Abstract
Background
Stress-induced dissociative states involving analgesia are a common feature of borderline personality disorder (BPD) and posttraumatic stress disorder (PTSD). Our aim was to investigate the psychologic, somatosensory (pain sensitivity) and neural correlates of dissociative states in patients with these disorders.
Methods
We included 15 women with BPD who were not taking medication; 10 of these women had comorbid PTSD. While undergoing functional magnetic resonance imaging at 1.5 Tesla, participants were exposed to a script describing a personalized dissociation-inducing situation and a personalized script describing a neutral situation. We assessed dissociative psychopathology and pain sensitivity.
Results
Dissociative psychopathology scores were significantly higher and pain sensitivity was lower after the dissociation-inducing script was read compared with the neutral script. The blood oxygen level–dependent (BOLD) signal was significantly increased in the left inferior frontal gyrus (Brodmann area [BA] 9) during the presentation of the dissociation-inducing script. Regression analyses revealed positive correlations between BOLD signal and dissociative psychopathology in the left superior frontal gyrus (BA 6) and negative correlations in the right middle (BA 21) and inferior temporal gyrus (BA 20). In the subgroup of participants with comorbid PTSD, we also found increased activity in the left cingulate gyrus (BA 32) during script-driven imagery-induced dissociation, a positive correlation between dissociation scores and activity in the right and left insula (BA 13) and a negative correlation in the right parahippocampal gyrus (BA 35).
Limitations
The main limitation of this pilot study is the absence of a control group. Therefore, the results may also reflect the neural correlates of non–BPD/PTSD specific dissociative states or the neural correlates of emotionally stressful or “loaded” memories. Another limitation is the uncorrected statistical level of the functional magnetic resonance imaging results.
Conclusion
Our results showed that the script-driven imagery method is capable of inducing dissociative states in participants with BPD with and without comorbid PTSD. These states were characterized by reduced pain sensitivity and a frontolimbic activation pattern, which resembles the findings in participants with PTSD while in dissociative states.
Introduction
Dissociation is characterized by a disintegration of perception, consciousness, identity and memory.1 Dissociative symptoms are part of the diagnostic criteria in 3 mental disorders: borderline personality disorder (BPD) and both acute and posttraumatic stress disorder (PTSD).1 Patients with BPD often have a variety of comorbidities, the most common being PTSD. Most epidemiologic studies report PTSD in 60%–70% of patients with BPD.2,3 Stress-related experience of dissociation is one of the DSM-IV criteria of BPD, and several studies revealed frequent dissociative states in this disorder.4–7 Stiglmayr and coworkers8 reported dissociative states to be significantly associated with everyday distress in patients with BPD. In patients with additional mental disorders, levels of dissociation were found to be elevated in comparison with healthy controls. These disorders include anxiety and panic disorders,9 affective disorders10 and obsessive– compulsive disorder.11
It is plausible that dissociative features such as disturbed consciousness or memory hamper learning and thereby impede the effectiveness of therapies that rely on learning (e.g., cognitive restructuring and exposure).12,13 Recently, both clinical13 and experimental research involving patients with BPD yielded empirical support for this hypothesis that links dissociation to psychotherapy response. Ebner-Priemer and colleagues14 conducted a differential aversive Pavlovian delay conditioning procedure in patients with BPD to investigate the impact of acute dissociative states on emotional learning processes. Patients with BPD with clinically relevant dissociation did not acquire any differential conditioning response, whereas both healthy controls and BPD patients without relevant dissociation demonstrated successful differential conditioning. Because basic learning processes, like classical conditioning, are the foundations of behavioural therapy treatment and because brain areas underlying conditioning processes are heavily influenced by dissociative experiences, it seems likely that learning processes in psychotherapy treatment are hampered by dissociation.
Despite the clinical importance of dissociative features, our knowledge about the neural correlates is sparse. Sierra and Berrios15 proposed a corticolimbic model of depersonalization which suggests that hypoemotionality and decreased arousal results from activation of the left medial prefrontal cortex with reciprocal amygdala inhibition, whereas dorsolateral prefrontal cortex activation with reciprocal anterior cingulate inhibition leads to hypervigilance and emptiness of mental contents.
In a functional magnetic resonance imaging (fMRI) study, Lanius and colleagues16 investigated neural activation patterns during dissociative states in patients with PTSD using a script-driven imagery paradigm. By comparing the neural responses of a personalized traumatic script and an implicit baseline, this group found significant activation of the medial prefrontal cortex, anterior cingulate cortex, middle temporal gyrus, superior temporal gyrus and medial parietal lobe in PTSD patients compared with a control group, which consisted of participants who met criterion A for PTSD but had no other DSM-IV criteria for PTSD. It is unclear whether the enhanced frontal activity during dissociative states reflects a control mechanism initiated to regulate an automatic process of hyperarousal involving limbic structures. Therefore, Felmingham and coworkers17 investigated the activation pattern of dissociative responses to conscious and nonconscious fear impact in patients with PTSD. They used the masked fear paradigm to compare conscious and unconscious processing of fearful facial expressions.18 By comparing the activation pattern among dissociative and nondissociative PTSD patients, they found enhanced activation in the ventral pre-frontal cortex for conscious fear and enhanced activity in the bilateral amygdala for nonconscious fear. Regression analyses revealed significant positive correlations between dissociation and activity in the left superior temporal gyrus and bilateral insula for conscious fear. For nonconscious fear, they found a positive correlation between dissociation and activity in the left parahippocampal gyrus.
