Abstract
Background
There are few data describing the relative height and weight patterns of children and adolescents in rural Nigeria, despite a prevalence of stunting of over 38% among children younger than five years.
Aim
To document the height and weight patterns relative to international standards among children and adolescents aged 5 to 20 years in rural Nigeria.
Subjects and methods
Children 5 to 20 years of age were enrolled from two rural villages. Height and weight were measured; body composition was estimated using bioelectrical impedance analysis. Z-scores and centiles for height and body mass index were calculated; prevalences of low relative height (ie, > 2 standard deviations below mean for age and sex) and weight by sex and age were estimated.
Results
A total of 623 participants (326 male and 297 female) were enrolled. The mean height-for-age z-score for males younger than 19 years was −2.1 and prevalence of low relative height was 50%. Among females, the mean height-for-age z-score was −1.2 during adolescence; only 15% of adolescent females were of low relative height. Based on BMI-for-age, 37% of the adolescent males and 23% of females were underweight. No children or adolescents were overweight based on BMI-for-age.
Conclusions
Low relative height and underweight occur in a large proportion of children and adolescents in rural Nigeria, with the lowest relative heights and weights occurring in mid-adolescence and among males.
Keywords: stunting, underweight, sub-Saharan Africa
Introduction
It is estimated that worldwide more than 200 million children under 5 years old are stunted, i.e., height more than two standard deviations below an accepted reference standard (Frongillo, 1999). Stunting is most commonly the result of protracted undernutrition often in combination with frequent infections and diarrhea (Allen, 1994). Linear growth faltering early in life has been linked to functional disadvantages such as poor cognitive and school performance (Mendez & Adair, 1999; Walker et al, 2000) and relatively poor reproductive outcomes later in life (Neumann & Harrison, 1994). Due to the associated reduction in years of schooling (Victora et al, 2003) as well as the decreased learning per year in school (Glewwe et al, 2001; Walker et al, 2005), significant losses in adult income have been reported (Grantham-McGregor et al, 2007). As expected, stunting and related consequences are most prevalent in developing countries (Frongillo, 1999).
National and international surveillance of stunting has tended to focus on early childhood, i.e., 5 years of age and younger. While patterns of stunting, as well as the potential for catch-up growth, are established early in life (Martorell et al, 1994b; Shrimpton et al, 2001), faltering of linear growth among adolescents has been documented in populations of sub-Saharan Africa, including South Africa (Cameron et al, 1994; Richardson, 1977), Senegal (Simondon et al, 1997; Simondon et al, 1998), Kenya (Leenstra et al, 2005) and Tanzania (Lwambo et al, 2000). A similar growth pattern was evident in each of these countries whereby increasingly negative z-scores in height-for-age were recorded into the teen years. Girls appeared to reach a nadir in relative height-for-age at 12–13 years; boys at age 15–16 (Cameron et al, 1994; Leenstra et al, 2005; Lwambo et al, 2000; Simondon et al, 1998). There typically followed a delayed and prolonged increase in height, termed “compensatory growth” by Cameron et al. resulting in adult heights that were not dramatically different from international means (Cameron, 1991). The concept of compensatory growth implies that the delay in linear growth observed among adolescents of developing countries is a long-term adaptation to the environment rather than the result of a change in diet or illness status (Cameron et al, 1994), whereas the phrase “catch-up growth” indicates that an acknowledged deficit has been corrected and the child has regained a normal growth trajectory.
Nigeria is the most populous African country with an estimated population of more than 140 million (Secretariat, 2008) and a reported prevalence of stunting in children under 5 years of age of 38.3% (National Population Commission (NPC) [Nigeria] and ORC Macro, 2004). The Nigeria Demographic and Health Survey documented significant regional variation in stunting, ranging from 19.7% in the southeast to 55.3% in the northwest (National Population Commission (NPC) [Nigeria] and ORC Macro, 2004). Higher rates were observed among rural children and the urban poor, whereas height among privileged school-aged children in urban areas did not differ from international means (Janes, 1974; Walker et al, 1996).
As in many developing countries, there is a need to document the health and nutrition status across the lifespan of Nigerians in order to provide the basis for future policy decisions regarding allocation of health care resources. Adolescents are frequently left out of the data mix as they typically are assumed to be healthy relative to more vulnerable age groups (Blum, 1991; Paxman & Zuckerman, 1987; Senderowitz, 1995). To date, there are few anthropometric data available for adolescents or young adults in Nigeria and no studies on relative height and weight for this age range. As a component of the International Collaborative Study of Hypertension in Blacks, we have previously reported anthropometric measurements in adults from Nigeria in relation to other populations of African origin (Rotimi et al, 1995); we have not, however, presented data for children and adolescents. In this report, we present anthropometric and body composition data for children, adolescents and young adults of rural southwestern Nigeria and compare these data against international references (Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS), 1996; Kuczmarski et al, 2000).
Methods
Participants
This study was conducted in two communities in rural southwestern Nigeria, Igbo-Ora and Idere, in which the predominant ethnic group is Yoruba. Igbo-Ora and Idere are the two main towns in Ibarapa Central Local Government Area of Oyo State and have a combined estimated population of 67,000. The primary source of income for most of the residents is the production and trade of agricultural products (Salami et al, 2003). Settlement patterns are based around extended family compounds of about 150 residents, which in turn are grouped into quarters associated with different founding ancestors. The local diet is based around starches, primarily cassava but also yam, which are both processed in various forms. A maize porridge, eko, is the basic weaning food and is often consumed by all ages for breakfast (Brieger, 1985).
