Abstract
Objective
Research has documented modest cognitive difficulties among women treated for breast cancer. The present study was designed to evaluate the effects of these subtle cognitive changes on quality of life after treatment.
Methods
Data are presented from women breast cancer patients who completed neuropsychological tests and questionnaires regarding quality of life six and twelve months post-chemotherapy (n's = 39 and 33). Neuropsychological test scores were examined for evidence of cognitive difficulties at each time point; repeated measures ANOVAs were used to identify changes over time. Regression analyses assessed relationships of quality of life outcomes with cognitive functioning, social support seeking, and fatigue.
Results
Small percentages of participants (< 20% across tests) evidenced deficits in delayed memory, processing speed, response inhibition, and verbal fluency at each time point. Reliable change index analyses suggested statistically reliable improvements in each cognitive domain for a modest portion of participants. Regressions revealed hesitation to seek social support and fatigue as the most consistent predictors of quality of life at six and twelve months post-chemotherapy. Cognitive complaints and verbal fluency difficulties were also significantly related to quality of life at twelve months.
Conclusions
In addition to confirming the importance of fatigue and social support in quality of life, these data offer preliminary indications that weaker verbal fluency skills and self-reported cognitive complaints may be associated with poorer functional outcomes among cancer survivors. Further research is needed to validate these potential relationships, which suggest that cognitive difficulties among cancer survivors may warrant monitoring and possible intervention.
Keywords: cancer, oncology, cognitive function, quality of life, neuropsychological tests, chemotherapy
Introduction
Research has documented statistically significant decrements in cognitive functioning among cancer survivors after treatment; however, the potential clinical relevance of these deficits has yet to be clarified. Sometimes referred to as “chemobrain” or “chemofog,” these cognitive difficulties have largely been considered secondary to chemotherapy. Several studies have shown neuropsychological difficulties in up to thirty-five percent of breast cancer survivors [1] across a wide range of domains [for reviews see 2, 3]. While research suggests that long-term survivors may experience a lessening of neuropsychological deficits over time [4], ongoing relative deficits have been documented among women 2 years and 5 years post-treatment [5, 6] and complaints of cognitive difficulties remain at 5−10 years after initial diagnosis [7]. Particularly susceptible skills appear to include executive functioning [8], attention/concentration [4, 9, 10, 11], and memory [5, 6, 11, 12]. Meta-analyses have revealed small to medium effect sizes across each of these neuropsychological domains [13, 14], with the largest effects for executive functioning and verbal memory [15]. A recent review of research in this area has noted growing evidence of frontal-subcortical effects of cancer treatment [16]. Consistent with this are the findings of executive functioning deficits in neuropsychological test performance, as well as neuroimaging findings of alterations in frontal and subcortical structure [17, 18] and function [19, 20] following chemotherapy.
On the other hand, controversies exist regarding this growing body of literature on cognitive difficulties following cancer treatment. First, some research has failed to document cognitive deficits following treatment for non-neurologic cancers [21, 22]. Second, some argue that deficits are not specific to chemotherapy, in part based on evidence of cognitive impairments among a subset of individuals prior to initiating cancer treatment [23, 24]. Finally, it has been suggested that the significance of the difficulties that have been observed may have been overstated [22], as a meta-analysis [14] documented effect sizes on cognitive test scores in the small to moderate range (−0.03 to −0.51 SD below controls). In fact, a review of raw and standardized neuropsychological test scores where available in these studies reveals that, despite suggestion of cognitive decline, many cancer survivors studied continue to function in the “average” (i.e., “normal”) range. Thus, in addition to uncertainty regarding causal factors, the clinical relevance of possible cognitive decline among survivors of non-neurological cancers remains undetermined.
Reflecting this, studies of the effects of neuropsychological difficulties on functional outcomes among cancer survivors are few. Difficulties concentrating have been identified as a significant stressor following cancer treatment [25]. Additionally, qualitative research has revealed that women cancer survivors perceive they are experiencing cognitive difficulties that affect their functioning at home and at work [26]. On the other hand, one longitudinal, quantitative study [22] found no association between quality of life and neuropsychological test performance either before chemotherapy or at two time points (6 and 18 months) post-chemotherapy. However, the ability of this study to detect a relationship may have been limited by the fact that participants had received low dose FEC (Fluorouracil, Epirubicin, Cyclophosphamide), which the authors suggested may have contributed to their evidencing minimal cognitive impairment.
Given uncertainty regarding the clinical significance of cognitive changes in cancer survivors, the present study was designed to assess the potential relationships between cognitive functioning and quality of life among breast cancer survivors post-chemotherapy. The primary aim was to determine whether, in addition to the previously documented effects of fatigue [27, 28, 29] and social support [30], there may be functional implications of cognitive difficulties among cancer survivors. Specifically, it was hypothesized that, among all cognitive domains typically assessed, executive functioning and memory deficits would be most likely to negatively affect social role functioning and quality of life.
