Abstract
Adolescents diagnosed with an alcohol use disorder show neurodegeneration in the hippocampus, a region important for learning, memory, and mood regulation. This study examines a potential mechanism by which excessive alcohol intake, characteristic of an alcohol use disorder, produces neurodegeneration. As hippocampal neural stem cells underlie ongoing neurogenesis, a phenomenon that contributes to hippocampal structure and function, we investigated aspects of cell death and cell birth in an adolescent rat model of an alcohol use disorder. Immunohistochemistry of various markers along with Bromo-deoxy-Uridine (BrdU) injections were used to examine different aspects of neurogenesis. After 4 days of binge alcohol exposure, neurogenesis was decreased by 33% and 28% at 0 and 2 days after the last dose according to doublecortin expression. To determine whether this decrease in neurogenesis was due to effects on neural stem cell proliferation, quantification of BrdU-labeled cells revealed a 21% decrease in the dentate gyrus of alcohol-exposed brains. Cell survival and phenotype of BrdU-labeled cells were assessed 28 days after alcohol exposure and revealed a significant, 50% decrease in the number of surviving cells in the alcohol-exposed group. Reduced survival was supported by significant increases in the number of pyknotic-, FluoroJade B positive-, and TUNEL-positive cells. However, so few cells were TUNEL-positive that cell death is likely necrotic in this model. Although alcohol decreased the number of newborn cells, it did not affect the percentage of cells that matured into neurons (differentiation). Thus, our data support that in a model of an adolescent alcohol use disorder, neurogenesis is impaired by two mechanisms: alcohol-inhibition of neural stem cell proliferation and alcohol effects on new cell survival. Remarkably, alcohol inhibition of neurogenesis may outweigh the few dying cells per section, which implies that alcohol inhibition of neurogenesis contributes to hippocampal neurodegeneration in alcohol use disorders.
Keywords: ethanol, alcoholism, neural stem cell, progenitor, cell death
The likelihood of developing an alcohol use disorder (AUD) triples in adolescents who begin drinking younger than 14 versus after age 18 (SAMHSA, Administration, 2008). Over 70 percent of teens have tried alcohol by the 12th grade with ”heavy drinking” (five or more drinks in one sitting in the last two weeks) or “having been drunk” reported in 25 to 30 percent of 17–18 year olds respectively (Johnston et al., 2007). Indeed, over five percent of 12–17 year olds meet the diagnostic criteria for an AUD (Harford et al., 2005). Adolescents drink quantities of alcohol similar to adults due to their pattern of intake (Deas et al., 2000). Approximately 60% of high school students who currently drink alcohol are binge drinkers (5 or more drinks/occasion; Zeigler et al., 2005). A binge drinking pattern is one of the few factors which predicts brain damage from alcohol (Hunt, 1993), which has led several groups to propose that binge drinking starts the downward spiral towards developing an AUD (Bechara, 2005; Crews, 1999; Koob and Le Moal, 1997). These statistics highlight that adolescence may be a window of vulnerability for developing an AUD (Spear, 2004). Thus, it is critical to investigate the distinct effects of binge alcohol exposure has on the adolescent brain.
The adolescent brain responds uniquely to alcohol across a variety of measures (Crews et al., 2007; Little et al., 1996; White and Swartzwelder, 2004). Adolescents are less sensitive to sedative and motor-impairing effects of alcohol - effects which may promote greater drinking versus adults - whereas animal models support that adolescence is a period of enhanced susceptibility to alcohol neurotoxicity (Crews et al., 2000; Evrard et al., 2006; Hargreaves et al., 2009; Silveri and Spear, 1998; Slawecki et al., 2001; White et al., 2002). Although adolescents with AUDs demonstrate cognitive deficits across a variety of spectrums, both animal models and human studies show that the hippocampus and its associated functions are particularly impaired in adolescents with an AUD (Moss et al., 1994; Tapert et al., 2001; Tapert et al., 2004; Tarter et al., 1995; see also White and Swartzwelder, 2004; Zeigler et al., 2005 for reviews). Indeed, hippocampal volume loss has been observed in adolescent humans with AUDs (De Bellis et al., 2000; Medina et al., 2007; Nagel et al., 2005). Despite the convincing literature that the adolescent hippocampus is vulnerable to alcohol impairment, little data exists as to what degenerates. In adolescent rats exposed to alcohol, hippocampal neurodegeneration is suggested by evidence of inflammation and gliosis (Evrard et al., 2006; Pascual et al., 2007) and a lone report of a small increase in apoptotic cell death in the dentate gyrus after acute injections of alcohol (Jang et al., 2002). And, although adolescent rats are reported to have greater alcohol-induced cell death, no data was shown for hippocampal regions (Crews et al., 2000).
Many groups now maintain that hippocampal structure and function relies upon hippocampal stem cells and constitutive neurogenesis (Imayoshi et al., 2008; Kempermann et al., 2004). The dentate gyrus subgranular zone (SGZ) of the hippocampus is one of two regions in the adult brain that contain neural stem cells (NSCs) that underlie adult neurogenesis (Palmer et al., 1997). The thousands of new cells added daily to the dentate gyrus suggest its role in hippocampal structure and/or function (Cameron and McKay, 2001). Neurogenesis consists of four main components: NSC proliferation followed by newborn cell migration, differentiation, and survival. In adolescent rats, adult neurogenesis has only been examined after an acute dose of alcohol. Although, adolescents appeared to be more susceptible to acute alcohol inhibition of NSC proliferation (Crews et al., 2006b), responses to an acute dose of alcohol may be very different than that which occurs during the course of an AUD. Acute doses of alcohol are less toxic and various adaptations occur as tolerance and dependence develop with more chronic exposure. Further, because neuromaturation continues into late adolescence, studies based on adults cannot necessarily generalize to adolescents (Spear, 2000). Therefore, as adolescents with AUDs show hippocampal neurodegeneration (De Bellis et al., 2000) and there are no reports to date on the effect of alcohol on adult neurogenesis within the context of an AUD, we investigated the effect alcohol has on the individual components of neurogenesis in a model of an AUD in adolescent rats.
Methods and Materials
Binge Alcohol Treatment
All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and strictly followed the Guidelines for the Care and Use of Laboratory Animals (NRC, 1996). Fifty-one adolescent male Sprague-Dawley rats (Charles River Laboratories; Portage, MI) were individually housed on a 12 h light/dark schedule. Rats arrived on approximately postnatal day 28 and were allowed to acclimate to the animal facility with food and water available ad libitum, except during alcohol treatment. At postnatal day 35, an age corresponding to mid adolescence (Spear and Brake, 1983), rats were administered a nutritionally complete alcohol diet (25% ethanol w/v in Vanilla Ensure®; n=26) or control diet (n=25) via intragastric gavage every 8h for 4d following the modified Majchrowicz (1975) binge model (Nixon and Crews, 2004). An initial 5 g/kg dose was given with subsequent doses titrated according to the behavioral intoxication state of the rat as assessed with a six point scale: 0-normal rat (5 g/kg), 1-hypoactive (4 g/kg), 2-ataxia (3 g/kg), 3-delayed righting reflex and ataxia with dragging abdomen (2 g/kg), 4-loss of righting reflex (1 g/kg) and 5-loss of eye blink reflex (0 g/kg). Control rats received an isocaloric diet of Vanilla Ensure® and dextrose. To verify intoxication, blood ethanol concentrations (BECs) were determined from tail blood. On Day 2 of the binge, tail blood was extracted approximately 90 m after the afternoon dose of alcohol, centrifuged and stored at −20°C until analysis. BECs were determined from plasma on a GM7 Alcohol Analyser (Analox, Lunenburg, MA).
