Abstract
Studies in animal models of Parkinson's disease have revealed that degeneration of noradrenaline neurons is involved in the motor deficits. Several types of adrenoceptors are highly expressed in neostriatal neurons. However, the selective actions of these receptors on striatal signaling pathways have not been characterized. In this study, we investigated the role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling by analyzing DARPP-32 phosphorylation at Thr34 (PKA-site) in mouse neostriatal slices. Activation of β1-adrenoceptors induced a rapid and transient increase in DARPP-32 phosphorylation. Activation of α2-adrenoceptors also induced a rapid and transient increase in DARPP-32 phosphorylation, which subsequently decreased below basal levels. In addition, activation of α2-adrenoceptors attenuated, and blockade of α2-adrenoceptors enhanced dopamine D1 and adenosine A2A receptor/DARPP-32 signaling. Chemical lesioning of noradrenergic neurons mimicked the effects of α2-adrenoceptor blockade. Under conditions of α2-adrenoceptor blockade, the dopamine D2 receptor-induced decrease in DARPP-32 phosphorylation was attenuated. Our data demonstrate that β1- and α2-adrenoceptors regulate DARPP-32 phosphorylation in neostriatal neurons. Gi activation by α2-adrenoceptors antagonizes Gs/PKA signaling mediated by D1 and A2A receptors in striatonigral and striatopallidal neurons, respectively, and thereby enhances D2 receptor/Gi signaling in striatopallidal neurons. α2-adrenoceptors may therefore be a therapeutic target for the treatment of Parkinson's disease.
Keywords: α2-adrenoceptor, D1 receptor, noradrenaline, phosphorylation, striatum
Introduction
Noradrenaline has been shown to interact with the dopaminergic system and regulate psychomotor functions. In animal models of Parkinson's disease, depletion of noradrenaline by degeneration of noradrenergic neurons or genetic deletion of dopamine β-hydroxylase potentiates the motor deficits of Parkinson's disease (Srinivasan and Schmidt 2003; Rommelfanger et al. 2007). These deficits can be improved by noradrenaline replacement (Rommelfanger et al. 2007), indicating a critical role for noradrenaline in the motor dysfunction of Parkinson's disease. Adrenoceptors are subdivided into three major classes by their differential coupling to G-proteins. α1-adrenoceptors (α1A, 1B, 1D), coupled to Gq, activate phospholipase C, α2-adrenoceptors (α2A-2C), coupled to Gi, inhibit adenylyl cyclase, and β-adrenoceptors (β1-3), coupled to Gs/olf, stimulate adenylyl cyclase. It is known that presynaptic α2-adrenoceptors negatively regulate dopamine release from dopaminergic terminals (Trendelenburg et al. 1994; Yavich et al. 1997; Gobert et al. 2004). It is likely that noradrenaline also modulates dopamine receptor signaling postsynaptically in medium spiny neurons. However, information on the interaction between noradrenaline and dopamine receptor signaling in medium spiny neurons is limited.
Despite the functional importance of noradrenaline in dopaminergic neurotransmission, the striatum receives only sparse noradrenergic innervation (Swanson and Hartman 1975; Aston-Jones 2004). However, certain types of adrenoceptors such as β1-, α2A- and α2C-adrenoceptors are expressed in the striatum (Nicholas et al. 1996; MacDonald et al. 1997). β1-adrenoceptors were found to be expressed in medium spiny neurons by radioligand binding (Nahorski et al. 1979; Waeber et al. 1991) and immunohistochemistry (Pisani et al. 2003), and the loss of β1-adrenoceptors in the striatum was reported in the late stages of Huntington's chorea (Waeber et al. 1991). α2A-adrenoceptors are widely distributed in the brain including the striatum (MacDonald et al. 1997), and mediate the inhibition of monoamine release and metabolism (Trendelenburg et al. 1994; Bucheler et al. 2002; Ihalainen and Tanila 2004). Generally, they are associated with functions such as sedation, analgesia and hypotension (MacMillan et al. 1996; Lakhlani et al. 1997; Altman et al. 1999; Philipp et al. 2002). In contrast, α2C-adrenoceptors show a unique distribution pattern, and are most abundantly expressed in the striatum, olfactory tubercle, hippocampus and cerebral cortex (Nicholas et al. 1996; MacDonald et al. 1997; Winzer-Serhan et al. 1997; Holmberg et al. 1999). α2C-adrenoceptors are expressed in medium spiny neurons in the striatum (Holmberg et al. 1999), and are negatively coupled to adenylyl cyclase via Gi (Lu and Ordway 1997; Zhang et al. 1999). α2C-adrenoceptors are also expressed in dopaminergic neurons in the substantia nigra (Rosin et al. 1996; Lee et al. 1998) and possibly at dopaminergic terminals in the striatum. Together with α2A-adrenoceptors, they inhibit the release of dopamine (Bucheler et al. 2002). In mice lacking α2C-adrenoceptors, amphetamine-induced locomotor activity, startle reactivity and aggressive behavior were enhanced, whereas pre-pulse inhibition was attenuated (Sallinen et al. 1998a, b). Opposite changes were reported in α2C-adrenoceptor overexpressing mice (Sallinen et al. 1998a, b). Thus, α2C-adrenoceptors likely play an inhibitory role in the regulation of motor and emotional functions and a modulatory role in the processing of sensory information (Scheinin et al. 2001).
DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa) is selectively enriched in medium spiny neurons in the striatum, and plays an essential role in dopaminergic neurotransmission (Greengard et al. 1999; Svenningsson et al. 2004). Dopamine activates dopamine D1 receptors coupled to Gs/olf, leading to activation of cAMP/protein kinase A (PKA) signaling and the phosphorylation of DARPP-32 at Thr34 (the PKA-site). When DARPP-32 is phosphorylated on Thr34, it is converted into a potent inhibitor of protein phosphatase-1, and thereby controls the phosphorylation state and activity of many downstream physiological effectors, including various neurotransmitter receptors and voltage-gated ion channels (Svenningsson et al. 2004). The state of DARPP-32 phosphorylation at Thr34 is also regulated by various other signaling molecules, providing a mechanism for integrating dopamine and other neurotransmitter signals.
Noradrenergic neurotransmission plays a critical role in the regulation of motor function by interacting with dopamine signaling, and noradrenaline replacement therapy has been proposed as a treatment for Parkinson's disease (Grimbergen et al. 2009). However, the molecular mechanisms by which adrenoceptors regulate dopamine signaling in the striatum have not been investigated. In this study, we investigated the regulation of DARPP-32 phosphorylation by β and α-adrenoceptors. We find that activation of both types of receptors affects the phosphorylation of DARPP-32 at Thr34. Furthermore, activation of Gi by α2-adrenoceptors and subsequent inhibition of adenosine A2A receptor/Gs/PKA signaling are required for the actions of Gi-coupled D2 receptors in striatopallidal neurons.
Materials and Methods
Preparation, incubation and processing of neostriatal slices
Male C57BL/6 mice at 6-8 weeks old were purchased from Japan SLC (Shizuoka, Japan). All mice used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. The Institutional Animal Care and Use Committee of Kurume University School of Medicine approved the specific protocols. Male C57BL/6 mice were sacrificed by decapitation. The brains were rapidly removed and placed in ice-cold, oxygenated Krebs-HCO3- buffer (124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 1.5 mM CaCl2, 1.25 mM KH2PO4, 1.5 mM MgSO4 and 10 mM D-glucose, pH 7.4). Coronal slices (350 μm) were prepared using a vibrating blade microtome, VT1000S (Leica Microsystems, Nussloch, Germany). Striata were dissected from the slices in ice-cold Krebs-HCO3- buffer. Each slice was placed in a polypropylene incubation tube with 2 ml fresh Krebs-HCO3- buffer containing adenosine deaminase (10 μg/ml). The slices were preincubated at 30°C under constant oxygenation with 95% O2/5% CO2 for 60 min. The buffer was replaced with fresh Krebs-HCO3- buffer after 30 min of preincubation. Adenosine deaminase was included during the first 30 min of preincubation. Slices were treated with drugs as specified in each experiment. Drugs were obtained from the following sources: isoproterenol, propranolol, CGP20712A, ICI118551, cirazoline, UK14304, yohimbine, nortriptyline, GR113808, SB258585, SKF81297, DSP-4 from Sigma-Aldrich (St. Louis, MO); CGS21680 from Tocris Cookson (Bristol, UK). After drug treatment, slices were transferred to Eppendorf tubes, frozen on dry ice, and stored at −80°C until assayed.