To our knowledge, there have been no studies investigating the neural activation patterns of dissociation in patients with BPD. In terms of the sensory aspects of dissociation in BPD, we found significant inverse correlations of subjective pain sensitivity with aversive inner tension and dissociation in patients with BPD but not in healthy controls.19 Subjective analgesia is closely correlated with the level of the dissociative state in BPD.19 Thus, the assessment of pain processing can be used to operationalize and quantify the level of acute dissociation independent from subjective experience and report. We found significant activation of the dorsolateral pre-frontal cortex and significant deactivation of the right amygdala and perigenual anterior cingulate gyrus during pain stimulation in BPD.20
To investigate the neural activation pattern of dissociative states in patients with BPD, we conducted an fMRI study using an advanced version of the script-driven imagery paradigm according to the methods of Lanius and colleagues.16 In contrast to that group,16 we did not use trauma scripts; we instead used personalized, autobiographical situations that were very stressful for the participant, which had previously induced dissociative states. The second script was a personalized, autobiographical script of a neutral situation. We compared the psychologic and neural reactions to the 2 scripts. The psychologic reactions (e.g., dissociation, aversive tension), which were different from those used in previous studies, were assessed instantly after each script was presented while the participant was in the scanner and not post hoc. In addition, we assessed pain sensitivity instantly after the presentation of each script. Because the following hypotheses refer to a neural pattern of dissociation in patients with PTSD,16 we performed an additional subgroup analysis including only borderline patients with comorbid PTSD.
We hypothesized that participants would have higher levels of psychometrically assessed dissociation and lower pain sensitivity after the dissociation-inducing scripts were presented, compared with the neutral scripts. Based on previous findings,16,17 we hypothesized that there would be increased activation in the prefrontal cortex and anterior cingulate cortex as well as altered activation in the temporal cortex during presentation of the dissociation-inducing scripts as compared with the neutral scripts. In addition, we hypothesized that the fMRI blood oxygen level–dependent (BOLD) signal in these brain areas would be correlated with the intensity of the dissociative experience.
Methods
Participants
We recruited participants at the Central Institute of Mental Health, Department of Psychosomatic Medicine and Psychotherapy in Mannheim, Germany. We included 15 women not taking medication who met the DSM-IV criteria for BPD, as assessed by the International Personality Disorder Examination21 (IPDE). A subgroup of 10 participants had comorbid PTSD, as assessed by the Structured Clinical Interview for DSM-IV Axis I disorders22 (SCID-I). We excluded women with a lifetime diagnosis of alcohol or substance dependence, lifetime diagnosis of psychotic disorder or bipolar I disorder and current major depression.22 Diagnostic interviews where administered by trained and experienced psychologists (P.L., G.V., and J.M.; interrater reliability IPDE: κ = 0.77, SCID: κ = 0.70). The characteristics and comorbid diagnoses of the participants are shown in Table 1.
Table 1.
Characteristics of patients with borderline personality disorder and patients with borderline personality disorder and comorbid posttraumatic stress disorder
| Group; mean (SD)* |
||
|---|---|---|
| Characteristic | BPD, n = 15 | BPD and PTSD, n = 10 |
| Age (SD) [range], yr | 28.1 (7.6) [19–43] | 28.8 (8.2) [22–43] |
| Trait dissociation score† | 28.8 (20.1) | 27.1 (20.1) |
| State dissociation score‡ | 1.1 (1.1) | 1.0 (0.9) |
| Symptom severity§ | 2.3 (1.1) | 2.4 (1.2) |
| Tension rating | 3.3 (2.4) | 3.9 (2.3) |
| Lifetime diagnoses (remitted), no. of patients | ||
| Major depressive disorder | 14 | 10 |
| Substance abuse | 10 | 6 |
| Social phobia | 6 | 4 |
| Eating disorder | 4 | 4 |
| Current diagnoses, no. of patients | ||
| Social phobia | 5 | 3 |
| Eating disorder | 4 | 4 |
The study protocol was approved by the ethics committee of the University of Heidelberg, and all participants gave verbal and written informed consent before participation.
