Abstract
Objective
Obesity is a prevalent condition in industrialized societies and is increasing around the world. We sought to assess the relative importance of resting energy expenditure (REE) and activity energy expenditure (AEE) in two populations with different rates of obesity.
Methods
Women of African descent between 18 and 60 were recruited from rural Nigeria and from metropolitan Chicago. Total energy expenditure (TEE) was measured using the doubly labeled water technique and REE by indirect calorimetry; AEE was calculated as the difference between TEE and the sum of REE plus a factor for the thermic effect of food. In the analyses all EE parameters were adjusted for body size using a regression method. Comparisons were made between the groups and associations between EE and adiposity examined.
Results
A total of 149 Nigerian and 172 African-American women completed the protocol. All body size measurements were lower in the Nigerian women. Adjusted TEE and REE were higher in the Nigerian cohort but adjusted AEE did not differ significantly. Adjustment for parity, seasonality and recent illness did not modify mean AEE or adiposity. In neither cohort was there a meaningful association between measures of AEE and adiposity.
Conclusion
In these cohorts of women from very different environments, AEE did not differ significantly nor was it associated cross-sectionally with adiposity. If generalizable, these findings suggest that reduction in AEE may have less of a role in the development of obesity than anticipated. The possibility remains that variation in type and duration of activity plays a role not captured by total AEE.
Keywords: Physical activity, resting metabolic rate, body composition, Africa
INTRODUCTION
Over the last 20 years societies as geographically and culturally distant as Barbados, Russia, Kuwait, and Japan have experienced rapid increases in relative weight, affecting both children and adults (1). Despite substantial heterogeneity in current prevalences of obesity in these populations, the trends have been remarkably similar. In the US the upturn in the slope of the trend for BMI occurred in the mid-1980’s and a 5-fold increase in the rate of change/year has been observed subsequently for every major sex/race/age/socioeconomic group (2,3). Clearly some “common source exposure” is shifting the population distribution of weight rightward and almost all segments of the societies that participate in the world economy are being affected. While it is obvious that long-term declines in EE and increases in relative calorie intake must be the cause, very little is known in a direct, quantitative way about their impact. Direct measurement of EE to evaluate its potential contribution to population risk has only been undertaken among the Pima Indians living in the southwest United States (4).
In the US, minority women have been at particular risk of obesity; the current prevalence among African-American women is 45% (5). In contrast, in contemporary populations in West Africa the prevalence remains low. Based on surveys in a rural Yoruba community in southwest Nigeria the prevalence among women is 6-8% (6,7). These and similar observations have led to the inference that mean EE in developing economies is likely to be greater than in industrialized societies as a result of mechanization of work, transport and domestic life (8-10). As noted, however, only the cross-cultural comparison of the Pima Indians in rural Mexico and on US reservations provides direct measurement of the various parameters of EE (4). While this study supports the hypothesis of declining physical activity additional studies among related populations with divergent rates of overweight and obesity will be required to confirm the generalizatiblity of this finding. Whether individual-level predictors of obesity vary across populations, and whether that variation is large enough to account for observed differences in obesity prevalence, are therefore important unresolved questions in the epidemiology of obesity.
Through an existing international collaborative study of chronic disease in populations of African descent we undertook a comparison of components of the energy budget in women from a rural community in southwest Nigeria and metropolitan Chicago, IL. The levels and distribution of parameters of energy expenditure, ie, total energy expenditure (TEE), resting (REE) and activity energy expenditure (AEE), were measured directly and the cross-sectional relationships between EE and measures of adiposity were determined.
METHODS
Participants
The Department of Preventive Medicine and Epidemiology at Loyola University School of Medicine and the University of Ibadan, Nigeria, initiated a cross-cultural, population-based research program on hypertension in 1992 involving communities in West Africa, the Caribbean and the US (11,12). The participants enrolled in the present women’s study of obesity and energy expenditure were recruited from the existing database of the prior participants who were sampled from households. The protocol was approved by the Institutional Review Board of Loyola University School of Medicine and the Ethics Committee of University College Hospital, University of Ibadan. Written informed consent was obtained from all participants and all relevant documents including the informed consent forms were translated into Yoruba for use at the Nigerian site.
In Nigeria the study sites was conducted in two rural villages in near the border with the Republic of Benin (Igbo-Ora and Idere). In the US participants were recruited from the predominantly African-American community of Maywood, a suburb adjacent to Chicago, and the Austin neighborhood, which is located just within the western boundary of the city. The primary occupation of the women in Nigeria was market trading (43%), with a substantial proportion also engaged in subsistence farming (21%). In contrast, the women in the US site were employed in a wide variety of occupations, with the most common being certified nurse assistant (8%), followed by customer service representative (6%); approximately 40% were unemployed at the time of the study.
