Abstract
Objectives
To evaluate symptoms and signs of genital irritation, vaginal leakage and acceptability of polystyrene sulfonate (PSS), which is being studied as a vaginal contraceptive and microbicide.
Methods
Forty-nine women applied 2.5 mL of either 5% PSS, 10% PSS, PSS vehicle, or Conceptrol® (a marketed spermicidal product containing 4% nonoxynol-9) for 6 consecutive days.
Results
All women completed the study except one in the Conceptrol group who experienced vaginal symptoms after her first use and was discontinued. After both the first use and after all uses, irritation was seen among more women in the Conceptrol group than in the PSS groups, reaching statistical significance with regard to any evidence of irritation, signs of irritation and product-related irritation. There were no adverse events that were serious, unexpected and related to product use in any group. The 5% concentration of PSS may be preferable in terms of leakage and acceptability.
Conclusion
The results suggest that PSS has a safety profile comparable to that of the marketed nonoxynol-9 product, Conceptrol, and appears to be associated with less genital irritation.
Keywords: Contraception, Female, Microbicide, Phase I clinical trial, Polystyrene sulfonate, Sexually transmitted infections, Spermicide, Vaginal, Vaginal irritation
1. Introduction
Polystyrene sulfonate (PSS) is a compound under development as a contraceptive and microbicide. In preclinical studies, PSS has been shown to inhibit sperm hyaluronidase, inhibit acrosin, promote acrosomal loss and impede cervical mucus penetration, but not to kill or immobilize sperm [1–3]. In rabbits, a gel containing 5% PSS prevented pregnancy when mixed with sperm prior to artificial insemination [1,3] or when applied vaginally prior to mating [2,3]. PSS gel appeared to retain its contraceptive activity over several hours [2,3].
Additionally, PSS has antimicrobial activity against a broad spectrum of sexually transmitted pathogens. In vitro, it was active against human immunodeficiency virus (HIV-1), herpes simplex viruses (HSV-1 and HSV-2), human papillomavirus [4], Neisseria gonorrhoeae, Chlamydia trachomatis, Gardnerella vaginalis and anaerobes [1,3–5]. PSS blocks HIV-1 (X4 and R5 tropic) entry into cells by binding to gp120 and gp41 [6]. At concentrations of 5% or less, PSS effectively inhibits herpes infection in mice [7,8]. Duration of action studies in the herpes infection mouse model suggest that 10% PSS concentrations were superior to 5% PSS when applied 5, 30 or 60 min prior to herpes inoculation [9].
Due to the high viscosity and stickiness of 20% PSS gel, the concentration was reduced to 10% for the early toxicology studies. Later vaginal irritation studies in the rat and rabbit indicated minimal irritation after exposure to 10% PSS for up to 14 days, with normal hematological and clinical chemistry parameters at a dose of at least five times the anticipated human vaginal dose [3]. In addition, primary eye irritation, primary skin irritation and penile muscle irritation studies in rabbits demonstrated no or only slight irritation after exposure to the 10% gel formulation. No acute oral toxicity in rats occurred at a dose of more than 5 g/kg, the highest concentration tested. The Bacterial Reverse Mutation Assay demonstrated no potential mutagenic effect. In vitro, PSS and sulfated polysaccharides have anticoagulant activity [10]. PSS has a very high molecular weight, and in vitro studies using human vaginal tissue [11] and skin [12] suggest that it is not absorbed. The daily vaginal doses used in this study (125 and 250 mg) were less than 2% of the lowest recommended daily dose of C-PSS, a cross-linked form of PSS marketed for treatment of hyperkalemia, administered orally as 15 g once to four times daily or rectally as 30 g once daily [13].
PSS appears to be a promising agent for both contraceptive and microbicidal purposes. To initiate human testing, we performed a randomized comparison of 5% and 10% PSS and two controls, PSS vehicle and Conceptrol® (a marketed spermicidal gel containing 4% nonoxynol-9 [N-9]), on symptoms and signs of genital irritation, vaginal leakage and acceptability.
2. Materials and methods
This was a randomized, double-blind, Phase I study performed at the CONRAD Clinical Research Center at the Eastern Virginia Medical School in Norfolk, VA, and Magee-Womens Research Institute of the University of Pittsburgh. The study was approved by the Institutional Review Boards at each site.
2.1. Selection of subjects
A total of 48 women were to be enrolled. The sample size was chosen as one that was considered reasonable and feasible for a Phase I study in which statistically significant differences in outcome between treatment groups are not usually sought. Eligible women were 18–50 years old, in good health and not at risk for pregnancy due to tubal ligation, partner vasectomy or abstinence. Women were not enrolled if they were pregnant or breastfeeding; were allergic to any component of the PSS system, Conceptrol or similar contraceptive products; had a history within the past 6 months of sexually transmitted infection; had known current drug or alcohol abuse or had recently used steroid hormones (oral contraceptives or implant in the past month, injectable or other steroid hormone therapy in the past 3 months).