The participants in the present study were recruited and enrolled in the Study of Hypertension in Populations of West African Origin (HL45508), a multi-site survey on hypertension and its associated risk factors (Cooper et al, 1997). Recruitment was based on compound-to-compound canvassing by local staff members. All families in Igbo-Ora and Idere with members of at least two generations available for measurement were invited to participate in the baseline survey. In the longitudinal component of the baseline survey, individuals and their family members, aged 14 years and older, had blood pressure and anthropometrics measured at baseline, with plans to re-examine them bi-annually. Children younger than 14 years were enrolled in a separate survey of body composition and insulin sensitivity; blood pressure and anthropometrics were measured using the same protocol as the larger family study (data unpublished). For the present analyses, data from the surveys of participants between the ages of 5 and 30 years were included. The protocols were reviewed and approved by the Ethics Committee of University College Hospital in Ibadan, Nigeria, and the Institutional Review Board of Loyola University, Maywood, IL, USA. The studies were explained in the Yoruba language, and informed consent was obtained from all participants. Parental consent and verbal assent from the child was obtained for children younger than 18 years.
Anthropometric measurements
Participants arrived at the clinic in Igbo-Ora between 7:00 a.m. and 8:00 a.m. having fasted from at least 10:00 p.m. the previous evening. Weight was measured to the nearest 0.1 kg on a calibrated beam balance (Health-O-Meter, Bridgeview, IL, USA) while participants were shoeless and wearing light clothing. Height was measured to the nearest 0.1 cm using a metal tape measure attached to a vertical wall with a wooden headboard. Body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters.
Body composition, i.e., fat-free mass and fat mass, was estimated using bioelectrical impedance analysis. A tetrapolar placement of electrodes was used, with current electrodes placed on the dorsal surfaces of the right hand and foot at the distal metacarpals and metatarsals, respectively, and the detector electrodes placed at the pisiform prominence of the right wrist and at the anterior surface of the true ankle joint (Lukaski & Bolonchuk, 1988). Total body water was calculated using an equation developed and validated in this sample (Leman et al, 2003). Fat-free mass (FFM) was calculated by dividing total body water by an age-specific hydration factor (Fomon et al, 1982; Wang et al, 1999). Fat mass was calculated as the difference between body weight and FFM.
Statistical analysis
Means and standard deviations were calculated for all anthropometric variables by age and sex. Differences between the sexes were assessed for significance by two-tailed t-tests. Participant height-for-age and BMI-for-age centiles and associated z-scores were calculated for participants 5 to 20 years of age based on standards from the National Center for Health Statistics and the World Health Organization (NCHS/WHO) (Kuczmarski et al, 2000) using EPI Info 2000 (Dean, 1999). Z-scores were calculated for participants between 21 and 30 years of age using the age- and sex-specific means and standard deviations for height and BMI for African Americans from the National Health and Nutrition Examination Survey (NHANES) III (Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS), 1996). Statistical significance was defined as p<0.05.
Results
A total of 623 children and adolescents, 326 males and 297 females aged 5 to 20 years, were recruited from the villages of Igbo-Ora and Idere. As all of the children were from the Yoruba ethnic group, the examinations were conducted in the local language. An additional 125 men and 111 women from the parent study, between the ages of 21 and 30 years, were included in order to extend the analysis of height and BMI into young adulthood. The characteristics of the participants are presented in Table 1 by age group and sex. As can be observed, until the age of 13 years there were few differences in mean height, weight, BMI or body composition between the sexes. Between the ages of 13 and 16 years, females were taller, weighed more and had higher body fat levels. While the males surpassed the females in height by 17-18 years of age, weights were comparable as adults, and the females continued to have significantly higher mean body fat levels.
Table 1.
