Abstract
Importance of the field
Autosomal dominant (AD) polycystic kidney disease (PKD) is the most common life-threatening hereditary disorder. There is currently no therapy that slows or prevents cyst formation and kidney enlargement in humans. An increasing number of animal studies have advanced our understanding of molecular and cellular targets of PKD.
Areas covered in the review
The purpose of this review is to summarize the molecular and cellular targets involved in cystogenesis and to update on the promising therapies that are being developed and tested based on knowledge of these molecular and cellular targets.
What the reader will gain
Insight into the pathogenesis of PKD and how a better understanding of the pathogenesis of PKD has led to the development of potential therapies to inhibit cyst formation and/or growth and improve kidney function.
Take home message
The results of animal studies in PKD have led to the development of clinical trials testing potential new therapies to reduce cyst formation and/or growth. A vasopressin V2 receptor antagonist, mTOR inhibitors, blockade of the renin–angiotensin system and statins that reduce cyst formation and improve renal function in animal models of PKD are being tested in interventional studies in humans.
Keywords: cAMP, MAP kinase, mTOR, polycystic kidney, renin-angiotensin-aldosterone, targets, therapy, vasopressin V2 receptor
1. PKD: a problematic genetic condition
Polycystic kidney disease (PKD) is a genetic disorder characterized by cyst formation and progressive enlargement of both kidneys, leading to end-stage renal disease (ESRD) [1,2]. The autosomal dominant form of PKD is by far the most common type of PKD, affecting approximately one in every 400–1000 live births. Autosomal dominant PKD (ADPKD) is more common than Huntington’s disease, hemophilia, sickle cell disease, Down’s syndrome, cystic fibrosis, and myotonic dystrophy combined. Yet, there is no cure for ADPKD and no therapy that is clinically proven to slow cyst and kidney enlargement.
2. Genes as targets of therapy in PKD
While gene therapy has shown great promise in diseases with single gene mutations like cystic fibrosis, it has not been favored in ADPKD largely because of the high genetic heterogeneity of ADPKD. The numerous ADPKD mutations identified on PKD1 and PKD2 genes are also highly variable. For instance, on the ADPKD database of the Mayo Clinic (http://pkdb.mayo.edu), as many as 333 truncating PKD1 mutations were identified on chromosome 16 p 13.3 in 417 families with 869 different variants, while 95 PKD2-truncating mutations were identified on chromosome 4q21 in 178 families with 128 variants [3,4].
PKD could in theory be treated by preventing the earliest initiating events. The initial and critical molecular event underlying cyst formation may be explained by the ‘double hit hypothesis’. In this theory, the first hit is an inherited mutation in the germ line (mutant allele) while the second hit is a somatic loss of the wild-type (normal) allele, leaving a single copy of the mutant allele. DNA analysis of cystic epithelia isolated from single cysts has provided support for the ‘double hit hypothesis’. Clonality of individual cysts and loss of heterozygosity (LOH) of microsatellites from within or close to the PKD1 gene has been demonstrated in a proportion of the cysts. Kidney and liver cysts have also demonstrated an intragenic somatic mutation and loss of heterozygosity [5]. The difficulty of targeting second-hit mutations in PKD is that somatic mutations are highly variable. Furthermore, it is also known that cysts develop at a more rapid rate when cilia are lost in newborn kidneys in which kidney development is not yet completed. Inactivation of ciliogenic genes (Kif3a) in newborn mice resulted in rapid cyst development, while inactivation of ciliogenic genes at postnatal day 10 or later resulted in a much slower rate of cyst formation [6]. These observations indicate that loss of cilia may also be implicated in the initiation of cystogenesis. Genetic modification resulting in imbalance in the expression of polycystin-1 and -2, the two functional proteins encoded by PKD1 and PKD2 respectively, may promote rather than prevent cyst development. Jiang and colleagues showed that progressive reduction of the PKD1 protein to levels that are not completely undetectable can induce cyst formation in two PKD1 animal models [7]. Further studies in transgenic mice overexpressing the PKD1 and PKD2 transgenes in the kidneys revealed that those mice developed renal cystic disease comparable to the human ADPKD phenotype [8,9]. It was concluded that partial inactivation of the genes may also initiate cystogenesis. This raised the question of how much inactivation is necessary for initiation or suppression of cyst formation. Thus, the topic of gene replacement in PKD is very complex.
3. Polycystins as targets of therapy in PKD
Polycystins are the protein products of the PKD1 and PKD2 genes, which respectively encode polycystin-1 (PC1, 460 kDa) and polycystin-2 (PC2, 110 kDa). PC1, a protein with a large extracellular domain, 11 transmembrane domains and a short intracellular C-terminal tail, functions as a mechanosensor. PC2, a less complex protein with a short N-terminal cytoplasmic region, six transmembrane domains, and a short C-terminal portion, has an important function as a cation-permeable transient receptor potential ion channel in kidney epithelial cells. Polycystins have a heterogeneous distribution with localization to the primary cilia expressed in epithelial cells of the kidney, liver, pancreas and breast, the smooth muscle as well as endothelial cells in the vasculature and astrocytes in the brain. Polycystins also have a non-ciliary localization, with PC1 detected at apical membranes, adherent and desmosomal junctions [10–13] and PC2 found in the cytoplasm as well as the apical and basolateral membranes of the kidney. PC1 and PC2 interact with each other through their C-terminal cytoplasmic domains [14,15].
Both PC1 and PC2 appear to play key roles in kidney development. PC1 expression is high in developing tissues and low in mature tissues [10]. Geng and co-workers showed that PC1 expression peaks at embryonic day 15 and falls thereafter to remain constantly low throughout adulthood [11]. The primary cilium appears to play a major role in PC1- and PC2-mediated mechanosensation and calcium signaling [16]. The cilium projects into the lumen in tubular epithelial cells and acts as a sensor. The PC1–PC2 complex translates mechanical or chemical stimulations into calcium influx through PC2 channels, allowing for release of calcium from intracellular stores.
Recently, investigators have targeted (PC1/PC2)-mediated calcium influx. Triptolide (Tripterygium wilfordii), the active diterpene in a traditional Chinese medicine isolated from the ‘Thunder God Vine’, was recently shown to activate PC2-mediated calcium release in the cilia, to cause cell growth arrest in murine pkd1-null cells, and to inhibit cyst formation in Pkd1−/− embryonic mice with ADPKD [17]. In another study, triptolide reduced cyst formation in a neonatal kidney-specific Pkd1(flox/-);Ksp-Cre mouse model of ADPKD [18]. A randomized clinical trial of triptolide in patients with ADPKD has been initiated at the Nanjing School of Medicine in China (see clinicaltrials.gov). Estimated enrollment is 150 patients and estimated completion date is June 2011. Primary outcome measures are renal volume on MRI scan and estimated glomerular filtration rate (eGFR). The secondary outcome measure is the development of ESRD. Triptolide has been shown to have antiproliferative or proapoptotic effects and has been used to treat inflammatory and autoimmune diseases [19]. In addition, other regulators of the PC2-mediated calcium influx are under investigation, with Nek8, a serine/threonine kinase that is mutated in the juvenile cystic kidney mouse, leading the way [20–24]. Thus, pharmacologic agents that induce PC2-mediated calcium release may potentially halt or delay cyst formation.
PC2 regulates the cell cycle through direct interaction with Id2, a member of the helix-loop-helix protein family that regulates cell proliferation [25]. The PC2–Id2 interaction is mediated by PC1-dependent phosphorylation of PC2 [25]. Inhibition of Id2 expression using RNA interference corrects the hyperproliferative response of PC1 mutant cells [25]. Thus, the effect of Id2 inhibitors, for example rosiglitazone, on cyst formation merits further study.
4. Molecular pathways in PKD
Researchers have focused the target of therapy towards the molecular pathogenesis of cyst formation and cyst progression. Mechanisms involved in cystogenesis include a secretory phenotype, increased proliferation and apoptosis, as well as loss of cellular differentiation and polarity. Some strategies have targeted cAMP-mediated processes of transepithelial fluid secretion and epithelial cell proliferation, while other strategies have focused on EGF-mediated cell proliferation as well as mammalian target of rapamycin (mTOR)-mediated processes of cell proliferation and apoptotic cell death. The current review focuses on therapeutic approaches to interfere with the molecular pathways of cystogenesis (Table 1 and Figure 1).