Frozen tissue samples were sonicated in boiling 1 % sodium dodecyl sulfate (SDS) containing 50 mM sodium fluoride and boiled for an additional 10 min. Small aliquots of the homogenate were retained for protein determination by the BCA protein assay method (Pierce, Rockford, IL). Equal amounts of protein (100 μg) were separated by SDS/polyacrylamide gel electrophoresis (10% polyacrylamide gels), and transferred to nitrocellulose membranes (0.2 μm) (Schleicher and Schuell, Keene, NH).
Immunoprecipitations of Flag- and Myc-tagged DARPP-32 in neostriatal slices from D1-DARPP-32-Flag/D2-DARPP-32-Myc mice
D1-DARPP-32-Flag/D2-DARPP-32-Myc transgenic mice express Flag- and Myc-tagged DARPP-32 under the control of dopamine D1 and D2 receptor promoters, respectively (Bateup et al., 2008). In the striatum, Flag-tagged DARPP-32 was shown to be expressed selectively in D1 receptor-enriched striatonigral neurons, and Myc-tagged DARPP-32 selectively in D2 receptor-enriched striatopallidal neurons. Using antibodies against Flag and Myc tags, we selectively immunoprecipitated DARPP-32 from D1 receptor- and D2 receptor-expressing neurons and analyzed the phosphorylation state of DARPP-32 in a neuronal subtype-specific manner. In each experiment, six striatal slices were prepared from one mouse, and were divided into three treatment conditions. In each treatment condition, six slices, collected from three mice (2 slices from each mouse), were used for the analysis of DARPP-32 phosphorylation. Six striatal slices were sonicated in 720 μl of IP lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% SDS, 100 nM okadaic acid, phosphatase inhibitor cocktail (#P5726; Sigma-Aldrich), and protease inhibitor cocktail (#11873580001; Roche, Basel, Switzerland)). After determination of protein concentration, 15 μg of protein was saved for the analysis of DARPP-32 phosphorylation in total striatal homogenate, and the residual homogenates were used for immunoprecipitations (IP). In each IP from striatal homogenate, 50 μl of washed EZView Red anti-Flag M2 affinity gel (Sigma-Aldrich) and 45 μl of anti-Myc antibody (Novus Biologicals, Littleton, CO) coupled to magnetic beads (3 μg of Myc antibody for every 5 μl of magnetic beads) (Dynabeads M-280 Tosylactivated; Invitrogen, Carlsbad, CA) were added. The homogenate/antibody mixture was gently rotated overnight at 4°C. Following the overnight incubation, the Myc magnetic beads were separated from the homogenate/antibody mixture using a magnetic particle concentrator (Invitrogen), and then the Flag affinity gels were separated by centrifugation. The Myc magnetic beads and Flag affinity gels were washed with 1× PBS three times. After the final wash, 30 μl of sample buffer was added, and samples were boiled for two minutes.
Flag IP, Myc IP and total striatal samples were loaded onto 4-12% polyacrylamide Bis-Tris gels (Bio-Rad, Hercules, CA), separated by electrophoresis, and transferred to nitrocellulose membranes (0.2 μM) (Schleicher and Schuell).
Lesioning of noradrenaline neurons
In some experiments, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4) was used to selectively lesion noradrenergic neurons in the locus coeruleus (LC) (Gesi et al. 2000; Rommelfanger et al. 2007). DSP-4 at a dose of 50 mg/kg or saline (0.1 ml/10 g body weight) was injected intraperitoneally (i.p.) to C57BL/6 mice. DSP-4 was dissolved in saline immediately before injection and used within 2 min to avoid degradation. After 7 days of DSP-4 or saline injection, neostriatal slices were prepared as described above, and treated with SKF81297 or CGS21680.
Immunoblotting
The membranes were immunoblotted using a phosphorylation state-specific antibody raised against DARPP-32 phospho-peptides: phospho-Thr34, the site phosphorylated by PKA (mAb-23, 1:750 dilution; CC500, 1:500-4,000) (Snyder et al. 1992). A monoclonal antibody (C24-5a, 1:7,500 dilution) generated against DARPP-32 (Hemmings and Greengard 1986), which is not phosphorylation state-specific, was used in order to determine the total amount of DARPP-32. None of the experimental manipulations used in the present study altered the total amount of DARPP-32.
The membrane was incubated with a goat anti-mouse or rabbit Alexa 680-linked IgG (1:5,000 dilution) (Molecular Probes, Eugene, OR) or a goat anti-mouse or rabbit IRDye™800-linked IgG (1:5,000 dilution) (ROCKLAND, Gilbertsville, PA). Fluorescence at infrared wavelengths was detected by the Odyssey infrared imaging system (LI-COR, Lincoln, NE), and quantified using Odyssey software. In an individual experiment, samples from control and drug-treated slices were analyzed on the same immunoblot. For each experiment, values obtained for slices were calculated relative to values for the control or drug-treated slices, as described in figure legends. Normalized data from multiple experiments were averaged and statistical analysis was carried out as described in the figure legends.
Immunohistochemistry
Under deep anesthesia induced with sodium pentobarbital, male C57BL/6 mice at 6-8 weeks old were perfused rapidly through the left ventricle with 50 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) at room temperature. Serial coronal sections 50 μm in thickness were cut with a vibrating microtome, VT1000S (Leica Microsystems). Sections were processed for immunohistochemistry with the use of the free-floating method, as described (Nishi et al. 2008). Sections were incubated with a rabbit anti-β1-adrenoceptor antibody (A-272; 1:500) (Sigma), a rabbit anti-α2C-adrenoceptor antibody (RA19064; 1:200 dilution) (Neuromics, Edina, MN), and a mouse anti-DARPP-32 antibody (C24-5a; 1:20,000 dilution) at 20°C for 7 days. Antibody binding was visualized with a fluorescein isothiocyanate-conjugated donkey anti-mouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA) and a rhodamine red-conjugated donkey anti-rabbit IgG (1:100; Jackson ImmunoResearch). Sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and examined with a confocal laser-scanning microscope, LSM 5 PASCAL (Zeiss, Oberkochen, Germany).
Results
Effects of β-adrenergic agonists on DARPP-32 Thr34 phosphorylation in neostriatal slices
Treatment of neostriatal slices with a non-selective β-adrenergic agonist, isoproterenol (10 μM), rapidly and transiently increased DARPP-32 Thr34 phosphorylation (Fig. 1A). Isoproterenol increased the level of phospho-Thr34 DARPP-32 by 4-fold within 1min of incubation, and the increased level of phospho-Thr34 DARPP-32 subsequently returned to basal values at 10 min. The stimulatory effect of isoproterenol on DARPP-32 Thr-34 phosphorylation was attenuated by a non-selective β-adrenergic antagonist, propranolol (10 μM) (Fig. 1B), although propranolol itself did not affect the level of phospho-Thr34 DARPP-32. To rule out the possibility that isoproterenol increases DARPP-32 Thr34 phosphorylation by activating dopamine D1 receptors (Vanderheyden et al. 1986), the effect of isoproterenol was examined in the presence of a dopamine D1 receptor antagonist, SCH23390 (1 μM). Pretreatment with SCH23390 did not affect the stimulatory effect of isoproterenol on DARPP-32 Thr34 phosphorylation. To determine the subtype of β-adrenoceptors involved in DARPP-32 Thr34 phosphorylation, neostriatal slices were pretreated with a β1-adrenergic antagonist, CGP20712A (10 μM), or a β2-adrenergic antagonist, ICI118551 (10 μM). Pretreatment with CGP20712A, but not ICI118551, attenuated the isoproterenol-induced increase in DARPP-32 Thr34 phosphorylation (Fig. 1C), suggesting that activation of β1-adrenoceptors induces the phosphorylation of DARPP-32 at Thr34 in neostriatal neurons.
