Abstract
Up-regulation and activation of epidermal growth factor receptor and/or urokinase-type plasminogen activator receptor in a variety of cancers have been shown to be associated with poor prognosis. High molecular weight kininogen can be hydrolyzed by plasma kallikrein to bradykinin and HKa. HKa and its domain 5 (D5) both have been demonstrated to have potent anti-angiogenic activity. We now show that HKa blocks human prostate cancer cell (DU145) migration by 76.0±2.4% at 300nM and invasion by 78.0±12.9% at 11.1nM. D5 inhibits tumor migration and invasion in a concentration-dependent manner. Stimulation by bFGF or VEGF results in clustering of uPAR and EGFR on the surface of DU145 cells. The co-localization of uPAR and EGFR is prevented by HKa. Immunoprecipitation suggests that uPAR, EGFR and α5β1 integrin formed a ternary complex. Immunoblotting demonstrates that HKa significantly decreases the bFGF-transactivated phosphorylation of EGFR at Tyr 1173 between 30min and 4hr. The phosphorylation of ERK and AKT, which are downstream effectors of EGFR, is also inhibited by HKa. These novel data indicate that HKa and D5 inhibit migration and invasion of human prostate cancer cells via a EGFR/uPAR pathway, suggesting the therapeutic potential of HKa and D5 to decrease metastasis of human prostate cancer.
Keywords: HKa, prostate cancer, EGFR, uPAR
INTRODUCTION
Urokinase plasminogen activator (uPA) is synthesized and secreted as a pro-enzyme, whose activation is markedly accelerated upon binding with high affinity (∼1 nM) to its receptor (uPAR). uPAR is a glycophosphatidylinositol (GPI)-anchored protein, consisting of three ∼90 amino acid repeats DI, DII and DIII (1). uPA and uPAR play a critical role in prostate cancer spread. First, elevated serum levels of uPA and uPAR are directly correlated with the serum level of prostate specific antigen (PSA) and the development of the prostate cancer (CaP) metastasis, and inversely correlated with overall survival rate among CaP patients (2). Second, the density of uPA and uPAR in prostate tumor tissues is significantly higher than in normal prostate from healthy individuals (3). Finally, the binding of uPA to its receptor uPAR can activate downstream signaling molecules, including the mitogen-activated protein kinase, signal transducer and activator of transcription (STAT), and the Ras/extracellular signal-regulated kinase pathway, which in turn, leads to cell proliferation, migration, and invasion (4, 5).
Epidermal growth factor receptor (EGFR) and its family members play a pivotal role in tumor development and their expression strongly affects the clinical outcome of cancer patients (6, 7). EGFR family consists of four transmembrane receptors belonging to the receptor tyrosine kinase (RTK) super family and includes EGFR (also known as ErbB1/HER-1), ErbB2/Neu/HER-2, ErbB3/HER-3, and ErbB4/HER-4 (8). In prostate cancer, EGFR expression was detected in 18% of cancers and was significantly associated with high grade, advanced stage, and high risk for PSA recurrence in univariate analysis (P < 0.0001) (9).
EGFR is a transducer of the urokinase receptor-initiated signal which is required for in vivo growth of a human carcinoma (10, 11). uPAR, EGFR and integrins form a ternary complex which promotes cancer cell migration, invasion, proliferation and survival(11). Specific ligands such as uPA or EGF working through paracrine or autocrine loops are well-established activators of EGFR (12). In cells expressing very low levels of uPAR, which are dormant in vivo (13), the α5β1 integrin exists in an inactive state and associates poorly with EGFR. In spite of its high expression, under both basal conditions or after cell adhesion to fibronectin (FN), EGFR is not phosphorylated. In contrast, in cells expressing high levels of uPAR, this receptor, in the presence of uPA, interacts frequently with and activates α5β1, leading to the formation of a multiprotein complex that contains FAK and EGFR, and that exhibits robust ERK activation. These results unveil a model whereby highly malignant human carcinoma cells, through overexpression of uPAR, are able to subvert and utilize a tightly regulated EGFR pathway to gain matrix-derived proliferative advantage.
High molecular weight kininogen (HK) is a multifunctional plasma protein that plays important roles in many pathophysiological processes, such as fibrinolysis, thrombosis, and inflammation (14, 15). Single-chain HK consists of 6 domains and is complexed in plasma with prekallikrein (16). On the endothelial cell surface prekallikrein is cleaved by prolylcarboxpeptidase to kallikrein which releases bradykinin from domain 4 of HK to generate two-chain high molecular weight kininogen (HKa). HKa undergoes extensive conformational changes to expose domain 5 (D5) and inhibits angiogenesis through these anti-adhesive sites (17). HKa and D5 bind uPAR and induce apoptosis in endothelial cells by disrupting uPAR association with integrins αvβ3 and α5β1 through cell detachment (18, 19). uPAR mediates adhesion and signaling in endothelial cells by binding to vitronectin. D5 of HK binds the soluble uPAR receptor with 10-fold higher affinity than Domain 3 (20). Therefore, exposure of D5 in HKa is consistent with HKa having a higher affinity for uPAR than HK. In this study, we hypothesize that the binding of HKa and D5 to uPAR inhibits EGFR phosphorylation and would therefore inhibit tumor cell migration and invasion in prostate cancer.
MATERIALS AND METHODS
Reagents and Antibodies
Two chain high molecular weight kininogen (HKa) was purchased from Enzyme Research Laboratories (South Bend, IN). Collagen solution (purified rat type 1 collagen) was purchased from BD Biosciences (Bedford, MA). Protease inhibitor cocktail was purchased from Sigma Co. (St. Louis, MO). Antibodies directed against total and phosphorylation-specific (S473) Akt, total and phosphorylation-specific (T202/Y204) extracellular signal-regulated kinase (ERK) were obtained from Cell Signaling Technology, Inc. Antibodies against total and phosphorylation-specific EGFR (Y1173), polyclonal antibodies against integrin αv and β1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies against αvβ3 integrin (LM609) and α5β1 were from Chemicon (Temecula, CA). Anti-uPAR mAb was from American Diagnostica Inc (Stamford, CT). Rabbit polyclonal anti-uPAR antibody (DIIDIII) was a gift kindly provided by Drs. Andrew Mazar and Graham Parry (Attenuon, L.L.C., San Diego, CA). Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) was obtained from Invitrogen Corporation (Carlsbad, CA). All other reagents were purchased from Sigma Chemical unless otherwise specified.
