Abstract
Background/Aims
Chronic infection with hepatitis C, genotype 2/3, responds better than other genotypes to peginterferon and ribavirin treatment. We hypothesized that a lower dose of peginterferon would be as effective, but less toxic than standard doses.
Methods
30 patients were treated with low-dose peginterferon alfa-2a (90 μg/week) and 27 patients with standard doses (180 μg/week) for 24 weeks in combination with 800 mg/day of ribavirin. Patients who failed treatment were offered 48 weeks of standard-dose treatment. Viral and serum IP-10 levels were measured and early viral kinetic parameters were calculated.
Results
Sustained virological response was achieved in 68% of the low-dose and 87% of the standard-dose patients (per-protocol, p=0.79 for non-inferiority). Retreatment was successful in all patients who tolerated full dose and duration. The standard-dose group had greater first phase declines of viral levels and faster time to negativity. The second phase slope was not dose-dependent. IP-10 induction was significantly greater with the standard dose. Although fatigue and general feeling during treatment were worse for standard dose, hematologic toxicity and depression did not differ between groups.
Conclusions
A lower dose of peginterferon is associated with some symptomatic benefit but the response is not equivalent to standard dosing.
Keywords: Liver, Viral hepatitis
Introduction
Chronic infection with hepatitis C virus (HCV) is estimated to affect 2% of the world population (1) and is a leading cause of liver-related mortality and HCC (2). Of the six major genotypes, genotype 1 is the most prevalent in the western world, while genotypes 2 and 3 affect a lesser proportion (3). These genotypes are more prevalent in south-east Asia and the Indian sub-continent, where they may have originated (4). Although genotypes do not differ in respect to pathogenicity, their response to interferon therapy is strikingly different; patients infected with genotype 2 or 3 achieve higher rates of sustained virological response (SVR) with a shorter course of therapy and a lower dose of ribavirin than that required for genotype 1 (5).
The greater responsiveness of genotypes 2 and 3 to interferon therapy prompted attempts to further modify their treatment regimen, attempting to reduce side-effects and costs and improve tolerability and compliance. Although a shortened, 16 week treatment course was initially suggested to be equivalent to 24 weeks of treatment, a recently published large randomized trial demonstrated that the shorter course was less effective (6, 7). The alternative approach is through reduction of medication dose. Several large studies, using peginteferon alfa-2a or alfa-2b alone (8, 9) or in combination with ribavirin (10), demonstrated similar response rates for standard and lower (135 μg/week, 1.0 or 0.5 μg/kg/week, respectively) doses of peginterferon in patients with genotype 2/3 infection.
We hypothesized that treatment with a low dose regimen of peginterferon combined with ribavirin can preserve the efficacy of the full dose, while reducing side effects and potentially, treatment cost.
Mathematical modeling of the early kinetics of interferon response demonstrated a typical biphasic pattern of response, and parameters derived from this model are thought to reflect different facets of interferon action. From these parameters one can estimate the efficiency of interferon in blocking viral replication and the rate of loss of infected cells (11). Interferon is thought to act through induction of interferon-stimulated genes (ISGs), although the actual effector ISGs are yet to be identified. It was previously shown that patients with baseline increased ISG expression are less likely to respond to treatment (12). This is thought to reflect near-maximal, but ineffective, activation of the interferon response system and inability to mount further responses to exogenous interferon (13-15). We hypothesized that dose-dependent changes in early viral kinetics and their effect on serum levels of a representative ISG will help to elucidate the mechanism of altered responsiveness and the effects of peginterferon dose. For that end we calculated parameters of early viral kinetics and measured serum interferon gamma inducible protein 10 (IP-10), an ISG for which baseline serum levels are known to be associated with response rates (16-18).
Patients and Methods
Study design
A prospective, open-label, controlled study to evaluate whether low-dose peginterferon and ribavirin would result in similar efficacy but improved tolerability compared to standard therapy. The primary end point was achievement of SVR, defined as HCV RNA negativity in the serum, 24 weeks after treatment cessation.
Entry criteria
Adult, treatment-naïve patients with HCV genotype 2 or 3 infection and detectable serum HCV RNA were eligible for inclusion. Exclusion criteria included severe systemic disease, human immunodeficiency virus (HIV) co-infection, other liver diseases, pre-existing bone marrow dysfunction and contraindications for peginterferon or ribavirin. An elevated level of alanine aminotransferase (ALT) was not required for eligibility.
Treatment and patients
Between December 2003 and December 2004, 31 patients were enrolled into the low-dose (LD) group, and were treated with peginterferon alfa-2a (Pegasys, Roche Pharmaceuticals, Nutley, NJ) 90 μg/week and ribavirin (Copegus, Roche Pharmaceuticals) 400 mg twice daily for 24 weeks.
In February 2005, after 31 patients were enrolled and 18 patients had finished 48 weeks of follow-up, only 9 patients (50%) had achieved SVR. A pre-defined analysis determined this to be significantly inferior to the published expected response rate of 78% with the standard dose. Enrollment to the LD group was halted and subsequent patients (from July 2005 to July 2007) were enrolled into a standard-dose (SD) group, and treated for 24 weeks with the doses of the approved regimen.