2.2. Study procedures
Each participant was seen in four visits. After obtaining informed consent at Visit 1, a medical history was obtained and a physical examination performed, including a wet mount from the posterior fornix, pH from the lateral vaginal wall, and cervical samples for detection of N. gonorrhea and C. trachomatis. If vaginal candidiasis, bacterial vaginosis or a urinary tract infection was diagnosed at this visit, the volunteer was treated, preferably with oral medication, and continued in the study. Resolution was demonstrated by repeating wet mount or urinalysis at Visit 2.
Participants with no written record of a Pap smear within the 12 months prior to enrollment had one taken at Visit 1. Laboratory testing included a complete blood count, chemistry panel, activated partial thromboplastin time (APTT) analysis, urinalysis and urine pregnancy test.
The participant was instructed not to use any intravaginal products other than the study product and to abstain from sexual intercourse beginning 72 h prior to the second visit and continuing until she completed the study or was discontinued. If the participant was not surgically sterile or with a partner who had undergone a vasectomy, she was instructed to abstain from intercourse from the start of her menstrual period prior to Visit 2 continuing through the last study visit (Visit 4).
Visit 2 was scheduled to fall within the follicular phase (cycle days 5–10) of the menstrual cycle that followed Visit 1. A urine specimen was taken for pregnancy testing, and cervical/vaginal colposcopy was carried out according to the CONRAD/World Health Organization colposcopy manual [14]. If the participant had findings involving complete disruption of the epithelium, she was not enrolled.
Eligible participants were randomly assigned to use 2.5 mL of one of the following vaginal gels on each of the six subsequent consecutive evenings: PSS vehicle, 5% PSS (125 mg), 10% PSS (250 mg) or Conceptrol (100 mg N-9). The dose volume was based on that of Conceptrol. The PSS vehicle contained the following ingredients: methylparaben, benzoic acid, hydroxyethyl cellulose and water. The PSS vehicle contained PSS (the active ingredient) plus vehicle. Conceptrol contained the following ingredients: glycerin, propylene glycol, methyl paraben, benzoic acid, hydroxyethyl cellulose, and water. All gels were clear, of similar consistency and packaged in the same applicators used for Conceptrol without the proprietary cap and symbol. The delivery volumes of the loaded applicators and the identity of the product contained therein were verified by the packager prior to shipping clinical supplies. The investigators, study coordinators and the sponsor (CONRAD) did not know which product a volunteer was testing. Investigators reported that the appearance of the four gels was similar and that they could not guess any participant’s assignment.
The participant was instructed to insert the study product at bedtime. She was given a diary card on which to record the time of product insertion, vaginal symptoms, use of medications and any other comments regarding the product; a single applicator and a preweighed sanitary pad enclosed in a resealable plastic bag. The participant was instructed to wear the pad for 10 h following the first insertion of the study product. The pad was then to be removed, put into the original bag and returned to the clinic at the next study visit.
Visit 3 was scheduled to take place between 10 and 18 h after the first insertion of study product. The participant was asked to verbally rate vaginal irritation and leakage on a 5-point ordinal scale, where 0 was “none” and 4 was “severe.” A pelvic examination followed by cervical/vaginal colposcopy was performed and evidence of remaining study product was noted. The participant was then given five additional applicators and diary cards for use on each of the 5 subsequent nights, and the process was repeated. The participant completed an acceptability questionnaire prior to exit.
2.3. Analysis of study outcomes
Data entry, data management and the statistical analysis were carried out by Family Health International. The primary safety outcome was the proportion of women with any signs and symptoms of genital irritation, after the first use and at any time during follow-up. Signs were defined as epithelial changes seen on pelvic examination and colposcopy, and symptoms were defined as self-reported itching, pain and abnormal bleeding. The percentage of women with signs and/or symptoms was evaluated as secondary endpoints. Adverse events (AEs) or findings judged by the investigator to be related to the product were a subset of the primary endpoint. All colposcopic findings were recorded and categorized as outlined in the World Health Organization/CONRAD colposcopy manual [14].
Leakage after the first product administration was evaluated quantitatively, by calculating the difference in pre- vs. post-use weight of the sanitary pad used during the first application, and qualitatively, using the 5-point ordinal self-report scale. The overall measure of leakage was obtained for each woman using her median self-reported leakage score across all nights of product use. The overall leakage endpoint was defined as whether the woman’s median value was 2.5 or greater, indicating moderate to severe leakage during product use.
Acceptability of the product was assessed using a questionnaire. The key acceptability item was prespecified as whether or not the participant said she would buy the product. A secondary acceptability item was prespecified as whether or not the participant reported having a problem with the study product.