Tissue Preparation
Rat brain tissue was harvested in a time course during and after binge exposure: 4D (directly after the last dose of alcohol on the fourth day), 4D+2 (2d after the last dose of alcohol), 4D+7, and 4D+28. To examine cell proliferation, rats were administered Bromo-deoxy-Uridine (BrdU; 300mg/kg, i.p; Fluka, Buchs, Switzerland) and sacrificed two hours later (4D injected 1 h after the last dose of alcohol). To investigate cell survival, BrdU (300mg/kg, i.p.) was administered at 4D and the rats were then sacrificed 28 days later (4D+28 days). The 300 mg/kg dose of BrdU was chosen as that which maximally labels dividing cells in PND 35 rats (Cameron and McKay, 2001). Identical to previous reports (Knapp and Crews, 1999; Nixon and Crews, 2004), rats were sacrificed by anesthetic overdose (Nembutal®; MWI Veterinary Supply, Nampa, Idaho) and transcardially perfused with 0.1M phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (pH 7.4 in 0.1M phosphate buffer). Brains were postfixed for 24 h in 4% paraformaldehyde then rinsed and stored in PBS until sectioning. Brains were sectioned coronally, at a thickness of 40 μm from Bregma 1.2 through −7.0, on a vibrating microtome (Leica VT1000S, Germany) and were collected and stored in cryoprotectant in 24 well plates at −20°C until use.
Neurogenesis Detection
Immunohistochemistry
To examine the various components of neurogenesis, immunohistochemistry with diaminobenzidine (DAB) detection was conducted on free-floating brain sections as described previously (Nixon and Crews, 2002; Nixon and Crews, 2004). Doublecortin (DCX, 1:400; Santa Cruz, Santa Cruz, CA), a well-accepted marker of immature neurons, was used to examine neurogenesis on every 12th section (Brown et al., 2003; Rao and Shetty, 2004). Cell proliferation and new cell survival were examined by manipulating the timing of BrdU injection versus sacrifice. BrdU (1μg/ml; Chemicon, Temecula, CA) immunohistochemistry was conducted on every 6th section of tissue and used a DNA denaturing step as described previously (Kuhn et al., 1996; Nixon and Crews, 2004). Ki-67 (1:200, Vector Labs, Burlingame, CA), an endogenous marker of proliferation that labels cells in all active phases of the cell cycle, was conducted on every 12th section of tissue (Scholzen and Gerdes, 2000). With the exception of the DNA denaturing step for BrdU, assays followed the same basic protocol as follows: cryoprotectant was rinsed off in tris buffered saline (TBS, Bio-Rad, Hercules, CA) and endogenous peroxidases were quenched by 0.3% H2O2 in TBS. Following washes, sections were blocked in TBS+ (TBS/0.1% or 0.2% of triton-X/3% normal serum as appropriate) and incubated in primary antibody at +4°C for 16–48 h, dependent upon the antibody. Primary antibody dilutions and durations were determined in dilution curves and antibody specificity was verified by control experiments that omitted the primary antibody, secondary antibody and/or BrdU injection. After incubation, sections were washed in TBS+ and then incubated in the appropriate biotinylated secondary antibody plus 1.5% serum for 1 h, followed by avidin-biotin-peroxidase complex (ABC elite kit, Vector Labs) for 1 h, and Nickel-enhanced DAB (Polysciences, Warrington, PA) as a chromagen. Sections were mounted onto glass slides and dried. For Ki-67 labeled tissue, mounted sections were counterstained in neutral red dye (Fisher Scientific, Waltham, MA) whereas the BrdU labeled tissue was counterstained with cresyl violet dye (Fisher Scientific). All sections were coverslipped with Cytoseal® (Stephens Scientific, Wayne, NJ)
Fluorescent Immunohistochemistry
To investigate BrdU-positive (BrdU+) cell phenotype, triple fluorescence immunohistochemistry for a neuron specific marker (neuronal nuclei: NeuN), astroglia specific marker (glial fibrillary acidic protein: GFAP), and BrdU was conducted on the 4D+28 tissue. Identical to procedures reported previously, every 12th section had DNA denatured before blocking in TBS+ (Nixon and Crews, 2004; Nixon et al., 2008). The sections were then incubated for 48 h at 4°C in the following primary antibodies in TBS+: rat anti-BrdU (1:400, Accurate, Westbury, NY), mouse anti-NeuN (1:500, Chemicon) and rabbit anti-GFAP (1:2500, DAKO, Glostrup, Denmark). Following rinses in TBS+, the sections were incubated for 1 h in fluorescent-conjugated secondary antibodies: goat anti-rat Alexa Fluor 488, goat anti-mouse Alexa Fluor 633, and goat anti-rabbit Alexa Fluor 555 (1:200, Molecular Probes, Invitrogen, San Diego, CA). Following washes in TBS, the sections were mounted on glass slides and coverslipped, using Pro-long® Gold anti-fade reagent (Molecular Probes, Invitrogen). Sections were kept in the dark at all points possible from secondary incubation forward.
Neurodegeneration Detection
Fluoro-Jade B
Every 12th section was processed for Fluoro-Jade B (FJB), as described (Obernier et al., 2002; Schmued and Hopkins, 2000). Sections were mounted on Superfrost Plus® slides (Fisher Scientific) and dried overnight. Slides were processed through 1% NaOH in 80% EtOH, 70% EtOH, dH2O and then 0.06% KMnO4 for 10 min. After a wash in dH2O, slides were incubated in the dark in FJB (Chemicon) for 20 minutes. Following washes in dH2O, sections were dried and coverslipped in Cytoseal® (Stephens Scientific).
Pyknotic Cells
To confirm cell death indicated by FJB staining, pyknotic cells were assessed in adjacent sections labeled for Ki-67 with neutral red counterstain. Pyknotic cells are conservatively defined by a shrunken nucleus and condensed chromatin, which is easily visualized with counterstains such as neutral red (He et al., 2005).
TUNEL staining
TUNEL identifies apoptotic cells by end-labeling the fragmented DNA present within apoptotic cells. TUNEL staining followed the manufacturer’s directions (Colormetric Kit PR-G7130 Promega, Madison, WI). Five sections per brain matched for level across the hippocampus were mounted onto Superfrost Plus® slides (Fisher Scientific) plus a positive control. Briefly, slides were washed in 0.85% NaCl then 0.1M PBS, permeabilized in Proteinase K, washed, equilibrated in equilibration buffer, and labeled by incubating in recombinant terminal deoxynucleotidyl transferase reaction mix for 1 hr at 37°C in a humidified chamber. Slides were immersed in standard sodium citrate, washed, and blocked in 0.3% H2O2. Following washes, sections were incubated in Streptavidin horseradish peroxidase, washed and colorized with DAB. Slides were dried then coverslipped with Cytoseal®.
Quantification
All brains were coded throughout staining and quantification procedures so that experimenters were blind to the treatment groups. Quantification of immunohistochemistry and staining was conducted on an Olympus BX-51 microscope with a motorized stage, and 12 MP camera (Olympus; Center Valley, PA). DCX-immunoreactivity (DCX+IR) was quantified at 100X in the dorsal dentate gyrus plus subgranular zone (an approximate 50μm ribbon between the granule cell layer and the hilus) as pixels/μm2 via image analysis system as described previously (Image Pro Plus, Bethesda, MD; Nixon and Crews, 2004). Profile counting methodology was used for all other histological measures and utilized a 40X lens for FJB (Olympus Plan Apo, numerical aperture 0.9) and a 60X oil immersion lens for all others (Olympus Plan Apo, numerical aperture 1.4). Profile counting methodology was chosen as these cell populations are nonhomogenously distributed and frequently total less than 100 profiles per region sampled, which suggests that stereology is not the best approach for quantification (Popken and Farel, 1997). Further, we have previously shown that profile counts and stereological estimates result in identical percent change and relative difference is the data of interest in these studies (Crews et al., 2004).