Patients (from both groups) who did not become HCV RNA negative by week 12 of treatment were defined for the purpose of this study as non-responders. This deviation from the standard definition was based on the observation that virtually all patients with genotype 2/3 infection become HCV RNA negative by 12 weeks. These non-responders, and patients who relapsed (i.e. had serum HCV RNA reappearance after treatment cessation) after treatment, were offered extended-therapy (ET) with standard doses of peginterferon and ribavirin for 48 weeks. The response to ET was analyzed separately. The study design is outlined on Supplementary Figure 1.
Patient monitoring
HCV RNA was qualitatively tested using PCR (Roche Amplicor, Roche Molecular Systems, Pleasanton, CA) with a lower limit of detection of 50 International Units (IU)/ml. Positive samples were quantified using a quantitative PCR assay (Cobas Amplicor HCV Monitor, Roche Molecular Systems) with a lower limit of detection of 600 IU/ml. After January 2007, HCV RNA was assessed using Taqman real-time PCR with a lower limit of detection of 15 IU/ml. A complete blood count was obtained on every visit. The peginterferon dose was reduced for an absolute neutrophil count < 500/ml or a platelet count < 50,000/ml; a hematocrit below 30% mandated ribavirin dose reduction. A visual analogue scale (VAS) was used at each visit to quantify the severity of common side-effects, including fatigue and general well-being, with scores ranging continuously from 0 (best) to 10 cm (worst). Depression was assessed at baseline and at weeks 12, 24 and 48 using the Center for Epidemiologic Studies Depression (CES-D) questionnaire (score range 0-60) (19).
Viral kinetics
Viral kinetic parameters were calculated as previously reported for standard interferon (11) and modified for pegylated interferon (20). The first phase decline was defined as the decrease in log-transformed viral levels from baseline to day 2. The second phase slope is usually defined as the slope of the linear regression of quantifiable log-transformed HCV RNA levels on days 7, 14 and 28. However, this method does not apply to rapid responders, who become HCV RNA negative (or non-quantifiable) at week 2 or earlier, for whom the “classic” slope calculation would underestimate the true slope and bias against detecting a difference between groups. To better estimate the true slope, we calculated the minimal possible slope, by assigning the sensitivity cut-off value of the relevant assay to the first non-quantifiable result. For example, a negative result with the real-time PCR assay (with a sensitivity of 15 IU/ml) was assigned a valued of 15 IU/ml for the calculation. When the first non-quantifiable value was on day 9, the slope was calculated from day 2 to day 9.
Liver biopsies
A liver biopsy was not required for enrollment, but was performed in some patients for clinical purposes, or as part of natural history protocols. A sample from all liver biopsies obtained at the NIH Clinical Center is routinely flash-frozen in liquid nitrogen and stored at −80°C.
IP-10 measurement
Serum samples, obtained before treatment and on days 0, 2 and 7, were stored at −80°C until assayed. IP-10 levels were measured using a solid-phase ELISA kit (Quantikine, R&D systems; Minneapolis, MN) according to the manufacturer's instructions. In preliminary analyses, a negative correlation was found between the measured IP-10 level and time in storage (Supplementary Figure 2), suggesting time-dependent degradation. To correct for this effect, we analyzed IP-10 levels from 50 untreated patients with chronic hepatitis C, genotype 1, collected during a similar time period, and fit a linear regression line to them. We used the parameters of this regression to correct the measured IP-10 levels from the genotype 2 or 3 patients using the equation: IP-10Corrected = IP-10Measured + 0.09854*t; where t represents the time (in days) in storage. All IP-10 levels reported are corrected values.
Frozen liver biopsy samples were thawed and RNA was extracted using EasyRNA mini kit (QIAGEN, Valencia, CA). After reverse transcription, Taqman real-time PCR was performed to quantitate levels of IP-10 and GAPDH mRNA (Applied Biosystems Inc., Foster City, CA).
Statistical analyses
Continuous variables were compared using Student's t-test (for normally distributed variables) or Mann-Whitney's U. Fisher's exact test and Pearson's X2 were used when appropriate. Cox regression was used for time-to-event analyses. Significance testing for non-inferiority was performed using the normal approximation solution corrected for continuity (21, 22). A maximal difference in efficacy of 10% was chosen as an acceptable margin. IP-10 levels were log-transformed to obtain a normal distribution. A two-tailed p-value of less than 0.05 was considered statistically significant for all secondary analyses.
Data were analyzed using Microsoft Excel, SPSS version 13 and GraphPad Prism version 4.0c.
Ethical approval and consent
The original design of the study and its modification on February 2005 were approved by the NIDDK Institutional Review Board and the protocol was conducted under an IND held by the senior investigator (JHH, IND #10168). All patients gave written informed consent. The study was registered on clinicaltrials.gov (NCT00056862).
Results
Patient characteristics
31 patients were enrolled in the LD and 27 in the SD group. One patient assigned to LD mistakenly injected the standard dose of peginterferon for several weeks and was omitted from further analysis. There were no significant differences at baseline between the two treatment groups, although there was a trend for greater proportion of HCV genotype 3 infection in the SD group (Table 1).
Table 1.