Psychometrics
To assess trait dissociation, participants were given the German adaptation of the Dissociative Experience Scale, the Fragebogen zu Dissoziative Symptomen23 (FDS), a self-rating instrument that consists of 44 items and 4 subscales (dissociative amnesia, absorption, derealization and conversion). We assessed state dissociation and aversive inner tension by use of the Dissociation Tension Scale-acute24 (Dissoziations-Spannungs-Skala-akut; DSS-acute). The DSS-acute consists of 21 items for dissociation and 1 for aversive inner tension with Likert scales ranging from 0 to 9. To assess state dissociation during the fMRI script-driven imagery procedure, we used a short version of the Dissociation Tension Scale25 (DSS-4). The DSS-4 consists of 4 Likert scales that assess reduced sensory perception (hearing), analgesia, depersonalization and derealization. We assessed symptom severity of BPD by use of the self-administered Borderline Symptom List26 (BSL). This list consists of 95 items and 7 subscales (self-perception, affect regulation, self-destruction, dysphoria, loneliness, intrusions and hostility).
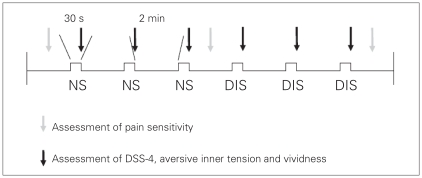
Immediately after each script was presented during the fMRI scan (Fig. 1), participants were asked to rate the intensity of the dissociative state, the aversive inner tension and the vividness on Likert scales ranging from 0 to 9.
Fig. 1.
Design of the study. We used the Dissociation Tension Scale (DSS-4; short version25) to assess the dissociation state and aversive inner tension in patients with borderline personality disorder with and without comorbid posttraumatic stress disorder. DIS = dissociation-inducing script; NS = neutral script.
Script-driven imagery
One day before the fMRI experiment, we prepared 1 neutral and 1 dissociation-inducing script with each participant. We instructed the participants to describe a situation they had experienced that was emotionally neutral for them and a situation that had previously induced a dissociative state. The investigator (P.L.) wrote down the situations in the first person singular and in the present tense.27–29 Contents of the dissociation-inducing scripts were interpersonal conflict–associated situations for 12 patients (e.g., being in an overcrowded department store or conflict with mother) and trauma-associated situations for 3 patients (e.g., meeting the former perpetrator of sexual abuse). Immediately after the assessment of the 2 autobiographical situations, we asked the participants about the intensity of the dissociation elicited by the situation when it was experienced (Likert scale from 0 [no dissociation] to 9 [most intense dissociation]. The mean intensity of dissociation in this posthoc rating was 7.4 (standard deviation [SD] 1.3, range 5–9).
The 2 scripts were recorded on a compact disc by the investigator and copied into a Presentation file including the Likert scales (DSS-4, aversive inner tension, vividness), which were presented in the scanner via projection onto a mirror on the head coil. The duration of each script was between 25 and 32 seconds. Each of the 2 scripts was played 3 times in the scanner in a fixed order (the neutral script followed by the dissociation-inducing script) at an interval of 2 minutes (Fig. 1). We did not permutate the order of the 2 scripts because the psychologic and neural reactions of the neutral script after dissociation induction would have been biased, because the time between the presentation of the 2 scripts was too short to return to a baseline state after experiencing an intense dissociative state and aversive inner tension. After the fMRI experiment, the patients were given the opportunity for a debriefing session with an investigator (P.L.) with extensive experience in psychotherapy.
Pain assessment
To assess pain sensitivity after the presentation of the scripts, a contact thermode (Thermal Sensory Analyzer; Medoc Ltd.) was fixed to the back of each participant’s left hand. The temperature of the thermode was increased constantly by 2°C per second up to 43°C, which was held for 30 seconds. This procedure was carried out 3 times before the scripts were presented, after the 3 presentations of the neutral situation and after the 3 presentations of the dissociation-inducing situation (Fig. 1). After each thermal stimulation, participants rated the intensity of the pain on a Likert scale ranging from 0 (not painful at all) to 9 (most intense pain imaginable).
Functional imaging
We performed fMRI scanning with a 1.5 Tesla whole body scanner (MAGNETOM Vision; Siemens). We used the following protocol parameters to acquire BOLD signals: repetition time 3 seconds, echo time 60 ms, flip angle 90°, field of view 220 + 220 mm2, matrix 64 × 64, slice thickness 5 mm, slice gap 1 mm, number of slices 25 and number of functional volumes 295.
Data analysis
Psychometrics
We obtained the clinical features of the participants through use of the BSL, FDS and DSS-acute. To compare the vividness and intensity of the dissociative experience (DSS-4), aversive inner tension and pain perception during the script-driven imagery, we performed 2-tailed t tests for dependent variables with a Bonferroni correction. We set the significance levels at p < 0.0125. All analyses were conducted with SPSS (version 14.0 for Windows; SPSS Inc.).
Functional data
We analyzed the functional data by use of SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5/). We discarded the first 4 images at the beginning of each trial to enable the signal to achieve steady state equilibrium between radiofrequency pulse and relaxation. The fMRI time series were realigned and unwarped to the mean to correct for movement of the participant’s head. We normalized the mean images to a Montreal Neurological Institute echoplanar imaging template with affine registration followed by nonlinear transformation with 25-mm cutoff, medium regularization and 16 iterations, resampled with trilinear interpolation and written in 3 × 3 × 3 mm3 isotropic voxels. We then applied the normalization parameters determined for the mean functional volume to the corresponding functional image volumes for each participant. Finally, we smoothed the images with a Gaussian kernel of 8-mm full-width at half-maximum. The data were high-pass filtered (1/128 Hz cutoff) to remove low-frequency signal drift.