Table 1.
Novel targets for the treatment of polycystic kidney disease (PKD).
| Target | Agent | Decreases PKD in animals | Human studies ongoing | Ref. |
|---|---|---|---|---|
| PC2-mediated calcium release | ||||
| Triptolide | Yes | Yes | [17,18] | |
| cAMP | ||||
| c-Src | SKI-606 | Yes | No | [54] |
| SSTR | Somatostatin | Yes | Yes | [32,33] |
| MEK | PD98059 | Yes | No | [34] |
| Vasopressin V2 receptor | Tolvaptan | Yes | Yes | [39,40,42] |
| EGFR tyrosine kinase | [45,46] | |||
| EGFR | EKI-785 | Yes | No | [49] |
| c-Src | SKI-606 | Yes | No | [54] |
| mTOR | ||||
| Sirolimus | Yes | Yes | [61] | |
| Everolimus | Yes | Yes | [62,65] | |
| CDK | ||||
| Roscovitine | Yes | No | [78] | |
| Pro-inflammatory cytokines | ||||
| TNF-α | Etanercept | Yes | No | [82] |
| RAAS | ||||
| Angiotensin-converting enzyme (ACE) | ACE inhibitors: enalapril, cilazapril | Yes | Yes | [87–91] |
| Angiotensin receptor (ATR) | ARB: losartan | Yes | Yes | [88] |
| HMG-CoA reductase | ||||
| Inhibitor: lovastatin | Yes | Yes | [89,98] | |
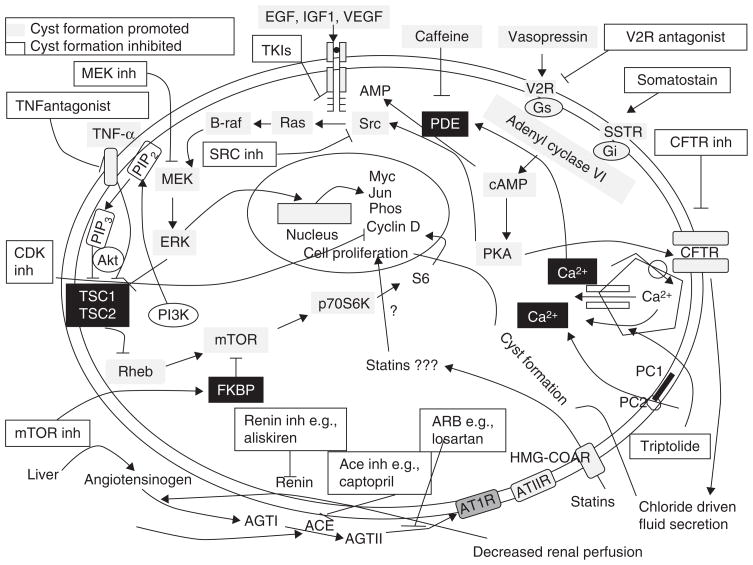
Figure 1. Schematic representation of the various molecular pathways up- or downregulated in polycystic kidney disease.
Potential targets for inhibition are depicted in light gray boxes. Potential inhibitors are depicted in clear boxes. The cAMP pathway promotes cell proliferation and fluid secretion in PKD. cAMP leads to fluid secretion via the CFTR. cAMP results in activation of the ERK-MAPK pathway leading to cell proliferation. cAMP accumulation is promoted by mechanisms of dysregulation of PC1 or PC2, as well as inhibition of Ca2+-dependent phosphodiesterase (PDE). Caffeine inhibits PDE, which normally degrades cAMP. Thus, caffeine results in an increase in cAMP. cAMP signaling also requires PKA, Src and Ras. Src is known to activate Ras. Activation of tyrosine kinase receptors also contributes to the stimulation of MAPK-ERK signaling with consequent cell proliferation. Potential therapeutic targets are the vasopressin V2 receptor, tyrosine kinases, MEK and c-Src. The PI3K-Akt pathway plays a major role in mTOR signaling (see Figure 2); mTOR inhibitors bind to FKBP, which subsequently inhibits mTOR. There is evidence for activation of the mTOR pathway in ADPKD. The RAAS (see Figure 3) is activated by a drop in renal perfusion that stimulates the JGA to make renin. ACE inhibitors block the conversion of angiotensin I to angiotensin II. Renin inhibitors directly block renin production by the JGA. Angiotensin receptor blockers block the ATIIR. There is evidence for activation of the RAAS in ADPKD patients.
ACE: Angiotensin-converting enzyme; AGT1: Angiotensin 1; ARB: Angiotensin receptor blocker; ATIIR: Angiotensin II receptor; Ca2+: Intracellular calcium; cAMP: Cyclic adenosine monophosphate; CDK: Cyclin-dependent kinase; CFTR: Cystic fibrosis transmembrane conductance regulator; EGF: Epidermal growth factor; ERK: Extracellular signal-regulated kinase; FKBP: FK506 binding protein; Gi,Gs: G protein; HMG-CoAR: 3-hydroxy-3-methyl-glutaryl-CoA reductase; IGF: Insulin-like growth factor; JGA: Juxtaglomerular apparatus; MAPK: Mitogen–activated protein kinase; MEK: Maperk kinase; mTOR: Mammalian target of rapamycin; PI3K: Phosphoinositide 3-kinase; PC1: Polycystin1; PC2: Polycystin2; PDE: Phosphodiesterase; PKA: Protein kinase A; Rheb: Ras homolog enriched in brain; Src: Sarcoma; SSTR: Somatostatin receptor; TKI: Tyrosine kinase inhibitor; TNF: Tumor necrosis factor; V2R: Vasopressin v2 receptor; VEGF: Vascular endothelial growth factor.
5. cAMP-targeted interventions
There is much evidence demonstrating a major role for cAMP in cyst fluid accumulation [26–28]. A number of agonists such as arginine vasopressin (AVP), prostaglandin E2 (PGE2), epinephrine, norepinephrine, adenosine and caffeine can result in cAMP accumulation. One of the agonists, AVP, has emerged as a powerful modulator of cystogenesis in recent years. Upon stimulation of cAMP, there is movement of chloride through the cystic fibrosis transmembrane conductance regulator (CFTR) and purinergic channels in the apical membrane of the renal tubules. Sodium enters the cyst cavity passively and water follows chloride and sodium osmotically. Blockade of the apical CFTR reduces cyst formation and renal failure in a mouse model [29]. In another study, it was shown that the basolateral KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease [30]. These studies demonstrate inhibition of chloride secretion from both the apical and basolateral sides of the tubule in PKD [31].
Somatostatin is thought to work by inhibiting cAMP accumulation (Table 1). The somatostatin analogue, octreotide, was shown to be effective in slowing progression in liver and kidney cystic disease in a rat model of PKD. Three of the five somatostatin receptor subtypes that bind octreotide (i.e., SSTR2, SSTR3, and SSTR5) were expressed in the kidneys of the PKD rats and octreotide significantly lowered cAMP [32]. In a pilot study of 13 ADPKD patients, octreotide was shown to reduce kidney volume [33]. A long-term 3-year follow-up study, with estimated enrollment of 66 patients, looking at the effect of long-acting somatostatin on nephropathy progression due to ADPKD has been initiated.
cAMP also plays a role in increased proliferation of cyst epithelial cells. Despite the evidence suggesting that cAMP is antimitogenic in normal cells, Yamaguchi and colleagues discovered that forskolin and cAMP increased cell proliferation in polycystic kidney cells to a greater degree than the mitogen, EGF [34]. These investigators also found that cAMP stimulates B-Raf, mitogen-associated/extracellular-regulated kinase (MEK) and extracellular signal-regulated kinase (ERK) in PKD cells but not normal cells (Figure 1), and that this ‘phenotypic switch’ in the cellular response to cAMP is a function of low intracellular calcium [35]. The response of B-Raf to cAMP appears to be the reason for the phenotypic switch [35]. In normal cells, B-Raf is suppressed by AKT in a calcium-dependent manner, but in ADPKD and calcium-restricted cells, AKT activity is decreased, allowing for activation of B-Raf by cAMP [35].