Patient baseline characteristics
| |
LD (n=30) |
SD (n=27) |
p value |
|---|---|---|---|
| Agea (years) |
48 (29-62) |
47 (26-69) |
0.821 |
| Male sex (%) |
15 (50%) |
13 (48%) |
1.002 |
| Ethnicity | |||
| Caucasian | 26 (87%) | 20 (74%) | |
| Asian | 2 (7%) | 5 (19%) | 0.322 |
| Hispanic | 1 (3%) | 0 (0%) | |
| African-American |
1 (3%) |
2 (7%) |
|
| ALT (U/L)b |
91 ± 69 |
97 ± 85 |
0.931 |
| HCV RNA (log IU/ml)b |
6.3 ± 0.82 |
5.9 ± 1.02 |
0.141 |
| Genotype | |||
| 2 | 21 (70%) | 13 (48%) | 0.1122 |
| 3 |
9 (30%) |
14 (52%) |
|
| Liver Histology | |||
| HAI inflammatory scorec | 8 (3-13) | 8 (5-14) | 0.363 |
| Ishak fibrosis scorec | 2 (0-6) | 1 (0-6) | 0.923 |
Mean (range)
Mean ± standard deviation
Median (range)
Student's t-test
Fisher's exact test
Mann-Whitney's U test
Treatment results
Patients in the SD group demonstrated numerically higher rates of SVR and lower rates of relapse on intention-to-treat and per-protocol analyses (Table 2). The null hypothesis of LD inferiority by more than 10% could not be rejected in either analysis (difference in SVR rates = 19.1%; 95% CI = −41.2 to +0.03%; p=0.79).
Table 2.
Treatment results
| Results | LD | SD |
|---|---|---|
| Intention-to-treat | ||
| No. of patients | 30 | 27 |
| SVR | 19 (63%) | 21 (78%) |
| Relapse/Breakthrough | 7 (23%) | 2 (7%) |
| Non-response | 3 (10%) | 1 (4%) |
| Treatment stopped for adverse event | 0 (0%) | 3 (11%) |
| Lost to follow-up | 1 (3%) | 0 (0%) |
| Per protocola | ||
| No. of patients | 28 | 23 |
| SVR | 19 (68%) | 20 (87%) |
| Relapse/Breakthrough | 7 (25%) | 2 (9%) |
| Non-response | 2 (7%) | 1 (4%) |
Excluded from analysis:
LD – 1 patient needed peginterferon dose reduction for neutropenia, 1 patient lost to follow-up
SD – 1 patient needed peginterferon dose reduction for neutropenia, 3 patients withdrawn from treatment for serious adverse events
Retreatment results
Of 11 patients who failed to achieve an SVR with LD treatment, 8 were retreated for 48 weeks using ET. Two (25%) patients could not tolerate the full dose and duration of ET and did not achieve an SVR. All 6 patients (2 non-responders and 4 relapsers) who were able to tolerate a full ET course achieved an SVR. Of the 3 patients who failed SD treatment, two (one relapser and one non-responder) received ET; both had an SVR. Including ET, the final SVR rate was 83% for the LD arm (25/30 patients) and 85% for SD (23/27, intention-to-treat). In both groups, all patients who failed to achieve an SVR were either intolerant of therapy or were lost to follow up.
Viral kinetics
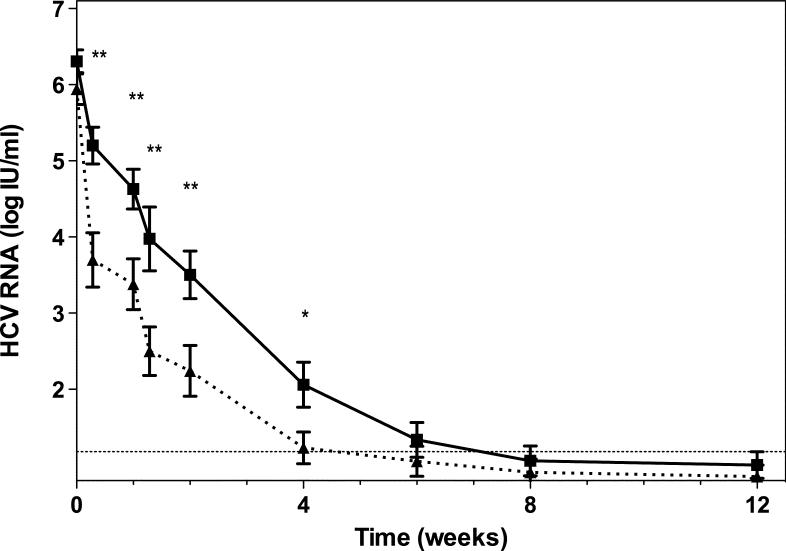
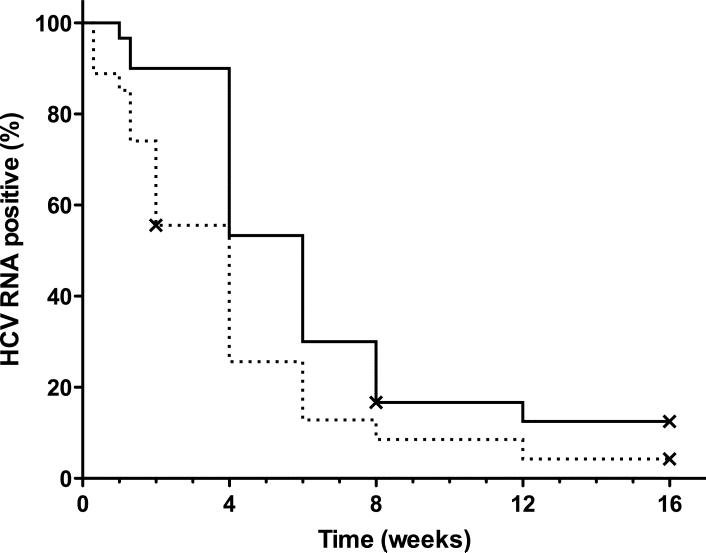
The change in HCV RNA levels over time is shown in Figure 1a. From day 2 to week 4 of treatment, the average viral level was significantly lower in the SD than the LD group. In subsequent days, as more patients from the LD group became HCV RNA negative, the difference disappeared. Patients treated with SD became HCV RNA negative earlier on treatment (Figure 1b; p=0.041 by Cox regression analysis). The first phase decline in HCV RNA levels was significantly greater for the SD group (Table 3). The second phase slope was calculable for 29/30 (97%) of LD patients and 21/27 (78%) of SD, and did not significantly differ between the groups (Table 3).