Fig. 1. Effect of a β-adrenergic agonist on DARPP-32 Thr34 phosphorylation in neostriatal slices.

(A) Neostriatal slices were treated with a non-selective β-adrenergic agonist, isoproterenol (10 μM), for the indicated times. Typical immunoblots for detection of phospho-Thr34 DARPP-32 and total DARPP-32 in the same membrane are shown at the left of panel. (B, C) Neostriatal slices were pre-treated for 10 min with (B) a non-selective β-adrenergic antagonist, propranolol (10 μM), or a dopamine D1 receptor antagonist, SCH23390 (1 μM), or (C) a β1-adrenergic antagonist, CGP20712A (10 μM), or a β2-adrenergic antagonist, ICI118551 (10 μM), followed by the addition of isoproterenol (10 μM) for 1 min. (D) Neostriatal slices from D1-DARPP-32-Flag/D2-DARPP-32-Myc mice were incubated with isoproterenol (Iso; 10 μM) for 1 min. Flag-tagged DARPP-32, expressed in D1 receptor-enriched striatonigral neurons, and Myc-tagged DARPP-32, expressed in D2 receptor-enriched striatopallidal neurons, were immunoprecipitated. The panel shows data from total striatal homogenate (Homog), Flag-tagged DARPP-32 in striatonigral neurons (D1-Flag) and Myc-tagged DARPP-32 in striatopallidal neurons (D2-Myc). Typical immunoblots for detection of phospho-Thr34 DARPP-32 and total DARPP-32 in the same membrane are shown at the left of panel. The levels of phospho-Thr34 DARPP-32 were quantified by the Odyssey infrared imaging system, and the data were normalized to values obtained with untreated slices. Data represent means ± SEM for 4-12 experiments. **p < 0.01, ***p < 0.001 compared with untreated slices; †p < 0.05, †††p < 0.001 compared with isoproterenol alone; §§§p < 0.001 compared with SCH23390 alone; §p < 0.05 compared with ICI118551 alone; one-way ANOVA followed by Newman-Keuls test.
We next examined whether activation of β1-adrenoceptors increases DARPP-32 phosphorylation in D1 receptor-enriched striatonigral and/or D2 receptor-enriched striatopallidal neurons, using neostriatal slices from D1-DARPP-32-Flag/D2-DARPP-32-Myc transgenic mice (Bateup et al, 2008). Flag- and Myc-tagged DARPP-32 were immunoprecipitated from striatonigral and striatopallidal neurons, respectively, and the phosphorylation state of DARPP-32 at Thr34 in the two types of neurons was analyzed. Treatment of neostriatal slices with isoproterenol (10 μM) for 1 min increased the level of phospho-Thr34 DARPP-32 by approximately 2 fold in total striatal homogenates (Fig. 1D). Isoproterenol increased the phosphorylation of both Flag- and Myc-tagged DARPP-32 at Thr34 by 2 fold, suggesting that isoproterenol activates the β1-adrenoceptor signaling cascades both in striatonigral and striatopallidal neurons. The results are consistent with immunohistochemical data, demonstrating that β1-adrenoceptors are expressed in all DARPP-32-positive striatal neurons (Fig. 3A).
Fig. 3. Expression of β1- and α2C-adrenoceptors in the striatum.

Double immunostaining of striatal tissues with (A) DARPP-32 and β1-adrenoceptor antibodies and (B) DARPP-32 and α2C-adrenoceptor antibodies. The expression of β1- and α2C-adrenoceptors was detected in many DARPP-32-positive neurons, suggesting their expression both in striatonigral and striatopallidal neurons. In agreement with Pisani et al. (Pisani et al. 2003), the expression of β1-adrenoceptors was also detected in DARPP-32-negative, large-sized neurons (A; arrows), presumably cholinergic interneurons. Scale bars, 10 μm.
Effects of α1- and α2-adrenergic agonists on DARPP-32 Thr34 phosphorylation in neostriatal slices
The role of α-adrenoceptors in the regulation of DARPP-32 Thr34 phosphorylation was examined in neostriatal slices. Treatment with a selective α1-adrenergic agonist, cirazoline (1 μM), did not affect the level of phospho-Thr34 DARPP-32 by 5 min of incubation (Fig. 2A). However, treatment with a selective α2-adrenergic agonist, UK14304 (1 μM), increased DARPP-32 Thr34 phosphorylation by 2.5-fold within 1 min of incubation, and the increased level of phospho-Thr34 DARPP-32 returned to basal values at 5 min (Fig. 2B). After 20 min of incubation with UK14304, the level of phospho-Thr34 DARPP-32 decreased slightly below basal values (81.0 ± 6.7 % of control; p < 0.05 with Student's t-test, but not significant with one-way ANOVA followed by Newman-Keuls test). The rapid and transient increase in DARPP-32 Thr34 phosphorylation induced by UK14304 was antagonized by an α2 adrenergic antagonist, yohimbine (1 μM) (Fig. 2C). Immunohistochemical data showed that α2C-adrenoceptors, the predominant subtype of a2-adrenoceptors in the striatum, were expressed in all DARPP-32-positive neurons (Fig. 3B).
Fig. 2. Effect of α1-adrenergic and α2-adrenergic agonists on DARPP-32 Thr34 phosphorylation in neostriatal slices.
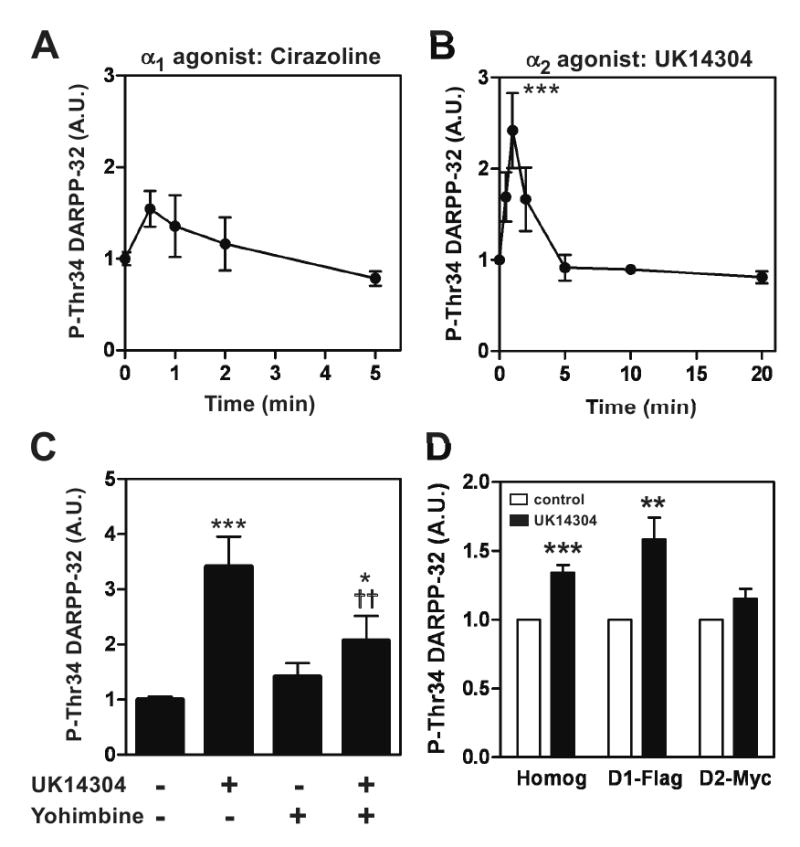
(A) Neostriatal slices were treated with a selective α1-adrenergic agonist, cirazoline (1 μM), for the indicated times. (B) Neostriatal slices were treated with a selective α2-adrenergic agonist, UK14304 (1 μM), for the indicated times. (C) Neostriatal slices were pre-treated with a selective α2-adrenergic antagonist, yohimbine (1 μM), for 15 min, followed by the addition of UK14304 (1 μM) for 1 min. (D) Neostriatal slices from D1-DARPP-32-Flag/D2-DARPP-32-Myc mice were incubated with UK14304 (10 μM) for 30 sec. Flag-tagged DARPP-32 and Myc-tagged DARPP-32 were immunoprecipitated. The panel shows data from total striatal homogenate (Homog), Flag-tagged DARPP-32 in striatonigral neurons (D1-Flag) and Myc-tagged DARPP-32 in striatopallidal neurons (D2-Myc). The levels of phospho-Thr34 DARPP-32 were quantified by the Odyssey infrared imaging system, and the data were normalized to values obtained with untreated slices. Data represent means ± SEM for 4-9 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated slices; ††p < 0.05 compared with UK14304 alone; one-way ANOVA followed by Newman-Keuls test.