Women were asked to participate if they were between the ages of 18 and 59 years, and were in general good health and not pregnant or lactating at the time of the baseline examination. Other exclusion criteria included: plans to travel outside the study area during the doubly labeled water (DLW) measurement period, active efforts to lose weight, a known thyroid condition, or orthopedic or other conditions which restricted movement. The same standardized examination protocol was used in both Nigeria and the US. This included measurement of total energy expenditure (TEE) using the DLW method, resting energy expenditure (REE) by indirect calorimetry, and body composition using isotope dilution. Additional anthropometric measurements and information on medical history were collected.
The research teams used clinic space convenient to the participant populations in both Nigeria (in Igbo-Ora) and the US (in Maywood). Participants arrived at the respective clinics around 7 a.m. following an 8- to 10-hour overnight fast. The baseline examination required a minimum of 5 hours to complete, after which the participants were provided with a light meal and instructions for completion of the DLW protocol.
Total energy expenditure
Free-living TEE (MJ·d−1) was measured over a period of 10 to 14 days using the DLW technique, as previously described in detail (13). A 10-day measurement period was used in Nigeria due to potentially more rapid water turnover in tropical regions, while a 14 day period was used in the US. All EE data are expressed per day, accounting for the differential length in measurement periods. Otherwise, sampling procedures were similar for both the Nigerian and US sites. Briefly, deuterium and 18O elimination rates were calculated by the 2-point method with the use of isotopic enrichment relative to baseline and the time difference between the third post-dose and final urine samples (14). Participants provided a baseline urine sample and drank a pre-made dose of DLW prior to the measurement of REE. The dose included 0.14 g deuterium and 0.20 g 18O per kg fat-free mass (FFM) (estimated). Urine samples were then collected at 2-, 4- and 5-hours following the isotope administration. In Nigeria, a midpoint spot urine sample was collected on day 5 and an endpoint sample was collected on day 10 after the isotope administration. In the US, two urine samples were collected on day 14, the first void of the morning and again 2 or more hours later in the clinic. Regardless of site, participants returned to the respective clinics on day 10 or 14 where they provided the final urine sample and their weights were recorded. All urine samples were analyzed for isotope abundance at the Stable Isotope Core Laboratory of the University of Wisconsin, Madison.
Resting energy expenditure
Following the collection of a baseline urine sample and the administration of the isotopes, REE and resting respiratory quotient (RQ) were measured using the same indirect calorimeter (Delta Trac II, Viasys Medical Systems, Palm Springs, CA) in both sites. Fasting 30-minute measurements were completed following a minimum of a 20-30 minute period of supine lying to acclimate the participant to the instrument. The DeltaTrac II operates as an open-circuit canopy system with a paramagnetic oxygen sensor, infrared carbon dioxide analyzer, and onboard computer. Briefly, rates of oxygen consumption and carbon dioxide production are calculated and used to calculate REE utilizing the modified equation of Weir (15). Prior to each measurement, the indirect calorimeter was calibrated using a gas of known concentration, and alcohol burn tests were performed monthly. These measures indicated that the unit operated within 2% at all times. The last 20-30 minutes of data was used to determine the average REE and was expressed as MJ·d−1.
Activity energy expenditure
Activity EE (MJ·d−1) was calculated as: AEE = (0.9*TEE – REE), where the term “(0.9*TEE)” represents the estimated 10% of TEE expended as the thermic effect of food (16). Expenditure in activity is also presented as the physical activity level (PAL), the ratio of TEE to REE, a metric used extensively in international studies (17).
Anthropometry and body composition
At each site height was measured, without shoes, to the nearest 0.1 cm using wall-mounted stadiometer (Seca Corp., San Jose, CA). Weight was measured without shoes and in light clothing to the nearest 0.1 kg using a calibrated electronic scale (Health-o-meter, Bridgeview, IL). Height and weight measurements were used to calculate body mass index (BMI) as weight (in kg)/height2 (in m). Waist circumference was measured to the nearest 0.1 cm at the narrowest part of the torso between the lowest rib and the iliac crest while hip circumference was measured to the nearest 0.1 cm at the point of maximum extension of the buttocks.
Body composition was measured using the isotope dilution method, as previously described by Schoeller et al. (18). Total body water (TBW) was calculated for both the deuterium and 18-oxygen dilution and the two values were averaged. FFM was calculated from TBW using a constant hydration factor (0.73)(19) and fat mass (FM) was calculated as the difference between body weight and FFM.
Medical history and other information
All participants were asked about their medical history, including presence of known chronic diseases, smoking and drinking habits, usual occupation, time since last illness requiring time off from work, and number of pregnancies. Additionally, the season in which the measurements were obtained was recorded in order to determine impact of seasonality on body weight and AEE. In Nigeria, the wet season was determined to be from the beginning of April to the end of October, and the dry season from the beginning of November to the end of March (20). A similar delineation of the seasons was used for the US cohort, with baseline examinations conducted between November to April designated as winter and those between May and October as summer.