Assessment of cell phenotype (triple Fluorescent IHC) was conducted on a laser scanning confocal microscope (Leica TCS SP5; Wetzlar, Germany) with a 63x oil lens. Fifty BrdU+ cells were sampled per dentate gyrus across a minimum of five tissue sections. Z plane image stacks (1024×1024 pixels) were taken at 0.8 μm for each cell analyzed. Z-stacks were reconstructed, viewed and analyzed for colabeling within the Leica software similar to that previously described (Nixon et al., 2008).
Analyses were conducted in Excel (2003, Microsoft) or Graphpad Prism (Graphpad Software; La Jolla, CA) and are reported as mean ± standard error of the mean (SEM). BECs (mg/dl) and ethanol dose (g/kg) were analyzed by ANOVA. All two group comparisons, specifically cell counts and immunoreactivity, were analyzed by t-test. Behavioral intoxication scores were analyzed by Kruskall-Wallis. In all analyses, differences were considered significant at p<0.05.
Results
Animal Model
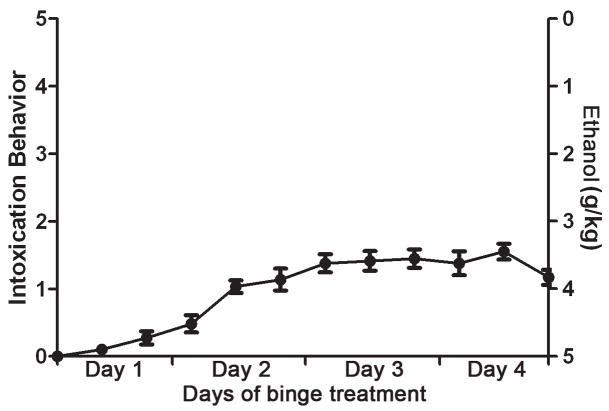
To investigate the effects of alcohol on neurogenesis within the context of an adolescent AUD model, ethanol was administered to adolescent rats in a binge model. Rats were approximately 35 days old at the start of alcohol administration, weighing 117.5 ±0.2 g. Rats eventually showed signs of intoxication, but they were limited on average to the lowest level of ataxia (level 1; see Figure 1). This decreased sensitivity of adolescent rats to behavioral intoxication effects of alcohol is consistent with previous reports (Little et al., 1996; White et al., 2002). This administration technique and dosing regimen were similar to past studies except that ethanol was administered three times a day instead of four as reported in Crews et al. (2000). The three doses per day paradigm have been used consistently in adults since 2000 and produces BECs that are more relevant to the human condition. Across all groups examined, the grand mean dose of ethanol was 12.4 ± 0.4 g/kg/day, which resulted in a grand mean BEC of 302.3 ± 12.1 mg/dl. All alcohol groups were similar in intoxication parameters as evidenced by BEC, mean behavioral intoxication score, and dose of ethanol per day (Table 1).
Figure 1.
Alcohol (ethanol) is administered in a four day binge model as reported previously (Nixon & Crews, 2004). At each feeding (7am, 3pm, 11pm), behavioral intoxication is scored using a scale (0–5) modified from Majchrowicz (1975) and a corresponding dose of ethanol (0g/kg–5g/kg) administered by gavage. The mean intoxication score (left axis) for all ethanol-exposed rats is plotted and shows that adolescent rats only show mild ataxia. Values shown are mean ± SEM.
Table 1. Intoxication parameters for the animal model.
The table above shows that all intoxication parameters are similar between the different time points. There were no significant differences between any of the four time points, thus differences in histological measures cannot be attributed to different intoxication levels between the groups.
| Intoxication Behavior (0–5 scale) | Dose (g/kg/day) | BEC (mg/dl) | |
|---|---|---|---|
| 4D (n= 6) | 1.0 ± 0.1 | 12.5 ± 0.8 | 349.6 ± 18.3 |
| 4D + 2 (n= 8) | 1.1 ± 0.2 | 11.8 ± 0.7 | 322.8 ± 18.7 |
| 4D + 7 (n= 8) | 1.0 ± 0.1 | 13.2 ± 0.4 | 286.5 ± 18.8 |
| 4D + 28 (n=4) | 1.0 ± 0.2 | 12.0 ± 0.5 | 231.9 ± 17.5 |
Decreased neurogenesis after binge alcohol exposure
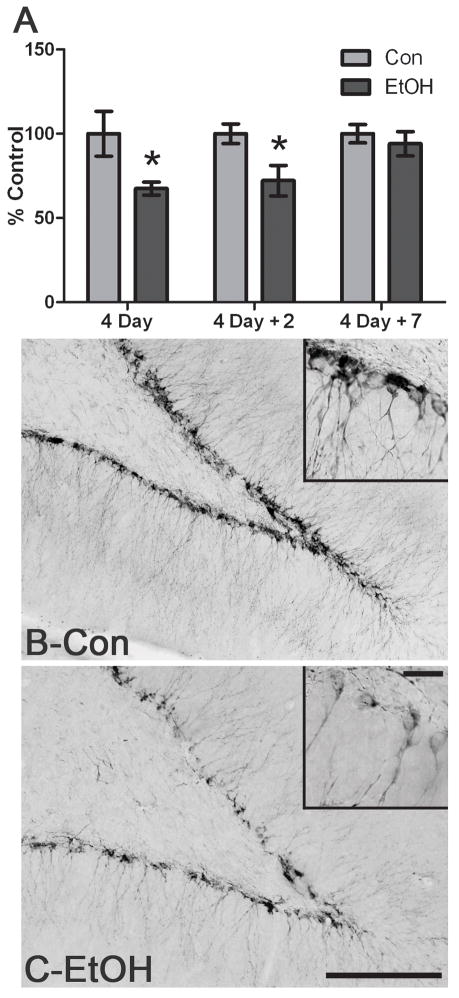
Well-accepted markers of adult neurogenesis were examined through immunohistochemistry on tissue from various time points during and after binge alcohol administration. To determine whether alcohol affected neurogenesis in an adolescent model of AUD, DCX expression was examined at various time points in the dorsal dentate gyrus. DCX is expressed transiently by migrating neuroblasts from a few to several days after a cell divides (Brown et al., 2003; Gleeson et al., 1999). As shown in Figure 2, DCX-positive cells were observed throughout the superior and inferior blades of the dentate gyrus of the hippocampus in both the control and alcohol exposed subjects. Because DCX immunohistochemistry was processed in batches by timepoint, each time point was analyzed independently for differences in pixels/μm2 but presented together as percent of control in Figure 2a. Assessment of DCX+IR showed, a 35 ± 3.9% decrease (p<0.05) after four consecutive days (4D) of alcohol exposure (Con= 0.159143 ± .021 pixels/μm2, n=6; EtOH= 0.107303 ± .006 pixels/μm2, n=6) and a 27.9 ± 9.1% decrease (p<0.05) for 4D+2 group (Con= 0.134994 ± .008 pixels/μm2, n=7; 4D+2= 0.097371 ± 0.012 pixels/μm2, n=8). DCX expression returned to control levels by one week following the last dose of alcohol (Con=0.18438 ± 0.010 pixels/μm2, n=7; EtOH=0.17263 ± 0.13 pixels/μm2, n=8). Thus, DCX labeling of new migrating neuroblasts, or neurogenesis, was reduced by binge alcohol exposure in an adolescent model of an AUD.