We next examined whether UK14304 induces DARPP-32 Thr34 phosphorylation selectively in striatonigral or striatopallidal neurons or both types of neurons. In neostriatal slices from D1-DARPP-32-Flag/D2-DARPP-32-Myc mice, treatment with UK14304 significantly increased the level of phopho-Thr34 DARPP-32 in total striatal homogenate, but the magnitude of the increase was less than that in C57BL/6 mice possibly due to strain differences. The rapid increase in DARPP-32 Thr34 phosphorylation induced by UK14304 was detected selectively in striatonigral but not striatopallidal neurons (Fig. 2D). These results demonstrate a cell-type specific effect of α2-adrenoceptors in the striatum, although α2C-adrenoceptors are present in all medium spiny neurons.
Interaction of α2-adrenoceptor signaling with dopamine D1 and adenosine A2A receptor signaling
We next examined whether α2-adrenoceptors could modulate Golf/PKA/DARPP-32 signaling activated by dopamine D1 and adenosine A2A receptors. Pretreatment with the α2-adrenergic antagonist, yohimbine (10 μM), did not affect the basal level of phospho-Thr34 DARPP-32. However, yohimbine enhanced the increase in DARPP-32 Thr34 phosphorylation induced by SKF81297 (1 μM) (Fig. 4A) or CGS21680 (5 μM) (Fig. 4B), suggesting that tonic activity of α2-adrenoceptors inhibits dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling in neostriatal neurons. To test this, we treated striatal slices with the α2-adrenergic agonist, UK14304 (1 μM for 20 min), which did not significantly affect the basal levels of phospho-Thr34 DARPP-32 in this series of experiments. As expected from antagonist experiments, UK14304 reduced the increase in DARPP-32 Thr34 phosphorylation induced by SKF81297 (1 μM) (Fig. 4C) or CGS21680 (5 μM) (Fig. 4D). These results suggest that α2-adrenoceptors negatively interact with dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling.
Fig. 4. Effect of α2-adrenoceptor blockade and activation on dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling in neostriatal slices.

(A, B) Neostriatal slices were pre-treated with an α2-adrenergic antagonist, yohimbine (10 μM for 15 min), followed by the addition of (A) a dopamine D1 receptor agonist, SKF81297 (1 μM for 5 min), or (B) an adenosine A2A receptor agonist, CGS21680 (5 μM for 2 min). (C, D) Neostriatal slices were pre-treated with an α2-adrenergic agonist, UK14304 (1 μM for 20 min), followed by the addition of (C) a dopamine D1 receptor agonist, SKF81297 (1 μM for 5 min), or (D) an adenosine A2A receptor agonist, CGS21680 (5 μM for 2 min). The levels of phospho-Thr34 DARPP-32 were quantified by the Odyssey infrared imaging system, and the data were normalized to values obtained with untreated slices. Data represent means ± SEM for 4 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 compared with untreated slices; †p < 0.05, ††p < 0.01 compared with SKF81297 or CGS21680 alone; one-way ANOVA followed by Newman-Keuls test.
We also examined the role of β-adrenoceptors in dopamine D1 and adenosine A2A receptor signaling. Pretreatment with the β-adrenergic antagonist, propranolol (10 μM), did not affect the SKF81297 (1 μM)-induced or CGS21680 (5 μM)-induced increase in DARPP-32 Thr34 phosphorylation (data not shown).
Interaction of α2-adrenoceptor signaling with dopamine D2 receptor signaling
We next examined whether α2-adrenoceptors also play a role in dopamine D2 receptor signaling. Treatment of slices with a dopamine D2 receptor agonist, quinpirole (1 μM), decreased the level of phospho-Thr34 DARPP-32, as previously reported (Nishi et al. 1997). Pretreatment with yohimbine (10 μM) attenuated the decrease in DARPP-32 Thr34 phosphorylation induced by quinpirole (Fig. 5), suggesting that activity of α2-adrenoceptors is required for the action of dopamine D2 receptor to downregulate DARPP-32 phosphorylation in neostriatal neurons.
Fig. 5. Effect of α2-adrenoceptor blockade on dopamine D2 receptor/PKA/DARPP-32 signaling in neostriatal slices.

Neostriatal slices were pre-treated with an α2-adrenergic antagonist, yohimbine (10 μM for 10 min), followed by the addition of a dopamine D2 receptor agonist, quinpirole (1 μM for 10 min). The levels of phospho-Thr34 DARPP-32 were quantified by the Odyssey infrared imaging system, and the data were normalized to values obtained with untreated slices. Data represent means ± SEM for 6-9 experiments. ***p < 0.001 compared with untreated slices; †††p < 0.001 compared with quinpirole alone; one-way ANOVA followed by Newman-Keuls test.
Effect of nortriptyline on DARPP-32 Thr34 phosphorylation in neostriatal slices
The expression of dopamine β-hydroxylase and noradrenaline transporters are reported to be low in the striatum (Swanson and Hartman 1975; Berridge et al. 1997; Moll et al. 2000; Moron et al. 2002; Arai et al. 2008), and therefore the striatum is thought to receive a sparse noradrenergic innervation. To determine whether the release and reuptake machinery of noradrenaline at noradrenergic terminals is functioning in the striatum, we examined the effect of nortriptyline on DARPP-32 phosphorylation. Nortriptyline inhibits the noradrenaline transporter with high potency (Ki 3.4 nM) and the serotonin transporter with low potency (Ki 161 nM), but does not affect the dopamine transporter (Ki 13,920 nM) (Gonzalo et al. 2003). Treatment of neostriatal slices with nortriptyline (100 nM) increased the level of phospho-Thr34 DARPP-32 by ∼5-fold within 30 seconds of incubation, and the increased level of phospho-Thr34 DARPP-32 returned to basal values at 2 min (Fig. 6A).
Fig. 6. Effect of nortriptyline on DARPP-32 Thr34 phosphorylation in neostriatal slices.

(A) Neostriatal slices were treated with nortriptyline (100 nM), an inhibitor of monoamine transporters relatively selective for noradrenaline transporters, for the indicated times. (B, C) Neostriatal slices were pre-incubated for 10 min with (B) CGP20712A (10 μM) plus yohimbine (1 μM) or (C) a 5-HT4 receptor antagonist, GR113808 (10 μM) plus a 5-HT6 receptor antagonist, SB258585 (10 μM), followed by the addition of nortriptyline (100 nM) for 30 sec. The levels of phospho-Thr34 DARPP-32 were quantified by the Odyssey infrared imaging system, and the data were normalized to values obtained with untreated slices. Data represent means ± SEM for 4-6 experiments. **p < 0.01, ***p < 0.001 compared with untreated slices; †p < 0.05 compared with nortriptyline alone; §p < 0.05 compared with CGP20712A plus yohimbine; one-way ANOVA followed by Newman-Keuls test.
The nortriptyline-induced increase in DARPP-32 Thr34 phosphorylation was partially but significantly antagonized by CGP20712A (10 μM) plus yohimbine (1 μM) (Fig. 6B); however, the antagonizing effect of either compound was not significant when slices were preincubated with CGP20712A or yohimbine alone (data not shown). We also examined the effect of serotonergic antagonists for 5-HT4 and 5-HT6 receptors, which were reported to be the serotonin receptor subtypes coupled to Gs/olf/cAMP/PKA signaling in the striatum (Svenningsson et al. 2002). Treatment with a 5-HT4 receptor antagonist, GR113808 (10 μM), plus a 5-HT6 receptor antagonist, SB258585 (10 μM), also partially attenuated the nortriptyline-induced increase in DARPP-32 Thr34 phosphorylation (Fig. 6C). Pretreatment with a dopamine D1 receptor antagonist, SCH23390 (1 μM), did not attenuate the effect of nortriptyline (data not shown).