Statistical analysis
All statistical analyses were completed using Stata, version 9.0 (College Station, TX). Energy expenditure data are presented both in absolute measures and after adjustment for body size and composition. TEE, REE and AEE were adjusted for FFM and FM using a regression method, as described previously (21). Age was not a significant determinant of any EE parameter thus was not used in the adjustment by the regression method. Means and standard deviations were calculated for all variables. Student’s t-test was used to assess differences between the two sites. Analysis of covariance was used to assess whether there was an association between AEE or percent body fat and number of pregnancies adjusting for age, and whether any difference in association existed between sites.
RESULTS
Two hundred six women were enrolled in Nigeria and 195 in the US. In both sites a small number of women younger than 18 years were inadvertently recruited (11 in Nigeria and 6 in the US), in addition, one woman from each site died within 12 months of enrollment (the Nigerian woman from cancer and the US woman from cardiovascular disease). These 19 women were excluded from the analyses, although the results did not differ with their inclusion. At the time this study was initiated there was a significant shortage of 18O-labeled water available for research (22) and, consequently, it was possible to assess TEE by DLW only in a subset of participants: 149 in Nigeria and 172 in the US. The TEE subset from Nigeria was younger (31.9 versus 33.7 y) and had a lower mean BMI (22.6 versus 23.1 kg/m2) than the total Nigerian cohort; there were no differences between the US participants with or without a TEE measurement. All subsequent analyses are restricted to the TEE subset.
Characteristics of participants are presented by site in Table 1. The mean age of the Nigerian participants was slightly younger than the US participants when analyses were restricted to those in the TEE subset. In contrast, every measure of body size and composition was significantly greater among the US women whether comparing the total sample or those in the TEE subset, as presented. The participants were classified according to the BMI categories for differing grades of under- and overweight (Table 2) (23). As can be observed, there was a marked rightward shift in the distribution by BMI categories in the US relative to Nigerian women. Overall, 6.7% of the Nigerian cohort had a BMI greater than 30, compared to 51.2% in the US.
Table 1.
Participant Characteristics by Site – mean ± SD
| Nigeria (n = 149) |
United States (n = 172) |
|
|---|---|---|
| Age (y) * | 31.9 ± 11.6 | 34.6 ± 10.6 |
| Height (cm) ** | 160.0 ± 6.2 | 164.5 ± 6.2 |
| Weight (kg) ** | 57.8 ± 11.6 | 83.5 ± 21.0 |
| Body Mass Index (kg/m2) ** | 22.6 ± 4.3 | 30.8 ± 7.3 |
| Waist Circumference (cm) ** | 78.3 ± 10.2 | 91.6 ± 16.8 |
| Hip Circumference (cm) ** | 93.4 ± 9.5 | 111.3 ± 13.7 |
| Fat-Free Mass (kg) ** | 40.4 ± 5.2 | 48.7 ± 8.0 |
| Fat Mass (kg) ** | 17.4 ± 7.8 | 34.9 ± 14.6 |
| Total Body Fat (%) ** | 28.9 ± 8.0 | 40.1 ± 8.5 |
Characteristics significantly different between Nigeria and the United States;
p<0.05
p<0.001
Table 2.
Distribution of Participants by BMI Category & Site – n (%)
| BMI Range | Nigeria (n = 149) |
United States (n = 172) |
|
|---|---|---|---|
| CED * II | 16.0 – 16.9 | 4 (2.7) | 1 (0.6) |
| CED * I | 17.0 – 18.4 | 17 (11.4) | 3 (1.7) |
| Normal Weight | 18.5 – 24.9 | 93 (62.4) | 36 (20.9) |
| Overweight | 25.0 – 29.9 | 25 (16.8) | 44 (25.6) |
| Obese | 30.0 – 34.9 | 7 (4.7) | 41 (23.8) |
| Severely Obese | 35.0 – 39.9 | 2 (1.3) | 27 (15.7) |
| Morbidly Obese | 40.0 – 49.9 | 1 (0.7) | 18 (10.5) |
| Super Morbidly Obese | ≥ 50.0 | 0 | 2 (1.2) |
Chronic energy deficiency
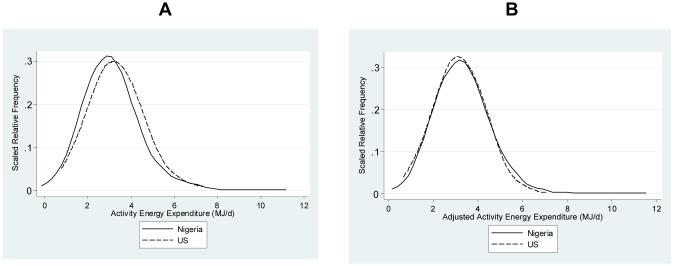
