Abstract
Background
Patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) often have chemotherapy resistant disease resulting in a poor prognosis. The aim of this study was to learn if inhibition of the mammalian target of rapamycin (mTOR) would produce tumor responses.
Methods
This was a phase II study of oral single-agent everolimus (10 mg/day) for relapsed/refractory indolent lymphoid malignancies including CLL.
Results
Four of 22 patients with CLL (18%, 95% CI: 5–40%) achieved a partial remission to therapy. An unanticipated finding in this study was an increase in the absolute lymphocyte count (ALC) associated with a decrease in lymphadenopathy in 8 (36%) patients. The ALC increased a median of 4.8 fold (range, 1.9 – 25.1), and the clinically measurable lymphadenopathy decreased a median of 75.5% (range, 38 – 93) compared to baseline measurements.
Conclusion
Everolimus has modest anti-tumor activity against CLL and can mobilize malignant cells from nodal masses into the peripheral circulation in a subset of CLL patients. Because CLL cells in lymphatic tissue and bone marrow can be more resistant to therapy than circulating CLL cells, the ability of everolimus to mobilize CLL cells into the circulation could be utilized in combination therapeutic regimens.
Keywords: CLL, mTOR, inhibitor, everolimus, rapamycins, mobilization
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL) is one of the most common lymphoid malignancies in the US with an estimated incidence of 20,000 new diagnoses per year and a prevalence of over 100,000.1 There is now highly effective initial therapy for previously untreated, advanced stage CLL with response rates of over 90%, complete responses (CR) in more than 40% of patients, and median response durations in excess of 3 years.2, 3 In contrast, outcome remains poor for patients with relapsed or treatment-refractory CLL.4, 5 More effective therapies for this group of patients are needed.
Rapamycin (sirolimus) is a highly specific inhibitor of the mammalian target of rapamycin (mTOR).6 mTOR is a multifunctional signal transduction kinase with a critical role in the signal transduction pathway linking growth stimuli with cell cycle progression.7 Rapamycin can inhibit cell growth and proliferation, induce apoptosis in some tumor cell lines, and inhibit tumor cell motility by impairing cell polarization and protrusion.9 Rapamycin has been demonstrated to induce apoptosis in CLL cells in vitro although the required concentration was higher than is achievable in vivo and the clinical significance of this finding remains uncertain.8 There are now two rapamycin analogs, temsirolimus and everolimus, which are currently approved by the US FDA for relapsed renal cancer. Temsirolimus also has demonstrated effectiveness in the treatment of relapsed/refractory mantle cell lymphoma.9, 10
Although a major pathogenic mechanism in development of CLL is defective apoptosis, recent research has shown that CLL is a dynamic malignancy with cellular turnover ranging between 0.1 and 1% per day.11 The CLL tumor burden has two distinct cellular populations, a proliferative compartment morphologically characterized by larger cells in the proliferation centers of the lymphoid tissues and bone marrow, and the kinetically inactive circulating cells.12, 13 A targeted inhibitor of cell division such as everolimus could be effective in the treatment of CLL, especially in patients with relapsed/refractory disease who often have more rapid cellular turnover.14 This hypothesis is supported by data from in vitro studies that showed that rapamycin and everolimus induce cell cycle arrest in activated CLL cells without inducing apoptosis.12, 15, 16 In addition, proliferation of CLL cells requires stromal support mediated through cytokines and adhesion molecules (e.g. integrins)17 and many of these supportive signals are transmitted by the PI3K and Akt pathways involving mTOR.13 Thus, there is a sound biological rationale for testing everolimus as a treatment for CLL.
We have recently completed a phase II clinical trial using everolimus to treat patients with relapsed/refractory indolent lymphoid malignancies that included 22 patients with CLL. In this paper we report the results of treatment of the CLL patients. The most striking finding was the increase in the absolute lymphocyte count (ALC) and concomitant decrease in the lymph node size observed in 8 of these patients.