Participant Characteristics by Sex & Age Group – Mean (SD)
| Height (cm) | Weight (kg) | Body Mass Index | FFM (kg) | Fat Mass (kg) | % Body Fat | |
|---|---|---|---|---|---|---|
| Males | ||||||
| 5–6 y (n = 10) | 115.7 (6.7) | 19.9 (2.7) | 14.8 (0.9) | 16.5 (1.6) | 2.7 (0.8) | 13.8 (3.8) |
| 7–8 y (n = 20) | 117.4 (7.7) | 20.6 (3.4) | 14.8 (1.1) | 17.6 (2.7) | 3.4 (1.4) | 16.1 (5.2) |
| 9–10 y (n = 20) | 126.4 (5.1) | 23.7 (2.6) | 14.8 (0.9) | 19.2 (2.3) | 3.6 (0.9) | 15.8 (3.9) |
| 11–12 y (n = 16) | 135.5 (6.2) | 28.5 (3.5) | 15.5 (1.1) | 24.1 (3.0) | 4.4 (1.7) | 15.2 (3.9) |
| 13–14 y (n = 54) | 143.1 (7.0) | 32.2 (4.7) | 15.7 (1.3) | 27.5 (4.0) | 4.9 (1.7) | 14.9 (4.4) |
| 15–16 y (n = 57) | 152.6 (10.4) | 39.7 (7.9) | 16.8 (1.8) | 34.6 (7.9) | 5.3 (2.5) | 13.5 (5.3) |
| 17–18 y (n = 85) | 160.6 (8.3) | 47.6 (8.6) | 18.3 (2.0) | 40.7 (6.9) | 7.1 (3.5) | 14.6 (5.5) |
| 19–20 y (n = 64) | 167.3 (5.8) | 54.5 (7.1) | 19.4 (1.9) | 46.0 (5.0) | 9.2 (3.7) | 16.4 (5.3) |
| 21–30 y (n =125) | 169.5 (8.3) | 60.0 (9.1) | 20.8 (2.7) | 48.6 (7.1) | 11.3 (4.9) | 18.7 (5.5) |
| Females | ||||||
| 5–6 y (n = 12) | 108.7 (7.6)* | 17.2 (2.1)* | 14.5 (1.1) | 14.6 (1.9) | 3.2 (0.8) | 17.7 (4.0) |
| 7–8 y (n = 23) | 118.9 (6.2) | 20.6 (2.7) | 14.5 (1.1) | 17.5 (2.6) | 3.1 (1.1) | 15.0 (5.1) |
| 9–10 y (n = 19) | 128.0 (6.8) | 24.4 (3.4) | 14.8 (1.0) | 19.9 (2.7) | 4.0 (1.3) | 16.4 (3.7) |
| 11–12 y (n = 24) | 133.9 (9.0) | 27.5 (5.0) | 15.2 (1.2) | 23.2 (5.4) | 5.0 (1.6) | 18.0 (5.6) |
| 13–14 y (n = 38) | 148.3 (8.5)** | 37.6 (7.8)*** | 16.9 (2.2)*** | 30.1 (5.6)** | 7.7 (3.9)*** | 19.7 (6.7)*** |
| 15–16 y (n = 78) | 155.6 (6.6)* | 44.0 (8.0)** | 18.1 (2.6)** | 34.3 (4.7) | 10.1 (4.4)*** | 22.0 (6.9)*** |
| 17–18 y (n = 56) | 156.4 (8.0)** | 50.9 (8.3)* | 21.1 (5.8)*** | 35.8 (4.5)*** | 15.2 (6.6)*** | 27.0 (7.0)*** |
| 19–20 y (n = 47) | 160.1 (6.6)*** | 52.6 (8.6) | 20.5 (2.8)* | 37.8 (6.0)*** | 14.4 (4.5)*** | 27.3 (5.9)*** |
| 21–30 y (n =111) | 160.8 (8.3)*** | 58.7 (11.6) | 22.9 (5.6)*** | 39.9 (5.3)*** | 18.3 (7.7)*** | 30.4 (7.6)*** |
Significant differences between males and females:
p<0.05,
p<0.01,
p<0.001
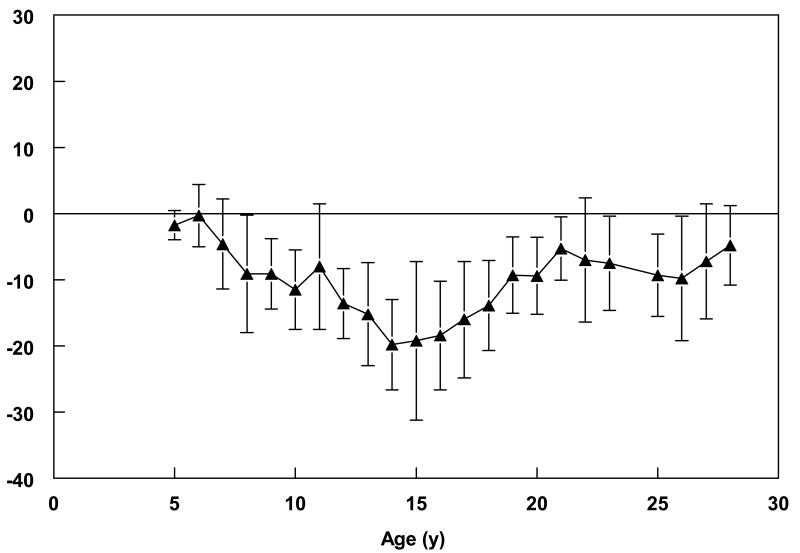
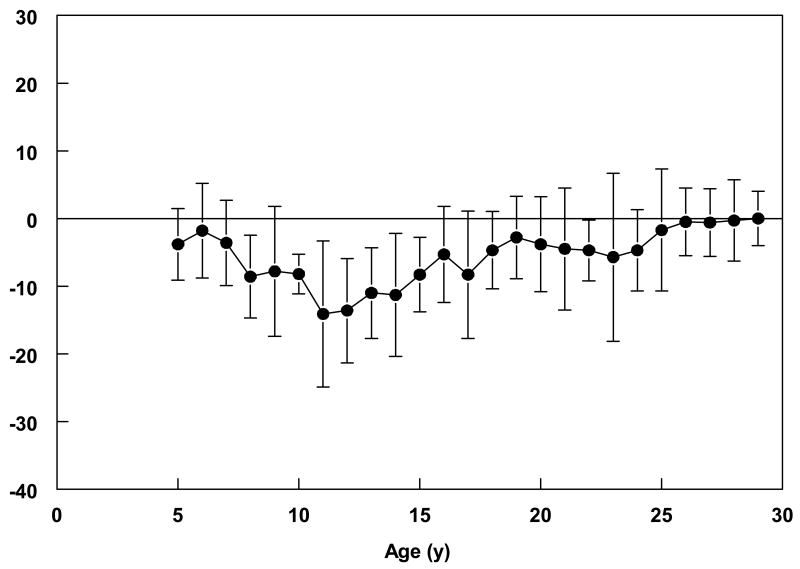
Data in Table 2 suggest that height stagnated in the teen years for both the males and females, with greater deficits relative to NCHS/WHO standards observed among the males. Mean height-for-age among males was between the 17th and 25th percentile of NCHS/WHO standards until the age of 11. At this age, it dropped to below the 5th percentile and did not increase to prior levels until the late teens. This pattern was mirrored in the mean z-scores across the age groups, being less than −2.0 between the ages of 11 and 18 in the males. Although the mean difference between measured height and international standards decreased after the age of 18 (mean difference of 7.4 cm), Nigerian males were much shorter than African-American males, as represented by NHANES III data, even in young adulthood (Figure 1). Height in the females remained, for the most part, between the 20–25th percentiles of the NCHS/WHO standards from 5 to 18 years, although a nadir was reached between 13–14 years (Table 2). The mean adult heights of the female participants, particularly after 25 years of age, were not different from NHANES III standards (Figure 2).