Preparation of recombinant D5 of HK
Glutathione-S-transferase (GST) and recombinant GST–D5 were prepared as previously described (19). Briefly, GST was removed from GST–D5 by digestion with thrombin, which was inactivated with d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (PPACK). Free GST was removed with Glutathione Sepharose 4 Fast Flow column (Amersham Pharmicia Biotech, Piscataway, NJ). Residual thrombin and PPACK were removed with Amicon Centriprep YM-30 (Millipore Corp., Bedford, MA). Using YM-10, D5 solution was exchanged into 50 mM HEPES, 150 mM NaCl, pH 7.5 buffer. Endotoxin levels in the preparations were determined with the chromogenic limulus amebocyte lysate assay by use of an endotoxin testing kit (Cambrex, Walkersville, MD). Endotoxin level in D5 was below detectable limits (< 0.1 U/ml). D5 was visualized on 20% SDS-PAGE and detected by Western blotting as a single band.
Cell Culture
DU145, a prostate cancer cell line, was purchased from ATCC (Manassas, VA). DU 145 cell line was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS), 2mmol/L glutamine, 100 units/mL penicillin, and 100 µg/mL streptomycin and cultured in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Zn++ (22.5 µmol/L) were added to the culture mix whenever HKa and D5 were involved, as Zn++ is required for HKa and D5 binding to tumor cells.
Cell Migration Assay
Cell migration was assessed in 48-well Boyden chambers. The under side of membrane of the upper chamber was coated with a collagen mixture (10 µg/ml, Calbiochem; San Diego, CA) and DU145 (2×104) cells in DMEM were seeded on the upper chamber. DMEM contained bFGF (20ng/ml) was added to the bottom chamber. Tumor cells were allowed to migrate for 6 hrs (21). Then, the cells that remained in the upper chamber were removed using a cotton swab. The cells that migrated to other side of membrane of the upper chamber were fixed with 4% paraformaldehyde and stained with 1% toluidine blue. We counted cells in 5 fields (100× magnification) per well that essentially covered 80% of the well surface. The average number of cells from each of the triplicates represents the average number of cells that migrated in that treatment group. Each experiment had triplicate wells for every treatment group and we repeated each experiment three times. The mean of all results from controls was considered as 100%.
Cell Invasion Assay
Cell invasiveness was determined by the ability to transmigrate through a layer of Matrigel in a Transwell chamber. Briefly, the 1:1 mixture (40 µl) of matrigel and DMEM was loaded on the top chamber of Transwell units. DU145 cells (20,000) were loaded on the top of matrigel. The medium + 10% FBS + Zn (15 µM) was added to the bottom chamber of Transwell units. Twenty-four hrs later, cells were fixed by formaldehyde and stained by 1% toluidine blue. The cells that remained in the upper chamber were removed using a cotton swab. Cells which migrated to the underside of a membrane were counted as described in Cell Migration Assay.
Cell Lysate Preparation, Immunoprecipitation (IP) and Immunoblotting (IB)
Protein extraction, SDS-PAGE separation of proteins and Western-blot analysis were performed as described previously(22). Cells were lyzed in an M-PER mammalian cell protein extraction buffer (Pierce, Rockford, IL) supplemented with Na3VO4 (1 mM) and protease inhibitor cocktail and followed by freeze and thaw three times. After being kept on ice for 40 min, the extracts were centrifuged at 15,000g for 15 min 4°C. The supernatant was designated as the cell lysate.
The complex formation of uPAR with other signaling molecules was determined by immunoprecipitation according to the methods described by Nykjaer et al (23) with some modifications. Cell lysate was incubated with corresponding antibodies followed by incubation of protein A/G beads. The immunoprecipitates were subjected to SDS-PAGE under non-reduced conditions, and immunoblot analysis was performed as described below.
Separately, the immunoprecipitated complex or the cell lysate containing equal amounts of protein (10–20 µg) were solubilized in Laemmli’s sample buffer and were subjected to SDS–PAGE. Separated proteins were then transferred onto nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in Tris-buffered-saline containing 0.05% Tween 20 and then probed with antibodies as indicated. Immunoblots were visualized by an enhanced chemiluminescence kit (Amersham Pharmacia Biotech) and analyzed by densitometry. Data were obtained from three independent experiments.
Immunofluorescence Microscopy
Cells grown on coverslips were treated as indicated in the figure 3 legend. Cells were fixed and processed as described(24). Cells were stained with anti-uPAR (murine monoclonal antibody, 4 µg/ml) and anti-EGFR (rabbit antibody, 2 µg/ml) antibodies in 0.1% BSA/PBS, or with vehicle alone. After washing and blocking, secondary antibody (FITC-conjugated anti-mouse IgG at 1:400, Sigma; AlexaRed-conjugated anti-rabbit IgG at 1:1000, Molecular Probes, Eugene, OR) in 0.1% BSA/PBS containing DAPI was added. Standard epifluorescence was captured with an Axioskop epifluorescence photomicroscope (Zeiss, Oberkochen, Germany).
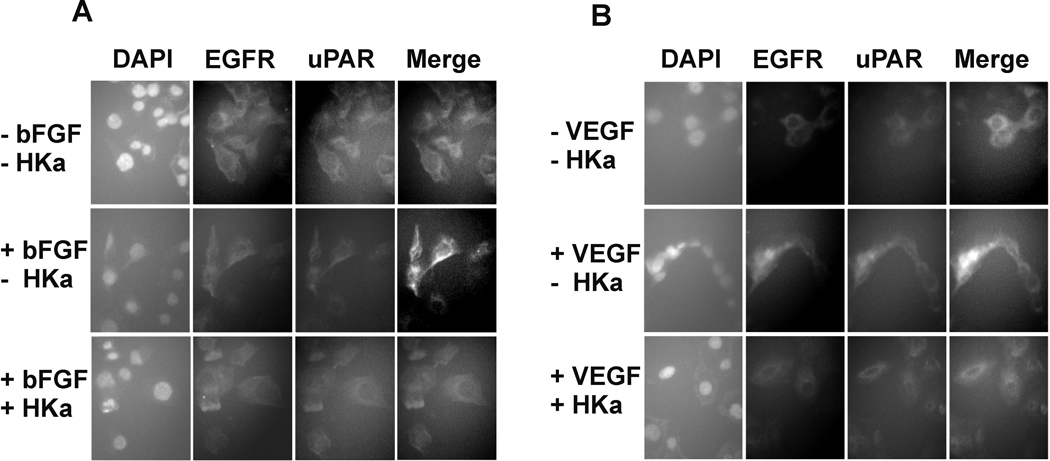
Figure 3. HKa prevents the association of uPAR and EGFR.