Figure 1.
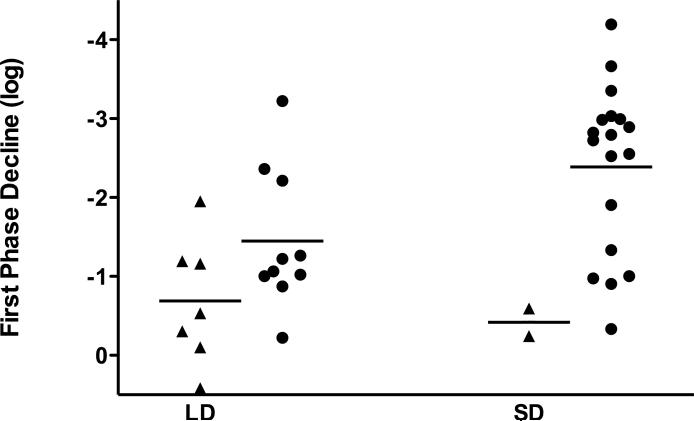
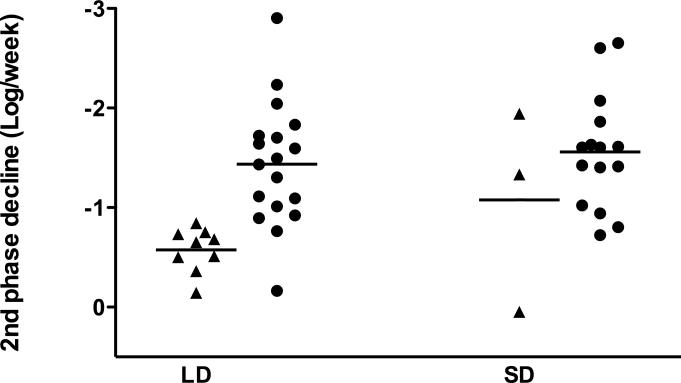
(a) The average level of HCV RNA (± standard error of the mean, SEM) during treatment. The solid line denotes SD, LD is marked by the dashed line. The dotted line marks the lower limit of detection. ** - p<0.01, * - p<0.05 (unpaired Student's t-test). (b) Kaplan-Meier analysis of time to negative serum HCV RNA on treatment (per protocol analysis). (c) First phase decline of HCV RNA levels. Patients who achieved SVR are marked with a circle, patients who did not achieve SVR by triangles (per-protocol analysis). (d) Second phase slope according to treatment group and result (per-protocol analysis).
Table 3.
Viral Kinetic Parameters
| LD | SD | p value | |
|---|---|---|---|
| First phase decline (Log10)1 | 1.15 ± 0.88 | 2.20 ± 1.14 | 0.002a |
| Second phase slope (Log10/week)1 | 1.14 ± 0.69 | 1.39 ± 0.64 | 0.20b |
| Time to negativity (days)2 | 42 (32-51) | 28 (19-37) | 0.047c |
Mean ± standard deviation
Median (95% confidence interval)
Student's t-test
Mann-Whitney U test
Mantel-Cox log rank test
The parameters of viral kinetics in both groups differed from those of a similar cohort of patients infected with HCV genotype 1 being treated in a parallel study (23). For the genotype 1 infected patients, the average first phase decline was −0.66 log (p=0.046 when compared with LD, p<0.001 when compared with SD) and the second phase slope was 0.46 log/week (p<0.001 and p<0.001 compared with LD and SD, respectively).
Viral kinetic parameters predicted SVR, irrespective of the peginterferon dose used. ROC analysis revealed that the first phase decline (AUC=0.853, p=0.02, Figure 1c) and the second phase slope (AUC=0.953, p<0.001, Figure 1d) significantly predicted SVR. The second phase was particularly predictive in the LD group; patients with a slope of less than −0.75 log/week were unlikely to achieve an SVR.
Patients who had failed initial LD treatment and were treated subsequently with ET had superior kinetics with the higher dose of peginterferon (Supplementary figure 3a-c). In contrast, a patient who relapsed after SD therapy and was retreated with ET, demonstrated similar response curves for both courses (Supplementary figure 3d).