Table 2.
Height & Body Mass Index Compared to Standards* by Sex & Age Group – mean (SD)
| Age Group | Height Centile | Height z-score | BMI Centile | BMI z-score |
|---|---|---|---|---|
| Males | ||||
| 5–6 y (n = 10) | 17.6 (21.0) | -1.8 (1.7) | 17.8 (17.9) | -1.4 (1.3) |
| 7–8 y (n = 20) | 18.7 (21.4) | -1.6 (1.5) | 22.0 (20.9) | -1.0 (0.8) |
| 9–10 y (n = 20) | 23.7 (27.1) | -1.0 (1.3) | 18.1 (17.4) | -1.2 (0.9) |
| 11–12 y (n = 16) | 4.4 (6.1) | -2.2 (0.9) | 14.5 (13.7) | -1.3 (0.7) |
| 13–14 y (n = 54) | 7.5 (16.2) | -2.1 (1.0) | 10.9 (13.8) | -1.7 (1.0) |
| 15–16 y (n = 57) | 9.5 (18.3) | -2.1 (1.2) | 11.6 (16.0) | -1.8 (1.1) |
| 17–18 y (n = 85) | 8.9 (14.4) | -2.0 (1.1) | 14.1 (17.7) | -1.6 (1.2) |
| 19–20 y (n = 64) | 15.8 (15.0) | -1.3 (0.8) | 13.2 (15.2) | -1.5 (1.2) |
| 21–30 y (n = 125) | - | -1.0 (1.1) | - | -0.9 (0.4) |
| Females | ||||
| 5–6 y (n = 12) | 30.7 (26.7) | -0.9 (1.2) | 33.6 (30.1) | -0.6 (1.1) |
| 7–8 y (n = 23) | 20.7 (22.1) | -1.2 (1.0) | 28.0 (20.3) | -0.7 (0.8) |
| 9–10 y (n = 19) | 19.8 (22.7) | -1.3 (1.1) | 16.3 (12.6) | -1.2 (0.7) |
| 11–12 y (n = 24) | 22.5 (24.7) | -1.1 (1.2) | 17.9 (17.8) | -1.3 (1.0) |
| 13–14 y (n = 38) | 16.8 (21.8) | -1.6 (1.3) | 20.7 (23.5) | -1.2 (1.1) |
| 15–16 y (n = 78) | 23.3 (24.7) | -1.0 (1.0) | 26.4 (25.1) | -1.1 (1.4) |
| 17–18 y (n = 56) | 22.3 (20.2) | -1.1 (1.3) | 40.7 (28.6) | -0.3 (1.1) |
| 19–20 y (n = 47) | 36.3 (29.1) | -0.5 (1.0) | 34.1 (27.8) | -0.6 (1.0) |
| 21–30 y (n = 111) | - | -0.3 (1.0) | - | -0.6 (0.6) |
For ages 5–20 years, centiles and z-scores were calculated using NCHS/WHO growth standards; for ages 21–30 years, mean height and BMI standards for African American adults from NHANES III were used to calculate z-scores.
Figure 1.
Mean (± SD) difference between male participants' measured height and mean reference standard of height-for-age. For participants aged 5 20 years, the reference standard was CDC/NCHS sex-specific height-for-age standards (reference); for participants 21 30 years, the reference standard was the National Health and Nutrition Examination Survey III age- and sex-specific height standards for African-Americans (reference).
Figure 2.
Mean (± SD) difference between female participants' measured height and mean reference standard of height-for-age. For participants aged 5 20 years, the reference standard was CDC/NCHS sex-specific height-for-age standards (reference); for participants 21 30 years, the reference standard was the National Health and Nutrition Examination Survey III age- and sex-specific height standards for African-Americans (reference).
With stunting defined as having a z-score for height of <−2.0, more than 60% of the males between the ages of 13 and 16 years could be considered stunted; yet, only 12% of the adult males between 21 and 30 years had heights more than 2 standard deviations below the reference mean (Table 3). A similar, though less striking pattern of low relative height was observed in the females with less than 5% being more than two standard deviations below the mean height-for-age in young adulthood—a proportion similar to that of the US. These data suggest that the males in rural southwestern Nigeria experience puberty-related growth quite late in adolescence relative to the pattern observed in the West.
Table 3.