MEK appears to be crucial in the cAMP and EGFR signaling pathways. The efficacy of MEK inhibitors has been examined in PKD and the results have been somewhat controversial. One of the MEK inhibitors, PD-98059, has been shown to effectively block EGF and cAMP proliferative effects in ADPKD epithelial cells in vitro (Figure 1) [34]. Furthermore, a recent paper by Omori and colleagues showed that the ERK inhibitor, PD-184352, slows cyst growth in the pcy mouse model of PKD [36]. In contrast, Shibazaki and coworkers reported conflicting evidence that inhibition of MEK in a Pkd1 conditional knockout model of PKD fails to inhibit disease progression [37]. Further studies looking at MEK inhibitors in other rat and mouse models of PKD are needed.
Conditionally immortalized renal epithelial cells prepared from ADPKD patients with known germ-line mutations in the PKD1 gene have an increased sensitivity to IGF-1 and cyclic AMP and require PI3K and ERK for enhanced growth [38]. Inhibition of Ras or Raf abolished the stimulated cell proliferation [38]. This study suggests that haploinsufficiency of polycystin-1 lowers the activation threshold of the Ras/Raf signaling system, leading to growth factor-induced hyperproliferation.
Gattone, Torres and colleagues took the knowledge of cAMP pathways a step further by demonstrating the upregulation of vasopressin and the inhibition of cystogenesis by V2 receptor antagonists in three different animal models of PKD [40,41]. The vasopressin V2 receptor antagonist, OPC-31260, reduced renal cAMP and inhibited kidney cyst development in the autosomal recessive (AR) PKD (PCK rat) and adolescent nephronophthisis (pcy mouse) models of ARPKD (Table 1) [41]. The vasopressin V2 receptor antagonist OPC-31260 also reduced renal cyclic AMP levels, downregulated the expression of V2 receptor–and cAMP-dependent genes (V2 receptor and aquaporin 2), inhibited renal cystogenesis and kidney enlargement, and protected against the loss of renal function in the PKD2WS25 mouse model of ADPKD [40]. In a recent study, OPC31260 had a similar effect to OPC-41061, another vasopressin V2 receptor antagonist, to decrease Ras-GTP and phosphorylated ERK levels and 95-kD/68-kD B-Raf ratios, in the PCK rat model of ARPKD and protect against the development of PKD [42].
It is interesting to note that in the PCK rat and PKDWS25 mouse models of PKD, cysts develop primarily in the distal tubule [43]. The presence of vasopressin receptors has been well characterized in the distal tubule. It is believed that the inhibition of vasopressin action in the collecting duct may be sufficient to reduce cyst formation. Interestingly, there have been no reported studies looking at the effect of vasopressin antagonists in the Han:SPRD rat model of ADPKD, which develops cysts exclusively in the proximal tubule. However, in PCK rats, increased water intake for 10 weeks reduced urinary AVP excretion, reduced urine osmolality, reduced renal expression of AVP V2 receptors, reduced the kidney/body weight ratio and improved renal function [44]. This study suggested that limiting serum AVP levels by increased water intake may be beneficial to some patients with PKD. It is also important to note that antagonists of the V2 receptor, OPC31260 and OPC-41061 had a significant effect in reducing cyst formation in three animal models of PKD. These results sparked the development of a clinical trial in humans with ADPKD using the orally active vasopressin V2 receptor antagonist, tolvaptan, which has a high affinity for the V2 receptor.
The Tolvaptan Efficacy and Safety in Management of PKD and Outcomes (TEMPO) programs are currently active [45,46]. A Phase II study to determine the response to increasing doses of tolvaptan (15, 30, 60, and 120 mg) in patients with ADPKD and normal kidney function has been completed. Pharmacokinetic profiles of tolvaptan were found to be similar to those of healthy control subjects. Urine output, frequency of nocturia, urine osmolality, and serum sodium changed in a dose-dependent manner [47]. In further studies, the minimum dose of tolvaptan required to decrease urinary osmolality but prevent hypernatremia and frequent nocturia was determined in 27 patients with ADPKD. Tolvaptan doses of 15 mg twice daily, 30 mg daily, 30 mg twice daily, and 30 mg in the morning/15 mg in the evening were well tolerated. After 5 days of tolvaptan therapy, patients were in fluid balance and had urine outputs of 6 l/day. The frequency of nocturia was increased by 0.5 voids per night. Urinary osmolality was suppressed for 24 h by tolvaptan [48].
A Phase III multicenter, double-blind, placebo-controlled, parallel-arm, 3-year trial to determine long-term safety and efficacy of oral tolvaptan tablets in adult subjects with ADPKD has been initiated. Enrollment of 2000 patients has been completed and the study completion date is estimated to be January 2011. The primary end point is to evaluate the long-term efficacy of tolvaptan in ADPKD through the rate of renal volume change (%) on MRI scan in tolvaptan-treated compared with placebo-treated subjects. Secondary outcome measures include the evaluation of the long-term efficacy of tolvaptan in ADPKD through a composite of ADPKD progression clinical markers (hypertension, kidney pain, albuminuria and kidney function). The tolvaptan Phase III study is the largest drug efficacy study in PKD patients to date. Tolvaptan is an agent of great promise in ADPKD because i) it is a powerful vasopressin V2 receptor antagonist that has been used in animal studies; ii) it has few side effects in Phase I and II studies [45,46,48] – a risk when using tolvaptan is dehydration, which can be prevented by patients maintaining adequate hydration; and iii) animal studies are very promising [40,42].
6. EGFR-targeted interventions
EGF plays an important role in cyst epithelial cell proliferation and cyst expansion. EGFR inhibition [49] reduces cyst formation in different animal models of PKD [49–53]. Specifically, the novel EGFR tyrosine kinase inhibitor, EKI-785, reduces cyst formation and mortality in the BPK mouse model of ARPKD [49]. In addition to the role of Src in the cAMP-mediated proliferation of cystic renal epithelial cells, there is strong evidence to suggest that the antiproliferative effect on human ADPKD cells that follows Src inhibition is EGFR-mediated. In the BPK murine model and the orthologous PCK rat model of ARPKD, greater Src activity was found to correlate with disease progression. Inhibition of Src activity with the pharmacologic inhibitor SKI-606 resulted in amelioration of renal cyst formation and biliary ductal abnormalities in both models [54]. SKI-606 (also known as bosutinib) is in clinical trials for breast cancer, advanced malignant tumors, and leukemia. Studies of SKI-606 have not yet been initiated in patients with ADPKD. Thus Src and the EGFR represent therapeutic targets in PKD.