Energy expenditure parameters are presented in Table 3. The absolute values for TEE and REE were significantly lower among the Nigerian women, reflecting their smaller body size, while absolute AEE levels did not differ significantly. In contrast, TEE and REE were greater among the Nigerian women after adjustment for FFM and FM (p<0.001), while neither adjusted AEE nor PAL differed significantly between the sites. The frequency distributions of unadjusted AEE and AEE adjusted for FFM and FM for both the Nigerian and US cohorts are presented in Figure 1. This figure illustrates the fact that not only do the two cohorts have the same mean adjusted AEE, they also have comparable distributions. The mean RQ was higher among the Nigerian women, reflecting the higher carbohydrate intake (Luke, unpublished data). The difference in mean adjusted TEE between the sites (0.53 MJ.d−1 or 125 kcal.d−1; p<0.001), was due to both higher REE (mean difference of 0.27 MJ.d−1 or 65 kcal.d−1; p<0.001) and higher AEE (mean difference of 0.20 MJ.d−1 or 50 kcal.d−1; p=0.12) in the Nigerian women; the remaining 0.06 MJ.d-1 is the difference in estimated thermic effect of food. In effect, therefore, while all three adjusted EE measures were 5-6% higher in Nigerians, the absolute differences in the means/variances by EE category determined the result of statistical tests. Preliminary analyses were performed both with and without participants with BMI <18.5 (suggestive of chronic energy deficiency) and, as there are no differences in any mean EE measure, the underweight women were kept in the analyses.
Table 3.
Energy Expenditure by Site – mean ± SD
| Nigeria (n = 149) |
United States (n = 172) |
|
|---|---|---|
| Total EE (MJ.d−1) ** | 9.49 ± 1.69 | 10.16 ± 1.81 |
| Resting EE (MJ.d−1) ** | 5.39 ± 0.67 | 5.82 ± 0.87 |
| Activity EE (MJ.d−1) | 3.15 ± 1.31 | 3.32 ± 1.14 |
| Adjusted TEE (MJ.d−1) ¶** | 9.90 ± 1.41 | 9.37 ± 1.18 |
| Adjusted REE (MJ.d−1) ¶** | 5.74 ± 0.43 | 5.47 ± 0.52 |
| Adjusted AEE (MJ.d−1) ¶ | 3.37 ± 1.30 | 3.17 ± 1.06 |
| Physical Activity Level ¶ | 1.77 ± 0.27 | 1.75 ± 0.20 |
| Respiratory Quotient * | 0.86 ± 0.01 | 0.84 ± 0.01 |
Energy expenditure significantly different between Nigeria and the United States;
p<0.05
p<0.001
Total EE, resting EE and activity EE adjusted for FFM and FM; physical activity level calculated as TEE/REE
Figure 1.
A) Frequency distributions of activity energy expenditure (MJ.d−1) for the Nigerian cohort ( ) and the US cohort (---). The y-axis values correspond to the relative frequency scaled so that the total area under the curve equals 1, as in theoretical probability density functions.
B) Frequency distributions of activity energy expenditure adjusted for fat-free mass and fat mass (MJ.d−1) for the Nigerian cohort ( ) and the US cohort (---). The y-axis values correspond to the relative frequency scaled so that the total area under the curve equals 1, as in theoretical probability density functions.
Due to the increased risk of acute infectious disease in Nigeria and its potential impact on body weight and activity levels, participants were asked to estimate the date of the last bout of illness severe enough to keep them from their occupation. Fourteen percent of the Nigerian women reported such an illness within the previous month (n=21); 57% of these individuals indicated they suffered from malaria and another 33% from “general body aches”. Mean BMI, percent body fat and adjusted AEE did not differ between the participants reporting illness in the previous month and the remaining Nigerian cohort, therefore, they were kept in the analyses.
The season in which the TEE measurements were completed had no impact on either adjusted AEE levels or body weight in Nigeria or the US. Adjusted AEE was 3.42 ± 1.34 vs 3.29 ± 1.17 MJ.d−1 (p=0.55) during the rainy and dry seasons, respectively, in Nigeria while mean weight was virtually identical. Likewise, adjusted AEE was 3.43 ± 1.12 MJ.d−1 in the winter and 3.17 ± 1.13 MJ.d−1 during the summer months, again with no differences in body weight, among the US women.