Methods and Materials
This was a two-stage, phase II study conducted to assess response in previously treated patients with lymphoid malignancies after treatment with single-agent everolimus. The study was conducted through the Mayo Clinic Cancer Center, was approved by the Mayo Clinic Institutional Review Board according to the principles of the Helsinki Declaration, and all patients provided written informed consent. Patients with CLL were eligible for this trial if they met the CLL diagnostic criteria defined by the National Cancer Institute-Working Group Criteria of 1996 (NCI-WG 1996)18 or the criteria for the small lymphocytic lymphoma (SLL) variant defined by the World Health Organization (WHO),19 had previously received therapy for their lymphoid malignancy, and had relapsed or were refractory to their last treatment. The relapse was required to be biopsy proven within 6 months prior to enrollment. There was no limit on the number of prior therapies. Patients were required to be ≥18 years old, and in addition to meeting diagnostic criteria, were also required to have pre-treatment measurable disease by computed tomography (CT) or magnetic resonance imaging (MRI) scanning with at least one lesion that had a single diameter of >2 cm, or an ALC >5 ×109/L. Patients were to have a life expectancy of ≥ 3 months; Eastern Cooperative Oncology Group performance status of 0, 1, or 2; absolute neutrophil count (ANC) ≥ 1 × 109/L; platelet count ≥ 75 × 109/L; hemoglobin ≥ 8 g/dL; serum creatinine ≤ 2× the upper limit of normal (UNL); serum bilirubin ≤ 2 UNL (if total bilirubin >2 then a direct bilirubin of <1.5 UNL was acceptable); AST ≤ 3 × ULN (≤ 5 × ULN if liver involvement was present). Patients could not have known HIV infection.
Patients were treated with 10 mg of everolimus by mouth in the fasting state every day and 4 weeks of treatment was considered one cycle. A complete blood count was performed weekly during the first cycle and then before each subsequent cycle. If the platelet count was ≥40 × 109/L, the ANC ≥1 × 109/L, and there were no grade 3 or 4 non-hematological toxicities (NCI Common Toxicity Criteria version 3.0), the full dose of everolimus was prescribed for the next cycle. Patients who did not meet the retreatment criteria had treatment held until recovery followed by a stepwise dose modification to 5 mg daily, 5 mg every other day, and 5 mg every third day. Patients did not receive prophylactic white blood cell growth factors to maintain dosing but could receive them at physician discretion if neutropenia developed. Erythropoietin treatment for anemia was also permitted at physician discretion.
Patients were restaged for tumor response after 2 and 6 cycles using the NCI-WG 1996 criteria18 for those with an ALC > 5 × 109/L and the International Workshop Criteria for patients who had never had an ALC > 5 × 109/L (SLL variant).20 The maximum decrease in the clinically measurable lymphadenopathy was determined from the sum of the products of the largest lymph nodes diameters on clinical examination of the largest cervical, axillary, and inguinal nodes.18 Patients who had disease progression or unacceptable toxicity at any time went off study. Patients with stable disease after 6 cycles continued treatment at their physician’s discretion. Patients who had a complete remission (CR) after cycle 6 were to receive 2 cycles past CR and then could discontinue everolimus and be observed or could continue on therapy at their physician’s discretion. Patients with a partial remission (PR) after 6 cycles of therapy continued on treatment until progression if tolerated.
Statistical analysis
This phase II study used a two-stage Simon design to assess the efficacy and tolerability of everolimus in patients with indolent lymphoid malignancies. Thirty-seven evaluable patients were required to test the null hypothesis that the true response rate for this regimen is at most 20% versus the alternative hypothesis that the true response rate is 40% or greater. The study had 90% power, with a 9% type I error rate. A patient was considered evaluable for response if they were eligible and received treatment. The response rate was estimated by the number of responses divided by the number of evaluable patients. A 95% binomial confidence interval for the true response rate was calculated. This report is limited to the 22 CLL patients enrolled on the study because of the unique characteristics of the response of these patients to treatment with everolimus.
Duration of response (DR) was defined for patients who achieved a response as the time from the date a response was first documented until the earliest date progression was documented. Progression-free survival (PFS) was defined for all patients as the time from the date of registration until the date of progression or death due to any cause. Overall survival (OS) was defined as the time from the date of registration until the date of death due to any cause. The distributions of time to event endpoints were estimated using the Kaplan- Meier method and patients who were event-free were censored on the date of last follow-up.
Adverse events were graded using the NCI Common Toxicity Criteria (version 3.0). Toxicity was defined as an adverse event classified as being possibly, probably, or definitely related to study treatment.
Results
Patient Characteristics
Twenty two patients with CLL (10 with the SLL variant) were enrolled on this study between January 2006 and July 2008 (Table 1). All patients were eligible and evaluable for response. The median time from diagnosis of CLL to registration was 7 years (range, 2 – 22 years).
Table 1.
