001;29:409–16. doi: 10.1002/eat.1037. [DOI] [PubMed] [Google Scholar]
- 44.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 45.Corning AF, Krumm AJ, Smitham LA. Differential social comparison processes in women with and without eating disorder symptoms. J Couns Psychol. 2006;53:338–49. [Google Scholar]
- 46.Fisher E, Dunn M, Thompson JK. Social comparison and body image: an investigation of body comparison processes using multidimensional scaling. J Soc Clin Psychol. 2002;21:566–79. [Google Scholar]
- 47.Festinger L. A theory of social comparison processes. Hum Relat. 1954;7:117–40. [Google Scholar]
- 48.Williamson DA, White MA, York-Crowe E, et al. Cognitive-behavioral theories of eating disorders. Int J Eat Disord. 2004;28:711–38. doi: 10.1177/0145445503259853. [DOI] [PubMed] [Google Scholar]
- 49.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–51. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maddock RJ, Buonocore MH. Activation of left posterior cingulate gyrus by the auditory presentation of threat-related words: an fMRI study. Psychiatry Res. 1997;75:1–14. doi: 10.1016/s0925-4927(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 51.Goethals I, Vervaet M, Audenaert K, et al. Does regional brain perfusion correlate with eating disorder symptoms in anorexia and bulimia nervosa patients? J Psychiatr Res. 2007;41:1005–11. doi: 10.1016/j.jpsychires.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17:1080–6. [PubMed] [Google Scholar]
- 53.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–35. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uher R, Treasure J. Brain lesions and eating disorders. J Neurol Neurosurg Psychiatry. 2005;76:852–7. doi: 10.1136/jnnp.2004.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bathgate D, Snowden JS, Varma A, et al. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 2001;103:367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- 56.Miller BL, Darby AL, Swartz JR, et al. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia. 1995;6:195–9. doi: 10.1159/000106946. [DOI] [PubMed] [Google Scholar]
- 57.Trampe D, Stapel DA, Siero FW. On models and vases: body dissatisfaction and proneness to social comparison effects. J Pers Soc Psychol. 2007;92:106–18. doi: 10.1037/0022-3514.92.1.106. [DOI] [PubMed] [Google Scholar]
- 58.Baker JD, Williamson DA, Sylve C. Body image disturbance, memory bias, and body dysphoria: effects of negative mood induction. Behav Ther. 1995;26:747–59. [Google Scholar]
- 59.Vocks S, Legenbauer T, Heil A. Food intake affects state body image: impact of restrained eating patterns and concerns about eating, weight and shape. Appetite. 2007;49:467–75. doi: 10.1016/j.appet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Fairburn CG, Cooper Z, Shafran R. Cognitive behavior therapy for eating disorders: a “transdiagnostic” theory and treatment. Behav Res Ther. 2003;41:509–28. doi: 10.1016/s0005-7967(02)00088-8. [DOI] [PubMed] [Google Scholar]
- 61.Vocks S, Wächter A, Wucherer M, et al. Look at yourself: Can body image therapy affect the cognitive and emotional response to seeing oneself in the mirror in eating disorders? Eur Eat Disord Rev. 2008;16:147–54. doi: 10.1002/erv.825. [DOI] [PubMed] [Google Scholar]