Taken together, these data reveal a significant contribution of striatal noradrenaline release to DARPP-32 T34 phosphorylation in medium spiny neurons. Since we also observed an attenuation of the nortriptyline effect by blocking serotonin receptors, it is likely that nortriptyline inhibited both noradrenaline and serotonin transporters in neostriatal slices, leading to increased extracellular noradrenaline and serotonin. Thus, nortriptyline increases phosphorylation of DARPP-32 via activation of β1- and α2-adrenoceptors and serotonin 5-HT4 and 5-HT6 receptors.
Dopamine D1 and adenosine A2A receptor signaling in neostriatal slices from DSP-4-lesioned mice
After determining that blockade of noradrenaline uptake in striatal slices affected DARPP-32 phosphorylation, we investigated whether depletion of noradrenaline in vivo had an effect on D1 or A2A receptor-mediated phosphorylation of DARPP-32. We injected mice with DSP-4 to selectively lesion noradrenergic neurons of the locus coeruleus (LC). The expression levels of tyrosine hydroxylase in the LC, determined by Western blotting, decreased to 45.7 ± 2.9 % of control (saline-treated mice) (p < 0.05; Student's t-test) after 7 days of DSP-4 administration, but the levels of tyrosine hydroxylase in the striatum were not affected (103.5 ± 5.2 % of control), indicating that DSP-4 selectively lesions noradrenergic neurons in the LC without lesioning dopaminergic neurons in the substantia nigra.
We examined the effect of dopamine D1 and adenosine A2A receptor agonists on DARPP-32 Thr34 phosphorylation in neostriatal slices from saline- or DSP-4-treated mice. Treatment of slices with SKF81297 (1 μM) increased DARPP-32 Thr34 phosphorylation 3-fold from 5 to 15 min of incubation in saline-treated mice (p < 0.001 compared with untreated slices; one-way ANOVA followed by Newman-Keuls test) (Fig. 7A). In slices from DSP-4-treated mice, the SKF81297-induced increase in DARPP-32 Thr34 phosphorylation was significantly higher than that in slices from saline-treated mice at 10 and 15 min of incubation (p < 0.05; two-way ANOVA followed by Bonferroni test).
Fig. 7. Dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling in neostriatal slices from saline- and DSP-4-treated mice.

Mice were intraperitoneally injected with saline or DSP-4, and neostriatal slices were prepared from the mice after 7 days of injection. Neostriatal slices from saline (closed circles)- or DSP-4 (open circles)-treated mice were treated with (A) a dopamine D1 receptor agonist, SKF81297 (1 μM), or (B) an adenosine A2A receptor agonist, CGS21680 (1 μM), for the indicated times. The levels of phospho-Thr34 DARPP-32 were quantified by the Odyssey infrared imaging system, and the data were normalized to values obtained from untreated slices in saline-treated mice. Data represent means ± SEM for 6 to 8 experiments. *p < 0.05, **p < 0.01 compared with values in saline-treated mice; two-way ANOVA followed by Bonferroni test.
Treatment of slices with CGS21680 (1 μM) increased DARPP-32 Thr34 phosphorylation 3-fold from 30 s to 2 min of incubation in saline-treated mice (p < 0.01 compared with untreated slices; one-way ANOVA followed by Newman-Keuls test) (Fig. 7B). In slices from DSP-4-treated mice, the CGS21680-induced increase in DARPP-32 Thr34 phosphorylation was significantly greater than that in slices from saline-treated mice at 1min of incubation. We also examined the effect of DSP-4-lesioning on dopamine D2 receptor signaling (p < 0.01; two-way ANOVA followed by Bonferroni test). Treatment with quinpirole (10 nM - 1 μM for 10 min) decreased DARPP-32 Thr34 phosphorylation similarly in slices from saline- and DSP-4-treated mice. The findings demonstrate that the chemical lesioning of noradrenergic neurons by DSP-4 results in the enhancement of dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling in striatal neurons.
Discussion
We have demonstrated that β1- and α2-adrenoceptors regulate PKA/DARPP-32 signaling in the striatum (see Fig. 8). Dopamine D1 and adenosine A2A receptor signaling was attenuated by prolonged activation of α2-adrenoceptors, whereas it was enhanced by pharmacological blockade of α2-adrenoceptors and chemical lesioning of noradrenergic neurons in the LC. This indicates that α2-adrenoceptors, coupled to Gi, are tonically active and counteract Gs/olf-coupled dopamine D1 and adenosine A2A receptor signaling in striatonigral and striatopallidal neurons, respectively. The tonic activity of Gi-coupled α2-adrenoceptors is also required for the action of dopamine D2 receptors, another Gi-coupled receptor expressed in striatopallidal neurons. Thus, α2-adrenoceptors induce two distinct effects on dopamine signaling: inhibition of dopamine D1 receptor signaling in striatonigral neurons and enhancement of dopamine D2 receptor signaling in striatopallidal neurons. Such functional features of α2-aderenoceptors suggest that α2-aderenoceptors could be a target of therapeutic drugs for Parkinson's disease.
Fig. 8. Interaction of β1- and α2A/C-adrenoceptor signaling with dopamine D1 and D2 receptor signaling in the striatum.
β1- and α2A/C-adrenoceptors regulate PKA/DARPP-32 signaling both in striatopallidal and striatonigral neurons, in addition to the inhibitory regulation of dopamine (DA) release by α2A/C-adrenoceptors. In striatopallidal neurons, activation of α2A/C-adrenoceptors, coupled to Gi, inhibits adenosine A2A receptor/Gs/olf/adenylyl cyclase (AC)/PKA/DARPP-32 signaling, and therefore enhances dopamine D2 receptor/Gi signaling. In striatonigral neurons, activation of α2A/C-adrenoceptors inhibits dopamine D1 receptor/Gs/olf/AC/PKA/DARPP-32 signaling. Thus, α2A/C-adrenoceptors differentially regulate dopamine signaling in two pathways, and activation of α2A/C-adrenoceptors by noradrenaline (NA) is required for dopamine to elicit its regulatory role in motor function via D2 receptors.
Noradrenergic innervation and the release of noradrenaline in the striatum
Despite the functional importance of noradrenaline in dopaminergic neurotransmission, striatum receives only sparse noradrenergic innervation (Swanson and Hartman 1975; Aston-Jones 2004). However, the presence of noradrenaline at the extracellular spaces in the striatum was demonstrated by microdialysis studies (Cenci et al. 1992; Li et al. 1998; Dawson et al. 2000; Gobert et al. 2004), and the synthesis of noradrenaline from dopamine in striatal tissues was detected in vitro (Udenfriend and Creveling 1959; Ross and Reis 1974) and in vivo (McGeer et al. 1963), suggesting the presence of dopamine β-hydroxylase activity in the striatum. Furthermore, the presence of noradrenaline transporter in the striatum was reported (Moll et al. 2000; Moron et al. 2002; Arai et al. 2008). Recently, Gobert et al. (Gobert et al. 2004) demonstrated that noradrenaline in the striatum was derived from noradrenergic terminals and its release was subject to inhibitory control by α2-adrenoceptors. It is also possible that noradrenaline may be synthesized by dopamine β-hydroxylase expressed in striatal cells other than noradrenergic terminals. We found that the relatively selective noradrenaline transporter inhibitor, nortriptyline, increased DARPP-32 Thr34 phosphorylation via activation of adrenergic as well as serotonergic receptors in neostriatal slices, indicating the presence of functional noradrenergic terminals in the striatum. This finding is supported by the fact that the lesioning of noradrenergic neurons by DSP-4 enhanced striatal dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling. Taken together, the presence of noradrenergic terminals in the striatum is supported by neuropharmacological and biochemical data, although contradictory data have also been reported (Versteeg et al. 1976; Oke et al. 1978).