To analyze activation patterns during the 2 different scripts, we defined for each participant 2 regressors, consisting of the neutral script sequence and the dissociation script sequence. The general linear model estimated the component of variance that could be explained by each of the regressors. We fit the following 2 contrasts for each participant: dissociation-related script minus the neutral script (activation pattern), and neutral script minus the dissociation-inducing script (deactivation pattern).
To compare activation between the neutral and dissociation-inducing scripts, we performed a 1-sample t test analysis (second-level random-effects analysis; t contrasts: 1. dissocia-tion script minus neutral script [0 1]; 2. neutral script minus dissociation script [1 0]; uncorrected p ≤ 0.001, cluster threshold κ = 10).
In addition, we performed a multiple regression analysis to determine the correlation patterns of BOLD signals with the intensity of the dissociative experience. We fit the means of the DSS-4 with the dissociation-inducing script as a covariate in the multiple regression analysis (t contrasts for positive correlation 1, negative correlation −1). Because dissociation and aversive inner tension are significantly correlated in patients with BPD,7,8 we also conducted a multiple regression analysis between BOLD signal and the intensity of aversive inner tension by fitting the means of the inner tension ratings with the dissociation-inducing script as a covariate.
Results
The characteristics of the participants are shown in Table 1. The trauma experienced by patients with PTSD was severe childhood sexual abuse (6 patients), physical abuse (3 patients) and physical neglect (1 patient). The psychopathological variables indicate that some of the participants with BPD had pronounced symptom severity and trait dissociation.23,26 However, state dissociation and aversive inner tension before the experiment were relatively low compared with previous findings.24 Among all patients with BPD, 5 also had social phobia and 4 met the criteria for an eating disorder. Of the participants with comorbid PTSD, 3 had social phobia and 4 had an eating disorder. Women with current major depression and current substance abuse and dependence were excluded from our study, but all except for 1 participant (who did not have PTSD) fulfilled the criteria for lifetime major depression. Two-thirds (10/15) of all participants and more than half of the participants with comorbid PTSD (6/10) had lifetime substance abuse. Pain sensitivity was not assessed for 5 participants because of technical failures of the Thermal Sensory Analyzer.
We assessed the intensity of the dissociative experience, vividness and aversive inner tension experienced after each script was presented in the scanner. For all patients, there were significantly higher levels of dissociation and aversive inner tension during presentation of the dissociation-inducing script than during the neutral script (Table 2). Pain sensitivity was significantly lower after the dissociation-inducing script than after the neutral script. There was no difference in vividness between the 2 situations.
Table 2.
Descriptive parameters and statistics of dependent t test analyses between psychometric assessments during presentation of the dissociation-inducing script and the neutral script
| BPD, n = 15 |
BPD and PTSD; n = 10 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Neutral script, mean (SD) | Dissociation-inducing script, mean (SD) | t value* | p value | Neutral script, mean (SD) | Dissociation-inducing script, mean (SD) | t value* | p value |
| Vividness rating† | 2.8 (1.6) | 2.9 (2.0) | t14 = −0.1 | 0.99 | 2.9 (1.4) | 3.0 (2.1) | t9 = −0.1 | 0.91 |
| DSS-4 score | 1.4 (0.8) | 3.0 (1.7) | t13 = −4.7 | < 0.001 | 1.5 (0.7) | 3.4 (1.4) | t9 = −4.5 | < 0.001 |
| Tension rating† | 3.4 (1.5) | 6.0 (1.2) | t14 = −6.5 | < 0.001 | 3.3 (1.6) | 6.0 (1.3) | t9 = −6.1 | < 0.001 |
| Pain rating† | 6.6 (1.58) | 4.3 (2.5) | t9 = 13.2 | 0.004 | 6.7 (1.86) | 3.7 (2.8) | t9 = 4.1 | 0.009 |
BPD = borderline personality disorder; DSS-4 = Dissociation Tension Scale 4;25 PTSD = posttraumatic stress disorder; SD = standard deviation.
Determined by use of 2-tailed t tests for dependent variables.
Vividness, tension and pain were rated on Likert scales ranging from 0 (low) to 9 (high)
We found increased BOLD signals in the left inferior frontal gyrus (BA 9) during the presentation of the dissociation-inducing script compared with the neutral script (Table 3). Compared with the dissociation-inducing script, we found activation in the right middle frontal gyrus (BA 46) during presentation of the neutral script. Regression analyses revealed significant positive correlations between BOLD signals and the intensity of the dissociative experience in the left superior frontal gyrus (BA 6). We found negative correlations for the right middle temporal gyrus (BA 21) and the right inferior temporal gyrus (BA 20). There was no correlation between BOLD signal and the intensity of aversive inner tension for any brain region (Table 3).