Figure 2.
Binge alcohol administration reduces DCX+IR during and following alcohol intoxication in adolescent rats. A) DCX+IR is significantly decreased following four days of binge exposure to alcohol (4D; Con n=6, EtOH n=6) and two days after the last dose of alcohol (4D+2; Con n=7, EtOH n=8). DCX+IR returns to control levels by 7 days after the last dose (4D+7; Con n=7, EtOH n=8). DCX is expressed between several hours to a few days after a cell divides such that its expression reflects activities that alter neurogenesis days prior (Brown et al., 2003). Representative photomicrographs of DCX expression in the dentate gyrus are shown for control (Con, B) and alcohol (EtOH, C) exposure at the 4D timepoint. Scale bar = 100μm. Inset scale bar = 20μm *p< 0.05
Decreased cell proliferation directly following alcohol
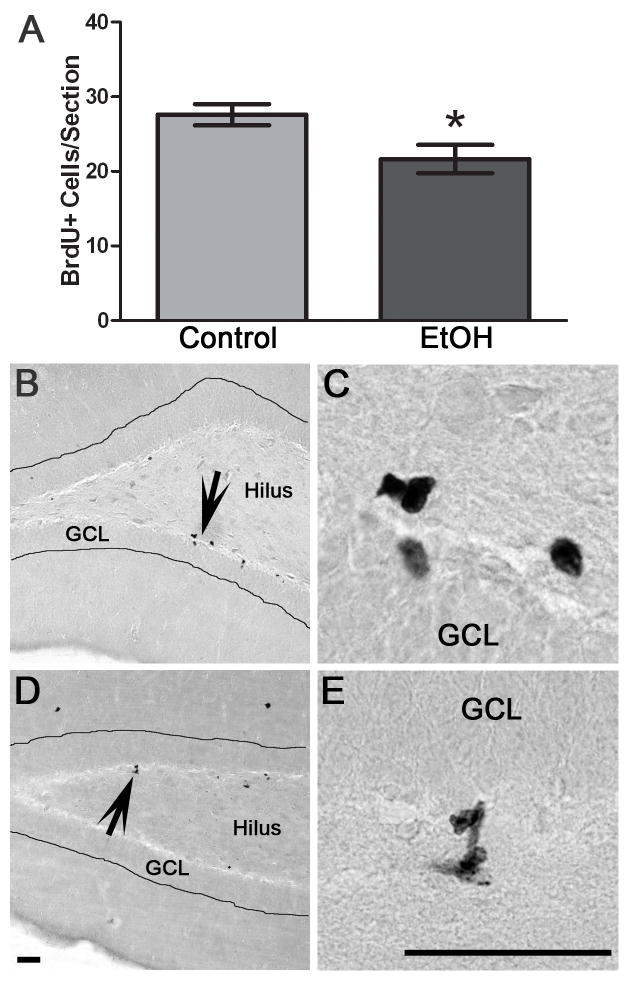
Because decreased neurogenesis could be due to effects on any of the components that comprise neurogenesis - proliferation, differentiation, migration or survival - we investigated the components of neurogenesis through the use of BrdU labeling of dividing cells. To examine cell proliferation, the 4D group had BrdU injected 1 h after the last dose of alcohol and rats sacrificed two hours later. Thus, BrdU was examined in adjacent sections. Two alcohol exposed and 1 control did not meet BrdU-labeling criteria (BrdU-labeled mitotic figures were not evident in any region of the brain, (Eisch et al., 2000) and were dropped from this assay. As can be seen in Figure 3, BrdU expression was punctate, with most cells in clusters that were scattered throughout the subgranular zone of both blades of the dentate gyrus as expected. The subgranular zone was defined as a two cell body width between the granule cell layer and hilus. Counting of BrdU-positive (BrdU+) cells revealed a 21.5% decrease (p<0.05) in 4D alcohol-exposed rats (n=4) versus controls (n=5). Therefore, four consecutive days of binge alcohol exposure decreased BrdU-labeling of the number of cells in the S-phase, or cell proliferation, in the dentate gyrus in an adolescent model of an AUD.
Figure 3.
Binge alcohol administration reduces the number of BrdU+ cells, or cell proliferation, during alcohol intoxication in adolescent rats. A) To label cell proliferation, BrdU was injected 1 h after the last dose of alcohol or control diet and rats were sacrificed 2 h later. The number of BrdU+ cells is significantly decreased in binge alcohol exposed rats (EtOH, n=4) versus controls (n=5). Representative photomicrographs of BrdU+ clusters scattered along both blades of the dentate gyrus of the hippocampus are shown for control (B) and alcohol rats (D). Higher magnification images show representative BrdU+ cells from control (C) and alcohol-treated rats (E). GCL= Granule cell layer. Scale bars = 50 μm. *p< 0.05
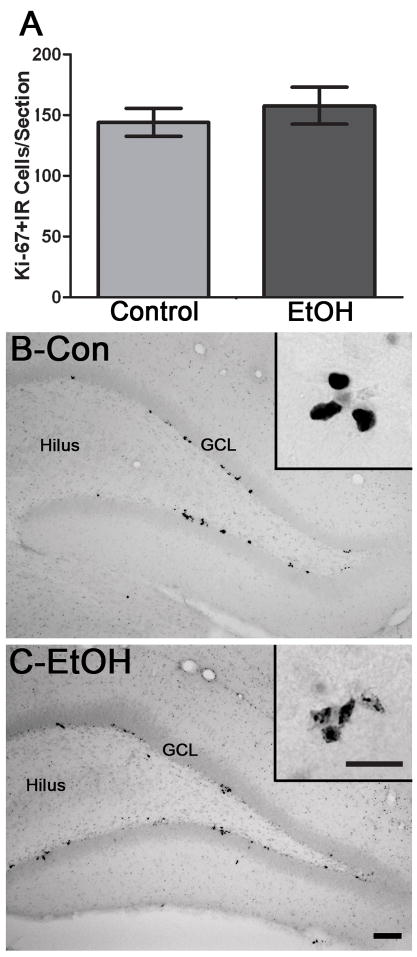
In order to compare the BrdU results to another cell cycle marker, Ki-67, an endogenous marker of all active phases of the cell cycle (excluding Go, the resting state), was examined in adjacent sections of 4D tissue. Ki-67 immunolabeling was similar to BrdU in that it was punctate with clusters spread throughout the subgranular zone (Figure 4). However, the number of Ki-67-positive (Ki-67+) cells was not different between the ethanol (n=6) and control groups (n=6). This lack of effect indicates that similar numbers of cells are in the active phase of the cell cycle despite binge alcohol intoxication.
Figure 4.