IP-10 levels
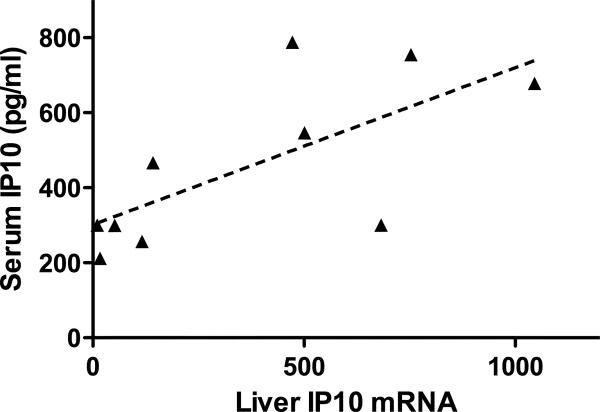
Baseline serum IP-10 levels did not differ between the two groups (p=0.218). The baseline IP-10 correlated with serum ALT, platelet count, inflammatory activity (HAI inflammatory score), Ishak fibrosis score and baseline viral levels (Table 4). The correlation with patient age reached borderline significance. On multivariate regression, only ALT level, platelet count and baseline HCV RNA level remained significant. IP-10 mRNA from liver biopsy samples was quantified for 10 patients who underwent biopsy shortly before therapy and correlated with the contemporaneous serum IP-10 levels for these patients (Figure 2a).
Table 4.
Association of baseline IP-10 (log transformed) and baseline features
| Parameter | R | p value |
|---|---|---|
| Univariate analysis1 | ||
| Age (years) | 0.249 | 0.07 |
| ALT (U/L) | 0.582 | <0.001 |
| Platelets (1000/μl) | −0.493 | <0.001 |
| HAI inflammatory score | 0.415 | 0.002 |
| Ishak fibrosis score | 0.433 | 0.002 |
| Baseline HCV RNA (log10 IU/ml)) | −0.403 | 0.003 |
| Multivariate analysis | ||
| ALT (U/L) | 0.454 | <0.001 |
| Baseline HCV RNA (log10 IU/ml)) | −0.289 | 0.01 |
| Platelets (1000/μl) | −0.258 | 0.03 |
Sex, genotype, bilirubin, albumin, white blood cells were not significantly correlated with IP-10
Figure 2.
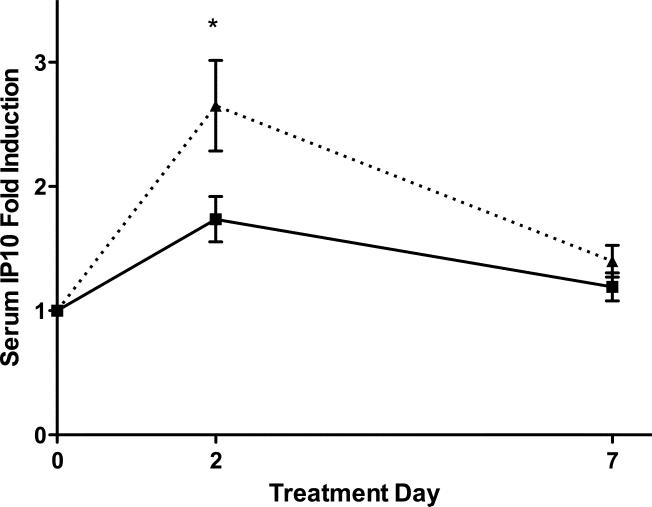
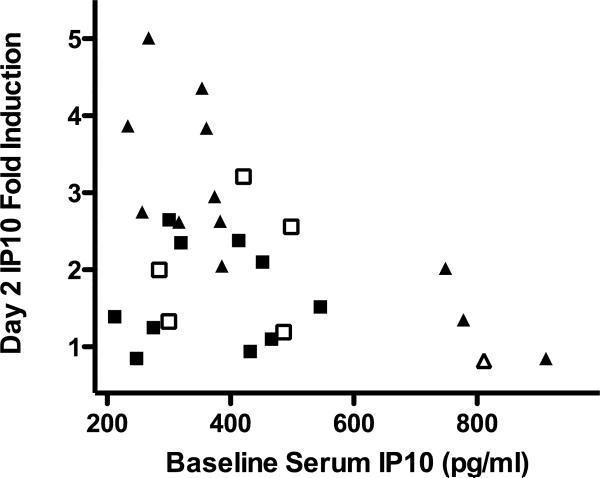
(a) Correlation of baseline serum IP-10 levels with liver IP-10 mRNA levels (normalized to GAPDH mRNA levels, arbitrary units). r2 = 0.489, p = 0.025. (b) Serum IP-10 level fold induction relative to day 0 for LD (solid line, squares) and SD (dashed line, triangles). * p<0.05. (c) Day 2 IP-10 induction vs. baseline levels. LD marked with squares, SD with triangles. Open symbols mark treatment failure and filled symbols mark SVR.
During treatment, serum IP-10 levels increased on day 2 and declined to near-baseline levels by day 7 (Figure 2b). The day 2 fold-induction was significantly higher in SD than LD patients (2.65 vs. 1.74, p=0.035) and correlated inversely with the baseline IP-10 levels (Pearson's r=−0.49, p=0.005), with little or no induction seen in patients with a high baseline IP-10 (Figure 2c).
In both treatment groups, baseline IP-10 levels did not predict treatment outcome. However, in the SD group, the baseline levels were strongly correlated with parameters of response kinetics (first phase decline, r=0.68, p=0.001; second phase slope, r=0.62, p=0.004). No correlation was seen with the response kinetics of the LD patients. As expected, the day 2 fold induction was highly correlated with the first phase response in both groups (r=−0.77, p<0.001).