Slightly over half of the Nigerian women reported having been pregnant (51.7%) compared to 75.6% of the US women. The number of pregnancies, however, was higher among the Nigerian women who had been pregnant (4.8 ± 2.3 and 3.9 ± 1.8, respectively) than among the US women (2.3 ± 1.5 and 2.3 ±1.5) (p<0.001). There was no observable association between number of pregnancies and BMI or percent body fat in either site. There was, however, a positive association between adjusted AEE and number of pregnancies among the US women (r=0.20, p<0.05) but not the Nigerian women. In a model including adjusted AEE as the outcome measure and number of pregnancies and age as the exposure measures, there was no difference in the association by site as assessed by analysis of covariance. Thus parity apparently had at most a minimal effect on adiposity or AEE in either Nigeria or the US.
There were no significant associations between adjusted TEE or REE and measures of adiposity in the Nigerian cohort; among the US women low-level positive associations between several variables were observed (TEE vs BMI, r = 0.19; TEE vs percent body fat, r = 0.15; REE vs % fat, r = 0.18; all p<0.05). In addition, there were only weak, non-significant associations between measures of adiposity and adjusted AEE, eg, correlations with BMI r = 0.03 and 0.10, respective, for Nigeria and with percent body fat r = −0.11 and −0.12, respectively, for the US. These correlations were not modified by the addition of age or parity to the models. The lack of association between adiposity and adjusted AEE persisted after stratifying the samples by BMI status as suggested by Hemmingsson and Ekelund (24), ie, participants with BMI ≥ 30 showed no stronger an association than those < 30.
In Table 4, mean adjusted TEE, REE and AEE and PAL values are presented by site for underweight, normal weight, overweight and obese participants. Due to small numbers in several categories, chronic energy deficiency (CED) I and II data were combined, as were data for all BMI categories ≥ 30. There were no significant differences in the measures of AEE between the Nigerian and US women for any category of body weight, while both TEE and REE differed between the cohorts at every BMI category except underweight.
Table 4.
Adjusted Energy Expenditure Parameters by BMI Category & Site – mean ± SD
| Nigeria | United States | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI Range | N | Adj. TEE (MJ.d−1) |
Adj. REE (MJ.d−1) |
Adj. AEE (MJ.d−1) |
PAL | N | Adj. TEE (MJ.d−1) |
Adj. REE (MJ.d−1) |
Adj. AEE (MJ.d−1) |
PAL | |
| CED I & II | <18.5 | 21 | 9.80±1.24 | 5.63±0.40 | 3.48±1.41 | 1.81 ±0.32 | 4 | 9.44±0.64 | 5.39±0.35 | 3.28±1.00 | 1.79±0.30 |
| Normal Wt | 18.5 – 24.9 | 93 | 9.79±1.43 | 5.71±0.44 | 3.33±1.31 | 1.76 ±0.28 | 36 | 8.95±1.08 | 5.34±0.49 | 3.01±0.95 | 1.73±0.20 |
| Overweight | 25.0 – 29.9 | 25 | 10.18±1.43 | 5.85±0.43 | 3.47±1.23 | 1.78 ±0.27 | 44 | 9.28±1.20 | 5.46±0.54 | 3.03±1.01 | 1.74±0.19 |
| Obese | ≥ 30.0 | 10 | 10.46±1.43 | 5.95±0.30 | 3.66±1.35 | 1.78 ±0.27 | 88 | 9.59±1.18 | 5.53±0.52 | 3.31±1.12 | 1.77±0.21 |
DISCUSSION
In this comparative study of EE and obesity we observed that while body composition differed dramatically between women living in rural Nigeria and Chicago, the levels of AEE were indistinguishable. As in previous reports (7,25), the Nigerian women were significantly shorter, weighed less and had less body fat than their US counterparts. After adjustment for body size and composition, TEE was higher in the Nigerian than the US cohort by 0.53 MJ.d−1 (125 kcal.d−1), more than half of which was due to a higher REE; neither AEE nor PAL differed significantly between the cohorts. Mean EE levels were not influenced by parity or season of measurement in either cohort. Unlike many previous studies of EE and adiposity, most of which were smaller in size or based on indirect measurement, there was no meaningful association between measures of EE and BMI or percent body fat in either the Nigerian or the US cohort. Since DLW is insensitive to variation in patterns of activity, it remains possible that intensity, duration or other characteristics of EE may contribute to adiposity differences between these cohorts of women. However, neither PAL nor AEE, as currently conceptualized and measured, appear to be important.