Prevalence of Low Relative Height and Underweight* - by Sex & Age Group (%)
| Age Group | Stunting | Underweight | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| 5–6 y | 30.0 | 16.7 | 10.0 | 16.7 |
| 7–8 y | 35.0 | 21.7 | 10.0 | 4.4 |
| 9–10 y | 25.0 | 15.8 | 20.0 | 21.1 |
| 11–12 y | 43.8 | 20.8 | 18.8 | 22.7 |
| 13–14 y | 63.0 | 36.8 | 31.5 | 15.8 |
| 15–16 y | 57.9 | 15.4 | 36.8 | 15.4 |
| 17–18 y | 44.7 | 3.6 | 32.9 | 7.3 |
| 19–20 y | 14.3 | 6.4 | 22.2 | 12.8 |
| 21–30 y | 11.9 | 4.5 | 0 | 0 |
Low relative height defined as more than 2 standard deviations below the standard mean height-for-age or a height z-score of <−2.0. Underweight defined as more than 2 standard deviations below the standard mean BMIfor-age or a BMI z-score of <−2.0. For ages 5–20 y, z-scores were calculated using NCHS/WHO growth standards; for ages 21–3 0 y, z-scores were calculated using mean height and BMI standards for African-American adults from NHANES III were used to calculate z-scores.
Among the males, BMI-for-age relative to NCHS/WHO standards did not exhibit as great a variation as height, with the mean z-score for BMI-for-age staying between −1 and −1.8, again with the lowest z-scores being between 13 and 18 years (Table 2). BMI was lower relative to standards for the females during the early teen years, catching up in the late teens and early adulthood. However, if underweight is defined as having a BMI-for-age z-score of <−2, a relatively high proportion of the participants in the present study were underweight in adolescence (Table 3). The prevalence of underweight peaked at 36.8% among the males at 13–14 years of age and at 22.7% among the girls at 11–12 years of age. Although a relatively large percentage of participants were underweight as defined by BMI-for-age, average % body fat was not excessively low in either males or females at any age (Table 1).
Fifteen percent of the adults had BMI's less than 18.5, indicating some level of chronic energy deficiency (James et al, 1988). However, most of the BMI's in this group fell between 17.5 and 18.4. Only 4.3% of the adult sample had BMI's less than 17.5, indicating moderate chronic energy deficiency, and none had a BMI z-score of <−2 relative to NHANES III. None of the children under 20 years of age were overweight or obese (Cole et al, 2000). However, 4% of the young adult males and 15.3% of the adult females were overweight (BMI ≥ 25 and < 30), and 0% and 5.4%, respectively, were obese (BMI ≥ 30).
Discussion
Children in rural southwestern Nigeria exhibited substantially shorter height compared to reference means as well as a relatively high degree of underweight in childhood, reaching the greatest prevalences in the adolescent years. The males continued to be shorter than African-American males up until the late teens and into their early 20's. By young adulthood, however, the entire deficit in height among the females and much of that in the males had been recovered. While heights of more than two standard deviations below the age-specific reference means occurred in over 60% of the boys and 35% of the girls aged 13 to 14 years, less than 10% would be considered ‘short’ as young adults. Although it was not possible to estimate peak height velocity from these cross-sectional data, it appeared that linear growth began to increase around 13 to 14 years of age in females and 16 to 17 years in males. The average z-score of BMI-for-age was consistently lower than standards in both males and females, but did not exhibit a faltering pattern as striking as that observed for height. By young adulthood there was a relatively low prevalence of underweight, or chronic energy deficiency as defined by BMI cut points, combined with an almost equal rate of overweight.
The patterns of low relative height and underweight documented in these rural Yoruba children and adolescents appear to be generalized phenomena in sub-Saharan Africa and have been reported among other rural and/or economically disadvantaged population samples (Cameron, 1991; Cameron et al, 1994; Cameron et al, 1992; Jooste et al, 1997; Lwambo et al, 2000; Neumann & Harrison, 1994; Oyemade et al, 1981; Prazuck et al, 1989; Richardson, 1977; Simondon et al, 1997; Stoltzfus et al, 1997; Walker et al, 1996). The lowest mean height-for-age z-scores consistently occurred during adolescence, between 14 and 17 years, with males exhibiting greater degrees of growth faltering and delayed compensatory growth. The etiology of the linear growth faltering observed in sub-Saharan adolescents is unclear, though the combination of poor nutrition and recurrent infection is most often cited as the primary cause among young children (Cole, 2000; Martorell et al, 1994a). Malaria and helminth infections and anemia from either nutritional or infectious causes are common in the children of sub-Saharan Africa (Abioye-Kuteyi et al, 1997; Beasley et al, 2002; Hautvast et al, 2000; Lwambo et al, 2000; Stoltzfus et al, 1997). Although the home environment for most children does not overtly change as they enter their teens, and the risk of infection remains high, the allocation and consumption of foods in households is likely modified as children increase in age and are more capable of procuring their own foods (Brieger, 1997). In the communities of Igbo-Ora and Idere, only about one-third of secondary school-aged children attend school; the remainder are apprentices in various trades, laborers, traders, farmers or drivers (Brieger, 1997). By their mid- to late-teens, some of these youths would have access to cash while others would still be dependent upon their families or employers for food or money for food.