Binge alcohol administration does not change the number of Ki-67+ cells during alcohol intoxication in adolescent rats. A) The number of Ki-67+ cells is identical between binge alcohol exposed rats (EtOH, n=6) and controls (n=6) which supports that binge alcohol exposure does not alter the number of cells in the active phases of the cell cycle. Representative photomicrographs show that Ki-67+ clusters are scattered along both blades of the dentate gyrus of the hippocampus in control (B) and alcohol-treated rats (C). GCL= Granule cell layer. Scale bars = 100μm. Inset scale bar = 30μm. *p< 0.05
Reduced cell survival following alcohol exposure
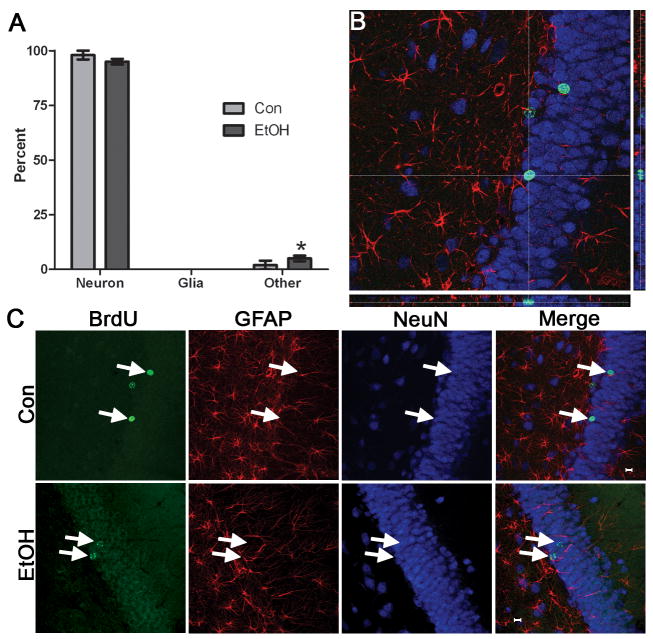
Newborn neurons in the dentate gyrus that survive to 28 d are considered to be permanently incorporated into hippocampal circuitry (Kempermann et al., 2003). To investigate long term effects of binge alcohol exposure on newly formed cells, cell fate (survival and phenotype) was analyzed 28 d after alcohol exposure. In this study, BrdU was administered 1 h following the last dose of ethanol, and the rats were maintained for 28 additional days (4D+28). To examine cell phenotype, tissue was subjected to triple immunofluorescent labeling for BrdU, a neuron-specific protein (NeuN) and astroglia-specific protein (GFAP; Figure 5). Fifty BrdU+ cells per dentate gyrus were examined for colabeling with either NeuN or GFAP and expressed as the percent of BrdU+ cells showing a neuronal, glia or other phenotype. The majority of BrdU+ cells in both groups (Con, n=5; EtOH, n=4) expressed a neuronal phenotype (Figure 5a). Also, a slight but significant increase was observed in the percentage of cells that did not colabel for either NeuN or GFAP, labeled as “other.” Two recent reports in adults suggest that these cells could be either microglia (Nixon et al., 2008) or undifferentiated cells (He et al., 2009). The cell phenotype percentages observed are similar to that reported in adolescent and juvenile rodents (Crews et al., 2006b; Ibi et al., 2008; Qiu et al., 2007) but remain consistently higher than that typically reported in adult rats (Nixon and Crews, 2004). The slightly lower percentage of glial differentiation observed in the current study may be due to GFAP and BrdU labeling different aspects of the cell (filaments versus nucleus) and therefore our conservative approach to analyzing colabeling could have biased the percentage of GFAP-labeled BrdU+ cells downward. Regardless, the majority of newborn cells become new neurons regardless of treatment.
Figure 5.
Binge alcohol administration does not alter the phenotype of cells born during alcohol intoxication. BrdU was injected at 4D and rats were sacrificed 28 days later (4D+28). A) Triple fluorescent labeling for BrdU, a neuron-specific marker (NeuN) and an astroglia specific marker (GFAP) was assessed for colabeling in 50 BrdU+ cells per subject. The percentage of BrdU+ cells that expressed a neuronal phenotype (BrdU+/NeuN+) or an astroglial phenotype (BrdU+/GFAP+) was similar in control (n=5) and alcohol-exposed tissue (n=4). There was only a significant increase in “other” cells (BrdU+/NeuN-/GFAP-) in the alcohol-exposed tissue, which merely shows that other types of cells are proliferating and remaining after binge treatment. These few other cells could be microglia, endothelial cells, or oligodendroglia. A representative orthogonal view of reconstructed Z-stacks is shown in B where BrdU is labeled in green, NeuN in blue, and GFAP in red. The crosshairs are placed atop the cell of interest such that colabeling can be assessed from all planes. C. Representative images of individual fluorochromes showing BrdU+ cells (green), GFAP+ cells (red), NeuN+ cells (blue), and merged in both the control and alcohol-exposed tissue.
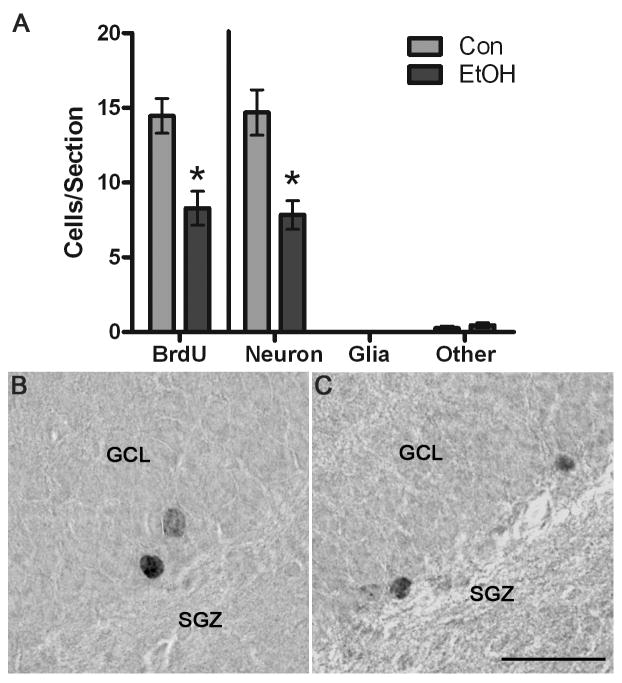
The number of remaining BrdU+ cells was then quantified in an adjacent series of tissue stained with DAB colorization due to greater stability of the labeling method. After 28 days, BrdU+ cells were larger and rounder in appearance than the cells labeled and viewed at the 4D proliferation timepoint (see Figures 5 and 6). The cells were dispersed individually throughout the subgranular zone and the inner portion of the granule cell layer of the dentate gyrus. As shown in Figure 6, a 42.7% decrease (p<0.05) was observed in the number of BrdU+ cells in the dorsal dentate gyrus of the ethanol group (n=4) when compared to the controls (n=5). This data suggests that after binge alcohol exposure, newborn cell survival is decreased. The effects of alcohol on neurogenesis was then estimated by multiplying the number of remaining BrdU+ cells at 4D+28 by the phenotype percentages shown in Figure 5a. “Calculated Neurogenesis” is shown in Figure 6 and specifically, that the fewer BrdU+ cells in the ethanol group (with a similar phenotype percentage) results in 53% fewer (p<0.01) new neurons in the ethanol group. There were no statistical effects of alcohol on the number of new glia or the number of “other” new cells not labeled by either NeuN or GFAP. Therefore, alcohol exposure in a model of an AUD results in decreased neurogenesis a month after exposure.
Figure 6.