Patient Characteristics
| N (%)1 | |
|---|---|
| Age (median, range) | 74 (46, 85) |
| Sex | |
| Male | 16 (73%) |
| Female | 6 (27%) |
| Pre-treatment blood count values (median, range) | |
| Lymphocyte count (× 109/L) Neutrophil count | 3.0 (0.4 – 292) |
| (× 109/L) | 2.8 (1.0 – 8.6) |
| Hemoglobin (g/dL) | 11.9 (9.6 – 16.4) |
| Platelet count (× 109/L) | 147 (78 – 269) |
| Absolute lymphocyte count > 5 × 109/L at baseline | |
| Yes | 9 (41%) |
| No | 13 (59%) |
| Lymph nodes ≥ 5 cm at baseline | 13 (59%) |
| Yes | 9 (41%) |
| No | |
| ECOG PS at baseline | |
| 0 | 11 (50%) |
| 1 | 9 (41%) |
| 2 | 2 (9%) |
| Number of prior treatments, (median, range) | 6 (1 –10) |
Unless otherwise noted
Response to Treatment
Four patients (18%, 95% CI: 5–40%) achieved a PR to therapy at 1.8, 6.4, 9.1, and 14.1 months of treatment and maintained a response for 2.2, 11.0, 19.8, and 8.9+ months, respectively. Seventeen patients had disease progression, and 15 patients have died. Causes of death included disease progression (n = 11), infection possibly attributable to treatment as detailed below (n = 2), infection unrelated to treatment (n=1), and was unknown in 1 patient. The median follow-up for patients still alive was 17 months (range, 6 – 30), the median OS was 10.5 months (95% CI: 4.9 – 20.7) and the median PFS was 5.1 months (95% CI: 2.3 – 8.3).
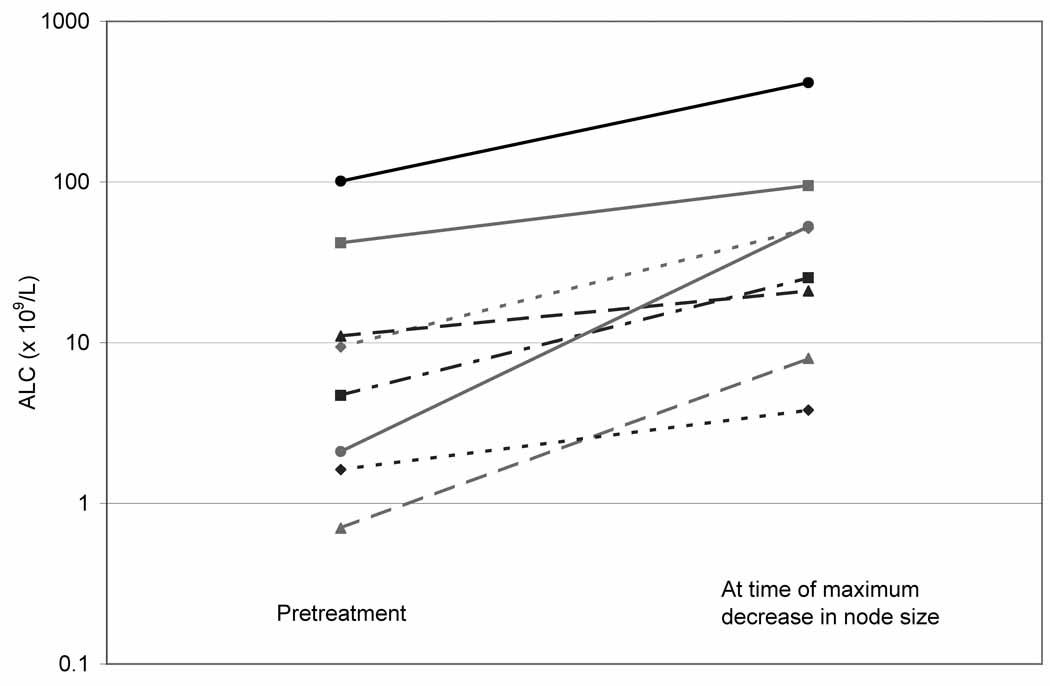
Increasing ALC Associated with Decreasing Lymph Node Size
An unanticipated and potentially important finding of this study was the increase in the ALC associated with a decrease in lymphadenopathy in 8 (36%) patients (Figure 1). This lymphocyte mobilization was observed in 6 (66%) of the 9 patients with an ALC > 5 × 109/L at baseline and 2 (15%) of the 13 patients with an ALC ≤ 5 × 109/L at baseline. Among the 8 patients in whom this phenomenon was observed, all had measurable lymphadenopathy at baseline and in 6 (75%) at least one node was ≥ 5 cm in diameter. The ALC increased from a median baseline level of 7.1 × 109/L (range, 0.7 – 101.2) to 18.3 × 109/L (range, 1.2 – 415.3) at the first post-treatment ALC (median interval from start of therapy 29 days, range, 7 – 49)(Figure 2). Patients achieved a median of 76% (range, 38 – 93) decrease in clinically measurable node size compared to baseline. This was associated with a median 4.8 fold (range, 1.9 – 25.1) increase in the ALC compared to baseline (median, 38.4 × 109/L; range, 3.8 – 415.3) which occurred at a median interval of 43 days (range, 29 – 242) from the start of therapy. Of the 8 patients with lymphocyte mobilization, 2 achieved a clinical PR (both had SLL), 4 had clinically stable disease, 1 had disease progression, and 1 was removed from the study because of an adverse event before clinical response could be evaluated.