Method
Participants
Women with Stage I –III breast cancer were recruited through three cancer treatment facilities in a mid-size Midwestern city. Exclusion criteria included: age younger than 18, previous chemotherapy treatment, chronic psychiatric illness, known neurological condition, and non-native English speaking. Of the 46 women who were evaluated within one month post-chemotherapy, 39 completed the six month follow-up and 33 remained for the one year follow-up (28% total attrition).1
The average age of all participants was 53.38 years (SD = 9.61), with an average of 14.87 years (SD = 2.56) of education. Sixty-four percent were married. A majority of participants were employed: 70.7% were employed 30 hours/week or more, and another 10.4% were employed 10−30 hours/week. All participants self-identified as Caucasian. Almost one-half of participants were diagnosed with Stage II breast cancer (47.4%), while 23.7% were diagnosed with Stage I and 28.9% were diagnosed with Stage III. Participants received adriamycin-cyclophosphamide with or without paclitaxel or taxotere or a variant of that program; the average number of chemotherapy cycles across all stages was 5.77 (SD = 1.93).2 Seventy-seven percent of participants received radiation therapy, while less than one-half received adjuvant hormone therapy (41%).
Predictor Variables
Demographic information
For each participant the following information was obtained: age, marital status, race/ethnicity, cancer stage, number of chemotherapy cycles, and types of treatment (e.g., chemotherapy, radiation, Tamoxifen).
Neuropsychological measures
Tests were used to evaluate skills in five neuropsychological domains: Immediate Memory (IM), Delayed Memory (DM), Processing Speed/Attention (PS), Response Inhibition (RI), and Verbal Fluency (VF). The selection of tests was based on previous neuropsychological studies with cancer patients [6, 9, 12] and includes those measures that have been identified as most sensitive to changes secondary to treatment for cancer. Tests comprising each domain were grouped based on a priori theory of neuropsychological domains [31], and the interrelatedness of grouped tests were confirmed via correlational analyses. To reduce the influence of practice effects, alternate forms were used where available (i.e., Rey AVLT, COWAT, Category Fluency). See Table 1 for information regarding specific tests in each neuropsychological domain.
Table 1.
Neuropsychological Measures
| Neuropsychological Domain | Measures | 1 Month Composite z-scoresb | 6 Month Composite z-scoresb | 12 Month Composite z-scoresb |
|---|---|---|---|---|
| Immediate Memory (r = 0.50, p = 0.001)a |
WMS-III Logical Memory I [43] Rey AVLT Trials 1−5 [44] |
0.82(0.68) | 1.12(0.71)c | 1.30(0.77)c |
| Delayed Memory (r = 0.46, p = 0.001)a |
WMS-III Logical Memory II [43] Rey AVLT Delayed Recall [44] |
0.68(0.69) | 0.95(0.72)c | 1.09(0.72)c |
| Processing Speed (r = 0.59, p < 0.001)a |
Trail Making Test A [45] Trail Making Test B [45] |
0.11(1.29) | 0.41(1.23) | 0.35(1.14) |
| Response Inhibition | Stroop Test [46] | 0.07(0.86) | 0.14(0.93) | 0.27(0.91)c |
| Verbal Fluency (r = 0.37, p = 0.01)a |
COWAT [47] Category Fluency [48] |
−0.23(0.81) | 0.06(0.93)c | 0.19(0.95)c |
Pearson correlation between tests comprising cognitive domains at 1 month post-treatment. These correlations remained significant (all p's < 0.05) at 6 and 12 months post-treatment.
Group means and standard deviations for composite scores at each time point for participants completing all 3 data collection sessions (n = 33).
Following significant repeated measures ANOVAs (all F's(1,31) > 3.19, p's < .05), subsequent paired t-test comparisons indicate significant difference (p < 0.05) compared to 1 month cognitive domain composite score.
Self-Reported Cognitive Difficulties
Because research suggests that self-reported cognitive difficulties may be more strongly associated with depression than actual neuropsychological impairment [9], a measure of cognitive complaints, the Confusion subscale of the Profile of Mood States – Short Form (POMS-SF) [32], was included.
Fatigue
Prior research has documented that fatigue following cancer treatment adversely affects survivors’ quality of life [27, 28, 29]. To evaluate fatigue in this sample, the Fatigue subscale of the POMS-SF was utilized [32].
Social Support Seeking
The availability and use of a positive social support network can have a substantial effect on functional outcomes among individuals with chronic illnesses [30]. Thus, the Hesitation Scale [33] was included as a measure of individuals’ willingness to seek social support.
Outcome variables
Psychological functioning
Symptoms of depression were documented with the Beck Depression Inventory-Second Edition (BDI-II) [34].
Social role functioning
Developed by Bettencourt and Sheldon [35, 36], the Social Role Functioning questionnaire was designed to assess individuals’ perceptions of their competency in fulfilling various social roles (e.g., spouse, parent, employee).
Quality of life
Two measures were used to assess quality of life. A single-item question (“In general, how satisfied are you with your overall quality of life?”) using a 5-point Likert scale was included based on research suggesting that such a question is a sensitive indicator to variability in quality of life experienced by cancer survivors [37]. Participants also completed the Functional Assessment of Cancer Therapy-Breast (FACT-B) [38], which has 44 items across six subscales, four of which were the focus of the present study: Physical Well-Being, Social/Family Well-Being, Emotional Well-Being, and Functional Well-Being.