Functional roles of β1-adrenoceptors in the striatum
β1-adrenoceptors are highly expressed in striatal neurons including cholinergic interneurons (Nahorski et al. 1979; Pazos et al. 1985; Pisani et al. 2003). Immunohistochemical analysis revealed the expression of β1-adrenoceptors both in striatonigral and striatopallidal neurons. Activation of β1-adrenoceptors, but not of β2-adrenoceptors, by isoproterenol induced the up-regulation of cAMP/PKA signaling, leading to the phosphorylation of DARPP-32 at Thr34 in the two types of striatal medium spiny neurons. The isoproterenol-induced phosphorylation of DARPP-32 at Thr34 was not due to the release of dopamine and subsequent activation of D1 receptors (Reisine et al. 1982) or the cross activation of D1 receptors by isoproterenol (Vanderheyden et al. 1986), since the effect of isoproterenol was not antagonized by the dopamine D1 receptor antagonist. Under physiological conditions in vivo, β1-adrenoceptors might be activated by noradrenaline released from noradrenergic terminals and/or by phasically released dopamine, resulting in increased excitability of both striatonigral and striatopallidal neurons due to activation of PKA/DARPP-32 signaling. Such changes in the activity of the basal ganglia circuit may play a role in psychomotor functions. The β-adrenergic antagonist, propranolol, has been used for the treatment of essential tremor and anxiety disorders (Emilien and Maloteaux 1998; Pahwa and Lyons 2003), although the mechanisms underlying its therapeutic effect are not understood. Whether the effects of β1-adrenoceptors on DARPP-32 phosphorylation in the two types of striatal neurons presented here relate to the pathophysiology of essential tremor or anxiety disorders remains to be determined.
Functional roles of α2-adrenoceptors in the striatum
Activation of α2-adrenoceptors by UK14304 attenuated the dopamine D1 and adenosine A2A receptor-induced increase in DARPP-32 Thr34 phosphorylation in striatal neurons. Our previous studies using striatal slices from D1-DARPP-32-Flag/D2-DARPP-32-Myc mice (Bateup et al. 2008; Kuroiwa et al. 2008) demonstrated that activation of dopamine D1 and adenosine A2A receptors selectively stimulates DARPP-32 Thr34 phosphorylation in striatonigral and striatopallidal neurons, respectively. Taken together, these findings indicate that activation of α2-adrenoceptors inhibits PKA/DARPP-32 signaling in both types of striatal neurons.
Pharmacological blockade of α2-adrenoceptors and chemical lesioning of noradrenergic neurons induced the enhancement of dopamine D1 and adenosine A2A receptor/PKA/DARPP-32 signaling, indicating that α2-adrenoceptors are activated under basal conditions and tonically inhibit PKA/DARPP-32 signaling in both striatopallidal and striatonigral neurons. It has been demonstrated that α2-adrenoceptors, specifically α2C-subtype adrenoceptors, are negatively coupled to adenylyl cyclase in the striatum using mice with infusion of antisense oligonucleotides against α2C-adrenoceptor mRNA (Lu and Ordway 1997) and with targeted inactivation of the α2C-adrenoceptor gene (Zhang et al. 1999). The possibility that dopamine is the endogenous activator of α2-adrenoceptors in the striatum has been proposed (Zhang et al. 1999), due to the sparse noradrenergic innervation. However, the data presented here suggest that α2-adrenoceptors are tonically activated by noradrenaline, because lesioning of noradrenergic neurons with DSP-4 mimics the effect of α2-adrenoceptor antagonism.
In behavioral studies, it has been shown that amphetamine-induced locomotor activity is enhanced in mice lacking α2C-adrenoceptors, whereas the opposite change is observed in mice over-expressing α2C-adrenoceptors (Sallinen et al. 1998b). Thus, α2C-adrenoceptors likely play an inhibitory role in the regulation of motor function under conditions of high dopamine tone (Scheinin et al. 2001). Our biochemical data demonstrate that activation of α2-adrenoceptors induces (1) down-regulation of dopamine D1 receptor/PKA signaling in striatonigral neurons, (2) down-regulation of adenosine A2A receptor/PKA signaling in striatopallidal neurons, and (3) up-regulation of dopamine D2 receptor signaling in striatopallidal neurons (Fig. 8). The role of α2C-adrenoceptors in amphetamine-induced locomotor activity can be explained by the modulation of dopamine D1 receptor/PKA signaling in striatonigral neurons. Removal of the inhibitory effect of α2C-adrenoceptors on dopamine D1 receptor/PKA signaling and the subsequent activation of striatonigral neurons presumably results in the enhancement of amphetamine-induced locomotor activity. If α2C-adrenoceptors were predominantly to affect striatopallidal neurons, then removal of the modulatory effect of α2C-adrenoceptors on striatopallidal neurons would induce activation of adenosine A2A receptor/PKA signaling and inhibition of dopamine D2 receptor signaling, resulting in activation of striatopallidal neurons and an attenuation of amphetamine-induced locomotor activity. Thus, when dopamine tone is high, the inhibitory role of α2C-adrenoceptors might be important to suppress the over-activation of dopamine D1 receptor/PKA signaling in striatonigral neurons. However, the role of α2C-adrenoceptors observed under conditions of amphetamine-induced high dopamine-tone seems to be different from that under conditions of low dopamine-tone in Parkinson's disease, as described below.
At early time points (30 s and 1 min), activation of α2-adrenoceptors induced a transient increase in DARPP-32 Thr34 phosphorylation selectively in striatonigral neurons. The effect was not mediated through activation of dopamine D1 receptors in striatonigral neurons by released dopamine, because a dopamine D1 receptor antagonist, SCH23390, failed to block the effect of α2-adrenoceptor activation (data not shown) and α2-adrenoceptors are known to presynaptically inhibit dopamine release in the striatum (Bucheler et al. 2002). Activation of α2-adrenoceptors has been reported to induce the release of GABA (Zhang and Ordway 2003) and phospholipase C-mediated activation of PKA (Karkoulias et al. 2007), both of which may result in the phosphorylation of DARPP-32 at Thr34 (Snyder et al. 1994). It is not clear why activation of α2-adrenoceptors induces DARPP-32 phosphorylation selectively in striatonigral neurons, since α2C-adrenoceptors are expressed in both striatonigral and striatopallidal neurons. It is possible that transient activation of dopamine D2 receptors counteracts the α2C-adrenoceptor-induced phosphorylation of DARPP-32 in striatopallidal neurons, although this is just speculation and requires further study. However, mechanisms for the activation of PKA/DARPP-32 signaling selectively in striatonigral neurons were not further investigated, because the α2-adrenoceptor-induced phosphorylation of DARPP-32 at early time points was relatively small and transient, and the physiological significance of this phenomenon is not clear.
Role of α2-adrenoceptors in Parkinson's disease
In animal models of Parkinson's disease, the degeneration of noradrenaline neurons in addition to dopamine neurons is involved in motor deficits of Parkinson's disease (Srinivasan and Schmidt 2003; Rommelfanger et al. 2007), and the symptoms of Parkinson's disease are improved by restoration of noradrenaline (Rommelfanger et al. 2007). In addition, in reserpine-induced akinesia, administration of L-DOPA improves akinesia, and the effect of L-DOPA is reported to be mediated in part by the synthesis of noradrenaline and activation of α2-adrenoceptors (Dolphin et al. 1976a; Dolphin et al. 1976b). These results indicate that noradrenaline plays a critical role in the regulation of motor functions by interacting with dopamine signaling pathways in the striatum.
α2-adrenoceptors expressed in striatopallidal neurons likely play a therapeutic role in Parkinson's disease. Here, we find that activation of α2-adrenoceptors in striatopallidal neurons induces down-regulation of adenosine A2A receptor/PKA signaling and up-regulation of dopamine D2 receptor signaling (Fig. 8). These modulatory effects of α2-adrenoceptors potentiate the action of dopamine through dopamine D2 receptors, which likely improves the symptoms of Parkinson's disease. Indeed, it has been shown that clonidine, an α2-adrenoceptor agonist, itself increases locomotor activity (Hill and Brotchie 1999), and enhances locomotor activity induced by dopamine receptor agonists (Pycock et al. 1977), muscarinic receptor antagonists (Carlsson et al. 1991), and a κ-opioid receptor agonist (Hill and Brotchie 1999) in monoamine-depleted animals.