Figure 1. Everolimus treatment resulted in an increase in the absolute lymphocyte count (ALC) and decrease in lymphadenopathy in 8 patients.
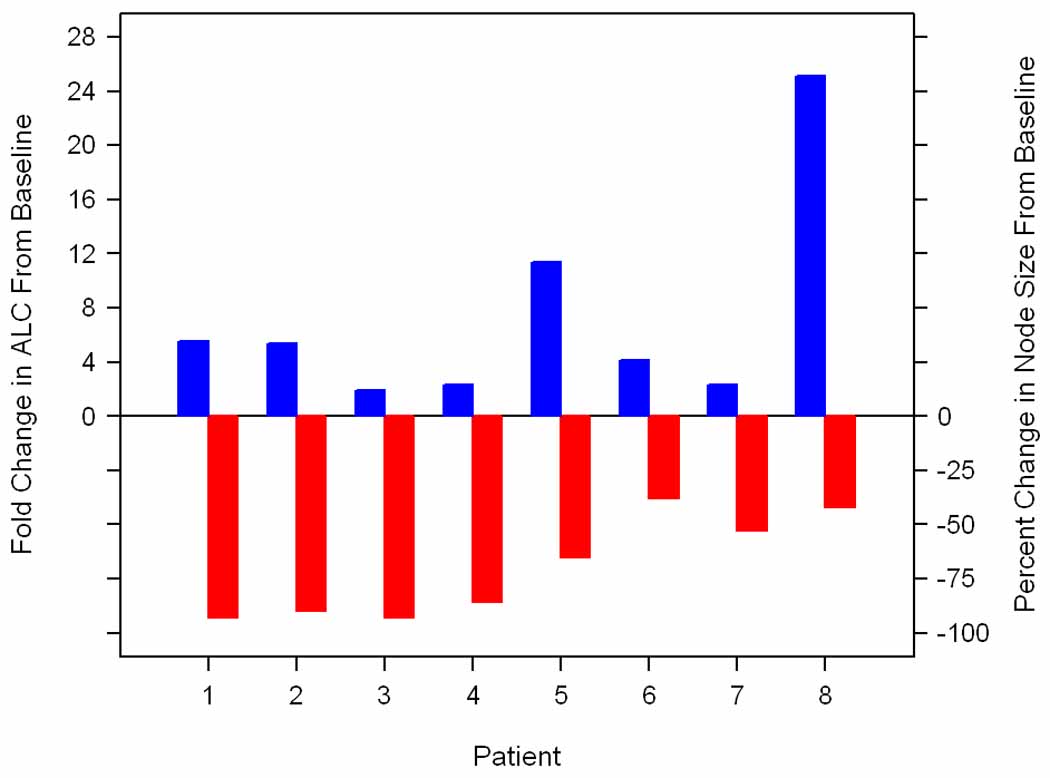
The maximum decrease in the clinically measurable lymphadenopathy (node size) is shown by the grey bars) and associated increase in the ALC by the black bars.
Figure 2. Increase in the absolute lymphocyte count (ALC) from the pretreatment level to the time of maximum decrease in lymphadenopathy.