The concept of the epidemiologic or nutrition transition has been widely used to provide a framework for the process of modernization of lifestyle. Although relatively little data exist on physical activity levels using objective measurement, lower EE in more industrialized societies is generally accepted as one of the driving factors in the increase of overweight and obesity in those societies (8-10). It seems apparent that as societies become more mechanized the EE associated with occupational activity and the chores of daily living would decrease markedly, and indirect evidence exists does support this notion (8). It is then reasonable to make the assumption that overall AEE, and by extension TEE, would also be decreased in more industrialized societies. Much of the research in developing countries, however, suggests that this scenario may not accurately reflect reality. Although most of the extant studies did not utilize the DLW technique, there is substantial experience measuring EE in developing countries in order to ascertain energy intake requirements (26). A comprehensive review of these data concluded that there was no evidence to suggest systematically higher EE in developing countries (27). The findings presented from this study of women in Nigeria and the US are obviously consistent with this general conclusion. In contrast, Esparza et al (28) reported dramatically lower TEE and AEE, ie, on the order of 2.1 MJ.d−1 (~500 kcal.d−1), and much higher adiposity among adult Pima Indians living in southern Arizona compared to Pima Indians living in the rural mountainous region in northern Mexico. Pima Indians living a more traditional lifestyle in Mexico were clearly expending more energy than a related population in the US, and this increased EE could have made an important contribution to the differences in obesity prevalence. Life in tropical regions may of course be different from that of northern Mexico. Ferro-Luzzi has suggested that individuals in environments of marginal energy supplies may take advantage of compensatory inactivity in order to conserve energy (29).
Although the difference in mean BMI between the Nigerian and US women was comparable to that between the two Pima Indian populations, the difference in TEE was only about 25% of that observed in the Pima Indian study. The major difference between the cohorts of women was in REE, with only a 0.20 MJ.d−1 (48 kcal.d−1) difference in AEE. In contrast to the present findings on REE, we previously reported no significant difference in REE adjusted for body composition between adults of both sexes in Nigeria and the US (30). A similar absolute difference in REE between the samples was present in the earlier data, however the sample size of the earlier study was not large enough to render this difference of 0.27 MJ.d−1 (65 kcal.d−1) significant. The explanation for lower REE among the US women is not obvious. These are two populations that are genetically-related, broadly speaking; population-specific allelic markers suggest that on average they share roughly 80% of their genetic material (31). It is possible that isotope dilution provided a less than optimal estimate of total body water, from which FFM and FM were estimated, which would be more pronounced in the much more obese US cohort. More likely, however, is that there is a slight, but significant, difference in the composition of the FFM component of the two cohorts. Recently Gallagher et al reported that lower REE among African-American than white adults might, in part, be due to a lower total volume of high metabolically active organs (32). The reverse may be happening here: the US women in the present study are much taller and heavier, with higher FFM, that their Nigerian counterparts. The relative proportion of low metabolically active tissue, ie, skeletal muscle and bone, may therefore be higher among the US women, contributing to a lower overall REE. Regardless of the etiology of the higher average REE of the Nigerian women, it is unlikely to play a significant role in the difference in obesity rates. In a large prospective cohort of Nigerian adults, we previously reported finding no association between REE and weight gain (33). With the exception of two influential studies among adult Pima Indians (4,34), there have been no reports supporting REE as a determinant of weight change (35).
Physical activity has been defined and measured differently in different studies, complicating generalizable statements about their potential relationship. The use of DLW for measuring TEE combined with measurement of REE lends itself to defining physical activity as either the physical activity level (PAL) or AEE in MJ/d, neither of which were found to be related to adiposity in our study. The absence of a relationship between adiposity and PAL, however, is not entirely unexpected since it is now recognized that PAL is highly confounded by body weight and only reflects differences in physical activity when the groups or persons being compared are of similar weight (36,37).
Our finding of no relationship between adiposity and AEE in both the Nigerian and the US cohorts is inconsistent with findings from many other studies, including our own; in general, of course, the reported observation is cross-sectional data is an inverse association between BMI and AEE (38,39). On the other hand, our findings are not completely unprecedented. In a combined analysis of 22 studies, Westerterp and Goran reported no association between AEE and body fat in women while in men the correlation was −0.35 (40). Our previous studies have also indicated a weaker relationship between AEE and adiposity in women than men (25). Neither inclusion of parity nor season of measurement improved the correlations appreciably, suggesting that factors other than EE are influencing adiposity in women living in rural Nigeria and in suburban US.
By default, a difference in energy intake between the cohorts is likely to be the most important determinant of differences in obesity prevalence. We have been unable to accurately assess dietary intake in our Nigerian participants because of an absence of a comprehensive macronutrient database. Careful measurement of quantity and composition of food in high vs. low risk settings represents an important additional challenge for the epidemiology of obesity.
Analysis of the body size-AEE relationship depends critically on the method of adjustment of the EE variables. As can be observed in table 4, there is a linear increase in mean expenditure by BMI classification for all EE variables except PAL; this increase is particularly striking for the Nigerian cohort. It may be that the accepted adjustments for body composition are simply not adequate and this becomes obvious when comparing two populations with such different body sizes and composition. Specifically, AEE is a product of the time spent in physical activity, the intensity of the physical activities, and the weight of the subject performing those physical activities (41,42). Thus a simple linear adjustment for weight may be insufficient. However, despite attempts to generate better models incorporating non-linear terms we could not improve on the basic adjustment procedures displayed above.