Study Procedure
Potential participants for the study were identified by their health care providers at three recruitment sites (an academic hospital, a private hospital, and a private oncology practice), and informed written consent for study participation was obtained per institutional guidelines. All participants were recruited prior to the completion of their last cycle of chemotherapy. Within 1 month of finishing chemotherapy, participants completed the battery of tests and questionnaires described above; results from this initial time point have been reported elsewhere [39]. The same measures were completed at 6 months and at 12 months after completing chemotherapy; these data form the basis for findings presented herein.
Results
Neuropsychological functioning
Individual test scores were converted to z-scores utilizing age-, education-, and gender-based normative data. Frequency analyses were conducted with these z-scores to identify the percent of individuals demonstrating subtle as well as more notable cognitive impairment on individual tests at 6 and 12 months post-chemotherapy. Subtle cognitive impairment was defined as more than 1 standard deviation (SD) below the normative data mean, while more severe impairment was defined as 1.5 to 2+ SD below the mean [40]. Using these criteria, analyses revealed that no participants demonstrated subtle or more severe cognitive impairments in: WMS-III Logical Memory I, WMS-III Logical Memory II, or Rey AVLT Trials 1−5 Total. A modest number of individuals were evidencing difficulties at 6 months and at 12 months on each of the other tests administered. Figure 1 details the percentage of individuals at 6 months and 12 months evidencing cognitive difficulties; each percentage utilized the number of participants in the 6 month group (n = 39) as the denominator. As anticipated, there was a decline in the percent of individuals evidencing difficulties on Rey Delay, Stroop Color Word, and Phonemic fluency measures from 6 months to 12 months post-chemotherapy. However, unexpectedly, there was a slight increase in the percent of individuals showing deficits on Trails A, Trails B, and Category fluency over this same time period.
Figure 1.
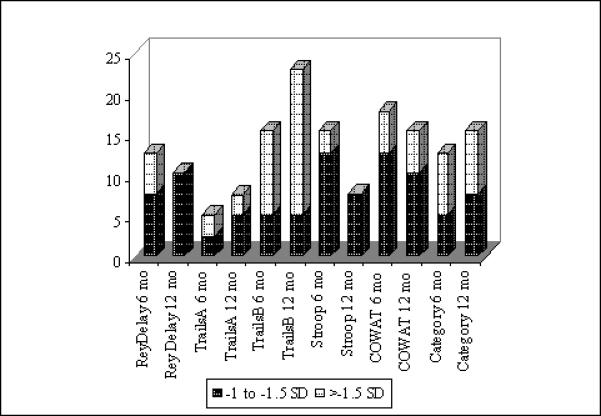
Percent of Participantsa with Test-Specific Cognitive Impairmentb at 6 and 12 Months
a Percent of participants for 6 months and 12 months was computed using the number of participants at 6 months (n = 39) as the denominator.
b Subtle cognitive impairment was defined as −1 to −1.5 SD, while more severe cognitive impairment was defined as below −1.5 SD [40].
Subsequent analyses sought to document changes in cognitive functioning during the first year following chemotherapy and to determine whether cognitive functioning at each time point was related to concurrent hormone (i.e., Tamoxifen) therapy. For these analyses, composite test scores for each neuropsychological domain were created by computing an average of each person's z-scores for tests comprising each domain. Then, Tamoxifen use was entered as a bi-level (yes/no) covariate in repeated measures ANOVAs assessing for changes in neuropsychological test composite scores over time. Subsequent paired samples t-tests were conducted to further clarify significant findings.
The results revealed that Tamoxifen use was not significant for any neuropsychological domain (all F's < 2.78, p's > 0.11). Within-subjects effects revealed statistically significant changes in test scores over time for Immediate Memory [F(2,62) = 13.35, p < 0.001], Delayed Memory [F(2,62) = 9.60, p < 0.001], Response Inhibition [F(2,62) = 3.20, p < 0.05], and Verbal Fluency [F(2,62) = 7.18, p = 0.002]. Changes in Processing Speed over time approached significance [F(2,62) = 2.99, p = 0.07].
As presented in Table 1, subsequent paired t-test analyses indicated statistically significant increases in composite Immediate Memory (IM), Delayed Memory (DM), and Verbal Fluency (VF) test scores for from 1 month to 6 months [IM: t = −3.33, p = 0.002; DM: t = −3.20, p = 0.003; VF: t = −2.78, p = 0.008] and from 1 month to 12 months [IM: t = 5.24, p < 0.001; DM: t = 3.79, p = 0.001; VF: t = 3.45, p = 0.002], as well as significant differences from 1 month to 12 months in Response Inhibition (t = 2.43, p = 0.021). Only for Immediate Memory did the difference between 6 month and 12 month data approach statistical significance (t = −2.00, p = 0.06).
Although these findings were suggestive of statistically significant changes in group means over time across some neuropsychological domains, further analyses to determine the reliability of these changes were undertaken using the Reliable Change Index [41]. Reliability coefficients were obtained from existing literature for individual tests where available. Utilizing a cutoff of RCI = 1.96 (reflecting statistical significance at the 0.05 level), none of the changes in group mean test scores met criteria for reliable change. See Table 2. Subsequently, RCIs were computed for each participant's test scores to determine whether reliable change in test scores was demonstrated by individual participants. Table 3 presents the percentage of individuals demonstrating reliable changes in test scores between each of the three data collections sessions (at 1 month, 6 months and 12 months post-chemotherapy).