A, B, Expression of uPAR and EGFR as monitored by immunofluorescence. Prostate cancer cells were grown on coverslips and starved for 2hours. Cells pretreated with or without 100 nM HKa for an additional hour. Cells were challenged with or without A) bFGF (20ng/ml) as well as B) VEGF (20ng/ml) for 30 minutes. Cells were fixed by 4.0% formaldehyde. Immunofluorescence was carried out as described in Materials and Methods.
Statistical Analysis
Statistical analyses were performed by One Way Analysis Of Variance (ANOVA) and all pairwise multiple comparison procedures (Student-Newman-Keuls Method). Results were considered significant when P=<0.05. The result presented as mean ± SEM.
RESULTS
HKa and D5 inhibit migration and invasion of prostate cancer cell
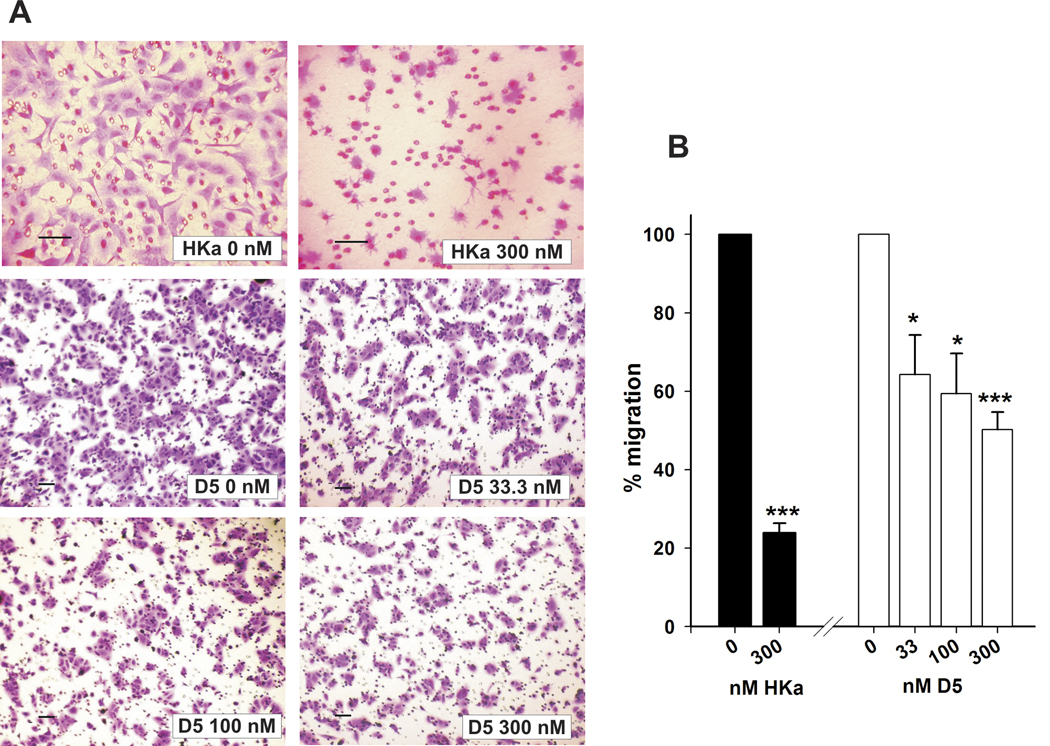
Growth factors induce uPAR internalization by initially activating pro-uPA followed by complex formation with PAI-1 and interaction of the ternary complex uPAR/uPA/PAI-1 with a member of the LDL receptor–like family (25). During cell migration, uPAR is redistributed to focal adhesions at the leading edge either by lateral movement or by internalization and recycling of the receptor. We previously showed (26) that binding of HKa or D5 to uPAR could prevent the process of uPAR internalization and inhibit endothelial cell migration. We postulated that HKa and D5 also would inhibit the migration of tumor cells expressing high levels of uPAR. We evaluated the inhibitory potential of HKa and D5 on a human prostate tumor cell line, DU 145, which expresses high levels of uPAR (19). In fig. 1, bFGF-induced cell migration was significantly decreased to 24±2.4% by HKa (300 nM) while D5 inhibition on cell migration at 33.3, 100 and 300 nM was 36±0.6, 41±3.4 and 50±5.7%, respectively. The inhibition of cell migration (76±2.4%) by HKa (300nM) is significantly greater than D5 (300nM) (P<0.0015).
Figure 1. HKa and D5 inhibit migration of prostate cancer cells in the presence of bFGF.
A, Inhibition of cell migration by HKa and D5 at different concentrations. Cell migration assay was performed as described in Materials and Methods. The medium in the lower chamber was DMEM + zinc (15µM) + bFGF (20ng/ml) with or without HKa or D5. DU145 (2×104) cells were premixed with the medium which was DMEM + zinc (15µM) with or without HKa or D5 and added in the upper chamber. The concentration of HKa and D5 is indicated in Figure 1. The migration assay took 6 hours. Magnification of the top panel is 200× while magnification of middle and bottom panels is 100×. Bar = 500 µm.
B, Quantification of prostate cancer cell migration. Migrated prostate cancer cells were counted and standardized as percentages. Controls were set to 100%. Columns, mean of three independent experiments done in triplicates; bars, SE. * = p<0.05, *** = p<0.001. Black column represents the HKa-treated group. White column represents the D5-treated group.