7. mTOR signaling pathway-targeted interventions
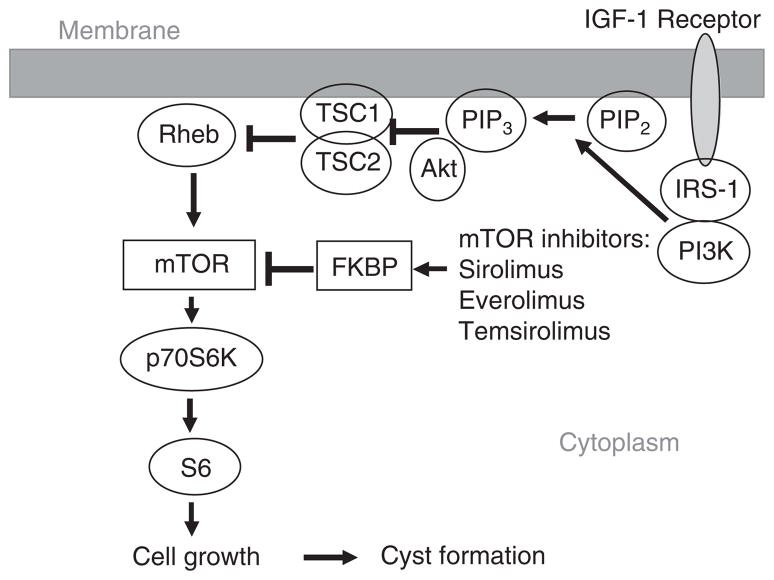
Dysregulated mTOR and AKT signaling may contribute to the pathophysiology of PKD [55,56]. The mTOR pathway involves five major players: IGF-1, AKT, tuberous sclerosis complex 1 and 2 (TSC1/2), mTOR, and p70S6K (Figure 2). IGF-1 activates the mTOR signaling pathway via stimulation of PI3K and AKT kinase. The tumor suppressor proteins, TSC1 and TSC2, link the PI3K and mTOR pathways. The TSC1 (hamartin) and TSC2 (tuberin) complex is required for mTOR signaling [57]. A mutation of either TSC1 or TSC2 results in the disease tuberous sclerosis complex (TSC). mTOR forms a complex with regulatory-associated protein of TOR (raptor) or rapamycin-insensitive companion of TOR (rictor) [58]. The mTOR–raptor complex, also known as mTORC1, promotes cell growth and is sensitive to inhibition by mTOR inhibitors. The mechanism of cell growth by mTORC1 involves phosphorylation of p70S6K [59]. mTOR inhibitors like sirolimus (rapamycin), which is a FDA-approved immunosuppressive agent, and everolimus (certican) and temsirolimus (torisel), which are FDA-approved for the treatment of advanced renal cell cancer, bind FK506-binding protein (FKBP). The binding of mTOR inhibitors to FKBP destabilizes the association between mTOR and raptor, preventing the downstream phosphorylation of p70S6 kinase [60].
Figure 2. The PI3K-AKT pathway plays a major role in mTOR signaling.
PI3K converts the lipid PIP2 into PIP3 (Phosphatidylinositol (3,4,5)-trisphosphate), which localizes AKT to the membrane. The tuberous sclerosis complex 1 (TSC1; hamartin) and TSC2 (tuberin) complex is inactivated by AKT-dependent phosphorylation. Inactivation of TSC2 results in activation of mTOR via the Ras-related small GTPase (Rheb). mTOR phosphorylates p70S6K1, resulting in cell proliferation. mTOR inhibitors bind to FKBP (FK506 binding protein), which subsequently inhibits mTOR. There is evidence for activation of the mTOR pathway in ADPKD.
Sirolimus (rapamycin) is a macrolide that was first discovered as a product of the bacterium Streptomyces hygroscopicus in a soil sample from Easter Island – an island also known as ‘Rapa Nui’, hence the trade name Rapamune. Sirolimus was originally developed as an antifungal agent. However, this was abandoned when it was discovered that it had potent immunosuppressive and antiproliferative properties. The drug is now FDA-approved for the prevention of organ transplantation rejection.
Everolimus and temsirolimus are FDA-approved for the treatment of renal cell cancer. Studies in rat and mouse models of PKD demonstrate that sirolimus or everolimus results in a decrease in renal cysts and improvement in kidney function [61–64]. As a result of the therapeutic effect of mTOR inhibition in animal studies of PKD, human studies have been initiated. Five interventional studies investigating the effect of mTOR inhibitors on PKD in humans are underway (Table 2). In a retrospective study of liver volumes in kidney transplant patients that received sirolimus, it was determined that sirolimus decreased polycystic liver volume and there was a trend toward a greater reduction in native kidney volume in the sirolimus group compared with the non-sirolimus group [65].
Table 2.
Human studies in autosomal dominant polycystic kidney disease.
| Drug | Location | Type of study | Primary end point |
|---|---|---|---|
| Octreotide | Mario Negri Institute, Italy | Randomized, single-blind, placebo-controlled | Kidney volume on MRI |
| Octreotide | Mayo Clinic, Novartis | Randomized, double-blind, placebo-controlled, crossover | Liver volume on MRI |
| Lanreotide | Radboud University, The Netherlands | Randomized, placebo-controlled | Liver volume on CT |
| Tolvaptan | Otsuka Pharm, Multicenter, USA | Open-label | Long-term safety |
| Tolvaptan | Otsuka Pharm, Multicenter, International | Double-blind, placebo-controlled | Kidney volume on MRI |
| Everolimus | Novartis Pharm, Germany | Randomized, placebo-controlled | Kidney volume on MRI |
| Rapamycin | University of Colorado, USA | Pilot study of rapamycin vs non-rapamycin immunosuppression in kidney transplant patients with ADPKD | Kidney and liver volume on MRI |
| Rapamycin | Mario Negri Institute, Bergamo, Italy | Randomized, placebo-controlled | Kidney volume on CT scan |
| Rapamycin | University Hospital, Zurich, Switzerland | Randomized, placebo-controlled | Kidney volume on MRI |
| Rapamycin | Cleveland Clinic, Cleveland, USA | Randomized, placebo-controlled | Iothalamate GFR |
| Lisinopril/termistan vs lisinopril/placebo and low BP vs standard BP | Multicenter, USA | Randomized, double-blind, placebo-controlled, 2 × 2 factorial assignment | Kidney volume on MRI |
| Lisinopril/termistan vs lisinopril/placebo | Multicenter, USA | Randomized, double-blind, placebo-controlled | Time to 50% decrease in eGFR, ESRD, or death |
| Pravastatin | University of Colorado, USA | Randomized, double-blind, placebo-controlled | Kidney volume, LVMI, urine albumin, endothelial-dependent vasodilation |
BP: Blood pressure; eGFR: Estimated glomerular filtration rate; ESRD: End-state renal disease; LVMI: Left ventricular myocardial infarction.
In animals and humans, mutations in the TSC gene results in kidney tumors. As the TSC1 and TSC2 protein complex is directly upstream of mTOR, it was possible to determine whether mTOR inhibition has an effect on the growth of kidney tumors. In fact, the rapamycin analogue, CCI-779, reduces kidney tumors in TSC2+/− mice [66]. In a 24-month, nonrandomized, open-label trial in patients with tuberous sclerosis and angiomyolipomas in the kidney, it was determined that angiomyolipoma volume partially regressed with sirolimus treatment but tended to increase in volume when sirolimus was stopped [67]. More recently, in a randomized clinical trial in ADPKD patients, sirolimus, at a dose of 1–2 mg/day, did not induce proteinuria, infection or renal dysfunction at 6 months of therapy [68]. It was concluded that sirolimus was safe and treatment adherence excellent in ADPKD [68].
There is evidence for IGF-1 signaling in animal models of PKD [69–72]. Hyperproliferation of PKD1 cystic cells is induced by IGF-1 activation of the Ras/Raf signaling system [38]. In hepatic cyst epithelium from patients with ADPKD, there is expression of the IGF-1 receptor (IGF-1R) [73]. Hepatic cyst-derived epithelial cells proliferated when exposed to IGF-1 and 17beta-estradiol. IGF-1R antagonists inhibited the proliferative effect of beta-estradiol and IGF-1. These findings may explain the effect of estrogens in accelerating the progression of hepatic cyst disease. The IGF-1R or estrogen may be novel therapeutic targets in ADPKD.
AKT is one of the major players in the mTOR signaling pathway. Phospho-AKT (p-AKT) was increased in cystic mouse kidneys compared to wild-type kidneys [74]. In 16-week-old Han:SPRD rat kidneys, constitutive expression of AKT-1, -2, and -3 mRNA was seen in both wild-type and PKD kidneys [63]; however, on immunoblot and ELISA, there was increased p-AKT (Ser473) in PKD kidneys compared with controls. p70S6K (Thr389) and total S6K were increased in 12-week-old Han:SPRD rat kidneys with PKD and inhibited with sirolimus treatment [61]. Phospho-mTOR and p70S6K are induced in cyst-lining epithelial cells in cysts from mouse and human kidneys [62].
In summary, the IGF-1, PI3K, AKT, TSC, mTOR pathway represents an important target for drug discovery in PKD. Sirolimus has emerged as a promising therapy for ADPKD in view of i) its efficacy in rat and mouse models of PKD [61–64]; ii) its potent anti-proliferative effect, which targets proliferation of tubular epithelial cells lining cysts that contributes to cyst formation; and iii) its favorable side-effect profile as determined in initial human studies in ADPKD [68].