Table 2.
Reliable Change Analyses of Group Means
| Neuropsychological Domain |
Test |
Reliability Coefficienta |
1 month (T1)b |
6 month (T2)b |
12 month (T3)b |
T1-T2 RCIc |
T2-T3 RCIc |
T1-T3 RCIc |
|---|---|---|---|---|---|---|---|---|
| Immediate Memory | WMS Log Mem I | .86 | .68(.81) | 1.24(.79) | 1.48(.75) | 1.06 | 0.46 | 1.52 |
| Delayed Memory | Rey AVLT Delay | .66 | .41(91) | .30(1.01) | .39(.95) | 0.74 | 0.11 | 0.63 |
| WMS Log Mem II | .86 | .89(.77) | 1.53(.66) | 1.75(.69) | 1.23 | 0.42 | 1.65 | |
| Processing Speed | Trails A | .79 | .25(1.04) | .59(1.12) | .53(.93) | 0.52 | 0.09 | 0.37 |
| Trails B | .79 | −.22(1.81) | .24(1.47) | .18(1.53) | 1.00 | 0.13 | 0.87 | |
| Response Inhibition | Stroop Color-Word | .73 | −.07(.88) | .06(.91) | .27(.91) | 0.18 | 0.28 | 0.46 |
| Verbal Fluency | COWAT | .83 | −.40(1.09) | −.02(1.08) | .05(1.07) | 0.67 | 0.12 | 0.79 |
| Category Fluency | .56 | −.31(.96) | .11(1.05) | .34(1.14) | 0.45 | 0.25 | 0.70 |
All reliability coefficients were obtained from Spreen, Sherman & Strauss [48], except those for WMS-III Logical Memory, for which WMS-III Auditory Memory reliability coefficient from Lineweaver and Chelune [49] was used.
Group mean and standard deviation z-scores.
RCI comparing 2 identified time points, using calculation from Jacobson and Traux [41].
Table 3.
Percent of Individuals Evidencing Reliable Changea between Sessions
| Neuropsychological Domain | Test | 1 mo – 6 mob | 6 mo – 12 moc | 1 mo – 12 moc |
|---|---|---|---|---|
| Immediate Memory | WMS Log Mem I | 17.9% | 6.1% | 33.3% |
| Delayed Memory | Rey AVLT Delay | 2.6% | 12.5% | 6.3% |
| WMS Log Mem II | 15.4% | 3.0% | 33.3% | |
| Processing Speed | Trails A | 7.7% | 3.0% | 9.1% |
| Trails B | 17.9% | 15.2% | 24.2% | |
| Response Inhibition | Stroop Color-Word | 2.6% | 3.0% | 3.0% |
| Verbal Fluency | COWAT | 7.7% | 0% | 9.1% |
| Category Fluency | 7.7% | 0% | 15.6% |
All changes reflect findings from RCI analyses [41] of improved test scores from the prior session.
Sample size limited by number of participants at 6 months (n=39).
Sample size limited by number of participants at 12 months (n=33).
Self-Reported Symptoms and Neuropsychological Functioning
Pearson correlation coefficients were computed to assess for relationships between neuropsychological composite test scores and concurrent fatigue, depression, and self-reported cognitive complaints at 6 months and 12 months post-chemotherapy. As data collected at 1 month following chemotherapy has been presented elsewhere [39], these correlations are presented for data from 6 and 12 months following chemotherapy. As shown in Table 4, these concurrent symptoms did not appear related to Processing Speed or Verbal Fluency at either 6 or 12 months. On the other hand, Immediate Memory scores were inversely correlated with these self-reported symptoms at both 6 and 12 months post-chemotherapy. Additionally, lower scores in Response Inhibition were associated with self-reported fatigue, depression and cognitive difficulties at 6 months but not at 12 months.
Table 4.
Correlations between Neuropsychological Test Composites and Other Concurrent Symptoms
| POMS Fatigue | POMS Confusion | BDI | ||
|---|---|---|---|---|
| Immediate Memory | 6 month | −.14 | −.36** | −.38** |
| 12 month | −.39** | −.36** | −.50*** | |
| Delayed Memory | 6 month | −.04 | −.16 | −.28 |
| 12 month | −.34* | −.14 | −.22 | |
| Processing Speed | 6 month | .02 | −.10 | −.05 |
| 12 month | −.05 | −.06 | −.02 | |
| Response Inhibition | 6 month | −.49*** | −.36** | −.47*** |
| 12 month | −.28 | −.26 | −.12 | |
| Verbal Fluency | 6 month | −.04 | −.12 | −.07 |
| 12 month | −.13 | −.03 | −.16 | |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
Correlation approached significance (p = 0.055).
Predicting Functional Outcomes Post-Chemotherapy
Regression analyses were conducted to determine what aspects of concurrent functioning predict depression, social role functioning, and quality of life at 6 and 12 months post-chemotherapy. To meet assumptions of normality for multiple regression analyses, all outcome variables were log transformed; subsequent Shapiro-Wilk tests of studentized residuals indicated that the assumption of normality was satisfied for all outcome variables. For each regression model, the following predictors from data collected concurrent to the outcome measures were entered simultaneously: fatigue (POMS-SF Fatigue subscale), self-reported neuropsychological complaints (POMS-SF Confusion subscale), willingness to seek help (Hesitation Scale), and neuropsychological test performances (composite test scores identified in Table 1). Results are presented by outcome domain for analyses of data collected at 6 and 12 months post-chemotherapy.