Side effects
Hematological toxicity, manifest as nadir blood counts, as cytopenic indication for dose reduction or the need for growth factor support, did not differ between groups. Fatigue and overall well-being VAS scores were significantly worse during SD treatment than with LD (p=0. 027 and p=0.033, respectively). In contrast, depression scores, measured by CES-D, were not different between the two groups (p=0.95). There were 2 serious adverse events in the LD group and 5 in the SD, of which four overall (1 in LD and 3 in SD) were considered treatment-related.
Discussion
In this study we demonstrate that the efficacy of 24 weeks of treatment with 90 μg/week of peginterferon alfa-2a and 800 mg/d of ribavirin was not equivalent to standard therapy, mostly due to higher rates of relapse (25% vs 11%).
Several studies have examined the response of HCV genotype 2 or 3 infected patients to lower doses of peginterferon. These studies differed in the peginterferon used, the dose of ribavirin, and duration of treatment, as well as in the methodology of analysis and interpretation. Sood et al (24) assessed the response of 103 patients infected with genotype 3 to a lower-dose regimen of peginterferon alfa-2b combined with weight-based ribavirin at 10-12 mg/kg/day.
The SVR rate for patients treated with peginterferon alfa-2b 1 μg/kg/week was 78.9%, compared to 92.6% with standard dose (1.5 μg/kg/week). The authors interpreted the results as showing equivalence; however, they defined a non-inferiority margin of up to 20% in response rates as the significance limit, a choice that could be debated. Similarly, Meyer-Wyss et al. (25) found a difference of 21% (65% vs. 86%) between patients treated with 800 mg/day of ribavirin and 1 or 1.5 μg/kg/week of peginterferon alfa-2b, a difference that was not statistically significant. A non-significant difference of 9% was observed in another study (26).
The consistent numeric difference between treatment groups (9% to 21%) in these studies suggests all had insufficient power to detect statistical significance. Furthermore, non-inferiority hypothesis testing is more appropriate than “classic” superiority analysis to address this question. In order to have a power of 80% to prove non-inferiority with the pre-determined margin of 10%, we would have had to include 270 patients in each group. Thus, our trial is obviously underpowered. However, the difference of 19% in SVR rates, which is in the same range as previously reported, suggests that the inability to prove non-inferiority hypothesis was not due to lack of power, but actually to true inferiority of the lower dose regimen.
In other studies, different doses of ribavirin and durations of therapy made the comparison to standard therapy difficult. An initial, dose-ranging study of peginterferon alfa-2a monotherapy (27) did not find a difference in efficacy in non-genotype 1 infection between 90 and 180 μg/week, although the number of patients was small. Abergel et al (28) found no difference in response rates of genotype 2 or 3 infected patients treated for 48 weeks with 800 mg/day of ribavirin and 0.75 or 1.5 μg/kg/week of peginterferon alfa-2b. A recently published uncontrolled study (29), assessed 135 μg/week of peginterferon alfa-2a combined with 11 mg/kg/day of ribavirin and observed SVR (85%) and rapid virological response (70%) rates remarkably similar to the results in our SD group. These results suggest that the inferior response to a lower dose of peginterferon may be overcome by prolonging treatment duration or by increasing the dose of ribavirin, but direct comparisons were not made.
Analyzing the early dynamics of viral response to treatment may shed light on the mechanism of the dose response and of the genotypic difference in sensitivity to treatment. We demonstrate a marked effect of peginterferon dose on first phase decline with no effect on the second phase slope. These findings are consistent with those of Neumann et al (11) as well as with the predictions of their model, in which the efficiency (ε) of interferon in blocking new virion production (the predominant determinant of the first phase) is dose-dependent. The second phase slope, although affected to a lesser degree by ε, is thought to mainly represent the rate of infected cell loss, by a mechanism yet unknown, and was not found to be dose-dependent. Similar to our results, a study comparing two doses of peginterferon alfa-2b combined with ribavirin in patients with genotype 1 infection (30), found the first phase to be affected by peginterferon dose. The second phase slope was not explicitly reported but estimation from the data shown suggests it was similar between doses.
To further examine the effect of peginterferon dose, we measured serum levels of IP-10, an interferon-stimuated gene. Baseline serum levels of IP-10 have been reported to correlate inversely with SVR rates and rapid virological response (16-18). In this study, this correlation was not found, probably because of the very high response rate achieved in genotypes 2 or 3 infection, and the fact that most treatment failures were due to relapse or intolerance to therapy. However, we did find a dose-dependent induction of IP-10, consistent with higher interferon effectiveness with higher doses of peginterferon. In both treatment groups, lower baseline IP-10 levels predicted a greater IP-10 induction on day 2 of treatment and, at least in the SD group, also predicted a greater virological response to peginterferon, manifested by a greater first phase decline and steeper second phase slope. These findings are concordant with reports showing that patients who have higher baseline expression of interferon-stimulated genes have a lower rate of response (15). Thus, patients with chronic activation of the endogenous interferon system appear to have a limited ability to mount a further response to exogenously administered interferon. The lack of an intrahepatic ISG response appears to result in lower response rates for genotype 1 infected patients. However, for patients with HCV genotype 2 or 3 infection, even a less-than-optimal ISG response appears to be sufficient to achieve viral eradication.