Lymphocyte mobilization was not an anticipated finding in this study and the mechanism of this response was not studied. In one patient with lymphocyte mobilization a bone marrow (BM) examination was performed on day 6 of everolimus treatment due to concern about the patient’s clinical status. Although the results of the BM biopsy showed minimal change compared to baseline, the majority of the BM and circulating lymphocytes had the same morphological appearance of CLL cells. This finding suggests that the increase in circulating lymphocytes was caused by an increase in CLL cells. No confirmatory immunophenotyping studies were done on the circulating lymphocytes.
Toxicity and Tolerability
Patients received a median of 2 months (range, 1 week – 29 months) and a median of 2 cycles (range, 1–18) of treatment. Twenty patients have gone off treatment, 13 due to disease progression, 3 due to toxicity (1 patient with grade 2 pancreatitis, 1 patient with grade 3 fatigue, 1 cytopenia), 2 died on-study (1 patient due to sepsis possibly related to treatment, 1 patient died from disease progression), and 2 patients refused further therapy without response or progression. Nine patients experienced dose delays on 12 cycles of therapy due to hematological adverse events (10 cycles), hospitalization (1 cycle), and a delay in getting laboratory results (1 cycle). Nine patients experienced dose reductions on 11 cycles due to hematological adverse events (9 cycles), mucositis (1 cycle), and infection (1 cycle).
Fifteen patients experienced a grade 3 or higher hematologic adverse event (7 grade 3, 8 grade 4) and 9 patients experienced a grade 3 or higher non-hematologic adverse event (5 grade 3, 1 grade 4, 3 grade 5). Focusing on adverse events at least possibly attributable to the study therapy (Table 2), 14 patients experienced a grade 3 or higher hematologic toxicity (6 grade 3, 8 grade 4) and 7 patients experienced a grade 3 or higher non-hematologic toxicity (5 grade 3, 2 grade 5). Grade 3 or 4 anemia, neutropenia, and thrombocytopenia occurred in 23%, 32%, and 50% of patients, respectively. The most common grade 3 non-hematologic toxicities were pneumonia (n = 2) and hypertriglyceridemia (n = 2). One patient, who died of infection with positive blood cultures, had grade 4 adult respiratory distress syndrome (ARDS) complicating sepsis and grade 3 neutropenia, both complications were considered to be probably related to treatment. Another patient with grade 2 neutropenia died of sepsis possibly related to treatment.
Table 2.
Treatment Toxicity: Grade 3+ adverse events possibly, probably, or definitely related to treatment with everolimus.
| Grade | |||||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | |||||
| N | % | N | % | N | % | ||
| Toxicity | 5 | 23 | |||||
| Hematology | Anemia | ||||||
| Leukopenia | 3 | 14 | |||||
| Neutropenia | 5 | 23 | 2 | 9 | |||
| Thrombocytopenia | 5 | 23 | 6 | 27 | |||
| Infection/febrile neutropenia | Pneumonia | 2 | 9 | ||||
| Sepsis | 2 | 9 | |||||
| Cellulitis | 1 | 5 | |||||
| Metabolic/Laboratory | Hypertriglyceridemia | 2 | 9 | ||||
| Pulmonary | ARDS (secondary to infection) | 1 | 5 | ||||
| Constitutional symptoms | Fatigue | 1 | 5 | ||||
| Gastrointestinal | Diarrhea | 1 | 5 | ||||
Discussion
Treatment of patients with relapsed/refractory CLL using single agent everolimus showed biological activity with 18% of patients achieving a PR. An additional finding was that 36% of patients had an unexpected and marked increase in the ALC with a concomitant decease in lymph node size. These results show that everolimus can be clinically active in some patients with relapsed/refractory CLL, and suggest that mTOR inhibition can mobilize CLL cells from tissue sites into the circulation. Both of these findings could have important implications for the treatment of CLL.
Patients with relapsed/refractory CLL, especially those with purine analogue refractory disease or loss of p53 function because of gene deletions or mutations, have a poor prognosis.4 The 18% PR rate achieved with everolimus therapy in this study, although modest, warrants further study in light of the heavily pretreated nature of the patient population, the fact that this study used single agent everolimus, and that tumor cell mobilization was observed. In addition, the modest response rate needs to be interpreted in the context of the NCI-WG 1996 criteria that requires a minimum 50% decrease in the ALC to qualify for a response to treatment. The increase in ALC observed in 6 of the patients with CLL who had a decrease in adenopathy, prevents them from being considered responsive to treatment. This could have resulted in an underestimation of the efficacy of single agent everolimus therapy in this group of patients.