Interestingly, α2-adrenoceptor antagonists such as idazoxan are also reported to have antiparkinsonian effects and attenuate L-DOPA-induced dyskinesia (Grondin et al. 2000; Srinivasan and Schmidt 2004; Fornai et al. 2007). α2-adrenoceptor antagonists have been demonstrated to enhance the release of noradrenaline by blocking presynaptic α2-adrenoceptors (Dennis et al. 1987). The increase in noradrenergic neurotransmission likely results in the potentiation of dopaminergic signaling (Dickinson et al. 1988; Mavridis et al. 1991), thereby inducing antiparkinsonian effects. Both the inhibition of presynaptic α2-adrenoceptors, which activates noradrenergic neurotransmission, and the stimulation of postsynaptic α2-adrenoceptors could be useful therapeutic approaches for the treatment of Parkinson's disease. The therapeutic potential of either α2-adrenergic antagonists or agonists may be determined by the stage of Parkinson's disease. When noradrenergic innervation is conserved in early stages, the α2-adrenoceptor antagonist is expected to improve the symptoms of Parkinson's disease, whereas the α2-adrenoceptor agonist may be useful in late stages. Indirect and direct activation of α2-adrenoceptors by α2-adrenoceptor antagonists and agonists, respectively, likely potentiates dopamine D2 receptor signaling in striatopallidal neurons and inhibits dopamine D1 receptor signaling in striatonigral neurons. The modulation of dopaminergic signaling by α2-adrenoceptor activation may improve the symptoms of Parkinson's disease and prevent the development of L-DOPA-induced dyskinesia (Santini et al. 2009). The long-term effects of α2-adrenoceptor antagonists and/or agonists in various stages of Parkinson's disease need to be clarified.
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (18300128 to A.N.) and grants from the U.S.P.H.S. (MH074866 and DA10044 to P.G.), the Michael Stern Parkinson's Research Foundation (to P.G.) and the Department of Defense (DOD/USAMRAA W81XWH-09-1-402 to P.G.). The authors thank Yukako Terasaki, Keiko Fujisaki and Michiko Koga for excellent technical assistance.
Abbreviations
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa
- LC
locus coeruleus
- PKA
protein kinase A
References
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, Kobilka BK, Hein L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Arai A, Tomiyama M, Kannari K, Kimura T, Suzuki C, Watanabe M, Kawarabayashi T, Shen H, Shoji M. Reuptake of L-DOPA-derived extracellular DA in the striatum of a rodent model of Parkinson's disease via norepinephrine transporter. Synapse. 2008;62:632–635. doi: 10.1002/syn.20535. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G. Locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The rat nervous system. Asademic Press; San Diego: 2004. pp. 259–294. [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11:932–939. doi: 10.1038/nn.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Stratford TL, Foote SL, Kelley AE. Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse. 1997;27:230–241. doi: 10.1002/(SICI)1098-2396(199711)27:3<230::AID-SYN8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bucheler MM, Hadamek K, Hein L. Two alpha(2)-adrenergic receptor subtypes, alpha(2A) and alpha(2C), inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Svensson A, Carlsson A. Synergistic interactions between muscarinic antagonists, adrenergic agonists and NMDA antagonists with respect to locomotor stimulatory effects in monoamine-depleted mice. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:568–573. doi: 10.1007/BF00184286. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Kalen P, Mandel RJ, Bjorklund A. Regional differences in the regulation of dopamine and noradrenaline release in medial frontal cortex, nucleus accumbens and caudate-putamen: a microdialysis study in the rat. Brain Res. 1992;581:217–228. doi: 10.1016/0006-8993(92)90711-h. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Li P. In vivo effects of the 5-HT(6) antagonist SB-271046 on striatal and frontal cortex extracellular concentrations of noradrenaline, dopamine, 5-HT, glutamate and aspartate. Br J Pharmacol. 2000;130:23–26. doi: 10.1038/sj.bjp.0703288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T, L'Heureux R, Carter C, Scatton B. Presynaptic alpha-2 adrenoceptors play a major role in the effects of idazoxan on cortical noradrenaline release (as measured by in vivo dialysis) in the rat. J Pharmacol Exp Ther. 1987;241:642–649. [PubMed] [Google Scholar]
- Dickinson SL, Gadie B, Tulloch IF. Alpha 1- and alpha 2-adrenoreceptor antagonists differentially influence locomotor and stereotyped behaviour induced by d-amphetamine and apomorphine in the rat. Psychopharmacology (Berl) 1988;96:521–527. doi: 10.1007/BF02180034. [DOI] [PubMed] [Google Scholar]
- Dolphin A, Jenner P, Marsden CD. Modification of the L-DOPA reversal of reserpine akinesia by inhibitors of dopamine-beta-hydroxylase. Eur J Pharmacol. 1976a;35:135–144. doi: 10.1016/0014-2999(76)90308-3. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Jenner P, Marsden CD. The relative importance of dopamine and noradrenaline receptor stimulation for the restoration of motor activity in reserpine or alpha-methyl-p-tyrosine pre-treated mice. Pharmacol Biochem Behav. 1976b;4:661–670. doi: 10.1016/0091-3057(76)90217-3. [DOI] [PubMed] [Google Scholar]
- Emilien G, Maloteaux JM. Current therapeutic uses and potential of beta-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998;53:389–404. doi: 10.1007/s002280050399. [DOI] [PubMed] [Google Scholar]
- Fornai F, di Poggio AB, Pellegrini A, Ruggieri S, Paparelli A. Noradrenaline in Parkinson's disease: from disease progression to current therapeutics. Curr Med Chem. 2007;14:2330–2334. doi: 10.2174/092986707781745550. [DOI] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Gobert A, Billiras R, Cistarelli L, Millan MJ. Quantification and pharmacological characterization of dialysate levels of noradrenaline in the striatum of freely-moving rats: release from adrenergic terminals and modulation by alpha2-autoreceptors. J Neurosci Methods. 2004;140:141–152. doi: 10.1016/j.jneumeth.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson's disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9:279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- Grondin R, Hadj Tahar A, Doan VD, Ladure P, Bedard PJ. Noradrenoceptor antagonism with idazoxan improves L-dopa-induced dyskinesias in MPTP monkeys. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:181–186. doi: 10.1007/s002109900167. [DOI] [PubMed] [Google Scholar]
- Hemmings JHC, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MP, Brotchie JM. The adrenergic receptor agonist, clonidine, potentiates the anti-parkinsonian action of the selective kappa-opioid receptor agonist, enadoline, in the monoamine-depleted rat. Br J Pharmacol. 1999;128:1577–1585. doi: 10.1038/sj.bjp.0702943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg M, Scheinin M, Kurose H, Miettinen R. Adrenergic alpha2C-receptors reside in rat striatal GABAergic projection neurons: comparison of radioligand binding and immunohistochemistry. Neuroscience. 1999;93:1323–1333. doi: 10.1016/s0306-4522(99)00260-2. [DOI] [PubMed] [Google Scholar]
- Ihalainen JA, Tanila H. In vivo regulation of dopamine and noradrenaline release by alpha2A-adrenoceptors in the mouse nucleus accumbens. J Neurochem. 2004;91:49–56. doi: 10.1111/j.1471-4159.2004.02691.x. [DOI] [PubMed] [Google Scholar]
- Karkoulias G, Mastrogianni O, Papathanasopoulos P, Paris H, Flordellis C. Alpha2-adrenergic receptors activate cyclic AMP-response element-binding protein through arachidonic acid metabolism and protein kinase A in a subtype-specific manner. J Neurochem. 2007;103:882–895. doi: 10.1111/j.1471-4159.2007.04852.x. [DOI] [PubMed] [Google Scholar]