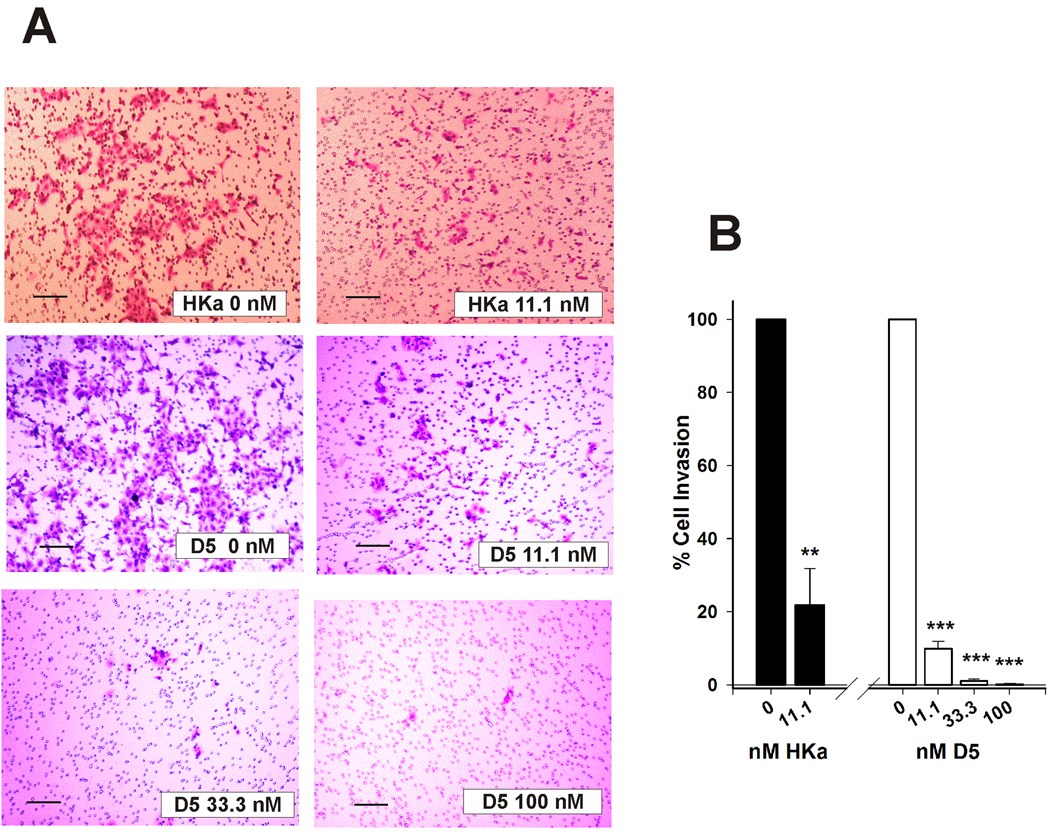
uPA is synthesized as a 55-kDa single-chain proenzyme and converted into the two-chain active form by a single cleavage at Lys158-Ile159. uPA efficiently converts the inactive zymogen, plasminogen, into the active serine protease, plasmin. Plasmin directly or indirectly cleaves ECM components including laminin, fibronectin, fibrin, vitronectin and collagen, which are initial steps to invasion (27). We have shown that binding of HKa to uPAR could prevent the association of uPA and uPAR (26). We tested whether binding of HKa to uPAR could interfere with this process and therefore inhibit cell invasion. As shown in fig. 2, HKa (11.1 nM) significantly inhibited neoplastic cell invasion by 78.0±12.9% while D5 at 11.1, 33.3 and 100 nM inhibited DU145 cell invasion by 90.2±1.7, 98.9±0.6 and 99.9±0.1%, respectively. These data showed that both HKa and D5 are potent inhibitors of tumor invasion and that the magnitude of their effects is similar.
Figure 2. HKa and D5 inhibit invasion of prostate cancer cells in a concentration-dependent manner.
A, Cell invasion assay was performed as described in Materials and Methods. The medium in the lower chamber was DMEM + 10% FBS+ zinc (15µM). DU145 (2×104) cells were premixed with the medium which was DMEM + zinc (15µM) with or without HKa or D5 and added in the upper chamber. Magnification is 100×. Bar is 1000 µm in length.
B, Quantification of prostate cancer cell migration. Prostate cancer cells were counted and standardized as percentages. Controls were set to 100%. Columns, mean of three independent experiments done in triplicates; bars, SE. * = p<0.05, *** = p<0.001. Black column represents the HKa-treated group. White column represents the D5-treated group.
HKa prevents the association of uPAR and EGFR in the presence of bFGF
We have demonstrated that prostate cancer cells expressed high levels of both uPAR and EGFR (19). EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma (10, 11). Recent data showed that uPAR, EGFR and integrins form a ternary complex which promotes cancer cell migration, invasion, proliferation and survival (11). We have observed that the binding of HKa and D5 to cells is mediated by uPAR in the presence of Zn++ (28). Thus, HKa and D5 could potentially inhibit the association of EGFR and uPAR in prostate cancer cells by targeting uPAR. In fig. 3A, expression of uPAR and EGFR in DU 145 cells were determined by immunofluorescence. In the quiescent DU 145 cells, uPAR and EGFR were partially co-localized (top panel). Stimulation with bFGF significantly enhanced the co-localization of uPAR and EGFR (middle panel).In contrast, the addition of HKa (100 nM) prevented the co-localization of uPAR and EGFR (bottom panel). Thus, HKa can block the association of uPAR and EGFR and therefore might inhibit uPAR and EGFR signaling pathways. Similar results were obtained in fig. 3B when VEGF is used instead of bFGF.
HKa disrupts the complex of EGFR and uPAR in the presence of bFGF
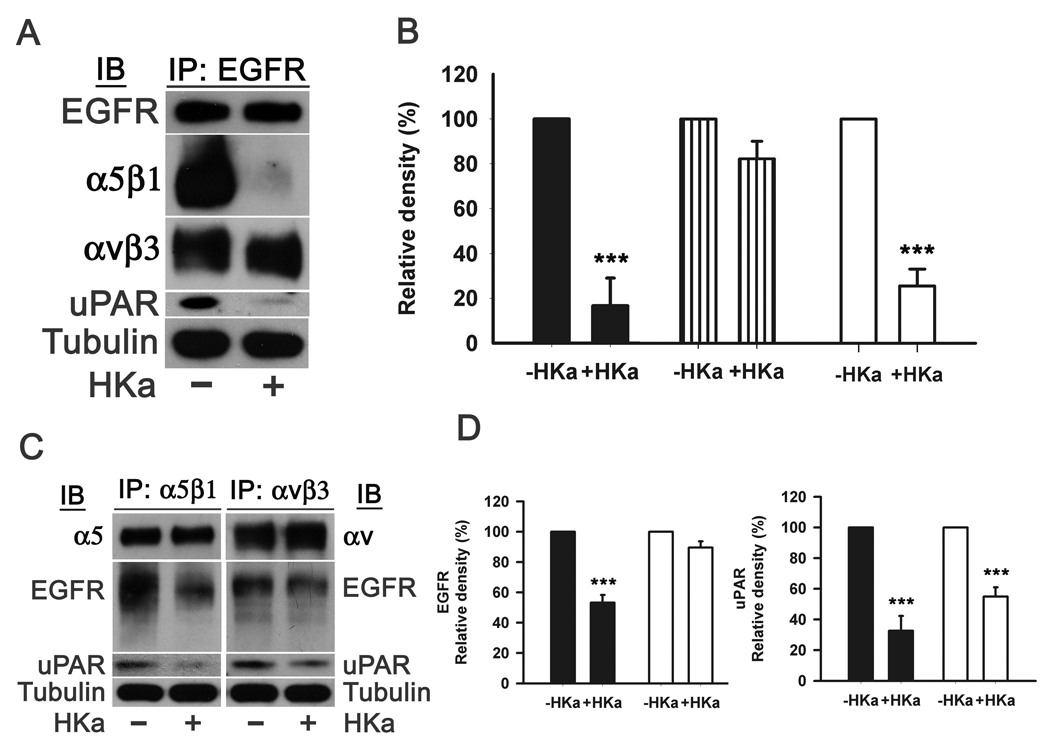
The data from fig. 3 indicated that uPAR and EGFR can form a complex in the presence of bFGF or VEGF. We postulated that HKa could disrupt this complex. Thus, we performed experiments in which lysates of DU145 cells were immunoprecipitated with an antibody to EGFR and the precipitates immunoblotted for uPAR (Figure 4A). The uPAR in cell lysates was precipitated by an antibody to the C-terminal of EGFR. HKa prevented the antibody to EGFR from precipitating uPAR by 74.8±8.2% (Figure 4B). The presence of EGFR was confirmed by probing the immunoprecipitates with anti-EGFR antibody.