Based on the prevalences of low relative height and underweight in these communities, the quantity and/or quality of energy and nutrient supplies available to younger children appear limited; whether this is due to economics or cultural practices is unknown. The fact that a smaller proportion of adults, especially women, were underweight compared to the children suggests that cultural dietary practices including mother-child interaction, length of breast-feeding and post-lactation childcare may contribute to delayed growth (Cole, 2000). Historically, children tend to follow the international growth standards during breastfeeding but begin to falter upon weaning (Martorell et al, 1994a). The primary weaning food in this region is eko, a maize porridge of relatively low nutrient density. After weaning, the main energy sources for all ages are cassava, yam, rice, and beans (Erinoso et al, 1992). Animal protein, including milk, is relatively limited particularly for children. Based on focus groups held among parents in the villages, it is commonly believed that meat fed to young children will induce them to become thieves (Salami, personal communication). In addition to a low protein diet, 24-hour dietary recall data from our group indicated a very limited variety in the foodstuffs available to most people. Limited variety in the diet is potentially associated with micronutrient deficiencies and, when combined with a low intake of protein-rich foods and milk, could negatively impact growth (Jooste et al, 1997).
In contrast to reports of very delayed menarche among stunted and underweight females in Senegal (Simondon et al, 1997), several studies conducted amongst the Yoruba indicated that menarche may not be significantly different from international means, with the average age being 13.6 years (Abioye-Kuteyi et al, 1997; Fakeye & Adegoke, 1994; Odujinrin & Ekunwe, 1991; Thomas et al, 1990). It is about this age that the difference in height between international standards and the girls in the present study begin to diminish. We did not collect data on puberty status due to cultural sensitivities and this is a limitation of the present study. It is likely that the late increase in height among the males may be due to delayed puberty. Zinc nutriture has a relatively sex-specific effect on maturation, with males being more negatively impacted by deficiency than females (Allen, 1994; Prasad, 1967). The best sources of zinc in an omnivore's diet are animal proteins.
Clearly, increased availability of foods and health services afforded by improved economic conditions impacts the nutritional status of children. As early as the 1960's, differences in growth were noted between advantaged and disadvantaged children in Nigeria (Janes, 1970). In 1974 Janes described a significant degree of stunting among poor urban children in southwest Nigeria while the growth curves of “elite” urban children did not differ from British Tanner growth charts (Janes, 1974). The same positive impact of improved social and economic status on the health and growth characteristics in African youths has been reported more recently. In Ibadan, Nigeria, low heights and weights relative to NCHS/WHO standards were found among rural and poor urban schoolchildren while comparable values were recorded among the middle and upper classes (Walker et al, 1996). Likewise, in South Africa “normal” growth curves were demonstrated among children of the black elite (Cameron et al, 1992).
The slow growth and development of the children, adolescents and young adults in rural southwest Nigeria may represent an adaptation to the relatively poor nutritional environment, typified by low nutrient-dense carbohydrates, small amounts of animal protein and very little variety of foodstuffs. Preliminary data suggest that dietary patterns may change in the early teen years to increase the protein intake of young adolescents. It may be that with or without a change in diet, the increased hormones in adolescence combine with increased sensitivity at target sites to overcome the adverse environment and allow more complete expression of genes dictating adult stature (Cameron et al, 1994). It is also quite possible that these patterns of height and weight represent the natural history of growth and development in these populations, rather than a pathological state.
The relatively better performance of females with respect to height and weight in the early years may be indicative of the child rearing practices in southwestern Nigeria. Even though male children are culturally preferred over females (Akande, 1989), these data could suggest that this preference does not translate into selective ‘over-feeding’ of the males compared to the females. It may be that female children tend to be trained by their mothers, especially in the kitchen, and simply have more access food.
In conclusion, there was a substantial prevalence of low relative height and low relative body mass among adolescents in rural southwestern Nigeria. These conditions were more severe in males than females. Based on these cross-sectional data, it appeared that compensatory growth occurred in late adolescence so that young adults were close to mean heights and weights of African Americans. More data are needed in order to determine the etiology of growth faltering in adolescence and its impact on future health.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (HL45508 and DK56781). We are very grateful to the parents and caregivers of these children as well as the whole communities of Igbo-Ora and Idere who participated in these studies. We acknowledge the diligence of our research nurses and assistants at the Institute of Child Health, University College Hospital, Ibadan, and our staff in Igbo-Ora and Idere.