Separate but parallel analyses compared 5% PSS and 10% PSS to either the PSS vehicle or Conceptrol. For testing differences in proportions across treatment groups, an exact permutation test, stratifying by center, was used unless the number of events was large enough to use an extended Mantel-Haenszel test, also stratifying by center and using table scores to maintain product group as an ordinal variable. The extended Mantel-Haenszel test was used to evaluate all proportions except the binary overall leakage endpoint and the secondary acceptability endpoint (problems with product) [15]. Because the sample size was not based on statistical considerations, the results of statistical tests should not be interpreted as hypothesis tests but only as supplementary information to guide clinical review. The tests have power to detect only very large differences among groups and, therefore, should at best be considered preliminary. For this reason, p values are reported (i.e., there is no reference to an α level for Type I error) and are not adjusted for multiple comparisons.
The 95% confidence intervals for proportions were calculated using exact methods. Approximate confidence intervals for median pad weight differences were computed using the method described by Sellers et al. [16]. Agreement between the participants’ subjective assessments of product leakage and the objective measurement (pre- vs. post-use weight of the sanitary pads) was evaluated by a weighted kappa [17].
3. Results
3.1. Enrollment
Forty-nine women, 13 in the 5% PSS group and 12 in each of the other three treatment groups, were enrolled. All but one subject completed the study using her assigned product for 6 nights and all were seen at each of the four scheduled office visits. One participant experienced vaginal symptoms judged to be product-related after her first use of Conceptrol and was discontinued.
3.2. Baseline characteristics
Participant characteristics are listed in Table 1. There were few abnormalities on the enrollment physical examination or laboratory tests, all but one of which, an eosinophil count, moved toward or into the normal range at Visit 4.
Table 1.
Selected demographic data and obstetrical and contraceptive history both centers pooled
| Characteristics | Vehicle (n = 12) | 5% PSS (n = 13) | 10% PSS (n = 12) | Conceptrol (n = 12) |
|---|---|---|---|---|
| Age (y) | ||||
| Mean | 38.6 | 32.6 | 36.4 | 32.6 |
| SD | (7.04) | (6.85) | (6.56) | (6.58) |
| Race, n, (%) | ||||
| Caucasian | 9 (75.0) | 7 (53.8) | 8 (66.7) | 7 (58.3) |
| Black | 2 (16.7) | 5 (38.5) | 3 (25.0) | 4 (33.3) |
| Hispanic | 1 (8.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Asian/Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| American Indian/Alaskan | 0 (0.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
| Other | 0 (0.0) | 1 (7.7) | 0 (0.0) | 1 (8.3) |
| Education (years in school) | ||||
| Mean | 14.0 | 14.4 | 14.0 | 13.7 |
| SD | (1.71) | (3.07) | (2.98) | (1.92) |
| Marital status, n, (%) | ||||
| Married/living w/partner | 4 (33.3) | 7 (53.8) | 5 (41.7) | 4 (33.3) |
| Married/not living w/partner | 2 (16.7) | 0 (0.0) | 1 (8.3) | 0 (0.0) |
| Unmarried/living w/partner | 1 (8.3) | 2 (15.4) | 1 (8.3) | 0 (0.0) |
| Unmarried/not living w/partner | 3 (25.0) | 1 (7.7) | 1 (8.3) | 5 (41.7) |
| Unmarried/no partner | 2 (16.7) | 3 (23.1) | 4 (33.3) | 3 (25.0) |
| Lifetime obstetrical history, n, (%) | ||||
| No pregnancy | 1 (8.3) | 3 (23.1) | 2 (16.7) | 3 (25.0) |
| Pregnancy | 11 (91.7) | 10 (76.9) | 10 (83.3) | 9 (75.0) |
| Vaginal delivery | 10 (83.3) | 8 (61.5) | 9 (75.0) | 7 (58.3) |
| Contraceptive method used while in study, n, (%) | ||||
| Tubal ligation | 2 (16.7) | 6 (46.2) | 3 (25.0) | 2 (16.7) |
| Partner vasectomy | 2 (16.7) | 1 (7.7) | 1 (8.3) | 1 (8.3) |
| Abstinence | 8 (66.7) | 6 (46.2) | 8 (66.7) | 9 (75.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Prior use of spermicide alone (all types), n, (%) | 7 (58.3) | 5 (38.5) | 3 (25.0) | 3 (25.0) |
| Prior use of spermicide alone (gel only), n, (%) | 3 (25.0) | 1 (7.7) | 1 (8.3) | 2 (16.7) |
Twenty (41%) women had a total of 37 colposcopic findings at baseline. Twenty (54%) findings involved intact epithelium and blood vessels (i.e., erythema), 13 (35%) involved intact epithelium with disrupted blood vessels (i.e., petechiae) and 4 (11%) involved superficially disrupted epithelium with intact blood vessels (i.e., peeling).