Table 3.
Brain areas showing activation during presentation of the dissociation-inducing script compared with the neutral script and significant correlations between blood oxygen level–dependent signal and dissociation
| Group; MNI coordinates | Side | Lobe | Gyrus | BA | T local maximum (voxel level) | κ | 1-sample t test* | Correlation between BOLD signal and DSS-4† | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BPD, n = 15 | ||||||||||
| −60 | 6 | 24 | L | Frontal | Inferior frontal | 9 | 5.64 | 39 | DIS > neutral | |
| 52 | 34 | 32 | R | Frontal | Middle frontal | 46 | 4.58 | 20 | Neutral > DIS | |
| −8 | −6 | 70 | L | Frontal | Superior frontal | 6 | 4.97 | 83 | Positive | |
| 38 | −2 | −38 | R | Temporal | Middle temporal | 21 | 5.02 | 46 | Negative | |
| 46 | −6 | −36 | R | Temporal | Inferior temporal | 20 | 4.74 | 46 | Negative | |
| BPD and PTSD, n = 10 | ||||||||||
| −16 | −6 | 68 | L | Frontal | Superior frontal | 6 | 6.30 | 24 | DIS > neutral | |
| 44 | −18 | 4 | R | Insula | 13 | 5.79 | 38 | DIS > neutral | ||
| −58 | 4 | 24 | L | Frontal | Inferior frontal | 9 | 5.31 | 11 | DIS > neutral | |
| −42 | −16 | 46 | L | Frontal | Precentral | 4 | 4.94 | 10 | DIS > neutral | |
| −58 | −22 | 16 | L | Parietal | Postcentral | 40 | 4.78 | 16 | DIS > neutral | |
| −8 | 12 | 38 | L | Frontal | Cingulate | 32 | 4.81 | 10 | DIS > neutral | |
| −8 | −6 | 66 | L | Frontal | Medial frontal | 6 | 7.40 | 29 | Positive | |
| 64 | −2 | 8 | R | Temporal | Superior temporal | 22 | 7.39 | 44 | Positive | |
| −42 | 10 | 8 | L | Frontal | Precentral | 44 | 6.36 | 60 | Positive | |
| −40 | 4 | 2 | L | Insula | 13 | 5.38 | 60 | Positive | ||
| 42 | −34 | 18 | R | Insula | 13 | 5.52 | 11 | Positive | ||
| −38 | 28 | 4 | L | Frontal | Inferior frontal | 47 | 4.95 | 13 | Positive | |
| 28 | −12 | −30 | R | Limbic | Parahippocampal | 35 | 4.83 | 11 | Negative | |
| 50 | −4 | −36 | R | Temporal | Inferior temporal | 20 | 8.52 | 35 | Negative | |
BA = Brodmann area; BOLD = blood oxygen level–dependent; BPD = bipolar disorder; DIS = dissociation-inducing script; DSS-4 = Dissociation Tension Scale-acute;25 L = left; MNI = Montreal Neurological Institute; PTSD = posttraumatic stress disorder; R = right.
All p ≤ 0.001
Regression analyses.
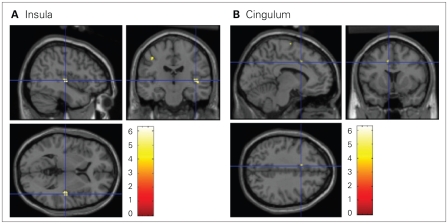
When we restricted our analyses to include only the 10 participants with BPD and PTSD, we found a significantly higher BOLD signal with the dissociation-inducing script than with the neutral script in left superior frontal gyrus (BA 6), right insula (BA 13), left inferior frontal gyrus (BA 9), left precentral gyrus (BA 4), left postcentral gyrus (BA 40) and left dorsal cingulate gyrus (BA 32) (Table 3, Fig. 2). There were no activations with the neutral script situation compared with the dissociation-inducing situation.
Fig. 2.
An increased blood oxygen level–dependent signal was observed in the (A) insula and (B) cingulum in participants with borderline personality disorder and comorbid posttraumatic stress disorder during the dissociation-inducing script compared with during the neutral script (t contrast 1 0, uncorrected p ≤ 0.001).
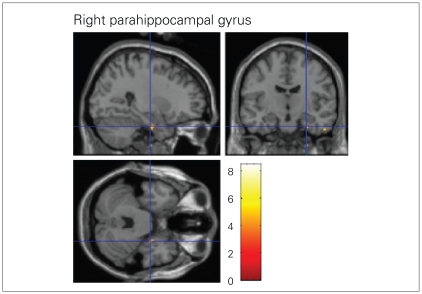
Regression analyses of data from the PTSD subgroup showed a positive correlation between DSS-4 scores and BOLD signal changes in the left medial frontal gyrus (BA 6), right superior temporal gyrus (BA 22), left precentral gyrus (BA 44), left and right insula (BA 13) and left inferior frontal gyrus (BA 47). We found negative correlations between DSS-4 scores and changes in BOLD signal in the right parahippocampal gyrus (BA 35) and right inferior temporal gyrus (BA 20, Table 3, Fig. 3).