8. Cyclin-dependent kinase-targeted interventions
Polycystin-1 and -2 proteins are localized in the primary cilium. Disruption of cilia proteins has been associated with dysregulated cell cycle progression [75,76]. Polycystin–1 has been shown to regulate the cell cycle by inhibiting CDK2 activity through upregulation of the CDK inhibitor p21waf1, arresting cells in G0/G1 phase and controlling terminal differentiation of tubular epithelial cells [77]. Therapeutic targeting of the dysregulated cell cycle with the CDK inhibitor roscovitine was tested in mouse models of PKD. Bukanov and colleagues [78] showed in jck and cpk mouse models of PKD that roscovitine effectively inhibits cystogenesis through cell cycle arrest and inhibition of apoptosis. In addition, CDK inhibition resulted in down-regulation of cAMP levels, suggesting that CDK inhibition targets the most proximal step in cystogenesis. Roscovitine (Seliciclib, CYC202, Cyclacel Pharmaceuticals, Inc.) has been shown to be effective in many in vitro and in vivo tumor models; it is currently in Phase II clinical trials in cancer patients [79,80]. The polycystic kidney may be likened to a slow-growing tumor; thus PKD may require life-long therapy. Life-long therapy with anticancer drugs like roscovitine may present side effects like bone marrow suppression [81]. However, these drugs may be able to be used in lower doses than are required for cancer therapy. The reported dose of roscovitine used to obtain a therapeutic effect in preclinical renal glomerular disease models is 2.8–120 mg/kg daily, compared with 50–150 mg/kg daily in PKD models and 100–500 mg/kg, three times daily in tumors [80].
9. TNF-α-targeted interventions
Interstitial inflammation may be important in the development of ESRD in ADPKD. Recently, Li and co-workers [82] discovered that TNF-α, a protein previously shown to have a role in inflammation, may also have a role in the formation of cysts in ADPKD [82]. It was found that TNF-α disrupted the localization of polycystin-2 to the plasma membrane and primary cilia through a scaffold protein, FIP2, which was induced by TNF-α. Treating mouse embryonic kidney organ cultures with TNF-α resulted in formation of cysts, which was exacerbated in the Pkd2+/− kidneys. TNF-α also stimulated cyst formation in vivo in Pkd2+/− mice. The TNF-α inhibitor etanercept prevented cyst formation in Pkd2+/− mice. It was concluded that there was activation of a novel pathway connecting TNF-α signaling, polycystins, and cystogenesis [82].
10. Renin angiotensin aldosterone system-targeted interventions
The RAAS is described in Figure 3. Human and experimental data provide strong evidence that abnormal proliferation in tubular epithelial cells plays a crucial role in cyst development and/or growth in PKD [83]. ACE inhibitors can directly reduce proliferation of renal epithelial cells in vitro and in vivo [84]. Infusion of angiotensin-II (AT-II) increases TNF-α expression and synthesis of pro-inflammatory cytokines in the kidney that is blocked by ACE inhibitors. It has been suggested that AT-II plays a pivotal role in promoting infiltration of macrophages/monocytes, cellular proliferation and apoptosis in the kidney [85]. ACE inhibitors suppress synthesis of TNF-α and IL-1β by human PBMC [86]. Thus blockade of the RAAS has antiproliferative and anti-inflammatory effects and represents a therapeutic avenue in PKD.
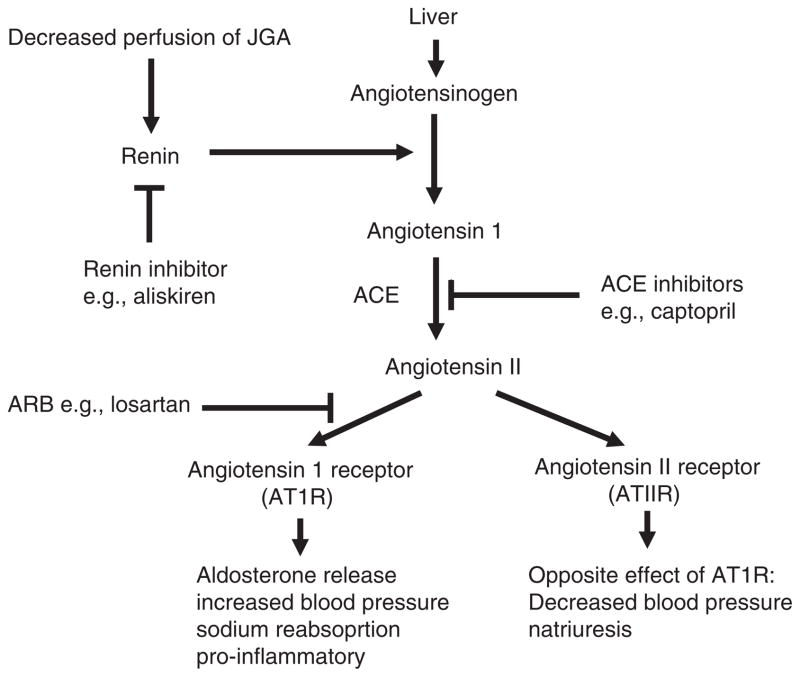
Figure 3. The renin–angiotensin–aldosterone system (RAAS).
The RAAS is activated by a drop in renal perfusion that stimulates the JGA to make renin. ACE inhibitors block the conversion of angiotensin 1 to angiotensin II. Renin inhibitors directly block renin production by the JGA. Angiotensin receptor blockers block the ATIIR. There is evidence for activation of the RAAS in ADPKD patients. Potential therapeutic targets in PKD are ACE, the angiotensin receptor and renin.
ACE: Angiotensin-converting enzyme; ARB: Angiotensin receptor blocker; JGA: Juxtaglomerular apparatus.
The renin–angiotensin system has been implicated in the functional and structural changes in ADPKD [87]. In 10-week-old Han:SPRD rats, both enalapril and hydralazine lowered blood pressure, but enalapril was superior in preserving renal function [87]. In other studies, enalapril or cilazapril decreased blood pressure, cyst volume and renal failure in male Han: SPRD rats [88,89]. In these studies, the effect of ACE inhibitors on epithelial cell proliferation and tubulointerstitial inflammation was not detailed. Numerous animal studies have shown that ACE inhibitors decrease cyst formation and improve renal function in animal models of PKD [89–92]. A study has also shown that the ARB losartan decreases cyst formation and improves kidney function in the Han: SPRD rat model of PKD [88].
Clinical data support evidence for activation of the RAAS in normotensive as well as hypertensive ADPKD patients. The RAAS contributes to hypertension in ADPKD, but may also independently accelerate renal cyst growth. Chapman and colleagues [93] found that plasma renin activity and aldosterone were increased in hypertensive ADPKD patients with normal renal function compared with well-matched essential hypertensives.
In the Modification of Diet in Renal Disease (MDRD) study in 222 patients with ADPKD, ACE inhibitor therapy or BP control did not reduce the rate of decline of kidney function [94]. In another study in 64 patients with ADPKD and chronic renal insufficiency, ramipril demonstrated no reduction of the rate of doubling of serum creatinine compared with placebo [95]. In a randomized trial in 72 patients with ADPKD and hypertension, ACE inhibitors reduced left ventricular hypertrophy compared to calcium channel blockers. However, ACE inhibitors were no better than calcium channel blockers in preserving kidney function [96]. These studies of ACE inhibitors in ADPKD tested small numbers of patients for short periods of time. In view of the impressive activation of the renin–angiotensin system in ADPKD, a larger study was undertaken. The HALT-PKD study is a prospective, NIH-sponsored, randomized, double-blind, placebo-controlled, multicenter interventional study sponsored to test the hypothesis that intensive blockade of the RAAS with combination of ACEIs and ARBs will delay the progression of renal disease independent of BP control. In addition, the HALT-PKD study will test the hypothesis that aggressive versus standard blood pressure control will be more effective in slowing PKD progression. Patients will be randomized to an ACE inhibitor, lisinopril, plus placebo versus lisinopril plus an angiotensin receptor blocker, telmisartan. The HALT-PKD study involves seven clinical centers and plans to recruit > 1000 ADPKD subjects.