Emotional functioning
The BDI was utilized to measure symptoms of depression during the year following cancer treatment. The model for 6 month data was significant (Adjusted R2 = 0.38; F[8, 26] = 3.57, p < .01). Examination of the individual betas showed that increased depression was associated with greater hesitation to seek support (b = .41, t = 2.43, p = .02). However, at 12 months, heightened levels of depression were associated with increased self-reported fatigue (Adjusted R2 = 0.66, F[8, 22] = 8.23, p = .004).
Social role functioning
Greater hesitation to seek social support was the sole significant predictor of poorer social role functioning at 6 months post-chemotherapy (Adjusted R2 = 0.49; F[8, 22] = 4.65, p = .002, b = .63, t = 3.79, p = .001). The model predicting social role functioning approached significance at 12 months post-chemotherapy (Adjusted R2 = 0.29; F[8, 18] = 2.30, p = .068), with greater hesitation to seek social support again being associated with poorer social role functioning (b = .61; t = 2.67, p = .01).
Quality of life
A single question of overall satisfaction with life and the FACT-B were utilized to measure quality of life in this study. Regarding the single question quality of life measure, the regression model at 6 months post-chemotherapy was significant (Adjusted R2 = .47; F[8, 27] = 4.85, p = .001). A review of the betas revealed that greater reluctance to seek social support was significantly associated with lower overall satisfaction (b = .59, t = 3.79, p = .001). At 12 months post-chemotherapy, the model was again significant (Adjusted R2 = 0.44; F(8,21) = 3.81, p = .007), with a review of betas revealing an inverse relationship between self-reported cognitive complaints and overall quality of life (b = .46, t = 1.82, p = .08).
Regression analyses were conducted for each FACT-B subscale separately; only models reaching significance are reported for each time point. At 6 months post-chemotherapy, the model for Functional Well-Being was significant (Adjusted R2 = .24, F[8, 27] = 2.35, p = .046). A review of the betas revealed that increased fatigue (b = .49, t = 2.27, p = .03) was significantly associated with poorer functional well-being. The model for Physical Well-Being at 6 months was also significant (Adjusted R2 = .65; F[8, 27] = 9.15, p < .001) ; a review of betas revealed that better physical well-being was significantly associated with lower self-reported fatigue (b = .83, t = 5.72, p < .001).
At 12 months post-chemotherapy, the model for Functional Well-Being was again significant (Adjusted R2 = .49; F[8, 21] = 4.54, p = .003), suggesting a continued association between poorer functional well-being and fatigue (b = .76, t = 3.40, p = .003). In addition, at 12 months, poorer functional well-being was associated with hesitation to seek social support (b = .38, t = 2.13, p = .045) and verbal fluency difficulties (b = −.42, t = −2.14, p = .04). Similar to the 6 months findings, the model for Physical Well-Being at 12 months post-chemotherapy achieved significance (Adjusted R2 = .41; F[8, 22] = 3.65, p = .008). In this model, greater self-reported fatigue was associated with poorer overall physical well-being (b = .58, t = 2.59, p = .017). Significant at 12 months only was the model for Emotional Well-Being (Adjusted R2 = .43; F[8, 22] = 3.84, p = .006). In this model, better emotional well-being was associated with decreased self-reported cognitive difficulties (b = .63, t = 2.71, p = .01). Finally, at 12 months post-chemotherapy, the model for Social Well-Being approached significance (Adjusted R2 = .23, F(8,22) = 2.14, p = .075), reflecting a significant relationship between hesitation to seek social support and poorer social well-being (b = .64, t = 3.00, p = .007).
Discussion
Consistent with other research, in this sample cognitive abilities remained within normal limits for a majority of breast cancer survivors in the year following treatment. A modest percentage of participants did evidence lower than expected performance in neuropsychological functioning, although a majority of these individuals were evidencing subtle deficits (defined as z = −1.00 to −1.49, per Vardy and colleagues [40]). There was some indication of improvements in neuropsychological test scores over the year following treatment, as RCI analyses of individuals’ data revealed that a small portion of individuals demonstrated statistically reliable improvements. This is commensurate with findings from other research and highlights the importance of looking at individual test scores rather than group means [16]. However, these findings are considered preliminary. While the RCI analyses help account for measurement error, the absence of a control group in this research precluded the use of RCI analyses that would take into account practice effects for this sample. It is possible that the improvements in test scores reflect the effects of repeated exposure to tests rather than clinical significant gains in cognitive functioning.