BrdU+ cells remain significantly decreased 28 d after the last dose of alcohol. A) In the dorsal dentate gyrus granule cell layer and subgranular zone, there were significantly fewer BrdU+ cells in the alcohol-exposed tissue (n=4), as compared to controls (n=5) as shown on the left side of the graph. Because BrdU alone does not indicate cell type, BrdU+ cell numbers were multiple multiplied by the phenotype percentage data (Figure 5) to estimate effects on neurogenesis, which is shown in the right portion of the graph. Thus, the significant decrease in BrdU+ cells, yet similar phenotypes, result in significantly fewer neurons. Representative photomicrographs of BrdU+ cells labeled with DAB are shown for control tissue (B) and in alcohol-exposed tissue (C). Scale bar = 30 μm. *p< 0.05
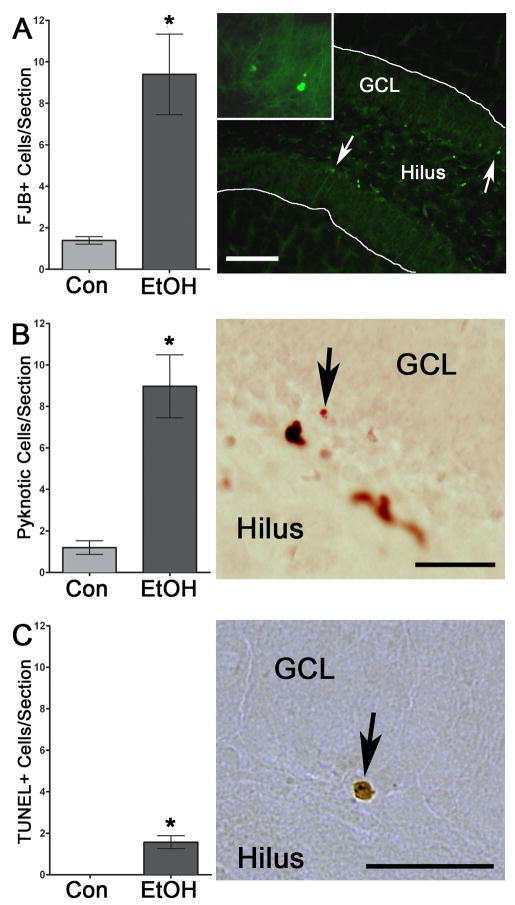
Notably, this 50% decrease in neurogenesis is greater than the 21% decrease in cell proliferation, which suggests that new neurons born during binge alcohol exposure do not survive at the same rate as in controls. Therefore, to further explore cell survival, “percent survival” was calculated for each subject: the number of BrdU+ cells at 28 days (4D+28) was divided by the mean number of BrdU+ cells at D4 for its treatment group which showed 54.6 ± 5.1% of BrdU+ cell survived in the control group and only 35.7 ± 4.9% of alcohol exposed BrdU+ cells survived 28d later (p<0.05). This difference in percent of new cells surviving suggests that the reduction in neurogenesis is due to effects other than alcohol altering cell proliferation or cell differentiation. Therefore, we examined cell death immediately after the last dose of alcohol. To assess the effects of alcohol on cell death, adjacent tissue sections were dyed for a common marker of cell death, FJB, and FJB-positive (FJB+) cells were counted in the dentate gyrus and subgranular zone of the hippocampus. As shown in Figure 7, FJB + cells were found throughout the dentate gyrus of the alcohol treated sections, and intriguingly most were along the subgranular zone, where proliferation occurs. Indeed, the number of FJB+ cells was significantly increased (p<0.05) in the ethanol-exposed subjects (n=6), as compared to controls (n=3). Capitalizing on the neutral red counterstain of the Ki-67 labeled sections, we then confirmed the cell death observed with FJB staining, by quantifying the number of pyknotic cells observed in the dentate gyrus. Similar to FJB, pyknotic cells were located sparsely throughout the granule cell layer but the majority were located along the subgranular zone. As shown in Figure 7, there was a significant increase (p<0.01) in the number of pyknotic cells in the tissue of the ethanol treated subjects (n=6) compared to controls (n=6). Thus, cell death is observed in the dentate gyrus immediately following binge alcohol exposure in adolescence. Previous work has suggested that binge alcohol exposure in adult rats results in a necrotic form of cell death (Obernier et al., 2002). However, perinatal alcohol exposure is thought to be apoptotic (Ikonomidou et al., 2000). Therefore, to specifically examine cell death due to apoptosis, TUNEL staining was conducted in a subset of subjects. As can be seen in Figure 7, very few TUNEL-positive cells were observed in ethanol-exposed tissue and essentially no TUNEL labeling was detected in controls. Although analysis of these data revealed a slight but significant increase (p<0.02) in TUNEL cells in ethanol exposed tissue (n=5) versus control (n=3), less than two TUNEL+ cells were observed per section at most. These data strongly support that alcohol-induced cell death is not likely apoptotic in adolescent rats after binge alcohol exposure.
Figure 7.
Cell death is significantly increased after four days of binge alcohol exposure according to three different measures. A) Binge alcohol exposure significant increased the number of FJB+ cells in the dentate gyrus as shown in representative photomicrographs of the alcohol-exposed tissue. Inset picture is of cells noted by the right most arrow. Scale bar = 100μm. B) Similarly, pyknosis was significantly increased after alcohol exposure in the dentate gyrus and subgranular zone. Arrows indicate a cell with condensed chromatin in the nucleus, characteristic of a pyknosis from a representative Ki67-labeled section (brown cells) that was counterstained in Neutral Red (pink). Scale bar = 30μm. C) The mechanism of cell death was examined by TUNEL staining for apoptosis. Very little TUNEL reactivity was observed, even though it was slightly but significantly increased after binge alcohol treatment, which suggests that cell death observed by FJB and pyknosis is not apoptotic. Past work in binge-exposed adults supports this observation (Obernier et al., 2002) as adult rats were similarly TUNEL negative and morphological assessments indicated dark cell degeneration, a necrotic form of cell death. Arrow indicates one of the very few TUNEL+ cells in the subgranular zone of the dentate gyrus. Scale bar = 30 μm. *p< 0.05
Discussion
These data are the first to show a decrease in neurogenesis in an adolescent rat model of an AUD. Specifically, binge alcohol exposure in adolescent rats reduced DCX+IR, a widely accepted marker of neurogenesis (Figure 2; Rao and Shetty, 2004). To further probe for mechanism of alcohol-induced inhibition of neurogenesis, a systematic assessment of the different aspects of neurogenesis was conducted. First, the decrease in DCX expression corresponded to reduced cell proliferation as evidenced by a decrease in BrdU-labeling of cells in S-phase immediately after the last dose of alcohol (Figure 3). Second, newborn cell survival was impacted as shown by the significantly reduced percentage of BrdU labeled cells that remain or survive to 28 days after the last dose of alcohol (Figure 6). As alcohol exposure did not alter the differentiation of newborn cells, when cell counts at 4D+28 (Figure 6) were combined with phenotype percentages (Figure 5), a nearly 50% reduction in neurogenesis was estimated at four weeks after the last dose of alcohol. Although the loss of BrdU+ signal at D4+28 days could in theory be due to signal dilution, other groups have shown that loss of BrdU is due to cell death (Cameron and McKay, 2001; Dayer et al., 2003). This interpretation is also consistent with prior work in adult rats where chronic alcohol ingestion reduces new cell survival specifically (He et al., 2005; Herrera et al., 2003). Furthermore, alcohol significantly increased three markers of cell death, FJB, Pyknosis, and TUNEL staining, which supports inhibited cell survival as the most plausible interpretation of decreased BrdU-labeled cells. In summary, this dual impact on hippocampal integrity - alcohol induced inhibition of cell proliferation and newborn cell survival - could contribute to hippocampal volume loss observed in adolescents diagnosed with an AUD (De Bellis et al., 2000).