Aliskiren (Tekturna, Novartis) is the first in a class of drugs known as direct renin inhibitors (Figures 1 and 3). Aliskiren is licensed for the treatment of hypertension. In a case report, a 47-year-old female patient with ADPKD, edema, hyperreninemia, hyperaldosteronism and hypertension was treated with aliskiren 300 mg/day [97]. The patient had an excellent lowering of blood pressure with reduction of edema and hyperaldosteronism. Direct renin inhibition in ADPKD merits further study.
11. 3 HMG-CoA reductase
Statins e.g., atorvastatin and simvastatin are drugs that lower cholesterol by inhibiting the enzyme HMG-CoA reductase, which is the rate-limiting enzyme of the mevalonate pathway of cholesterol synthesis. Statins are widely used to lower cholesterol in humans. Statins have effects independent of cholesterol lowering that make them attractive for use in PKD (e.g., anti-inflammatory, antiproliferative and protecting against endothelial dysfunction). Lovastatin decreases renal cyst formation and improves renal function in a rat model of ADPKD [89,98]. The precise mechanism of the protective effect of statins in animal models of ADPKD is not known, but may be related to an increase in renal blood flow and an anti-inflammatory effect or antiproliferative effect [89]. In a short-term 4-week study in 10 ADPKD patients, it was demonstrated that simvastatin improved both GFR and effective renal plasma flow [99]. In a study of 16 ADPKD patients with normal kidney function, it was determined that simvastatin 40 mg treatment for 6 months improved endothelial dysfunction as measured by high-resolution brachial artery ultrasound [100]. A 3-year interventional randomized, double-blind, placebo-controlled efficacy study has been initiated in children and young adults with ADPKD. Primary end points are total renal volume, left ventricular mass index, urinary albumin excretion and endothelial-dependent vasodilation.
12. MMPs and tissue inhibitors of MMPs
The extracellular matrix is thought to undergo extensive remodeling as PKD develops [101]. The conditioned medium from the cystic kidney tubules derived from the C57BL/6J cpk/cpk mouse contains higher than normal levels of MMP-9, -2, and -3 as well as TIMP-1 and -2 [102]. In a mouse model of PKD, kidney protein extracts, mRNA and tissue sections had high expression of MMP-2 [101]. To determine whether MMPs play a role in cyst development, Han:SPRD rats were treated with the MMP inhibitor, batimastat, for 8 weeks [103]. Batimastat resulted in a significant reduction in cyst number and kidney weight. MMP inhibitors as a potential therapy for PKD merits further study.
13. Expert opinion
It has been determined that inhibitors of cAMP, mTOR, CDK, TNF-α, the RAAS and HMG-CoA reductase reduce cyst formation and improve renal function in rat and mouse models of PKD. Thus cAMP, mTOR, CDK, TNF-α, the RAAS and HMG-CoA reductase are potential therapeutic targets in PKD. Current developments in the field of PKD research are very exciting. The results of studies in rat and mouse models of PKD have been translated to the bedside. Tolvaptan, sirolimus and everolimus, ACE inhibitors and ARBs, and statins that reduce cyst formation and improve renal function in animal models of PKD, are being tested in interventional studies in humans.
Tolvaptan is a promising drug for the treatment of ADPKD in humans for the following reasons: i) in robust studies, tolvaptan was extremely effective in reducing cyst formation in rat and mouse models of PKD [40,42]; ii) in the Phase I and II studies in PKD patients, tolvaptan had a very low side-effect profile [45,46,48]; and iii) a large multicenter study with the goal of FDA approval is nearing completion. However, the extent to which inhibition of V2 receptors, which are mainly present in the collecting ducts, is sufficient to halt a disease that manifests in the entire nephron, remains to be proven. mTOR inhibition reduces cyst formation in rat and mouse models of PKD and in a small study of patients with ADPKD. No study has compared the mTOR inhibitors, rapamycin and everolimus, with tolvaptan in terms of efficacy or safety profile.
Statins are widely used to lower cholesterol and they have anti-inflammatory and antiproliferative qualities. However there are only two reported animal studies, both in Han:SPRD rats, that demonstrate that statins reduce cyst formation. Triptolide, a traditional Chinese medicine, and octreotide, a somatostatin analogue, reduce cyst formation in animal models of PKD. Small studies of triptolide or somatostatin in patients with ADPKD have been initiated. However, a larger study of triptolide or somatostatin in ADPKD patients will be required for FDA approval of these drugs for use in ADPKD patients.
SKI-606 (also known as bosutinib), a Src inhibitor, reduces cyst formation in animal models of PKD. SKI-606 has shown promise in human studies of cancer. However, SKI-606 has not yet been tested in patients with ADPKD. Roscovitine, a CDK inhibitor, reduces cyst formation in mouse models of PKD and Phase II human studies in cancer have been initiated. However, the bone marrow toxicity of long-term roscovitine treatment in humans with ADPKD remains to be tested.
Small studies of the effect of ACE inhibitors in patients with ADPKD did not show an effect on the progression of kidney disease. However, ACE inhibitors show promise in reducing cyst formation in animal models of PKD. Activation of the renin–angiotensin system in PKD kidneys is prominent, and a larger study of ACE inhibitors or ARBs in 1200 ADPKD patients (the HALT-PKD study) has been initiated. The results of these interventional studies are eagerly awaited.
Future developments include the development of new drugs for human use in PKD, such as roscovitine, somatostatin, etanercept and other cytokine inhibitors. It is likely that current or future interventional studies in patients with ADPKD will result in the discovery of an agent that can slow the growth of the polycystic kidneys and delay the onset of renal failure.
Article highlights
Autosomal dominant polycystic kidney disease (ADPKD) is more common than Huntington’s disease, hemophilia, sickle cell disease, Down’s syndrome, cystic fibrosis, and myotonic dystrophy combined.
Triptolide (Tripterygium wilfordii), a traditional Chinese medicine, activates PC2-mediated calcium release in the cilia and inhibits cyst formation.
There is upregulation of vasopressin and the inhibition of cystogenesis by V2 receptor antagonists in three different animal models of PKD.
Mammalian target of rapamycin (mTOR) inhibition is a promising therapy for ADPKD in animal models.
The renin–angiotensin system has been implicated in the functional and structural changes in ADPKD.
Tolvaptan, sirolimus and everolimus, ACE inhibitors and angiotensin receptor blockers (ARBs), as well as statins that reduce cyst formation and improve renal function in animal models of PKD, are being tested in interventional studies in humans.
This box summarizes key points contained in the article.
Acknowledgments
Declaration of interest
CL Edelstein was funded by a grant (DK 2RO1 DK056851) from the National Institutes of Health.