The mobilization effect observed could be especially useful for selecting drugs to be tested in combination with everolimus. In the only previously published report of the treatment of patients with CLL using everolimus,21 the pilot trial was stopped after treatment of only 7 patients because of infectious complications. Of the treated patients, one achieved a PR and 3 had stable disease. Although not commented on by the authors, review of the published data shows that 6 of the 7 patients appear to have had progressive lymphocytosis in response to initiation of therapy with everolimus at a dose of 5 mg/day; there was no information on the effect of everolimus on lymphadenopathy.
The stromal microenvironment of the lymphoid tissues and bone marrow provides critical support for CLL cell proliferation, survival, and resistance to therapeutic drugs.22 Pre-clinical data suggests that disruption of the interaction between CLL cells and stroma can decrease drug resistance in CLL cells.17 In addition, the therapeutic unconjugated monoclonal antibodies alemtuzumab and rituximab, which are highly effective against circulating CLL cells, have limited efficacy against bulky adenopathy and splenomegaly.23, 24 Mobilization of CLL cells from tissue into the circulation is likely to remove these CLL cells from the protective stromal microenvironment and enhance the cytotoxicity of drugs such as alemtuzumab which have the additional advantage of being effective in patients with purine analogue refractory CLL with defective p53 function.25, 26 Combinations of everolimus and drugs such as alemtuzumab could potentially be synergistic in patients with bulky adenopathy resulting in improved treatment outcomes.
Everolimus is an immunosuppressive drug that can also be myelosuppressive. Thus, it was not surprising that infections were observed in this study of CLL patients already immunocompromised by their underlying disease and prior therapy. Indeed, infection resulted in 2 deaths. Neutropenia could have contributed to the risk of infection, but thrombocytopenia was not associated with bleeding complications. The other grade 3–4 toxicities responded well to appropriate management. These data suggest that CLL patients treated with everolimus in the future will likely benefit from prophylaxis and surveillance for opportunistic infections, and that combination therapies including everolimus should be designed to minimize marrow toxicity in this patient population.
The mTOR has been shown to be an integral component of important signaling pathways in CLL cells, and inhibition of these pathways by everolimus could be expected to decrease cell growth and be cytotoxic.12, 15, 16 In contrast, the mechanism by which everolimus causes CLL cell mobilization into the circulation is unknown and will need to be investigated in order to develop interventions to optimize this effect. These investigations were not performed in this study because the finding was unexpected. The extent of mobilization and the concomitant decrease in the clinically detectable lymphadenopathy was only fully appreciated at the time of analysis of the study results. The study was designed to evaluate ALC each cycle and measurable lymph nodes after cycles two and six and every three cycles thereafter. The study was not designed to measure or record ALC more frequently; therefore, the kinetics and magnitude of the CLL cell mobilization could actually have been underestimated in this study population. Future studies should be designed to more frequently measure the mobilization effect of everolimus in CLL patients, study the cell surface immunophenotype of the mobilized cells, and determine if the CLL cells mobilized into the circulation are as sensitive to monoclonal antibody mediated cytotoxicity as cells collected prior to treatment with everolimus.
In conclusion, this study shows that everolimus could be a valuable drug for use in combination therapy for CLL patients. Further understanding of the mechanism of action of everolimus in CLL will be very useful in designing trials to improve therapy for this incurable disease.
Acknowledgments
Research Support
This study was supported by University of Iowa/Mayo Clinic NIH SPORE Grant CA97274 and Novartis Oncology.
Footnotes
Financial Disclosures
Dr. Zent has research funding from Bayer and Genentech and Drs. Witzig and Johnston have served as uncompensated consultants for Novartis Oncology.