As previously noted, the intent of this project was to bring attention to the question of whether subtle neuropsychological difficulties evidenced by breast cancer survivors were clinically/functionally significant. To this end, analyses were conducted to determine whether neuropsychological test performance was associated with concurrent self-reported emotional functioning, social role functioning, and quality of life. In addition, recognizing that cognitive difficulties do not occur in isolation, the potential contributions of fatigue, self-reported cognitive complaints, and social support seeking were also considered important in evaluating quality of life outcomes. The resulting regression models revealed that, consistent with other research [30, 42], social support was significantly related with several aspects of quality of life and social role functioning at 6 months and 12 months post-chemotherapy. Also consistent with other work [27, 28, 29] were findings that fatigue continued to negatively affect physical well-being and functional well-being among breast cancer survivors 6 and 12 months post-chemotherapy.
Of greatest relevance in terms of the aims of the present research were findings suggesting that self-reported cognitive complaints and neuropsychological test performance may be associated with some aspects of quality of life in the year following chemotherapy. Self-reported cognitive complaints have been associated with emotional distress in other studies [9], but this is one of the few studies that documents its potential relationship to a related construct, quality of life. Specifically, these data indicated that self-reported cognitive difficulties were associated with poorer emotional well-being among this sample of breast cancer survivors at 12 months post-chemotherapy.
In addition, although only a small portion of study participants evidenced cognitive abilities that were below expectations over the year following chemotherapy, regression analyses indicated that ongoing difficulties with verbal fluency may be associated with poorer functional well-being 12 months post-chemotherapy. In considering these findings, it is important to recognize that < 20% of study participants’ test scores were considered below expectations (i.e., z < −1.00) on any given test at each time point, suggesting that the frequency of cognitive difficulties was low. However, from a treatment perspective, it could be argued that this finding may be noteworthy nonetheless. Specifically, if these findings are replicated in future studies, it would suggest that providers may have an opportunity to improve survivors’ outcomes by monitoring cognitive functioning over time and considering treatment if indicated.
Despite some potentially thought-provoking findings, the present study has several limitations that deserve mention. In terms of the study sample, although consistent with the methodology of approximately one-half of published studies in this area that were recently reviewed [16], the absence of a control group limits the extent to which these findings can be identified as specific to the population of women cancer survivors status post chemotherapy. In other words, factors unrelated to cancer and/or its treatments may be contributing to subjective cognitive complaints as well as documented neuropsychological test performance difficulties. On the other hand, from a purely pragmatic perspective, if cognitive difficulties are leading to poorer quality of life in cancer survivors, it could be argued that they warrant attention regardless of the etiology.
In addition, findings from the regression analyses would be considered more conclusive if the relationships between neuropsychological tests scores and/or subjective cognitive complaints were significantly associated with a greater percentage of our outcome measures. This highlights the preliminary nature of these findings and the need for follow-up research to determine whether these relationships are replicated with other samples. However, it is also worth noting that our ability to detect cognitive decline and its relationship to functional outcomes may have been hampered by the nature of our sample. First, because this particular sample was relatively well educated, it is possible that their baseline cognitive abilities were higher than average, which could obscure evidence of cognitive decline (i.e., modest cognitive decline could result in performances that remained within normal limits). This issue may be minimized in future studies by including a pre-treatment baseline assessment of cognitive abilities. In addition, analyses indicated that individuals who dropped out after 1 month were evidencing significantly poorer verbal fluency (see footnote), suggesting that we may not have retained individuals who were experiencing greater difficulties after treatment.
The relatively small size of the sample, particularly by the 12 month time point, is another limitation of the study. Substantial efforts were made to retain participants, but this was a difficult task given the nature of patients served in the cancer centers in our community (many come from rural populations far from the facilities where they are treated). Future studies can endeavor to minimize the impact of this issue by reducing burden on participants (e.g., by offering to travel to their homes for data collection). Related to this, a relatively high number of variables were included in the analyses given the small sample size. Efforts were made to lessen the risk of a Type II error by creating composite test scores for the neuropsychological domains and minimizing the number of other predictor variables included in each equation. Additionally, all variables were selected based on a priori hypotheses derived from an understanding of prior research in this area.
An additional statistical concern that occurs with longitudinal studies involving neuropsychological testing lies in our ability to account for practice effects on tests. Efforts were made to minimize the risk of practice effects by utilizing alternate forms where possible (e.g., Rey AVLT, COWAT, Category fluency), but practice effects likely played a role in some of the improvements documented, particularly in WAIS-III scores. Future studies can address concerns regarding practice effects by: (1) minimizing their occurrence through the use of alternate forms, and (2) including a control group that would allow for RCI analyses that can statistically account for these effects.
Given these limitations, findings from this study are considered preliminary. However, when considered in the context of other research documenting women's concerns that cognitive difficulties following treatment are affecting their quality of life [26], they highlight the need for further exploration of possible relationships between cognitive difficulties following cancer treatment and functional outcomes. In addition, future research may focus on understanding factors that contribute to the experience of neuropsychological test deficits and self-reported cognitive complaints among cancer survivors. This important work of identifying risk factors can lead to interventions offered before and/or during the course of treatment that could potentially lessen the impact of cancer and/or its treatment on cognitive functioning, thereby improving quality of life outcomes for cancer survivors.
Acknowledgments
This manuscript was supported with funding from the National Institutes of Health, National Cancer Institute (R03 CA108340; PI: S. Reid-Arndt).
Footnotes
One-way ANOVAs were conducted to determine whether individuals who dropped out of the study at each time point differed from those who remained in terms of demographic factors, medical factors, distress, self-reported cognitive complaints, and neuropsychological test performance.