Figure 4. HKa disrupts the complex of uPAR, EGFR and integrins.
A, Prostate cancer cells were grown on 60 cm2 dishes and treated with or without HKa (100nM) for 4 hours and harvested by adding extraction buffer. Immunoprecipitation procedures were performed as described in Materials and Methods using an antibody to EGFR. Total α5β1, αvβ3 and uPAR were probed by corresponding antibodies. α-tubulin showed equal protein loading.
B, Total α5β1, αvβ3 and uPAR to EGFR were quantified by densitometry. Controls were set to 100%. Comparisons were made with control. Black column represents total α5β1 to EGFR. Stripped column represented total αvβ3 to EGFR. White column represents total uPAR to EGFR. Data were obtained from three independent experiments. Date plotted as mean ± SEM. ***=P<0.005.
C, HKa disruption of the integrins, uPAR and EGFR was confirmed by reciprocal experiments. Immunoprecipitation procedures were performed as described in Materials and Methods using antibodies to α5β1 or αvβ3. Total EGFR and uPAR were probed by corresponding antibodies. α-tubulin showed equal protein loading.
D, Total EGFR and uPAR to either α5β1 or αvβ3 were quantified by densitometry. Controls were set to 100%. Comparisons were made with control. Data were obtained from three independent experiments. Black column and white column represent that the immunoprecipitation were performed with the antibodies to α5β1 and αvβ3, respectively. Date plotted as mean ± SEM. ***=P<0.005.
It has been suggested that the association of uPAR and EGFR requires α5β1 integrin(29). This observation raises the question whether uPAR directly binds to EGFR or via α5β1 integrin in prostate cancer cells. As shown in fig. 4C, antibodies to α5β1 and αvβ3 precipitated uPAR and EGFR from cell lysates. Consistent with our previous observations (18, 19), HKa prevented the antibody to α5β1 from precipitating uPAR by 67.4±9.7% and EGFR by 46.8±5.1% (Figure 4D) while HKa only prevented the antibody to αvβ3 from precipitating uPAR by 45.1±6.0% (Figure 4D) but not EGFR. Reciprocal experiments revealed that the antibody to EGFR precipitated α5β1 and αvβ3 integrin (Figure 4A), suggesting that uPAR, EGFR and integrins formed a complex. HKa blocked the antibody to EGFR from precipitating α5β1 by 83.3±12.3% (Figure 4B) but not αvβ3. Based on the data above, we propose that uPAR, EGFR and α5β1 or αvβ3 form two different complexes. In one complex, uPAR bridges EGFR and α5β1 together while in the other one αvβ3 brings uPAR and EGFR in close proximity. Thus, HKa can completely disrupt the EGFR-uPAR-α5β1 complex but only partially block the EGFR-αvβ3-uPAR complex became the binding of EGFR to αvβ3 is not inhibited by HKa.
HKa suppresses the signaling pathway of EGFR in the presence of bFGF
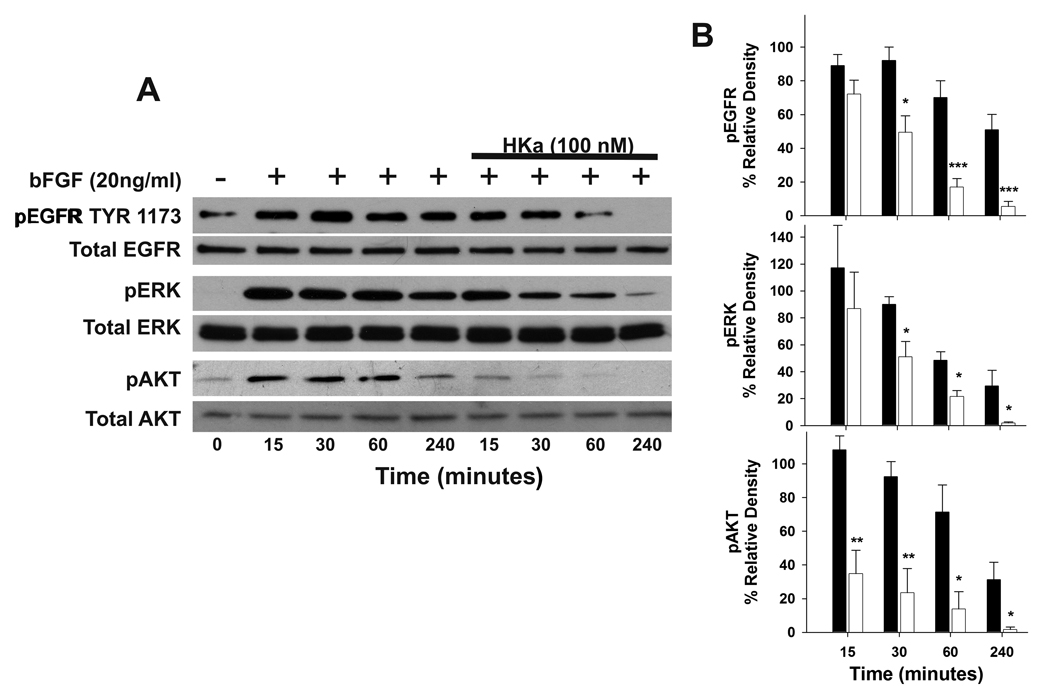
Prevention of the association of uPAR and EGFR by HKa suggested that it might inhibit downstream signaling events via the EGFR pathway. Western blotting showed that HKa inhibited the phosphorylation of EGFR at Tyr 1173 (Figure 5A). The inhibition of EGFR phosphorylation by HKa (100 nM) was time dependent, 18.9±6.7, 46.4±8.0, 75.8±9.9 and 89.5±9.1% at 15min, 30min, 1h and 4hrs, respectively (Figure 5B top). The differences between the untreated group and HKa-treated group at 30min, 1h and 4hrs were significant. The phosphorylation of ERK and AKT (Ser 473) was also inhibited by HKa (Figure 5A). The inhibition of ERK phosphorylatiion by HKa mimicked HKa inhibition of EGFR phosphorylation, which was 25.9±27.1, 43.3±5.7, 55.3±6.5 and 93.9±11.7 at 15 min, 30 min, 1hr and 4hrs, respectively (Figure 5B middle). However, HKa almost completely prevented AKT phosphorylation from 15min to 4hrs. HKa inhibition on AKT phosphorylation was progressed with 67.9±8.3, 74.5±9.0, 80.7±16.0 and 94.6±10.3% at 15min, 30 min, 1hr and 4hrs, respectively (Figure 5B bottom).