Weinsier and coworkers (43) compared different measures of physical activity, including intensity and duration in addition to PAL, AEE, and concluded that these measures represent different domains and have different relationships with body weight and adiposity. It may therefore be inappropriate to equate any single measure with the term physical activity. For example, we found that the values for AEE were not significantly different despite a 44% greater weight in the US cohort as well as an 11% greater percent body fat. Because the energy costs of a given activity increase proportionally with weight (41), our findings would suggest that the time spent in physical activities in the US cohort was less. Unfortunately, we do not have any reliable measures of the time domains of physical activity. It is worth noting that Weinsier et al (44) and Levine et al (45) identified differences in some of these domains between lean and obese samples in which AEE was similar. Finally, it should also be noted that the 6% lower AEE in the US would, if extended over a period of years, lead to substantial excess in calories stores. However, this interpretation is unwarranted given the lack of statistical significance in the two-group comparison and the absence of within-group associations.
While alternative explanations of our results may be plausible, the most parsimonious interpretation suggest that our expectation of high EE in developing countries may, in fact, be erroneous. While we did observe higher adjusted TEE among the Nigerian cohort of women, this was driven primarily by increased REE; AEE did not differ significantly between the cohorts. A better understanding of energy intake and time domains in physical activity in both populations will be necessary to form a complete picture of energy balance.
Acknowledgements
This research was supported in part by grants from the National Institutes of Health (DK56781 and HL45508).
REFERENCES
- 1.World Health Organization WHO Global NCD InfoBase. The SuRF Report 1. http://www.who.int/ncd_surveillance/infobase/en.
- 2.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960-2002. Advance data from vital and health statistics. 347. National Center for Health Statistics; Hyattsville, MD: 2004. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System. http://www.cdc.gov/brfss/technical_infodata/surveydata.htm.
- 4.Tataranni PA, Harper IT, Snitker S, et al. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Disord. 2003;27:1578–83. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Rotimi CN, Cooper RS, Ataman SL, et al. Distribution of anthropometric variables and the prevalence of obesity in populations of West African origin: the International Collaborative Study on Hypertension in Blacks (ICSHIB) Obes Res. 1995;3(Suppl 2):95s–105s. doi: 10.1002/j.1550-8528.1995.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman JS, Durazo-Arvizu RA, Rotimi CN, McGee DL, Cooper RS. Obesity and hypertension in populations of African origin. Epidemiol. 1996;7:398–405. doi: 10.1097/00001648-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Ferro-Luzzi A, Martino L. Obesity and physical activity. In: Chadwick DJ, Cardew G, editors. The Origins and Consequences of Obesity. John Wiley & Sons; Chichester: 1996. pp. 207–27. [Google Scholar]
- 9.Prentice A, Jebb S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nut Rev. 2004;62:S98–S104. doi: 10.1111/j.1753-4887.2004.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 10.Popkin BM. The nutrition transition: an overview of world patterns of change. Nut Rev. 2004;62:S140–S143. doi: 10.1111/j.1753-4887.2004.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooper R, Rotimi C, Ataman S, et al. Hypertension prevalence in seven populations of African origin. Am J Public Health. 1997;87:160–8. doi: 10.2105/ajph.87.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper R, Rotimi C, Kaufman JS, et al. Prevalence of NIDDM among populations of the African diaspora. Diabetes Care. 1997;20:343–8. doi: 10.2337/diacare.20.3.343. [DOI] [PubMed] [Google Scholar]
- 13.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculations. Am J Physiol. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 14.Schoeller DA, Taylor PB. Precision of the doubly labeled water method using the two-point calculation. Hum Nutr Clin Nutr. 1987;41C:215–23. [PubMed] [Google Scholar]
- 15.Weir JBV. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63:164–9. doi: 10.1093/ajcn/63.2.164. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Energy and protein requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organization; Geneva: 1985. WHO, Technical Report Series 724. [PubMed] [Google Scholar]
- 18.Schoeller DA, Kushner RF, Taylor WB, Dietz WH. Measurement of total body water: isotope dilution techniques. Columbus, OH: 1985. Report. [Google Scholar]