- Kuroiwa M, Bateup HS, Shuto T, Higashi H, Tanaka M, Nishi A. Regulation of DARPP-32 phosphorylation by three distinct dopamine D1-like receptor signaling pathways in the neostriatum. J Neurochem. 2008;107:1014–1026. doi: 10.1111/j.1471-4159.2008.05702.x. [DOI] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci U S A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Wissekerke AE, Rosin DL, Lynch KR. Localization of alpha2C-adrenergic receptor immunoreactivity in catecholaminergic neurons in the rat central nervous system. Neuroscience. 1998;84:1085–1096. doi: 10.1016/s0306-4522(97)00578-2. [DOI] [PubMed] [Google Scholar]
- Li XM, Perry KW, Wong DT, Bymaster FP. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl) 1998;136:153–161. doi: 10.1007/s002130050551. [DOI] [PubMed] [Google Scholar]
- Lu L, Ordway GA. Alpha2C-adrenoceptors mediate inhibition of forskolin-stimulated cAMP production in rat striatum. Brain Res Mol Brain Res. 1997;52:228–234. doi: 10.1016/s0169-328x(97)00257-x. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- Mavridis M, Colpaert FC, Millan MJ. Differential modulation of (+)-amphetamine-induced rotation in unilateral substantia nigra-lesioned rats by alpha 1 as compared to alpha 2 agonists and antagonists. Brain Res. 1991;562:216–224. doi: 10.1016/0006-8993(91)90624-5. [DOI] [PubMed] [Google Scholar]
- McGeer EG, Ling GM, McGeer PL. Conversion of tyrosine to catecholamines by cat brain in vivo. Biochemical and biophysical research communications. 1963;13:291–296. [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahorski SR, Howlett DR, Redgrave P. Loss of beta-adrenoceptor binding sites in rat striatum following kainic acid lesions. Eur J Pharmacol. 1979;60:249–252. doi: 10.1016/0014-2999(79)90226-7. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Hokfelt T, Pieribone VA. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol Sci. 1996;17:245–255. doi: 10.1016/0165-6147(96)10022-5. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller DB, O'Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke A, Keller R, Adams RN. Dopamine and norepinephrine enhancement in discrete rat brain regions following neonatal 6-hydroxydopamine treatment. Brain Res. 1978;148:245–250. doi: 10.1016/0006-8993(78)90398-0. [DOI] [PubMed] [Google Scholar]
- Pahwa R, Lyons KE. Essential tremor: differential diagnosis and current therapy. Am J Med. 2003;115:134–142. doi: 10.1016/s0002-9343(03)00259-6. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios JM. Beta-adrenoceptor subtypes in the human brain: autoradiographic localization. Brain Res. 1985;358:324–328. doi: 10.1016/0006-8993(85)90977-1. [DOI] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Martorana A, Fusco F, Sancesario G, De Persis C, Bernardi G, Calabresi P. Activation of beta1-adrenoceptors excites striatal cholinergic interneurons through a cAMP-dependent, protein kinase-independent pathway. J Neurosci. 2003;23:5272–5282. doi: 10.1523/JNEUROSCI.23-12-05272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ, Jenner PG, Marsden CD. The interaction of clonidine with dopamine-dependent behaviour in rodents. Naunyn Schmiedebergs Arch Pharmacol. 1977;297:133–141. doi: 10.1007/BF00499922. [DOI] [PubMed] [Google Scholar]
- Reisine TD, Chesselet MF, Lubetzki C, Cheramy A, Glowinski J. A role for striatal beta-adrenergic receptors in the regulation of dopamine release. Brain Res. 1982;241:123–130. doi: 10.1016/0006-8993(82)91235-5. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Talley EM, Lee A, Stornetta RL, Gaylinn BD, Guyenet PG, Lynch KR. Distribution of alpha 2C-adrenergic receptor-like immunoreactivity in the rat central nervous system. J Comp Neurol. 1996;372:135–165. doi: 10.1002/(SICI)1096-9861(19960812)372:1<135::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Ross RA, Reis DJ. Effects of lesions of locus coeruleus on regional distribution of dopamine-beta-hydroxylase activity in rat brain. Brain Res. 1974;73:161–166. doi: 10.1016/0006-8993(74)91016-6. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. Adrenergic alpha2C-receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J Neurosci. 1998a;18:3035–3042. doi: 10.1523/JNEUROSCI.18-08-03035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. D-amphetamine and L-5-hydroxytryptophan-induced behaviours in mice with genetically-altered expression of the alpha2C-adrenergic receptor subtype. Neuroscience. 1998b;86:959–965. doi: 10.1016/s0306-4522(98)00100-6. [DOI] [PubMed] [Google Scholar]
- Santini E, Alcacer C, Cacciatore S, Heiman M, Herve D, Greengard P, Girault JA, Valjent E, Fisone G. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–633. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Sallinen J, Haapalinna A. Evaluation of the alpha2C-adrenoceptor as a neuropsychiatric drug target studies in transgenic mouse models. Life Sci. 2001;68:2277–2285. doi: 10.1016/s0024-3205(01)01016-5. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fisone G, Greengard P. Phosphorylation of DARPP-32 is regulated by GABA in rat striatum and substantia nigra. J Neurochem. 1994;63:1766–1771. doi: 10.1046/j.1471-4159.1994.63051766.x. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Girault JA, Chen JYC, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: Regulation by factors other than dopamine. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci. 2003;17:2586–2592. doi: 10.1046/j.1460-9568.2003.02684.x. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Schmidt WJ. The effect of the alpha2-adrenoreceptor antagonist idazoxan against 6-hydroxydopamine-induced Parkinsonism in rats: multiple facets of action? Naunyn Schmiedebergs Arch Pharmacol. 2004;369:629–638. doi: 10.1007/s00210-004-0929-2. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Liu F, Fienberg AA, Nomikos GG, Greengard P. DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc Natl Acad Sci U S A. 2002;99:3188–3193. doi: 10.1073/pnas.052712699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Starke K, Limberger N. Presynaptic alpha 2A-adrenoceptors inhibit the release of endogenous dopamine in rabbit caudate nucleus slices. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:473–481. doi: 10.1007/BF00173016. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Creveling CR. Localization of dopamine-beta-oxidase in brain. J Neurochem. 1959;4:350–352. doi: 10.1111/j.1471-4159.1959.tb13216.x. [DOI] [PubMed] [Google Scholar]
- Vanderheyden P, Ebinger G, Kanarek L, Vauquelin G. Epinephrine and norepinephrine stimulation of adenylate cyclase in bovine retina homogenate: evidence for interaction with the dopamine D1 receptor. Life Sci. 1986;38:1221–1227. doi: 10.1016/0024-3205(86)90177-3. [DOI] [PubMed] [Google Scholar]
- Versteeg DH, Van Der Gugten J, De Jong W, Palkovits M. Regional concentrations of noradrenaline and dopamine in rat brain. Brain Res. 1976;113:563–574. doi: 10.1016/0006-8993(76)90057-3. [DOI] [PubMed] [Google Scholar]
- Waeber C, Rigo M, Chinaglia G, Probst A, Palacios JM. Beta-adrenergic receptor subtypes in the basal ganglia of patients with Huntington's chorea and Parkinson's disease. Synapse. 1991;8:270–280. doi: 10.1002/syn.890080405. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of alpha 2 adrenoceptors during rat brain development--II. Alpha 2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1997;76:261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Yavich L, Lappalainen R, Sirvio J, Haapalinna A, MacDonald E. Alpha2-adrenergic control of dopamine overflow and metabolism in mouse striatum. Eur J Pharmacol. 1997;339:113–119. doi: 10.1016/s0014-2999(97)01375-7. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ordway GA. The alpha2C-adrenoceptor modulates GABA release in mouse striatum. Brain Res Mol Brain Res. 2003;112:24–32. doi: 10.1016/s0169-328x(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Zhang W, Klimek V, Farley JT, Zhu MY, Ordway GA. alpha2C adrenoceptors inhibit adenylyl cyclase in mouse striatum: potential activation by dopamine. J Pharmacol Exp Ther. 1999;289:1286–1292. [PubMed] [Google Scholar]