Bibliography
- 1.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–45. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1:148–57. doi: 10.2215/CJN.00330705. [DOI] [PubMed] [Google Scholar]
- 3.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149–68. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18(5):1374–80. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 5.Harris PC. Autosomal dominant polycystic kidney disease: clues to pathogenesis. Hum Mol Genet. 1999;8:1861–186. doi: 10.1093/hmg/8.10.1861. [DOI] [PubMed] [Google Scholar]
- 6.Davenport JR, Watts AJ, Roper VC, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17(18):1586–94. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang ST, Chiou YY, Wang E, et al. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol. 2006;168(1):205–20. doi: 10.2353/ajpath.2006.050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thivierge C, Kurbegovic A, Couillard M, et al. Overexpression of PKD1 causes polycystic kidney disease. Mol Cell Biol. 2006;26(4):1538–48. doi: 10.1128/MCB.26.4.1538-1548.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park EY, Sung YH, Yang MH, et al. Cyst formation in kidney via B-Raf signaling in the PKD2 transgenic mice. J Biol Chem. 2009;284(11):7214–22. doi: 10.1074/jbc.M805890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng L, Segal Y, Peissel B, et al. Identification and localization of polycystin, the PKD1 gene product. J Clin Invest. 1996;98(12):2674–82. doi: 10.1172/JCI119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng L, Segal Y, Pavlova A, et al. Distribution and developmentally regulated expression of murine polycystin. Am J Physiol. 1997;272(4 Pt 2):F451–9. doi: 10.1152/ajprenal.1997.272.4.F451. [DOI] [PubMed] [Google Scholar]
- 12.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest. 1999;104(10):1459–68. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheffers MS, van der BP, Prins F, et al. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum Mol Genet. 2000;9(18):2743–50. doi: 10.1093/hmg/9.18.2743. [DOI] [PubMed] [Google Scholar]
- 14.Tsiokas L, Kim E, Arnould T, et al. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA. 1997;94(13):6965–70. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian F, Germino FJ, Cai Y, et al. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16(2):179–83. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 16.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33(2):129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 17.Leuenroth SJ, Okuhara D, Shotwell JD, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci USA. 2007;104:4389–94. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leuenroth SJ, Bencivenga N, Igarashi P, et al. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol. 2008;19:1659–62. doi: 10.1681/ASN.2008030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8(8):2666–74. [PubMed] [Google Scholar]
- 20.Cai Y, Somlo S. Too much of a good thing: does Nek8 link polycystic kidney disease and nephronophthisis? J Am Soc Nephrol. 2008;19(3):418–20. doi: 10.1681/ASN.2008010084. [DOI] [PubMed] [Google Scholar]
- 21.Sohara E, Luo Y, Zhang J, et al. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol. 2008;19(3):469–76. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trapp ML, Galtseva A, Manning DK, et al. Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatr Nephrol. 2008;23(3):377–87. doi: 10.1007/s00467-007-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valkova N, Yunis R, Mak SK, et al. Nek8 mutation causes overexpression of galectin-1, sorcin, and vimentin and accumulation of the major urinary protein in renal cysts of jck mice. Mol Cell Proteomics. 2005;4(7):1009–18. doi: 10.1074/mcp.M500091-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Lu W, Obara T, et al. A defect in a novel Nek-family kinase causes cystic kidney disease in the mouse and in zebrafish. Development. 2002;129(24):5839–46. doi: 10.1242/dev.00173. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Luo Y, Starremans PG, et al. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol. 2005;7:1102–12. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- 26.Schwiebert EM, Wallace DP, Braunstein GM, et al. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol. 2002;282:F763–75. doi: 10.1152/ajprenal.0337.2000. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DP, Rome LA, Sullivan LP, Grantham JJ. cAMP-dependent fluid secretion in rat inner medullary collecting ducts. Am J Physiol Renal Physiol. 280:F1019–29. doi: 10.1152/ajprenal.2001.280.6.F1019. [DOI] [PubMed] [Google Scholar]
- 28.Wallace DP, Christensen M, Reif G, et al. Electrolyte and fluid secretion by cultured human inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2002;283:F1337–50. doi: 10.1152/ajprenal.00165.2002. [DOI] [PubMed] [Google Scholar]
- 29.Yang B, Sonawane ND, Zhao D, et al. Small-molecule CFTR inhibitors slow cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19(7):1300–10. doi: 10.1681/ASN.2007070828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albaqumi M, Srivastava S, Li Z, et al. KCa3.1 potassium channels are critical for cAMP-dependent chloride secretion and cyst growth in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;74(6):740–9. doi: 10.1038/ki.2008.246. [DOI] [PubMed] [Google Scholar]
- 31.Alper SL. Let’s look at cysts from both sides now. Kidney Int. 2008;74(6):699–702. doi: 10.1038/ki.2008.357. [DOI] [PubMed] [Google Scholar]
- 32.Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132:1104–16. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–16. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi T, Pelling JC, Ramaswamy NT, et al. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000;57:1460–71. doi: 10.1046/j.1523-1755.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi T, Wallace DP, Magenheimer BS, et al. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004;279:40419–30. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 36.Omori S, Hida M, Fujita H, et al. Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J Am Soc Nephrol. 2006;17:1604–14. doi: 10.1681/ASN.2004090800. [DOI] [PubMed] [Google Scholar]
- 37.Shibazaki S, Yu Z, Nishio S, et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet. 2008;17(11):1505–16. doi: 10.1093/hmg/ddn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker E, Newby LJ, Sharpe CC, et al. Hyperproliferation of PKD1 cystic cells is induced by insulin-like growth factor-1 activation of the Ras/Raf signalling system. Kidney Int. 2007;72:157–65. doi: 10.1038/sj.ki.5002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Gao X, Saucedo LJ, et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–81. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 40.Torres VE, Wang X, Qian Q, et al. Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med. 2004;10:363–4. doi: 10.1038/nm1004. [DOI] [PubMed] [Google Scholar]
- 41.Gattone VH, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–6. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Gattone V, Harris PC, Torres VE. Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol. 2005;16:846–51. doi: 10.1681/ASN.2004121090. [DOI] [PubMed] [Google Scholar]
- 43.Guay-Woodford LM. Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol. 2003;285:F1034–49. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- 44.Nagao S, Nishii K, Katsuyama M, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17(8):2220–7. doi: 10.1681/ASN.2006030251. [DOI] [PubMed] [Google Scholar]
- 45.Grantham JJ, Chapman AB, Torres VE, et al. Acute and chronic osmostasis after vasopressin V2 receptor inhibition with tolvaptan in ADPKD [abstract] J Am Soc Nephrol. 2005;16:361A. [Google Scholar]
- 46.Torres VE, Wang X, Ward CJ, et al. Urine aquaporin -2 and cyclic AMP responses to tolvaptan administration in autosomal dominant kidney disease [abstract] J Am Soc Nephrol. 2005;16:361A. [Google Scholar]
- 47.Chapman AB, Torres VE, Grantham JJ, et al. A phase IIB pilot study fof the safety and efficacy of tolvaptan, a vasopressin V2 receptor antagonist (V2RA) in patients with ADPKD [abstract] J Am Soc Nephrol. 2005;16:68A. [Google Scholar]
- 48.Chapman AB. Autosomal dominant polycystic kidney disease: time for a change? J Am Soc Nephrol. 2007;18:1399–407. doi: 10.1681/ASN.2007020155. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney WE, Chen Y, Nakanishi K, et al. Treatment of polycystic kidney disease with a novel tyrosine kinase inhibitor. Kidney Int. 2000;57:33–40. doi: 10.1046/j.1523-1755.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakanishi K, Gattone VH, Sweeney WE, Avner ED. Renal dysfunction but not cystic change is ameliorated by neonatal epidermal growth factor in bpk mice. Pediatr Nephrol. 2001;16:45–50. doi: 10.1007/s004670000495. [DOI] [PubMed] [Google Scholar]
- 51.Sweeney WE, Futey L, Frost P, Avner ED. In vitro modulation of cyst formation by a novel tyrosine kinase inhibitor. Kidney Int. 1999;56:406–13. doi: 10.1046/j.1523-1755.1999.00577.x. [DOI] [PubMed] [Google Scholar]