Notably, the observation of cell death in the adolescent rat hippocampus following binge alcohol exposure is consistent with recent reports of morphological and inflammatory indices of neurodegeneration in adolescent rat models of AUDs and a lone report of apoptotic cell death after acute ethanol injections (Evrard et al., 2006; Jang et al., 2002; Pascual et al., 2007). Although neurodegeneration has been examined with deOlmos cupric silver stain in the brains of adolescent rats exposed to binge alcohol, only regions with visually distinct silver stain were quantified (Crews et al., 2000). Eight to ten dead cells per section, as observed here, may not be remarkably distinct at the magnification used for image analysis of silver stain. Further, the very low numbers of TUNEL+ cells compared to the FJB and Pyknotic data, suggest that the mechanisms of cell death is not likely apoptotic. FJB labeling and pyknotic nuclei are observed throughout the apoptosis-necrosis spectrum of cell death and cannot be used to differentiate the mechanism of cell death. However, TUNEL is a well-accepted marker of apoptosis (Gavrieli et al., 1992) and the lack of TUNEL labeling is consistent with necrosis as the mechanism of cell death in binge alcohol exposure (Obernier et al., 2002). Intriguingly, this level of cell death, i.e. a few cells per section, would not be expected to contribute significantly to volume loss. However, a sustained 50% decrease in neurogenesis could, especially if neurogenesis were decreased for several days. Alcohol induced inhibition of neurogenesis results in an estimated cell loss that is identical to that reported after chronic alcohol exposure (Nixon and Crews, 2002; Walker et al., 1980). Thus, these data when considered altogether strongly support that inhibition of neurogenesis - both reduced proliferation and reduced cell survival - may be a mechanism that contributes to volume loss in adolescents with an AUD.
The data presented in this study contribute to a growing body of literature that alcohol use and abuse detrimentally affects the various aspects of hippocampal neurogenesis depending on timing dose, pattern and duration of exposure (Goodlett et al., 2005). Most reports have shown that alcohol intoxication inhibits neurogenesis in adult rats (see Nixon, 2006 for review). Although binge alcohol inhibited NSC proliferation in adolescent rats, it was to a lesser extent than that reported for adult rats (21% in adolescents versus the fairly consistent 40–50% inhibition in adults; see Table 1 in Nixon et al., 2006 for review). Within the context of an AUD, perhaps adolescent rats were showing a slight tolerance to alcohol inhibition of NSC proliferation. Acute alcohol administration to adolescent rats dose dependently inhibited NSC proliferation up to 78% with a 5g/kg dose (Crews et al., 2006b) whereas only a 21% decrease was observed in NSC proliferation in adolescent rats after four days of binge alcohol. Although one might jump to speculate on the perceived mechanism of this compensatory effect, so little is known about the different effects of alcohol on adults versus adolescents, let alone the effects of alcohol on the components of neurogenesis, that this would be pure conjecture.
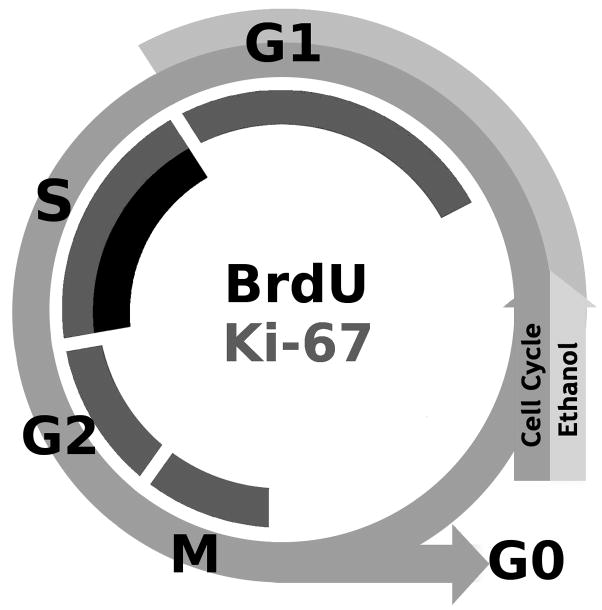
We explored the mechanism of alcohol inhibition of neurogenesis by systematically assessing the different components that contribute to the birth of new neurons. Several subtleties in the data reveal potential mechanisms of alcohol effects. First, alcohol inhibition of NSC proliferation in adolescent rats appears to be due to alcohol effects on cell cycle, and possibly cell cycle arrest. Proliferation is reduced by either altering the cell cycle or killing progenitor cells (Crews et al., 2003; Luo and Miller, 1998). Several pieces of these data suggest the former. For example, by the 4D+7 time point, DCX+IR had returned to control levels (Figure 2), which suggests that NSCs are not lost permanently. This interpretation is supported further by the Ki-67 data, which was not significantly different between the control and alcohol groups. As illustrated in Figure 8, Ki-67 and BrdU label different aspects of the cell cycle: Ki-67 is expressed during all active phases of the cell cycle (M G2, S and G1; Scholzen and Gerdes, 2000) whereas BrdU is incorporated into cells when the DNA is singled stranded, during the S-phase. Comparison of the Ki67 and BrdU data suggests that alcohol may arrest cells in the G1 phase. Arresting cells in G1 or increasing the length of the cell cycle would result in decreased numbers of BrdU+ cells (S-phase) and similar numbers of Ki-67+ cells (all active phases) similar to the pattern observed. Alcohol- induced effects on cell proliferation by increasing the length of G1 or inhibiting the progression of cells from G1 to S-phase of the cell cycle has long been suggested as an effect of alcohol on fetal brain development (Jacobs, 2001; Luo and Miller, 1998; Miller and Nowakowski, 1991). Further, other drugs of abuse impact adult neurogenesis via cell cycle alterations (Mandyam et al., 2008).
Figure 8.
A representation of the two markers used to assess proliferating cells in comparison with the stages of the cell cycle. Ki-67, an endogenous marker of proliferation is expressed during all active phases of the cell cycle (M, G2, S, and G1; Scholzen and Gerdes, 2000). BrdU, an exogenous marker, is incorporated into cells when the DNA is single stranded, which occurs during the S-phase of the cell cycle. Although cells arrested in G1 or G2 phases are considered mitotically active and express Ki67, they would not incorporate BrdU. The outer arrow depicts the cell cycle in ethanol-exposed cells arrested in G1 phase, where cells would express Ki-67 but not incorporate BrdU.
The lack of alcohol effect on Ki-67+ cells following binge alcohol exposure in adolescents differs from that observed in binge alcohol-exposed adults, where Ki-67+ cells are reduced by 30% (Crews et al., 2006a). This implies a developmental difference, though subtle, in the mechanism of alcohol effects on neurogenesis. For example, the reduction in Ki-67+ cells in adults (e.g. Crews et al., 2006a) reflects that fewer cells are in the active portion of the cycle, which could be due to either loss of progenitors or cells driven to quiescence (G0 phase). As discussed above, adolescent data suggest that alcohol is arresting or altering the cell cycle, an effect that is more similar to that observed during early development (Luo and Miller, 1998). This is consistent with developmental differences in the amount of neurogenesis or increased numbers of proliferating cells in the adolescents versus adults (He and Crews, 2007). Although the length of the cell cycle is similar between adults and adolescents (Cameron and McKay, 2001), little is known about the cell cycle or the reaction of NSC to various factors during adolescence. Obviously, further studies need to be conducted on the effects of alcohol on cell cycle dynamics. Unfortunately, a well-designed comparison is hampered by the dissimilar alcohol pharmacokinetics between adults and adolescents (Walker and Ehlers, 2009). Collectively, these data support that adolescents have a unique response to alcohol: some observations were quite similar to the adult condition (e.g. alcohol intoxication inhibits neurogenesis, cell death does not appear to be apoptotic) but other observations are more similar to development (e.g. alcohol targeting the cell cycle). Although these data do not suggest greater effects of alcohol on neurogenesis in adolescent rats than adults, these observations support that the adolescent brain reacts uniquely to alcohol and warrants independent investigation (Crews et al., 2007; Spear, 2000).