Figure 5. Effects of HKa on the phosphorylation of EGFR, ERK and AKT.
A, HKa inhibited the phosphorylation of EGFR, ERK and AKT. Prostate cancer cells (DU 145) were sub-confluent. Cells were starved for 2hours and pretreated with or without HKa (100nM) for an additional hour and stimulated with bFGF (20ng/ml) at different time points. Cells were lyzed and subjected to Western blotting. The activation of EGFR, ERK and PI3 kinase was probed by antibodies to phosphorylated EGFR, ERK and AKT, respectively.
B, Phosphorylation of top) EGFR, middle) ERK and bottom) AKT were quantified by densitometry. The data were normalized and converted into percentage. Comparisons were made with control. Black column represents the untreated group. White column represents the HKa-treated group. Data plotted as mean ± SEM. *=P<0.05, **=P<0.01 and ***=P<0.005.
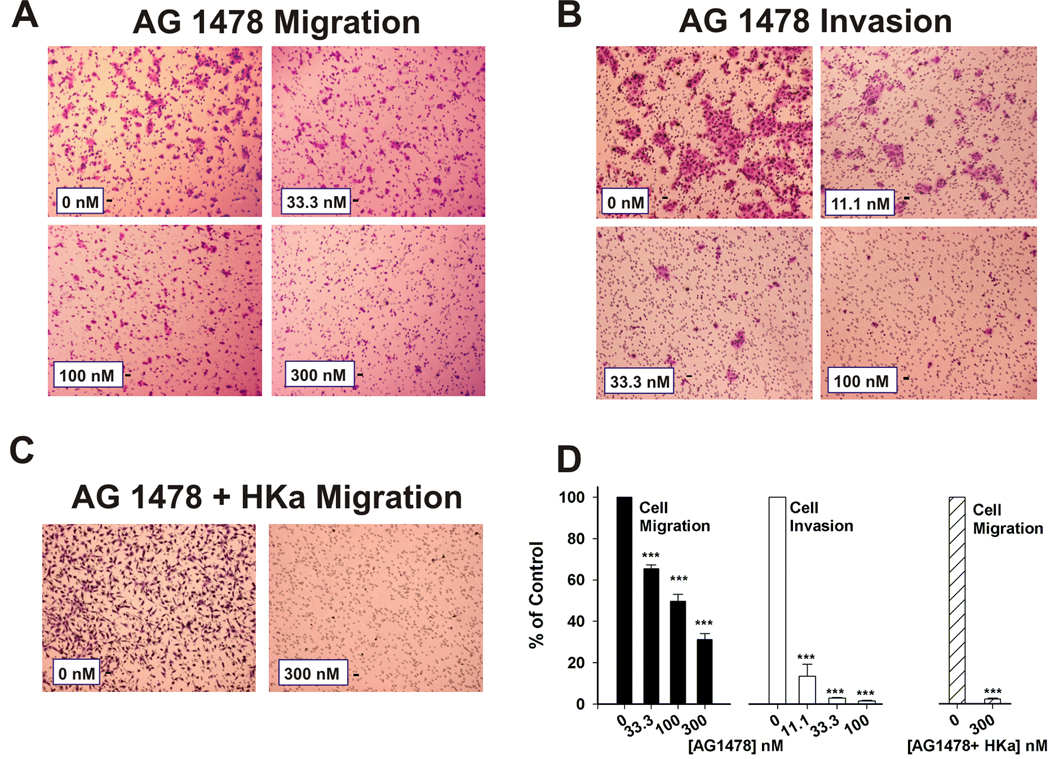
AG 1478 inhibits migration and invasion of prostate cancer cell
EGFR regulates cell migration and invasion in a variety of cells. This observation was further confirmed by both migration and invasion assays as shown in fig. 6, AG 1478, an EGFR inhibitor, concentration-dependently inhibited both migration (Figure 6A) and invasion (Figure 6B) of prostate cancer cells. AG 1475 at 33.3, 100 and 300 nM inhibited cell migration about 34.6±1.3, 50.5±2.3 and 68.7±3.5%, respectively (Figure 6D, black bar). AG 1478 even more potently suppressed cell invasion about 88.1±17.3, 97.1±0.8 and 98.5±0.4% at 11.1, 33.3 and 100 nM, respectively (Figure 6D, white bar). Although HKa and AG 1478 inhibited cell migration, it was not potent as it did on cell invasion. We wondered if HKa and AG 1478 would synergistically inhibit cell migration. As shown in fig. 6C, combination of HKa (300 nM) plus AG 1478 (300 nM) almost completely inhibited cell migration. Inhibition of HKa plus AG 1478 was about 97.7% (Figure 6D, strip bar).
Figure 6. Effects of EGFR inhibitor, AG 1478, on migration and invasion of prostate cancer cells.
A, B, Inhibition of cell migration A) and invasion B) by AG 1478 at different concentrations.
C, Inhibition of AG 1478 (300 nM) plus HKa (300 nM) on cell migration. Cell migration and invasion assay were performed as described in Materials and Methods as well as figure 1 and figure 2 except AG 1478 or AG 1478 plus HKa in place of D5. Magnification is 100×. Bar is 100 µm in length.
D, Quantification of prostate cancer cell migration and invasion. Prostate cancer cells were counted and standardized as percentages. Controls were set to 100%. Black column and white column represent the cell migration group and the cell invasion group treated with AG 1478, respectively. Strip column represents the cell migration group treated with HKa plus AG 1478. *** = p<0.001. (n=3).