Although many ISGs are induced by endogenous and exogenous interferon, it is unclear which specific ISGs mediate the antiviral activity and IP-10 is not necessarily one of those. Most of the published data on ISGs is derived from measurements of mRNA levels in the liver (15) or peripheral blood mononuclear cells. There is little data on serum levels of secreted ISGs in chronic hepatitis C, apart from IP-10, for which baseline and on-treatment levels were shown to correlate with treatment response (16-18). Thus, we selected IP-10 as a representative serum marker of baseline and on-treatment interferon responsiveness.
All patients who failed initial treatment responded to a retreatment course of 48 weeks with standard doses of peginterferon and ribavirin (per-protocol analysis). A qualitative comparison of the dynamics of viral response for individual patients to the two doses further supported the differences in viral responses observed between LD and SD groups. Furthermore, patients re-treated with the same dose of peginterferon had identical viral responses, during both the first and second phases, demonstrating the reproducibility of this antiviral response. These findings imply that interferon does not exert a selective pressure on the virus and that resistance to treatment (whether viral- or host-mediated) exists prior to therapy.
In conclusion, we demonstrate a dose-dependent response of patients with HCV genotype 2 or 3 infection, evident in the clinical response rate, viral dynamics and cytokine (IP-10) induction. As the lower dose was only marginally better in terms of tolerability, this regimen is not justified for most patients. However, LD therapy still yields reasonably high response rates, meaning that therapy need not be abandoned if patients cannot tolerate full dose therapy. Furthermore, failures can be successfully treated by a second, prolonged course using the standard dose of peginterferon. The genotypic difference in viral sensitivity to interferon allows patients infected with HCV genotype 2 or 3 to overcome barriers such as low peginterferon dose or pre-existing activation of the interferon system, barriers that play a greater role in the treatment of the more resistant genotype 1.
Supplementary Material
Supplementary Figure 1 – Trial design. Patients were enrolled into the low dose (LD) or standard dose (SD) groups, for a planned duration of 24 weeks. At week 12 of treatment, patients who did not become HCV RNA negative were defined as non-responders and received extended therapy (ET) with full dose for another 36 weeks. Patients who relapsed after the end of treatment were given ET for 48 weeks.
Supplementary Figure 2 – Decay of IP-10 levels in stored serum samples. Pre-treatment IP-10 levels (all measured on December 2007) are displayed against the date the sample was drawn. Linear regression slope −0.24pg/(ml*day) (dashed line), r2=0.13.
Supplementary Figure 3 - HCV RNA levels during initial therapy (solid line) and ET course (dashed line) for individual patients. ND - non-detectable. (a, b) Relapsers after initial LD treatment. (c) Non-responder to initial LD treatment. Switched to ET on week 16. (d) Relapser after initial SD treatment.
Supplementary Table 1 – Hematologic toxicity parameters
Acknowledgments
This study was funded by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
The medications for the study were kindly supplied by Roche Pharmaceuticals, Nutley, NJ under a Clinical Trial Agreement with the National Institutes of Health.
Abbreviations
- ALT
alanine aminotransferase
- AUC
area under the curve
- ET
extended therapy
- HAI
histological activity index
- IU
international units
- LD
low dose
- ROC
receiver-operator characteristics
- SD
standard dose
- SVR
sustained virological response
- VAS
visual analog scale
References
- 1.Perz JF, Farrington LA, Pecoraro C, Hutin YJ, Armstrong GL. Estimated global prevalence of hepatitis C virus infection. Annual meeting of the Infectious Diseases Society of America.; Boston. 2004. [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J. Gastroenterol. 2007;42(7):513–21. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 4.Forns X, Bukh J. The molecular biology of hepatitis C virus. Genotypes and quasispecies. Clin Liver Dis. 1999;3(4):693–716, vii. doi: 10.1016/s1089-3261(05)70234-8. [DOI] [PubMed] [Google Scholar]
- 5.Hadziyannis SJ, Sette H, Jr., Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 2004;140(5):346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, et al. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N. Engl. J. Med. 2007;357(2):124–34. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- 7.Andriulli A, Mangia A, Iacobellis A, Ippolito A, Leandro G, Zeuzem S. Meta-analysis: the outcome of anti-viral therapy in HCV genotype 2 and genotype 3 infected patients with chronic hepatitis. Aliment. Pharmacol. Ther. 2008;28(4):397–404. doi: 10.1111/j.1365-2036.2008.03763.x. [DOI] [PubMed] [Google Scholar]