The integration of new neurons into the existing hippocampal circuitry is now considered crucial for hippocampal function (Imayoshi et al., 2008), which implicates altered hippocampal neurogenesis in neurodegenerative and psychiatric disorders, such as drug and alcohol abuse, depression and anxiety (Canales, 2007; Eisch et al., 2008; Nixon, 2006). These results show that this process remains impaired during intoxication in a rodent model of an AUD, although a single, acute dose of alcohol (5 g/kg) appears more potent (Crews et al., 2006b). Although several studies have shown that the adolescent hippocampus may be particularly susceptible to alcohol-induced neurodegenerative events in the long term (Evrard et al., 2006; Hargreaves et al., 2009; Pascual et al., 2007), these data do not suggest an enhanced sensitivity to alcohol inhibition of neurogenesis in adolescent rats within the context of alcohol dependence. However, many aspects of how new neurons develop, become integrated in to dentate gyrus and function have not been investigated. Perhaps those cells that are born and survive though alcohol intoxication have impaired functions due to inappropriate synaptic connections or any number of altered neurotransmitter or signaling cascades. In summary, binge alcohol exposure disrupts neurogenesis by decreasing the number of proliferating cells, increasing cell death and thus reducing new cell survival in the dentate gyrus of the hippocampus. Emerging theories on the contribution of neurogenesis to hippocampal function imply that alcohol dysregulation of neurogenesis likely contributes to impairments in hippocampus associated behaviors such as learning, memory, and mood, and may have implications for the development of alcohol abuse or addiction (Canales, 2007; Eisch and Mandyam, 2004).
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (AA16307 to KN), the National Institute on Drug Abuse (T32 DA16176 to SAM), the Alcoholic Beverage Medical Research Foundation (KN), and the University of Kentucky Chandler Medical Center. We gratefully thank J.L. Leasure for her insightful comments on the manuscript.
Grant Sponsors: NIAAA, NIDA, ABMRF
Grant Number: R01 AA16307, T32 DA16176
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–35. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Canales JJ. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci. 2007 doi: 10.1007/s00406-007-0730-6. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30(11):1938–49. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Reviews. 1999;5(4):379–394. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24(11):1712–23. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim DH, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006b;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Miller MW, Ma W, Nixon K, Zawada WM, Zakhari S. Neural stem cells and alcohol. Alcohol Clin Exp Res. 2003;27(2):324–35. doi: 10.1097/01.ALC.0000052581.46630.C5. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33(1):63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460(4):563–72. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157(5):737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Deas D, Riggs P, Langenbucher J, Goldman M, Brown S. Adolescents are not adults: developmental considerations in alcohol users. Alcohol Clin Exp Res. 2000;24(2):232–7. [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–84. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28(46):11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Mandyam CD. Drug dependence and addiction, II: Adult neurogenesis and drug abuse. Am J Psychiatry. 2004;161(3):426. doi: 10.1176/appi.ajp.161.3.426. [DOI] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A. A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol. 2006;200(2):438–59. doi: 10.1016/j.expneurol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. Journal of Cell Biology. 1992;199(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–71. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230(6):394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Harford TC, Grant BF, Yi HY, Chen CM. Patterns of DSM-IV alcohol abuse and dependence criteria among adolescents and adults: results from the 2001 National Household Survey on Drug Abuse. Alcohol Clin Exp Res. 2005;29(5):810–28. doi: 10.1097/01.alc.0000164381.67723.76. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, Quinn H, Kashem MA, Matsumoto I, McGregor IS. Proteomic analysis demonstrates adolescent vulnerability to lasting hippocampal changes following chronic alcohol consumption. Alcohol Clin Exp Res. 2009;33(1):86–94. doi: 10.1111/j.1530-0277.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol Biochem Behav. 2007;86(2):327–33. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21(10):2711–20. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- He J, Overstreet DH, Crews FT. Abstinence from moderate alcohol self-administration alters progenitor cell proliferation and differentiation in multiple brain regions of male and female p rats. Alcohol Clin Exp Res. 2009;33(1):129–38. doi: 10.1111/j.1530-0277.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100(13):7919–24. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10(6):559–61. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Ibi D, Takuma K, Koike H, Mizoguchi H, Tsuritani K, Kuwahara Y, Kamei H, Nagai T, Yoneda Y, Nabeshima T, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105(3):921–32. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jacobs JS, Miller MW. Proliferation and death of cultured fetal neocortical neurons: effects of ethanol on dynamics of cell growth. Journal of Neurocytology. 2001;30:391–401. doi: 10.1023/a:1015013609424. [DOI] [PubMed] [Google Scholar]
- Jang MH, Shin MC, Jung SB, Lee TH, Bahn GH, Kwon YK, Kim EH, Kim CJ. Alcohol and nicotine reduce cell proliferation and enhance apoptosis in dentate gyrus. Neuroreport. 2002;13(12):1509–13. doi: 10.1097/00001756-200208270-00004. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2006: Volume I, Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2007. [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14(2):186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23(4):633–43. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20(8):1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27(2):157–67. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64(11):958–65. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29(1):141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Effect of prenatal exposure to ethanol on the cell cycle kinetics and growth fraction in the proliferative zones of fetal rat cerebral cortex. Alcoholism: Clinical & Experimental Research. 1991;15(2):229–32. doi: 10.1111/j.1530-0277.1991.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcohol Clin Exp Res. 1994;18(1):159–63. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139(3):181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16(3):287–95. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–93. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24(43):9714–22. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31(2):218–29. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Guide for the Care and Use of Laboratory Animals. Washington, D.C: The National Academies Press; 1996. [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26(4):547–557. [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25(2):541–50. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Popken GJ, Farel PB. Sensory neuron number in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol. 1997;386(1):8–15. [PubMed] [Google Scholar]
- Qiu L, Zhu C, Wang X, Xu F, Eriksson PS, Nilsson M, Cooper-Kuhn CM, Kuhn HG, Blomgren K. Less neurogenesis and inflammation in the immature than in the juvenile brain after cerebral hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27(4):785–94. doi: 10.1038/sj.jcbfm.9600385. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–46. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- SAMHSA (Substance Abuse and Mental Health Services Administration) Results from the 2007 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2008. [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Research. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128(1):63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24(2):115–23. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescence and the trajectory of alcohol use: introduction to part VI. Ann N Y Acad Sci. 2004;1021:202–5. doi: 10.1196/annals.1308.025. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–45. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28(10):1577–86. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Mezzich AC, Hsieh YC, Parks SM. Cognitive capacity in female adolescent substance abusers. Drug Alcohol Depend. 1995;39(1):15–21. doi: 10.1016/0376-8716(95)01129-m. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009;91(4):560–5. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DW, Barnes DE, Zornetzer SF, Hunter BE, Kubanis P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–713. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci. 2004;1021:206–20. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73(3):673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Zeigler DW, Wang CC, Yoast RA, Dickinson BD, McCaffree MA, Robinowitz CB, Sterling ML. The neurocognitive effects of alcohol on adolescents and college students. Prev Med. 2005;40(1):23–32. doi: 10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]