- 52.Sweeney WE, Jr, Hamahira K, Sweeney J, et al. Combination treatment of PKD utilizing dual inhibition of EGF-receptor activity and ligand bioavailability. Kidney Int. 2003;64:1310–19. doi: 10.1046/j.1523-1755.2003.00232.x. [DOI] [PubMed] [Google Scholar]
- 53.Torres VE, Sweeney WE, Jr, Wang X, et al. Avner ED: EGF receptor tyrosine kinase inhibition attenuates the development of PKD in Han:SPRD rats. Kidney Int. 2003;64:1573–9. doi: 10.1046/j.1523-1755.2003.00256.x. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney WE, Jr, von Vigier RO, Frost P, Avner ED. Src inhibition ameliorates polycystic kidney disease. J Am Soc Nephrol. 2008;19:1331–41. doi: 10.1681/ASN.2007060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edelstein CL. Mammalian target of rapamycin and caspase inhibitors in polycystic kidney disease. Clin J Am Soc Nephrol. 2008;3:1219–26. doi: 10.2215/CJN.05611207. [DOI] [PubMed] [Google Scholar]
- 56.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 57.Gao X, Zhang Y, Arrazola P, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 58.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–89. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 59.Burnett PE, Barrow RK, Cohen NA, et al. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA. 1998;95:1432–7. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oshiro N, Yoshino K, Hidayat S, et al. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells. 2004;9:359–66. doi: 10.1111/j.1356-9597.2004.00727.x. [Erratum appears in Genes Cells 2004;9(5):497] [DOI] [PubMed] [Google Scholar]
- 61.Wahl PR, Serra AL, Le Hir M, et al. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 62.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–71. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu M, Wahl PR, Le Hir M, et al. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30:253–9. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 64.Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease (PKD) J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 65.Qian Q, Hui D, King BF, et al. Sirolimus reduces polycystic liver volume in ADPKD patients. J Am Soc Nephrol. 2008;19:631–8. doi: 10.1681/ASN.2007050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee L, Sudentas P, Donohue B, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer. 2005;42:213–27. doi: 10.1002/gcc.20118. [DOI] [PubMed] [Google Scholar]
- 67.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–51. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serra AL, Kistler AD, Poster D, et al. Safety and tolerability of sirolimus treatment in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2009;24(11):3334–42. doi: 10.1093/ndt/gfp280. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura T, Ebihara I, Nagaoka I, et al. Growth factor gene expression in kidney of murine polycystic kidney disease. J Am Soc Nephrol. 1993;3:1378–86. doi: 10.1681/ASN.V371378. [DOI] [PubMed] [Google Scholar]
- 70.Aukema HM, Housini I. Dietary soy protein effects on disease and IGF-I in male and female Han:SPRD-cy rats. Kidney Int. 2001;59:52–61. doi: 10.1046/j.1523-1755.2001.00465.x. [DOI] [PubMed] [Google Scholar]
- 71.Mehls O, Irzynjec T, Ritz E, et al. Effects of rhGH and rhIGF-1 on renal growth and morphology. Kidney Int. 1993;44:1251–8. doi: 10.1038/ki.1993.376. [DOI] [PubMed] [Google Scholar]
- 72.Du J, Wilson PD. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol. 1995;269:C487–95. doi: 10.1152/ajpcell.1995.269.2.C487. [DOI] [PubMed] [Google Scholar]
- 73.Alvaro D, Onori P, Alpini G, et al. Morphological and functional features of hepatic cyst epithelium in autosomal dominant polycystic kidney disease. Am J Pathol. 2008;172:321–32. doi: 10.2353/ajpath.2008.070293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishio S, Hatano M, Nagata M, et al. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J Clin Invest. 2005;115:910–18. doi: 10.1172/JCI22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otto EA, Schermer B, Obara T, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet. 2003;34:413–20. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–36. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 77.Bhunia AK, Piontek K, Boletta A, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–68. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 78.Bukanov NO, Smith LA, Klinger KW, et al. Long lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–52. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 79.MacCallum DE, Melville J, Frame S, et al. Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res. 2005;65:5399–407. doi: 10.1158/0008-5472.CAN-05-0233. [DOI] [PubMed] [Google Scholar]
- 80.McClue SJ, Blake D, Clarke R, et al. In vitro and in vivo antitumor properties of the cyclin dependent kinase inhibitor CYC202 (R-roscovitine) Int J Cancer. 2002;102:463–8. doi: 10.1002/ijc.10738. [DOI] [PubMed] [Google Scholar]
- 81.Song H, Vita M, Sallam H, et al. Effect of the Cdk-inhibitor roscovitine on mouse hematopoietic progenitors in vivo and in vitro. Cancer Chemother Pharmacol. 2007;60(6):841–9. doi: 10.1007/s00280-007-0431-x. [DOI] [PubMed] [Google Scholar]
- 82.Li X, Magenheimer BS, Xia S, et al. A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med. 2008;14:863–8. doi: 10.1038/nm1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–64. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 84.Thomas GL, Yang B, Wagner BE, et al. Cellular apoptosis and proliferation in experimental renal fibrosis. Nephrol Dial Transplant. 1998;13:2216–26. doi: 10.1093/ndt/13.9.2216. [DOI] [PubMed] [Google Scholar]
- 85.Cao Z, Cooper ME. Role of angiotensin II in tubulointerstitial injury. Semin Nephrol. 2001;21:554–62. doi: 10.1053/snep.2001.26794. [DOI] [PubMed] [Google Scholar]
- 86.Schindler R, Dinarello CA, Koch KM. Angiotensin-converting-enzyme inhibitors suppress synthesis of tumour necrosis factor and interleukin 1 by human peripheral blood mononuclear cells. Cytokine. 1995;7:526–33. doi: 10.1006/cyto.1995.0071. [DOI] [PubMed] [Google Scholar]
- 87.Kennefick TM, Al-Nimri MA, Oyama TT, et al. Hypertension and renal injury in experimental polycystic kidney disease. Kidney Int. 1999;56:2181–90. doi: 10.1046/j.1523-1755.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 88.Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am J Kidney Dis. 1994;24:491–8. doi: 10.1016/s0272-6386(12)80907-3. [DOI] [PubMed] [Google Scholar]
- 89.Zafar I, Tao Y, Falk S, et al. Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol Renal Physiol. 2007;293:F854–9. doi: 10.1152/ajprenal.00059.2007. [DOI] [PubMed] [Google Scholar]
- 90.Jafar TH, Stark PC, Schmid CH, et al. ACE Inhibition in Progressive Renal Disease (AIPRD) Study Group. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67:265–71. doi: 10.1111/j.1523-1755.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 91.Ogborn MR, Sareen S, Pinette G. Cilazapril delays progression of hypertension and uremia in rat polycystic kidney disease. Am J Kidney Dis. 1995;26:942–6. doi: 10.1016/0272-6386(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 92.Rubanyi GM. Endothelium-dependent pressure-induced contraction of isolated canine carotid arteries. Am J Physiol. 1988;255:H783–8. doi: 10.1152/ajpheart.1988.255.4.H783. [DOI] [PubMed] [Google Scholar]
- 93.Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–6. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 94.Levey AS, Greene T, Beck GJ, et al. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol. 1999;10:2426–39. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 95.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–56. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 96.Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–9. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 97.Amico P, Kalbermatter S, Kiss D. Aliskiren corrects recurrent hyperreninemia and hyperaldosteronism in autosomaldominant polycystic kidney disease. Clin Nephrol. 2009;72(3):237–9. doi: 10.5414/cnp72237. [DOI] [PubMed] [Google Scholar]
- 98.Gile RD, Cowley BD, Jr, Gattone VH2, et al. Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis. 1995;26:501–7. doi: 10.1016/0272-6386(95)90497-2. [DOI] [PubMed] [Google Scholar]
- 99.van Dijk MA, Kamper AM, van Veen S, et al. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2001;16:2152–7. doi: 10.1093/ndt/16.11.2152. [DOI] [PubMed] [Google Scholar]
- 100.Namli S, Oflaz H, Turgut F, et al. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren Fail. 2007;29:55–9. doi: 10.1080/08860220601038892. [DOI] [PubMed] [Google Scholar]
- 101.Rankin CA, Itoh Y, Tian C, et al. Matrix metalloproteinase-2 in a murine model of infantile-type polycystic kidney disease. J Am Soc Nephrol. 1999;10(2):210–7. doi: 10.1681/ASN.V102210. [DOI] [PubMed] [Google Scholar]
- 102.Rankin CA, Suzuki K, Itoh Y, et al. Matrix metalloproteinases and TIMPS in cultured C57BL/6J-cpk kidney tubules. Kidney Int. 1996;50(3):835–44. doi: 10.1038/ki.1996.383. [DOI] [PubMed] [Google Scholar]
- 103.Obermuller N, Morente N, Kranzlin B, et al. A possible role for metalloproteinases in renal cyst development. Am J Physiol Renal Physiol. 2001;280(3):F540–50. doi: 10.1152/ajprenal.2001.280.3.F540. [DOI] [PubMed] [Google Scholar]