- 8.Pockros PJ, Carithers R, Desmond P, Dhumeaux D, Fried MW, Marcellin P, et al. Efficacy and safety of two-dose regimens of peginterferon alpha-2a compared with interferon alpha-2a in chronic hepatitis C: a multicenter, randomized controlled trial. Am. J. Gastroenterol. 2004;99(7):1298–305. doi: 10.1111/j.1572-0241.2004.30306.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, et al. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology. 2001;34(2):395–403. doi: 10.1053/jhep.2001.26371. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 11.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, et al. Hepatitis C Viral Dynamics in Vivo and the Antiviral Efficacy of Interferon-Therapy. Science. 1998;282(5386):103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Borozan I, Feld J, Sun J, Tannis LL, Coltescu C, et al. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128(5):1437–44. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MW, Tsukahara T, Brodsky L, Schaley J, Sanda C, Stephens MJ, et al. Changes in gene expression during pegylated interferon and ribavirin therapy of chronic hepatitis C virus distinguish responders from nonresponders to antiviral therapy. J. Virol. 2007;81(7):3391–401. doi: 10.1128/JVI.02640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, et al. Interferon signaling and treatment outcome in chronic hepatitis C. Proc. Natl. Acad. Sci. U. S. A. 2008;105(19):7034–9. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, et al. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46(5):1548–63. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diago M, Castellano G, Garcia-Samaniego J, Perez C, Fernandez I, Romero M, et al. Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55(3):374–9. doi: 10.1136/gut.2005.074062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagging M, Romero AI, Westin J, Norkrans G, Dhillon AP, Pawlotsky JM, et al. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44(6):1617–25. doi: 10.1002/hep.21407. [DOI] [PubMed] [Google Scholar]
- 18.Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, et al. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J. Infect. Dis. 2006;194(7):895–903. doi: 10.1086/507307. [DOI] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 20.Zeuzem S, Herrmann E, Lee JH, Fricke J, Neumann AU, Modi M, et al. Viral kinetics in patients with chronic hepatitis C treated with standard or peginterferon alpha2a. Gastroenterology. 2001;120(6):1438–47. doi: 10.1053/gast.2001.24006. [DOI] [PubMed] [Google Scholar]
- 21.Dunnett CW, Gent M. Significance testing to establish equivalence between treatments, with special reference to data in the form of 2X2 tables. Biometrics. 1977;33(4):593–602. [PubMed] [Google Scholar]
- 22.Christensen E. Methodology of superiority vs. equivalence trials and non-inferiority trials. J. Hepatol. 2007;46(5):947–54. doi: 10.1016/j.jhep.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Feld JJ, Ko MS, Hara K, Lutchman GA, Rotman Y, Heller T, et al. Ribavirin improves second phase kinetics through enhanced interferon signaling in genotype 1 HCV infection.. AASLD Liver Meeting; San Francisco, CA. 2008; 2008. [Google Scholar]
- 24.Sood A, Midha V, Hissar S, Kumar M, Suneetha PV, Bansal M, et al. Comparison of low-dose pegylated interferon versus standard high-dose pegylated interferon in combination with ribavirin in patients with chronic hepatitis C with genotype 3: an Indian experience. J. Gastroenterol. Hepatol. 2008;23(2):203–7. doi: 10.1111/j.1440-1746.2007.05057.x. [DOI] [PubMed] [Google Scholar]
- 25.Meyer-Wyss B, Rich P, Egger H, Helbling B, Mullhaupt B, Rammert C, et al. Comparison of two PEG-interferon alpha-2b doses (1.0 or 1.5 microg/kg) combined with ribavirin in interferon-naive patients with chronic hepatitis C and up to moderate fibrosis. J. Viral Hepat. 2006;13(7):457–65. doi: 10.1111/j.1365-2893.2005.00709.x. [DOI] [PubMed] [Google Scholar]
- 26.Krawitt EL, Gordon SR, Grace ND, Ashikaga T, Ray MA, Palmer M, et al. A study of low dose peginterferon alpha-2b with ribavirin for the initial treatment of chronic hepatitis C. Am. J. Gastroenterol. 2006;101(6):1268–73. doi: 10.1111/j.1572-0241.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 27.Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33(2):433–8. doi: 10.1053/jhep.2001.21747. [DOI] [PubMed] [Google Scholar]
- 28.Abergel A, Hezode C, Leroy V, Barange K, Bronowicki JP, Tran A, et al. Peginterferon alpha-2b plus ribavirin for treatment of chronic hepatitis C with severe fibrosis: a multicentre randomized controlled trial comparing two doses of peginterferon alpha-2b. J. Viral Hepat. 2006;13(12):811–20. doi: 10.1111/j.1365-2893.2006.00768.x. [DOI] [PubMed] [Google Scholar]
- 29.Weiland O, Hollander A, Mattsson L, Glaumann H, Lindahl K, Schvarcz R, et al. Lower-than-standard dose peg-IFN alfa-2a for chronic hepatitis C caused by genotype 2 and 3 is sufficient when given in combination with weight-based ribavirin. J. Viral Hepat. 2008;15(9):641–5. doi: 10.1111/j.1365-2893.2008.00999.x. [DOI] [PubMed] [Google Scholar]
- 30.Buti M, Sanchez-Avila F, Lurie Y, Stalgis C, Valdes A, Martell M, et al. Viral kinetics in genotype 1 chronic hepatitis C patients during therapy with 2 different doses of peginterferon alfa-2b plus ribavirin. Hepatology. 2002;35(4):930–6. doi: 10.1053/jhep.2002.32150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 – Trial design. Patients were enrolled into the low dose (LD) or standard dose (SD) groups, for a planned duration of 24 weeks. At week 12 of treatment, patients who did not become HCV RNA negative were defined as non-responders and received extended therapy (ET) with full dose for another 36 weeks. Patients who relapsed after the end of treatment were given ET for 48 weeks.
Supplementary Figure 2 – Decay of IP-10 levels in stored serum samples. Pre-treatment IP-10 levels (all measured on December 2007) are displayed against the date the sample was drawn. Linear regression slope −0.24pg/(ml*day) (dashed line), r2=0.13.
Supplementary Figure 3 - HCV RNA levels during initial therapy (solid line) and ET course (dashed line) for individual patients. ND - non-detectable. (a, b) Relapsers after initial LD treatment. (c) Non-responder to initial LD treatment. Switched to ET on week 16. (d) Relapser after initial SD treatment.
Supplementary Table 1 – Hematologic toxicity parameters