Fig. 3.
A negative correlation was observed between the intensity of the dissociative experience and the blood oxygen level–dependent signal change in the right parahippocampal gyrus in participants with borderline personality disorder and comorbid posttraumatic stress disorder (t contrast –1, uncorrected p ≤ 0.001).
Discussion
To our knowledge, this is the first study to investigate the neural, somatosensory and psychologic correlates of dissociative states in patients with BPD. We used a script-driven imagery method to induce dissociative states during fMRI scanning, and we assessed dissociative psychopathology and pain sensitivity. Dissociative states were characterized by significantly elevated levels of depersonalization, derealization and subjectively reduced sensory perception. We found that BOLD signals were significantly increased in the left inferior frontal gyrus during the presentation of a dissociation-inducing script as compared with a neutral script. Regression analyses revealed positive correlations between BOLD signal and dissociative psychopathology in the left superior frontal gyrus and negative correlations in the right middle and inferior temporal gyrus. In the subgroup of 10 participants with co-morbid PTSD, we also found increased activity in the left cingulate gyrus during induced dissociation, a positive correlation between dissociation scores and activity in the right insula and a negative correlation between dissociation scores and activation in the right parahippocampal gyrus.
Our hypotheses about psychometrically assessed dissociation were confirmed, suggesting that the induction of dissociative states by use of autobiographical script-driven imagery is possible in patients with BPD. We found no difference in the vividness of the recalled experiences, which supports the assumption that differences in the psychometric assessments were not because of a lower ability to imagine the situation or because of avoidance of the dissociative experience. Nevertheless, the requirement of a minimum of attention and readiness to answer the questions during the experiment may provide an explanation as to why the relatively moderate intensities of dissociation (mean 3.0) during the presentation of the dissociation-inducing script were not as high as the retrospectively assessed values of the real situations (mean 7.4). Also, the artificial environment of the scanner may have prevented the development of full-blown dissociative states. Another explanation might be that patients overestimated the intensity of dissociation in the real situation. Because we did not assess heart rate or skin conductance, we do not have additional physiologic markers of hyper- or hypoarousal.
Our results in terms of pain perception in BPD support previous findings that pain perception was lower during states of intense aversive tension than during baseline conditions,30 as well as previously reported correlations between pain perception and aversive inner tension and between pain perception and dissociation.19 Furthermore, the current study provides strong evidence that reduced pain sensitivity is a somatic marker of dissociative states in patients with BPD.
Our findings of increased BOLD signal during dissociative states in the inferior frontal gyrus are in agreement with those of Lanius and coworkers.16 However, in contrast with their results, we observed inferior frontal gyrus activation in the left cerebrum.
Regression analyses revealed a positive correlation between BOLD signal and the intensity of the dissociative experience during presentation of the dissociation-inducing script in the left superior frontal gyrus (BA 6). We found negative correlations in the right middle and inferior temporal gyrus. Regression analyses of BOLD signal and aversive inner tension revealed no significant results, suggesting that the results are related to dissociation rather than to states of inner tension.
The inverse correlations between temporal areas and the intensity of dissociation are in agreement with the corticolimbic model of dissociation that postulates a corticolimbic disconnection in patients with depersonalization disorder.31,32 Röeder and colleagues33 found reduced amygdala activity during induced depersonalization in healthy control participants. Taken together, these findings suggest that reduced temporal activity is a potential correlate of dissociation and reduced pain sensitivity. Despite these correlative results and the reduced pain sensitivity during presentation of the dissociation-inducing script, we did not find any deactivation of the amygdala. One explanation might be the relatively moderate intensity of dissociation as mentioned above.
When we included data from only the participants with BPD and PTSD, we found significantly increased BOLD signal in the left inferior and superior frontal gyrus, right insula and left anterior cingulate gyrus. The activation patterns in this group resembled those from earlier studies involving PTSD patients; these studies also found activation of the inferior frontal gyrus and dorsal cingulate gyrus.16 Interestingly, Lanius and colleagues34 showed that, in patients with dissociative PTSD, thalamic activity was positively correlated with activity in the right insula. Because the insula plays an important role in monitoring internal states,35 increased insula activity during dissociative states may be a correlate of an attentional shift from external to internal foci. Increased cingulate activity has been suggested to resemble a super-suppression of affective arousal during dissociative states.36
Positive correlations between the intensity of dissociation and activation of the left medial frontal gyrus as found in our study are consistent with the corticolimbic model of depersonalization.15 This model postulates that the medial frontal regions inhibit limbic structures during states of depersonalization. Also, in agreement with this model, we found a negative correlation between dissociation and activation in the right parahippocampal gyrus. This is also in keeping with deactivation of the right parahippocampal gyrus in patients with dissociative PTSD.16 In contrast, Felmingham and coworkers17 found a significant positive correlation between dissociative responses and activation in the left parahippocampal gyrus during nonconscious fear in patients with PTSD. Our results are consistent with previous findings;16,17 nevertheless, there are differences in hemispheres or contrary correlations that might result from the different patient sample, the fact that we did not use explicit trauma scripts to induce dissociation, or the different study design (e.g., compared with Felmingham and colleagues17). The fact that we did not use explicit trauma scripts in our study may be seen as an advantage because our findings are not confounded by the effects of traumatic remembrance. Another strength of our study is the concurrent assessment of dissociative psychopathology and pain sensitivity.