- 19.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free mass: review and critique of a classic body-compositon constant. Am J Clin Nutr. 1999;69:833–41. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- 20.Stern RD, Dennett MD, Garbutt DJ. The start of the rains in West Africa. J Climatol. 1981;1:59–68. [Google Scholar]
- 21.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 22.Schoeller DA. The shortage of O-18 water (Letter) Obes Res. 1999;7:519. doi: 10.1002/j.1550-8528.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 23.James WPT, Ferro-Luzzi A, Waterlow JC. Definition of chronic energy deficiency in adults. Report of a working party of the International Dietary Energy Consultative Group. Eur J Clin Nutr. 1988;42:969–81. [PubMed] [Google Scholar]
- 24.Hemmingsson E, Ekelund U. Is the association between physical activity and body mass index obesity dependent? Int J Obes Relat Disord. 2007;31:663–8. doi: 10.1038/sj.ijo.0803458. [DOI] [PubMed] [Google Scholar]
- 25.Luke A, Durazo-Arvizu RA, Rotimi CN, et al. Activity energy expenditure and adiposity among black adults in Nigeria and the United States. Am J Clin Nutr. 2002;75:1045–50. doi: 10.1093/ajcn/75.6.1045. [DOI] [PubMed] [Google Scholar]
- 26.Ferro-Luzzi A. The conceptual framework for estimating food energy requirement. Public Health Nutr. 2005;8:940–52. doi: 10.1079/phn2005789. [DOI] [PubMed] [Google Scholar]
- 27.Ferro-Luzzi A. In: Taylor TG, Jenkins NK, editors. Range of variation in energy expenditure and scope for regulation; Proc of the XIII International Congress of Nutrition; London: J. Libbey Press. 1986.pp. 393–9. [Google Scholar]
- 28.Esparza J, Fox C, Harper IT, et al. Daily energy expenditure in Mexican and USA Pima Indians: low physical activity as a possible cause of obesity. Int J Obes Relat Disord. 2000;24:55–9. doi: 10.1038/sj.ijo.0801085. [DOI] [PubMed] [Google Scholar]
- 29.Ferro-Luzzi A, Scaccini C, Taffese S, Aberra B, Demeke T. Seasonal energy deficiency in Ethiopian rural women. Eur J Clin Nutr. 1990;44(Suppl 1):7–18. [PubMed] [Google Scholar]
- 30.Luke A, Rotimi CN, Adeyemo AA, et al. Comparability of resting energy expenditure in Nigerians and U.S. blacks. Obes Res. 2000;8:351–9. doi: 10.1038/oby.2000.42. [DOI] [PubMed] [Google Scholar]
- 31.Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–51. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher D, Albu J, He Q, et al. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than white adults. Am J Clin Nutr. 2006;83:1062–7. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr. 2006;83:1076–81. doi: 10.1093/ajcn/83.5.1076. [DOI] [PubMed] [Google Scholar]
- 34.Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. New Engl J Med. 1988;318:467–72. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 35.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16:667–74. [PubMed] [Google Scholar]
- 36.Ekelund U, Yngve A, Brage S, Westerterp K, Sjöström M. Body movement and physical activity energy expenditure in children and adolescents: how to adjust for differences in body size and age. Am J Clin Nutr. 2004;79:851–6. doi: 10.1093/ajcn/79.5.851. [DOI] [PubMed] [Google Scholar]
- 37.Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB. Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Disord. 1995;19:644–52. [PubMed] [Google Scholar]
- 38.Ball K, Owen N, Salmon J, Bauman A, Gore CJ. Associations of physical activity with body weight and fat in men and women. Int J Obes Relat Metab Disord. 2001;25:914–9. doi: 10.1038/sj.ijo.0801622. [DOI] [PubMed] [Google Scholar]
- 39.Bernstein MS, Costanza MC, Morabia A. Association of physical activity intensity levels with overweight and obesity in a population-based sample of adults. Prev Med. 2004;38:94–104. doi: 10.1016/j.ypmed.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 40.Westerterp KR, Goran MI. Relationship between physical activity related energy expenditure and body composition: a gender difference. Int J Obes Relat Metab Disord. 1997;21:184–8. doi: 10.1038/sj.ijo.0800385. [DOI] [PubMed] [Google Scholar]
- 41.Schoeller DA, Jeffords G. Determinants of the energy costs of light activities: inferences for interpreting doubly labeled water data. Int J Obes Relat Disord. 2002;26:97–101. doi: 10.1038/sj.ijo.0801851. [DOI] [PubMed] [Google Scholar]
- 42.Forsum E, Löf M, Schoeller DA. Calculation of energy expenditure in women using the MET system. Med Sci Sports Exerc. 2006;38:1520–5. doi: 10.1249/01.mss.0000228947.93270.2e. [DOI] [PubMed] [Google Scholar]
- 43.Schutz Y, Weinsier RL, Hunter GR. Assessment of free-living physical activity in humans: an overview of currently available and proposed new measures. Obes Res. 2001;9:368–79. doi: 10.1038/oby.2001.48. [DOI] [PubMed] [Google Scholar]
- 44.Weinsier RL, Hunter GR, Desmond RA, Byrne NM, Zuckerman PA, Darnell BE. Free-living activity energy expenditure in women successful and unsuccessful at maintaining a normal body weight. Am J Clin Nutr. 2002;75:499–504. doi: 10.1093/ajcn/75.3.499. [DOI] [PubMed] [Google Scholar]
- 45.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]