Participants who withdrew from the study between the 1 month and the 6 month sessions did not differ in terms of demographic factors [all F's < 1.15, p's > 0.29], BDI scores [F(1,41) = 0.65, n.s.], or POMS-SF Fatigue [F(1, 41) = 0.51, n.s.]. Individuals who discontinued the study after the 1 month session were reporting slightly greater cognitive complaints (POMS-SF Confusion) at a level approaching significance [F(1,41) = 3.66, p = 0.06]. There were generally no differences in self-reported quality of life [all F's < 2.60, p's > 0.11], with the exception that individuals discontinuing the study after the 1 month session were reporting poorer Physical Well-being at a level approaching significance [F(1,44) = 3.46, p = 0.07]. Finally, z-scores on the verbal fluency composite neuropsychological measure were significantly lower [F(1,44) = 5.00, p < 0.05] among those who dropped out of the study after the 1 month session [M = −0.99 (0.80)] compared to those who returned for the 6 month session [M = −0.24 (0.81)]. There were no other significant differences in neuropsychological test composite scores among those who dropped out of the study after completing the 1 month session.
Similar analyses were conducted to evaluate for differences between individuals who discontinued between the 6 month and 12 month sessions. One-way ANOVAs revealed no significant differences in terms demographic factors, quality of life at 6 months, or neuropsychological test scores at 6 months (all F's < 1.84, p's > 0.18) between those who discontinued after the 6 month session and those who remained in the study for the 12 month session.
There were no significant correlations between number of chemotherapy cycles and any of the predictor or outcome variables at any of the three time points (Neuropsychological composite score variables: all r's < 0.22, p's > 0.20; Other predictor variables: all r's < 0.28, p's > 0.14; All outcome variables: all r's < 0.27, p's > 0.13).
References
- 1.Ahles TA, Saykin A. Cognitive Effects of Standard-Dose Chemotherapy in Patients with Cancer. Cancer Investigation. 2001;19:812–820. doi: 10.1081/cnv-100107743. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Ahles TA, Ganz PA, Van Dam FS. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. Journal of Clinical Oncology. 2003;22(11):2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 3.Reid-Arndt SA. The Potential For Neuropsychology To Inform Functional Outcomes Research With Breast Cancer Survivors. NeuroRehabilitation. 2006;21(1):51–64. [PubMed] [Google Scholar]
- 4.Schagen SB, van Dam FSAM, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive Deficits after Postoperative Adjuvant Chemotherapy for Breast Carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Brezden CB, Phillips K, Abdolell M, Bunston T, Tannock IF. Cognitive Function in Breast Cancer Patients Receiving Adjuvant Chemotherapy. Journal of Clinical Oncology. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin A, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic Impact of Standard-Dose Systemic Chemotherapy in Long-Term Survivors of Breast Cancer and Lymphoma. Journal of Clinical Oncology. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 7.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz B, Belin TR. Quality of Life in Long-Term, Disease-Free Survivors of Breast Cancer: A Follow-up Study. Journal of the National Cancer Institute. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- 8.Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J. A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function. Cancer. 2005;104(10):2222–2233. doi: 10.1002/cncr.21469. [DOI] [PubMed] [Google Scholar]
- 9.Van Dam FSAM, Schagen SB, Muller MJ, Boogerd W, v.d. Wall E, Fortuyn MED, Rodenhuis S. Impairment of Cognitive Function in Women Receiving Adjuvant Treatment for High-Risk Breast Cancer: High Dose Versus Standard-Dose Chemotherapy. Journal of the National Cancer Institute. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 10.Van Oosterhout AGM, Ganzevles PGJ, Wilmink JT, de Geus BWJ, van Vonderen RGMW, Wijnstra A. Sequelae in Long-Term Survivors of Small Cell Lung Cancer. International Journal of Radiation Oncology. 34:1037–1044. doi: 10.1016/0360-3016(95)02257-0. 1996l. [DOI] [PubMed] [Google Scholar]
- 11.Wieneke MH, Dienst ER. Neuropsychological Assessment of Cognitive Functioning Following Chemotherapy for Breast Cancer. Psycho-Oncology. 1995;41:61–66. [Google Scholar]
- 12.Meyers CA, Byrne KS, Komaki R. Cognitive deficits in patients with small cell lung cancer before and after chemotherapy. Lung Cancer. 1995;12:231–235. doi: 10.1016/0169-5002(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 13.Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A Meta-Analysis of the Neuropsychological Effects of Adjuvant Chemotherapy Treatment in Women Treated for Breast Cancer. The Clinical Neuropsychologist. 2006;20:76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 14.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips K. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: A meta-analysis of the current literature. Brain and Cognition. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. Journal of the International Neuropsychological Society. 2003;9(7):967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- 16.Vardy J, Wefel JS, Ahles T, Tannock IF, Schagen SB. Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Annals of Oncology. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 17.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Seminars in Clinical Neuropsychiatry. 2003;8(4):201–216. [PubMed] [Google Scholar]