Limitations
A main limitation of our study is that we did not include a control group. Therefore, it is possible that our results instead reflect the neural correlates of non–borderline specific dissociation states or the neural correlates of emotionally stressful or “loaded” memories. The fact that the regression analyses of BOLD signal and aversive inner tension revealed no significant differences suggests that the results are related to dissociation rather than to aversive inner tension or stress. Also, in the case of tension effects, we would expect a different activation pattern with decreased prefrontal and increased medial temporal activity. Nevertheless, the comparison of the results with a control group would have been useful.
Second, we have reported all results at an uncorrected level of p ≤ 0.001, which weakens the robustness of the data. We emphasize that this was a first attempt to assess neural correlates of dissociation in patients with BPD using the script-driven imagery method; the results of this study may help to design further studies on this topic. A further limitation is the sample size, particularly the number of patients with BPD but not PTSD. This group of 5 patients was too small to conduct a subgroup comparison, which might have revealed more specific reaction and neural activation patterns. The small sample size of 10 patients with PTSD may have prevented detection of further differences.
We used the same order of scripts (neutral script followed by the dissociation-inducing script) for each participant. This was done to prevent carry-over effects from dissociation induction to the neutral scripts, but it may have led to order effects. Furthermore, we did not assess any additional physiologic markers that may have given more insight into the psychophysiology of dissociation. Finally, we did not assess the awareness of fear, which appears to play a crucial role in the activation pattern of dissociation.17 Future studies should examine whether the enhanced activation in frontal regions is a correlate of a control mechanism following the presentation of the script or a neural reflection of dissociation.
Conclusion
Taken together, our findings support the use of the script-driven imagery method as a method to induce dissociative states in patients with BPD. These states are characterized by reduced pain sensitivity and a specific pattern of frontolimbic activation.
Acknowledgements
The authors thank Gunilla Oberthuer for help with the fMRI experiment.
Footnotes
Competing interests: None declared.
Contributors: Drs. Ludäscher, Valerius, Lanius, Bohus and Schmahl designed the study. Drs. Ludäscher and Mauchnik acquired the data, which all authors analyzed. Drs. Lusdächer, Lanius and Schmahl wrote the article. All authors reviewed the article and approved it for publication.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revised. Washington (DC): The Association; 2000. [Google Scholar]
- 2.Zanarini MC, Frankenburg FR, Dubo ED, et al. The axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733–9. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman MJ. Axis I diagnostic comorbidity and borderline personality disorder. Compr Psychiatry. 1999;40:245–52. doi: 10.1016/s0010-440x(99)90123-2. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky BS, Cloitre M, Dulit RA. Relationship of dissociation to self-mutilation and childhood abuse in borderline personality disorder. Am J Psychiatry. 1995;152:1788–92. doi: 10.1176/ajp.152.12.1788. [DOI] [PubMed] [Google Scholar]
- 5.Ebner-Priemer UW, Badeck S, Beckmann C, et al. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: a startle response study. J Psychiatr Res. 2005;39:85–92. doi: 10.1016/j.jpsychires.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Philipsen A, Schmahl C, Lieb K. Naloxone in the treatment of acute dissociative states in female patients with borderline personality disorder. Pharmacopsychiatry. 2004;37:196–9. doi: 10.1055/s-2004-827243. [DOI] [PubMed] [Google Scholar]
- 7.Stiglmayr CE, Shapiro DA, Stieglitz RD, et al. Experience of aversive tension and dissociation in female patients with borderline personality disorder: a controlled study. J Psychiatr Res. 2001;35:111–8. doi: 10.1016/s0022-3956(01)00012-7. [DOI] [PubMed] [Google Scholar]
- 8.Stiglmayr CE, Ebner-Priemer UW, Bretz J, et al. Dissociative symptoms are positively related to stress in borderline personality disorder. Acta Psychiatr Scand. 2008;117:139–47. doi: 10.1111/j.1600-0447.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 9.Ball S, Robinson A, Shekhar A, et al. Dissociative symptoms in panic disorder. J Nerv Ment Dis. 1997;185:755–60. doi: 10.1097/00005053-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Oedegaard KJ, Neckelmann D, Benazzi F, et al. Dissociative experiences differentiate bipolar-II from unipolar depressed patients: the mediating role of cyclothymia and the type A behaviour speed and impatience subscale. J Affect Disord. 2008;108:207–16. doi: 10.1016/j.jad.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Watson D, Wu KD, Cutshall C. Symptom subtypes of obsessive-compulsive disorder and their relation to dissociation. J Anxiety Disord. 2004;18:435–58. doi: 10.1016/S0887-6185(03)00029-X. [DOI] [PubMed] [Google Scholar]