This data confirm that EGFR plays a critical role in cell migration and invasion while HKa inhibition of EGFR activation by disrupting the complex of uPAR and EGFR could suppress tumor cell migration and invasion, therefore it predicts to inhibit tumor metastasis.
DISCUSSION
The over-expression of uPAR and EGFR is associated with poor prognosis in patients with prostate cancer. We have previously demonstrated that HKa and D5 could inhibit cell motility and proliferation by binding to the domain II and III of uPAR. We also observed that the core sequence of HKa in which exerts its inhibitory effects on cell motility is G486–G496 (19). In this study, we show that HKa and D5 also inhibited both prostate cancer cell motility and invasion. We hypothesize that this observation is due to the binding of HKa to uPAR. As shown in fig. 3 and fig. 4, HKa prevents the association of uPAR and EGFR and disrupts the complex of EGFR and uPAR. Finally, we show that HKa inhibits the activation of ERK and PI3 kinase signaling by disrupting the complex of uPAR, EGFR with integrins
The X-ray structure of uPAR has been solved recently and has revealed that uPAR binds uPA in a pocket comprised by all of its three domains. This conformation presents the entire external surface of uPAR free for interactions with other proteins, e.g. integrins, EGFR and FPR receptors (27). We initially observed that prostate cancer expressed high levels of uPAR and EGFR (19). We tested whether HKa could inhibit EGFR signaling pathway because HKa can bind to domain II and III of uPAR. Immunofluorescence revealed that HKa could prevent the co-localization of uPAR and EGFR. By immunoprecipitation, we proved that HKa could directly disrupt the complex of uPAR, integrins and EGFR. Mazzieri (30) suggested that human cleavage resistant uPAR does not activate ERK and does not engage FPRL1, but it activates an alternative pathway initiated by the formation of a ternary complex (uPAR-α3β1-EGFR) and resulting in the tyrosine autophosphorylation of EGFR. Gangliosides are thought to regulate epithelial cell adhesion and migration by inhibiting alpha(5)beta(1) integrin and epidermal growth factor receptor (EGFR) signaling. Wang (29) reported that gangliosides inhibited the uPA-dependent cell migration by preventing the association of uPAR with alpha(5)beta(1) integrin or uPAR/alpha(5)beta(1) integrin with the EGFR. Moreover, a direct association of uPAR with α5β1 has been described and a 9-amino acid peptide composed of amino acids 240–248 of uPAR can directly bind to α5β1 (31). Substitution of a single amino acid within this region by alanine (S245A) in cell surface-expressed uPAR impaired its interaction with α5β1. Our data showed that uPAR was co-immunoprecipitated by both anti-EGFR antibody and anti-α5β1 and αvβ3 antibodies while EGFR was co-immunoprecipitated by anti-α5β1 and αvβ3 antibodies. The reverse experiments precipitating with anti-EGFR and then Western blotting for uPAR and integrins corroborated these results. HKa prevented the antibody to EGFR from precipitating uPAR and α5β1, suggesting that HKa completely disrupted EGFR-uPAR-α5β1 complex because EGFR and α5β1 might directly bind to uPAR. This observation was confirmed by reciprocal experiments. In contrast, HKa did not prevent the antibody to EGFR from precipitating αvβ3 and vice versa, indicating that EGFR, uPAR and αvβ3 formed a different complex in which EGFR and uPAR bind to αvβ3 integrin.
In the process of transformation of a benign tumor to a malignant tumor, assembling of the local proteolytic machinery is a prerequisite. Prostate cancer cells can up-regulate uPAR expression, which is the high affinity receptor for pro-uPA (∼1nM), allowing uPAR to form a ternary complex with pro-uPA and EGFR. uPA not only serves as a component of the cell protease system, but also initiates the survival signals via EGFR pathway, which may be critical for tumor resistance to hormone ablation. In both cases, uPA could utilize either uPAR-EGFR or uPAR-integrin complexes to auto-activate and initiate a signaling pathway. This observation can explain that a single antagonist of EGFR produces a limited benefit in patient with prostate cancer. The disruption of the uPAR-EGFR-integrins complex by HKa might interfere with this transduction and suppress the activation of pro-uPA and signaling pathways initiated by uPA, which underscore its potential in prevention of tumor metastasis.
The metastatic spread of cancer cells is a dreaded complication of malignant neoplasms. Metastasis is a multistep process in which malignant cells must initially migrate from the primary tumor, invade the surrounding tissue, and enter the vascular circulation (27). If they are able to survive in the blood stream, they must then successfully arrest at a secondary target site, cross the vascular barrier, and migrate into the extravascular connective tissues. Subsequently, tumor cells may proliferate to form a clinically relevant metastatic colony. In the fig. 1 and fig. 2, we showed that HKa and D5 both inhibited cell migration and invasion of prostate cancer cells in a dose-dependent manner, which strongly indicated the potential of HKa and D5 to prevent the metastasis of prostate cancer cells since cell migration and invasion are initial steps of tumor metastasis.
In this study, we first compared the inhibitory potency of HKa and D5 on tumor cell motility and invasion. We found that both HKa and D5 were potent inhibitors of tumor cell invasion, since they at 11.1 nM inhibited tumor invasion about 90%. As shown in fig. 1, the inhibitory effect of HKa on tumor migration is more potent than that of D5 but both significantly slowed down the tumor motility. HKa and D5 mimicked the inhibitory effects of AG 1478 on tumor motility and invasion (Figure 6), indicating HKa and D5 are alternative EGFR inhibitors. The molecular mechanism of HKa and D5 for exerting its inhibitory effects on tumor motility and invasion is that both HKa and D5 can bind to uPAR and block the association of uPAR and EGFR. This observation was verified by both immunofluorescence and immunoprecipitation experiments. Thus, our data revealed the potential of HKa and D5 on the inhibition of prostate cancer metastasis.
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA83121, R01 AR051713 and T32 HL07777 to R.W. Colman.
Abbreviations
- CaP
carcinoma of the prostate
- HK
high molecular weight kininogen
- HKa
cleaved high molecular weight kininogen
- EGFR
epidermal growth factor receptor
- uPA
urokinase-type plasminogen activator
- uPAR
urokinase-type plasminogen activator receptor.