References
- Abioye-Kuteyi EA, Ojofeitimi EO, Aina OI, Kio F, Aluko Y, Mosuro O. The influence of socioeconomic and nutritional status on menarche in Nigerian school girls. Nutr Health. 1997;11:185–95. doi: 10.1177/026010609701100304. [DOI] [PubMed] [Google Scholar]
- Akande B. Some socio-cultural factors influencing fertility behaviour: a case study of Yoruba women. Biol Soc. 1989;6:165–70. [PubMed] [Google Scholar]
- Allen LH. Nutritional influences on linear growth: a general review. Eur J Clin Nutr 48 Suppl. 1994;48 1:S75–89. [PubMed] [Google Scholar]
- Beasley M, Brooker S, Ndinaromtan M, Madjiouroum EM, Baboguel M, Djenguinabe E, Bundy DA. First nationwide survey of the health of schoolchildren in Chad. Trop Med Int Health. 2002;7:625–30. doi: 10.1046/j.1365-3156.2002.00900.x. [DOI] [PubMed] [Google Scholar]
- Blum RW. Global trends in adolescent health. Jama. 1991;265:2711–9. [PubMed] [Google Scholar]
- Brieger WR. Food groups in cultural perspective. Trop Doct. 1985;15:42–3. doi: 10.1177/004947558501500124. [DOI] [PubMed] [Google Scholar]
- Brieger WR. The health and social needs of adolescents and youth in rural Nigerian communities. In: Dare L, et al., editors. The Status of Adolescents and Young Adults in Nigeria. Lagos, Nigeria: CSS Press for Centre for Health Sciences Training, Research and Development, Ibadan; 1997. pp. 428–449. [Google Scholar]
- Cameron N. Human growth, nutrition, and health status in Sub-Saharan Africa. Yearb Phys Anthropol. 1991;34:211–50. doi: 10.1002/ajpa.1330340611. [DOI] [PubMed] [Google Scholar]
- Cameron N, Gordon-Larsen P, Wrchota EM. Longitudinal analysis of adolescent growth in height, fatness, and fat patterning in rural South African black children. Am J Phys Anthropol. 1994;93:307–21. doi: 10.1002/ajpa.1330930304. [DOI] [PubMed] [Google Scholar]
- Cameron N, Kgamphe JS, Leschner KF, Farrant PJ. Urban-rural differences in the growth of South African black children. Ann Hum Biol. 1992;19:23–33. doi: 10.1080/03014469200001892. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) The Third National Health and Nutrition Examination Survey Data (NHANES III 1988-1994) Hyattsville, Maryland: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1996. [Google Scholar]
- Cole TJ. Secular trends in growth. Proc Nutr Soc. 2000;59:317–24. doi: 10.1017/s0029665100000355. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Rotimi C, Ataman S, McGee D, Osotimehin B, Kadiri S, Muna W, Kingue S, Fraser H, Forrester T, Bennett F, Wilks R. The prevalence of hypertension in seven populations of west African origin. Am J Public Health. 1997;87:160–8. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AG. Epi InfoTM and Epi Map: Current status and plans for Epi InfoTM 2000. J Pub Health Management and Practice. 1999;5:54–57. doi: 10.1097/00124784-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Erinoso HO, Olusanya O, Atinmo T. Nutrient Intakes of Children in a Rural Nigerian Community. Journal of Tropical Pediatrics. 1992;38:329. doi: 10.1093/tropej/38.6.329. [DOI] [PubMed] [Google Scholar]
- Fakeye O, Adegoke A. The characteristics of the menstrual cycle in Nigerian schoolgirls and the implications for school health programmes. Afr J Med Med Sci. 1994;23:13–7. [PubMed] [Google Scholar]
- Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35:1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- Frongillo EA., Jr Symposium: Causes and Etiology of Stunting. Introduction. J Nutr. 1999;129:529S–530S. doi: 10.1093/jn/129.2.529S. [DOI] [PubMed] [Google Scholar]
- Glewwe P, Jacoby H, King E. Early Childhood Nutrition and Academic Achievement: A Longitudinal Analysis. Journal of Public Economics. 2001;81:345–368. [Google Scholar]
- Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautvast JL, Tolboom JJ, Kafwembe EM, Musonda RM, Mwanakasale V, van Staveren WA, van 't Hof MA, Sauerwein RW, Willems JL, Monnens LA. Severe linear growth retardation in rural Zambian children: the influence of biological variables. Am J Clin Nutr. 2000;71:550–9. doi: 10.1093/ajcn/71.2.550. [DOI] [PubMed] [Google Scholar]
- James WP, Ferro-Luzzi A, Waterlow JC. Definition of chronic energy deficiency in adults. Report of a working party of the International Dietary Energy Consultative Group. Eur J Clin Nutr. 1988;42:969–81. [PubMed] [Google Scholar]
- Janes MD. The effect of social class on the physical growth of Nigerian Yoruba children. Bulletin of the International Epidemiological Association. 1970;20:127. [Google Scholar]
- Janes MD. Physical growth of Nigerian Yoruba children. Trop Geogr Med. 1974;26:389–98. [PubMed] [Google Scholar]
- Jooste PL, Langenhoven ML, Kriek JA, Kunneke E, Nyaphisi M, Sharp B. Nutritional status of rural children in the Lesotho Highlands. East Afr Med J. 1997;74:680–9. [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States Advance data from vital and health statistics; no314. Hyattsville, Maryland: National Center for Health Statistics; 2000. pp. 1–27. [PubMed] [Google Scholar]
- Leenstra T, Petersen LT, Kariuki SK, Oloo AJ, Kager PA, ter Kuile FO. Prevalence and severity of malnutrition and age at menarche; cross-sectional studies in adolescent schoolgirls in western Kenya. Eur J Clin Nutr. 2005;59:41–8. doi: 10.1038/sj.ejcn.1602031. [DOI] [PubMed] [Google Scholar]