References
- 1.Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer. 2001;92:1325–1330. doi: 10.1002/1097-0142(20010901)92:5<1325::aid-cncr1454>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 2.Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B-chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keating MJ, O'brien S, Albitar M, Lerner S, Plunkett W, Giles F, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 4.Gribben JG. Salvage Therapy for CLL and the Role of Stem Cell Transplantation. Hematology Am Soc Hematol Educ Program. 2005:292–298. doi: 10.1182/asheducation-2005.1.292. [DOI] [PubMed] [Google Scholar]
- 5.Wierda W, O'Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 6.Kurmasheva RT, Huang S, Houghton PJ. Predicted mechanisms of resistance to mTOR inhibitors. Br J Cancer. 2006;95:955–960. doi: 10.1038/sj.bjc.6603353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19:6680–6686. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 8.Aleskog A, Norberg M, Nygren P, Rickardson L, Kanduri M, Tobin G, et al. Rapamycin shows anticancer activity in primary chronic lymphocytic leukemia cells in vitro, as single agent and in drug combination. Leuk Lymphoma. 2008;49:2333–2343. doi: 10.1080/10428190802475295. [DOI] [PubMed] [Google Scholar]
- 9.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM, Inwards DJ, Rowland KM, Jr, Flynn PJ, Morton RF, Moore DF, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messmer BT, Messmer D, Allen SL, Kolitz JE, Kudalkar P, Cesar D, et al. In vivo measurements document the dynamic cellular kinetics of chronic lymphocytic leukemia B cells. J Clin Invest. 2005;115:755–764. doi: 10.1172/JCI23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringshausen I, Peschel C, Decker T. Mammalian target of rapamycin (mTOR) inhibition in chronic lymphocytic B-cell leukemia: a new therapeutic option. Leuk Lymphoma. 2005;46:11–19. doi: 10.1080/10428190400005353. [DOI] [PubMed] [Google Scholar]
- 13.Bennett F, Rawstron A, Plummer M, de Tute R, Moreton P, Jack A, et al. B-cell chronic lymphocytic leukaemia cells show specific changes in membrane protein expression during different stages of cell cycle. Br J Haematol. 2007;139:600–604. doi: 10.1111/j.1365-2141.2007.06790.x. [DOI] [PubMed] [Google Scholar]
- 14.Panwalkar A, Verstovsek S, Giles FJ. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer. 2004;100:657–666. doi: 10.1002/cncr.20026. [DOI] [PubMed] [Google Scholar]
- 15.Decker T, Hipp S, Ringshausen I, Bogner C, Oelsner M, Schneller F, et al. Rapamycin-induced G1 arrest in cycling B-CLL cells is associated with reduced expression of cyclin D3, cyclin E, cyclin A, and survivin. Blood. 2003;101:278–285. doi: 10.1182/blood-2002-01-0189. [DOI] [PubMed] [Google Scholar]
- 16.Decker T. Is mTOR inhibition a therapeutic option in chronic lymphocytic leukemia? Leuk Lymphoma. 2008;49:2235–2236. doi: 10.1080/10428190802531295. [DOI] [PubMed] [Google Scholar]
- 17.Kay NE, Shanafelt TD, Strege AK, Lee YK, Bone ND, Raza A. Bone biopsy derived marrow stromal elements rescue chronic lymphocytic leukemia B-cells from spontaneous and drug induced cell death and facilitates an "angiogenic switch". Leuk Res. 2007;31:899–906. doi: 10.1016/j.leukres.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukemia: Revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 19.Muller-Hermelink HK, Catovsky D, Montserrat E, Harris NL. Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: Jaffe E, Harris N, Stein H, Vardiman J, editors. Tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 127–130. [Google Scholar]
- 20.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 21.Decker T, Sandherr M, Goetze K, Oelsner M, Ringshausen I, Peschel C. A pilot trial of the mTOR (mammalian target of rapamycin) inhibitor RAD001 in patients with advanced B-CLL. Ann Hematol. 2009;88:221–227. doi: 10.1007/s00277-008-0582-9. [DOI] [PubMed] [Google Scholar]
- 22.Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J Intern Med. 2008;264:549–562. doi: 10.1111/j.1365-2796.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 23.Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H. Humanized CD52 monoclonal antibody Campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol. 1996;93:151–153. doi: 10.1046/j.1365-2141.1996.450989.x. [DOI] [PubMed] [Google Scholar]
- 24.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 25.Lozanski G, Heerema NA, Flinn IW, Smith L, Harbison J, Webb J, et al. Alemtuzumab is an Effective Therapy for Chronic Lymphocytic Leukemia with p53 Mutations and Deletions. Blood. 2004;103:3278–3281. doi: 10.1182/blood-2003-10-3729. [DOI] [PubMed] [Google Scholar]
- 26.Stilgenbauer S, Döhner H. Campath-1H-Induced Complete Remission of Chronic Lymphocytic Leukemia despite p53 Gene Mutation and Resistance to Chemotherapy. N Engl J Med. 2002;347:452–453. doi: 10.1056/NEJM200208083470619. [DOI] [PubMed] [Google Scholar]