References
- 1.Nykjaer A, Christensen EI, Vorum H, Hager H, Petersen CM, Roigaard H, Min HY, Vilhardt F, Moller LB, Kornfeld S, Gliemann J. Mannose 6-phosphate/insulin-like growth factor-II receptor targets the urokinase receptor to lysosomes via a novel binding interaction. J Cell Biol. 1998;141:815–828. doi: 10.1083/jcb.141.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchheimer JC, Pfluger H, Ritschl P, Hienert G, Binder BR. Plasminogen activator activity in bone metastases of prostatic carcinomas as compared to primary tumors. Invasion Metastasis. 1985;5:344–355. [PubMed] [Google Scholar]
- 3.Miyake H, Hara I, Yamanaka K, Arakawa S, Kamidono S. Elevation of urokinase-type plasminogen activator and its receptor densities as new predictors of disease progression and prognosis in men with prostate cancer. Int J Oncol. 1999;14:535–541. doi: 10.3892/ijo.14.3.535. [DOI] [PubMed] [Google Scholar]
- 4.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–3396. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- 6.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 7.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 8.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 9.Schlomm T, Kirstein P, Iwers L, Daniel B, Steuber T, Walz J, Chun FH, Haese A, Kollermann J, Graefen M, Huland H, Sauter G, Simon R, Erbersdobler A. Clinical significance of epidermal growth factor receptor protein overexpression and gene copy number gains in prostate cancer. Clin Cancer Res. 2007;13:6579–6584. doi: 10.1158/1078-0432.CCR-07-1257. [DOI] [PubMed] [Google Scholar]
- 10.Mamoune A, Kassis J, Kharait S, Kloeker S, Manos E, Jones DA, Wells A. DU145 human prostate carcinoma invasiveness is modulated by urokinase receptor (uPAR) downstream of epidermal growth factor receptor (EGFR) signaling. Exp Cell Res. 2004;299:91–100. doi: 10.1016/j.yexcr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L. EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell. 2002;1:445–457. doi: 10.1016/s1535-6108(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 12.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 13.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colman RW, Sartor RB, Adam AA, DeLa Cadena RA, Stadnicki A. The plasma kallikrein-kinin system in sepsis, inflammatory arthritis, and enterocolitis. Clin Rev Allergy Immunol. 1998;16:365–384. doi: 10.1007/BF02737657. [DOI] [PubMed] [Google Scholar]
- 15.Colman RW. Plasma and tissue kallikrein in arthritis and inflammatory bowel disease. Immunopharmacology. 1999;43:103–108. doi: 10.1016/s0162-3109(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 16.Schmaier AH, McCrae KR. The plasma kallikrein-kinin system: its evolution from contact activation. J Thromb Haemost. 2007;5:2323–2329. doi: 10.1111/j.1538-7836.2007.02770.x. [DOI] [PubMed] [Google Scholar]
- 17.Colman RW. Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des. 2006;12:2599–2607. doi: 10.2174/138161206777698710. [DOI] [PubMed] [Google Scholar]
- 18.Cao DJ, Guo YL, Colman RW. Urokinase-type plasminogen activator receptor is involved in mediating the apoptotic effect of cleaved high molecular weight kininogen in human endothelial cells. Circ Res. 2004;94:1227–1234. doi: 10.1161/01.RES.0000126567.75232.46. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Sainz IM, Wu Y, Pixley R, Espinola RG, Hassan S, Khan MM, Colman RW. The inhibition of tube formation in a collagen-fibrinogen, three-dimensional gel by cleaved kininogen (HKa) and HK domain 5 (D5) is dependent on Src family kinases. Exp Cell Res. 2008;314:774–788. doi: 10.1016/j.yexcr.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahdi F, Shariat-Madar Z, Kuo A, Carinato M, Cines DB, Schmaier AH. Mapping the interaction between high molecular mass kininogen and the urokinase plasminogen activator receptor. J Biol Chem. 2004;279:16621–16628. doi: 10.1074/jbc.M313850200. [DOI] [PubMed] [Google Scholar]
- 21.Katkade V, Soyombo AA, Isordia-Salas I, Bradford HN, Gaughan JP, Colman RW, Panetti TS. Domain 5 of cleaved high molecular weight kininogen inhibits endothelial cell migration through Akt. Thromb Haemost. 2005;94:606–614. doi: 10.1160/TH04-12-0834. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Pelekanakis K, Woolkalis MJ. Thrombin and tumor necrosis factor alpha synergistically stimulate tissue factor expression in human endothelial cells: regulation through c-Fos and c-Jun. J Biol Chem. 2004;279:36142–36147. doi: 10.1074/jbc.M405039200. [DOI] [PubMed] [Google Scholar]
- 23.Nykjaer A, Conese M, Christensen EI, Olson D, Cremona O, Gliemann J, Blasi F. Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. Embo J. 1997;16:2610–2620. doi: 10.1093/emboj/16.10.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12:863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prager GW, Breuss JM, Steurer S, Mihaly J, Binder BR. Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood. 2004;103:955–962. doi: 10.1182/blood-2003-07-2214. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Cao DJ, Sainz IM, Guo YL, Colman RW. The Inhibitory Effect of HKa in Endothelial Cell Tube Formation Is Mediated by Disrupting the uPA-uPAR Complex and Inhibiting its Signaling and Internalization. Am J Physiol Cell Physiol. 2008 doi: 10.1152/ajpcell.00569.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Cozzi PJ. Targeting uPA/uPAR in prostate cancer. Cancer Treat Rev. 2007;33:521–527. doi: 10.1016/j.ctrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Pixley RA, Lin Y, Isordia-Salas I, Colman RW. Fine mapping of the sequences in domain 5 of high molecular weight kininogen (HK) interacting with heparin and zinc. J Thromb Haemost. 2003;1:1791–1798. doi: 10.1046/j.1538-7836.2003.00291.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang XQ, Sun P, Paller AS. Gangliosides inhibit urokinase-type plasminogen activator (uPA)-dependent squamous carcinoma cell migration by preventing uPA receptor/alphabeta integrin/epidermal growth factor receptor interactions. J Invest Dermatol. 2005;124:839–848. doi: 10.1111/j.0022-202X.2005.23669.x. [DOI] [PubMed] [Google Scholar]
- 30.Mazzieri R, D'Alessio S, Kenmoe RK, Ossowski L, Blasi F. An uncleavable uPAR mutant allows dissection of signaling pathways in uPA-dependent cell migration. Mol Biol Cell. 2006;17:367–378. doi: 10.1091/mbc.E05-07-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaurasia P, Aguirre-Ghiso JA, Liang OD, Gardsvoll H, Ploug M, Ossowski L. A region in urokinase plasminogen receptor domain III controlling a functional association with alpha5beta1 integrin and tumor growth. J Biol Chem. 2006;281:14852–14863. doi: 10.1074/jbc.M512311200. [DOI] [PubMed] [Google Scholar]