- Leman CR, Adeyemo AA, Schoeller DA, Cooper RS, Luke A. Body composition of children in south-western Nigeria: validation of bio-electrical impedance analysis. Ann Trop Paediatr. 2003;23:61–7. doi: 10.1179/000349803125002887. [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Bolonchuk WW. Estimation of body fluid volumes using tetrapolar bioelectrical impedance measurements. Aviat Space Environ Med. 1988;59:1163–9. [PubMed] [Google Scholar]
- Lwambo NJ, Brooker S, Siza JE, Bundy DA, Guyatt H. Age patterns in stunting and anaemia in African schoolchildren: a cross-sectional study in Tanzania. Eur J Clin Nutr. 2000;54:36–40. doi: 10.1038/sj.ejcn.1600890. [DOI] [PubMed] [Google Scholar]
- Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. Eur J Clin Nutr. 1994a;48 1:S45–57. [PubMed] [Google Scholar]
- Martorell R, Khan LK, Schroeder DG. Reversibility of stunting: epidemiological findings in children from developing countries. Eur J Clin Nutr. 1994b;48 1:S45–57. [PubMed] [Google Scholar]
- Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129:1555–62. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- National Population Commission (NPC) [Nigeria] and ORC Macro. Nigeria Demographic and Health Survey 2003. Calverton, Maryland: National Population Commission and ORC Macro; 2004. [Google Scholar]
- Neumann CG, Harrison GG. Onset and evolution of stunting in infants and children. Examples from the Human Nutrition Collaborative Research Support Program. Kenya and Egypt studies. Eur J Clin Nutr. 1994;48 1:S90–102. [PubMed] [Google Scholar]
- Odujinrin OM, Ekunwe EO. Epidemiologic survey of menstrual patterns amongst adolescents in Nigeria. West Afr J Med. 1991;10:244–9. [PubMed] [Google Scholar]
- Oyemade A, Olugbile A, Janes MD. Health of Nigerian rural school children. J Trop Pediatr. 1981;27:101–5. doi: 10.1093/tropej/27.2.101. [DOI] [PubMed] [Google Scholar]
- Paxman JM, Zuckerman RJ. Laws and Policies Affecting Adolescent Health. Geneva: World Health Organization; 1987. [Google Scholar]
- Prasad AS. Importance of zinc in human nutrition. Am J Clin Nutr. 1967;20:648–52. doi: 10.1093/ajcn/20.6.648. [DOI] [PubMed] [Google Scholar]
- Prazuck T, Fisch A, Pichard E, Sidibe Y, Gentilini M. Growth of adolescents in Mali (West Africa) J Trop Pediatr. 1989;35:52–4. doi: 10.1093/tropej/35.2.52-a. [DOI] [PubMed] [Google Scholar]
- Richardson BD. Underweight, stunting and wasting in black and white South African schoolchildren: malnutrition or adaptation? Trans R Soc Trop Med Hyg. 1977;71:210–6. doi: 10.1016/0035-9203(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Rotimi CN, Cooper RS, Ataman SL, Osotimehin B, Kadiri S, Muna W, Kingue S, Fraser H, McGee D. Distribution of anthropometric variables and the prevalence of obesity in populations of west African origin: the International Collaborative Study on Hypertension in Blacks (ICSHIB) Obes Res. 1995;3 2:95s–105s. doi: 10.1002/j.1550-8528.1995.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Salami KK, Brieger WR, Olutayo L. Stress and coping among mothers of twins in rural southwestern Nigeria. Twin Res. 2003;6:55–61. doi: 10.1375/136905203762687906. [DOI] [PubMed] [Google Scholar]
- Secretariat PDotDoEaSAotUN. World Population Prospects: The 2006 Revision 2008 [Google Scholar]
- Senderowitz J. Adolescent health: reassessing the passage to adulthood. World Bank discussion paper No 272 1995 [Google Scholar]
- Shrimpton R, Victora CG, de Onis M, Lima RC, Blossner M, Clugston G. Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics. 2001;107:E75. doi: 10.1542/peds.107.5.e75. [DOI] [PubMed] [Google Scholar]
- Simondon KB, Simon I, Simondon F. Nutritional status and age at menarche of Senegalese adolescents. Ann Hum Biol. 1997;24:521–32. doi: 10.1080/03014469700005282. [DOI] [PubMed] [Google Scholar]
- Simondon KB, Simondon F, Simon I, Diallo A, Benefice E, Traissac P, Maire B. Preschool stunting, age at menarche and adolescent height: a longitudinal study in rural Senegal. Eur J Clin Nutr. 1998;52:412–8. doi: 10.1038/sj.ejcn.1600577. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Albonico M, Tielsch JM, Chwaya HM, Savioli L. Linear growth retardation in Zanzibari school children. J Nutr. 1997;127:1099–105. doi: 10.1093/jn/127.6.1099. [DOI] [PubMed] [Google Scholar]
- Thomas KD, Okonofua FE, Chiboka O. A study of the menstrual patterns of adolescents in Ile-Ife, Nigeria. Int J Gynaecol Obstet. 1990;33:31–4. doi: 10.1016/0020-7292(90)90651-z. [DOI] [PubMed] [Google Scholar]
- Victora CG, Barros FC, Lima RC, Behague DP, Gon alves H, Horta BL, Gigante DP, Vaughan JP. The Pelotas birth cohort study, Rio Grande do Sul, Brazil, 1982-2001. Cad Saude Publica. 2003;19:1241–56. doi: 10.1590/s0102-311x2003000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MB, Omotade OO, Walker O. Height and weight measurements of Ibadan school children. Afr J Med Med Sci. 1996;25:273–6. [PubMed] [Google Scholar]
- Walker SP, Chang SM, Powell CA, Grantham-McGregor SM. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet. 2005;366:1804–7. doi: 10.1016/S0140-6736(05)67574-5. [DOI] [PubMed] [Google Scholar]
- Walker SP, Grantham-Mcgregor SM, Powell CA, Chang SM. Effects of growth restriction in early childhood on growth, IQ, and cognition at age 11 to 12 years and the benefits of nutritional supplementation and psychosocial stimulation. J Pediatr. 2000;137:36–41. doi: 10.1067/mpd.2000.106227. [DOI] [PubMed] [Google Scholar]
- Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol. 1999;276:E995–E1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]