- 12.Michelson L, June K, Vives A, et al. The role of trauma and dissociation in cognitive-behavioral psychotherapy outcome and maintenance for panic disorder with agoraphobia. Behav Res Ther. 1998;36:1011–50. doi: 10.1016/s0005-7967(98)00073-4. [DOI] [PubMed] [Google Scholar]
- 13.Rufer M, Held D, Cremer J, et al. Dissociation as a predictor of cognitive behavior therapy outcome in patients with obsessive-compulsive disorder. Psychother Psychosom. 2006;75:40–6. doi: 10.1159/000089225. [DOI] [PubMed] [Google Scholar]
- 14.Ebner-Priemer UW, Mauchnik J, Kleindienst N, et al. Emotional learning during dissociative states in borderline personality disorder. J Psychiatry Neurosci. 2009;34:214–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Sierra M, Berrios GE. Depersonalization: neurobiological perspectives. Biol Psychiatry. 1998;44:898–908. doi: 10.1016/s0006-3223(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 16.Lanius RA, Williamson PC, Boksman K, et al. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–11. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 17.Felmingham K, Kemp AH, Williams L, et al. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med. 2008;38:1771–80. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- 18.Williams LM, Lidell BJ, Rathjen J, et al. Mapping the timecourse of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp. 2004;21:64–74. doi: 10.1002/hbm.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludäscher P, Bohus M, Lieb K, et al. Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry Res. 2007;149:291–6. doi: 10.1016/j.psychres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Schmahl C, Bohus M, Esposito F, et al. Neural correlates of an-tinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63:659–67. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- 21.Loranger AW. IPDE: International personality disorder examination; DSM-IV module. In: Mombour W, Zaudig M, Berger P, et al., editors. Weltgesundheitsorganisation WHO. Deutschsprachige Ausgabe. Bern (Germany): Huber; 1996. [Google Scholar]
- 22.First MB, Frances AJ, Pincus HA, et al. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp Community Psychiatry. 1994;45:18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Freyberger HJ, Spitzer C, Stieglitz RD. Fragebogen zu Dissoziative Symptomen (FDS) In: Bernstein U, Putnam FW, editors. German adaptation of the Dissociative Experience Scale (DES) Bern (Germany): Huber; 1986. [Google Scholar]
- 24.Stiglmayr CE, Braakmann D, Haaf B, et al. Development and characteristics of Dissociation-Tension Scale acute (DSS-Acute) Psychother Psychosom Med Psychol. 2003;53:287–94. doi: 10.1055/s-2003-40495. [DOI] [PubMed] [Google Scholar]
- 25.Stiglmayr C, Schmahl C, Bremner JD, et al. Development and psycho-metric characteristics of the DSS-4 as a short instrument to assess dissociation during neuropsychological experiments. Psychopathology. 2009;42:370–4. doi: 10.1159/000236908. [DOI] [PubMed] [Google Scholar]
- 26.Bohus M, Limberger MF, Frank U, et al. Psychometric properties of the Borderline Symptom List (BSL) Psychopathology. 2007;40:126–32. doi: 10.1159/000098493. [DOI] [PubMed] [Google Scholar]
- 27.Pitman RK, Orr SP, Forgue DF, et al. Psychophysiological assessment of post-traumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–5. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 28.Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without post-traumatic stress disorder. Am J Psychiatry. 1999;156:1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanius RA, Williamson PC, Boksman K, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158:1920–2. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- 30.Bohus M, Limberger M, Ebner U, et al. Pain perception during self-reported distress and calmness in patients with borderline personality disorder and self-mutilating behavior. Psychiatry Res. 2000;95:251–60. doi: 10.1016/s0165-1781(00)00179-7. [DOI] [PubMed] [Google Scholar]
- 31.Simeon D, Guralnik O, Hazlett EA, et al. Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry. 2000;157:1782–8. doi: 10.1176/appi.ajp.157.11.1782. [DOI] [PubMed] [Google Scholar]
- 32.Phillips ML, Medford N, Senior C, et al. Depersonalization disorder: thinking without feeling. Psychiatry Res. 2001;108:145–60. doi: 10.1016/s0925-4927(01)00119-6. [DOI] [PubMed] [Google Scholar]
- 33.Röder CH, Michal M, Overbeck G, et al. Pain response in depersonalization: a functional imaging study using hypnosis in healthy subjects. Psychother Psychosom. 2007;76:115–21. doi: 10.1159/000097970. [DOI] [PubMed] [Google Scholar]
- 34.Lanius RA, Williamson PC, Bluhm RL, et al. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2005;57:873–84. doi: 10.1016/j.biopsych.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Critchley HD, Wiens S, Rotshtein P, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 36.Frewen PA, Lanius RA. Toward a psychobiology of posttraumatic self-dysregulation reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci. 2006;1071:110–24. doi: 10.1196/annals.1364.010. [DOI] [PubMed] [Google Scholar]