- 18.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 19.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5−10 years after chemotherapy. Breast Cancer Research and Treatment. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 20.Saykin AJ. Altered brain activation following systemic chemotherapy for breast cancer: interim analysis from a prospective study. Journal of International Neuropsychological Society. 2006;12:131. [Google Scholar]
- 21.Mehlsen M, Pedersen AD, Jensen AB, Zachariae R. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psycho-Oncology. 2008 doi: 10.1002/pon.1398. Published online in Wiley InterScience ( www.interscience.wiley.com) DOI: 10.1002. [DOI] [PubMed]
- 22.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-Oncology. 2005;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 24.Wefel JS, Lenzi R, Theriault R, et al. ‘Chemobrain’ in breast carcinoma?: a prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 25.Lauver DR, Connolly-Nelson K, Vang P. Stressors and Coping Strategies Among Female Cancer Survivors After Treatments. Cancer Nursing. 2007;30(2):101–111. doi: 10.1097/01.NCC.0000265003.56817.2c. [DOI] [PubMed] [Google Scholar]
- 26.Downie FP, Mar Fan HG, Houédé-Tchen N, Yi Q, Tannock IF. Cognitive Function, Fatigue, and Menopausal Symptoms in Breast Cancer Patients Receiving Adjuvant Chemotherapy: Evaluation with Patient Interview after Formal Assessment. Psycho-Oncology. 2006;15(10):921–930. doi: 10.1002/pon.1035. [DOI] [PubMed] [Google Scholar]
- 27.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 28.Beijer S, Kempen GI, Pijls-Johannesma MC, de Graeff A, Dagnelie PC. Determinants of overall quality of life in preterminal cancer patients. International Journal of Cancer. 2008;123(1):232–235. doi: 10.1002/ijc.23497. [DOI] [PubMed] [Google Scholar]
- 29.Bower JE. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain, Behavior, & Immunity. 2007;21(7):863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanton AL, Revenson TA, Tennen H. Health Psychology : Psychological Adjustment to Chronic Disease. Annual Review of Psychology. 2007;58:565–592. doi: 10.1146/annurev.psych.58.110405.085615. [DOI] [PubMed] [Google Scholar]
- 31.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. Fourth Edition Oxford University Press; New York, New York: 2004. [Google Scholar]
- 32.Baker F, Denniston M, Zabora J, Polland A, Dudley WN. A POMS short form for cancer patients: psychometric and structural evaluation. Psycho-Oncology. 2002;11(4):273–281. doi: 10.1002/pon.564. [DOI] [PubMed] [Google Scholar]
- 33.Farmer JE, Clark MJ, Sherman AK. Rural versus urban social support seeking as a moderating variable in traumatic brain injury outcome. Journal of Head Trauma Rehabilitation. 2003;18(2):116–127. doi: 10.1097/00001199-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Beck AT, Steer RA, Brown GK. Beck Depression Inventory. Second Edition The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 35.Bettencourt BA, Sheldon K. Social roles as mechanism for psychological need satisfaction within social groups. Journal of Personality and Social Psychology. 2001;81(6):1131–1143. [PubMed] [Google Scholar]
- 36.Sheldon K, Bettencourt BA. Psychological Need-Satisfaction and Subjective Well-Being within Social Groups. British Journal of Social Psychology. 2002;41:25–38. doi: 10.1348/014466602165036. [DOI] [PubMed] [Google Scholar]
- 37.Bernhard J, Sullivan M, Hurny C, Coates AS, Rudenstam CM. Clinical relevance of single item quality of life indicators in cancer clinical trials. British Journal of Cancer. 2001;84(9):1156–1165. doi: 10.1054/bjoc.2001.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy – Breast quality of life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 39.Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and Psychological Factors Associated with Early Post-Treatment Functional Outcomes in Breast Cancer Survivors. Journal of Psychosocial Oncology. doi: 10.1080/07347330903183117. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vardy J, Rourke S, Tannock IF. Evaluation of Cognitive Function Associated with Chemotherapy: A Review of Published Studies and Recommendations for Future Research. Journal of Clinical Oncology. 2007;25(17):2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 41.Jacobson NS, Traux P. Clinical significance: A statistical approach to defining the meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 42.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast Cancer in Older Women: Quality of Life and Psychosocial Adjustment in the 15 Months After Diagnosis. Journal of Clinical Oncology. 2003;21(21):4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 43.Wechsler D. WMS-III administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 44.Taylor EM. Psychological appraisal of children with cerebral deficits. Harvard University Press; Cambridge, MA: 1959. [Google Scholar]
- 45.Reitan RM. The relation of the trail making test to organic brain damage. Journal of Consulting Psychology. 1955;16:383–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 46.Golden CJ. Stroop color and word test. Stoelting; Chicago, IL: 1978. [Google Scholar]
- 47.Benton AL, Hamsher KdeS. Multilingual Aphasia Examination. AJA Associates; Iowa City, Iowa: 1989. [Google Scholar]
- 48.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Third Edition Oxford University Press; New York: 2006. [Google Scholar]
- 49.Lineweaver TT, Chelune GJ. Use of the WAIS-III and WMS-III in the Context of Serial Assessments: Interpreting Reliable and Meaningful Change. In: Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, Prifitera A, Ledbetter MF, editors. Clinical Interpretation of the WAIS-III and WMS-III. Elsevier Science; San Diego, California: 2003. [Google Scholar]