3.3. Genital irritation
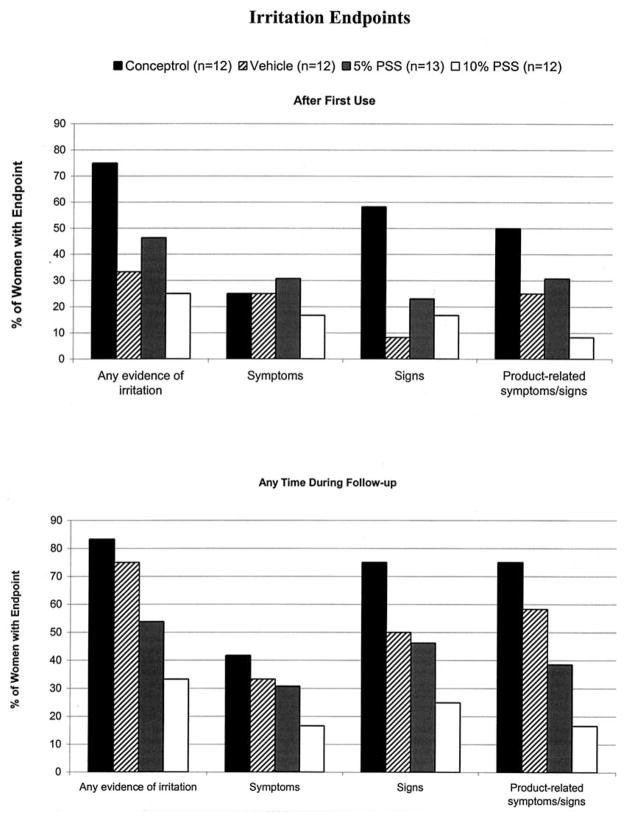
After the first use, genital irritation was seen among more women in the Conceptrol group than in the PSS groups, reaching statistical significance with regard to any self-reported symptoms or clinical signs of irritation (p = 0.011), clinical signs only of irritation (p = 0.034), and irritation determined to be product-related (p = 0.027) (Fig. 1).
Fig. 1.
Percentage of women with evidence of irritation, after first use and at any time in follow-up.
During continued product use over 6 days, irritation was also significantly higher in the Conceptrol group compared to the PSS groups with regard to any self-reported symptoms or clinical signs of irritation (p = 0.014), clinical signs of irritation (p = 0.017) and product-related irritation (p = 0.005). More women in the vehicle group experienced irritation than in the PSS groups, reaching statistical significance with regard to any self-reported symptoms or clinical signs of irritation (p = 0.044) and product-related irritation (p = 0.041). Symptoms were reported by two to five women in each group with the fewest complaints in the 10% PSS group and the most in the Conceptrol group. There were few abnormalities on pelvic examination after 6 days of product use, with no obvious differences between groups.
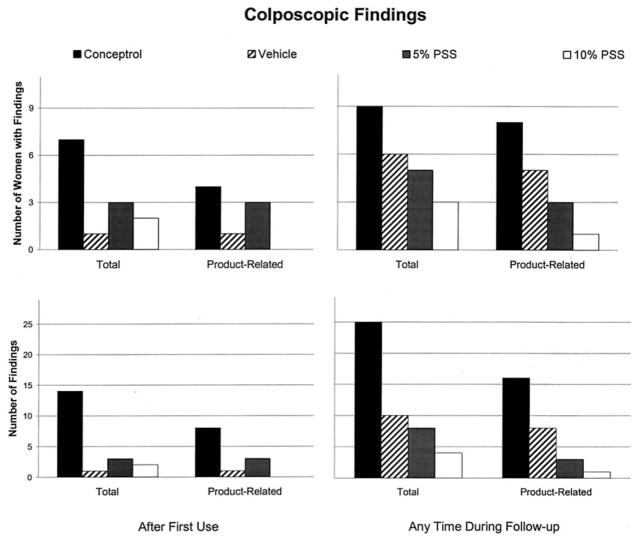
With regard specifically to colposcopic findings, the Conceptrol group had the greatest number of women with any colposcopic findings and the greatest number of such findings both after first use and during 6 days of follow-up (Fig. 2). Among the three remaining groups, there were few findings after first use. However, during continued follow-up, the number of women with findings as well as the number of findings among these three groups decreased as the concentration of PSS increased.
Fig. 2.
Number of women with colposcopic findings and number of colposcopic findings, total and product-related, after first use and at any time in follow-up. Findings present at the follow-up visits that were also present at baseline are not included. Findings that were present at more than one follow-up visit are counted only once.
The number of women with product-related colposcopic findings (which, by definition, were deemed related by the investigator and excluded findings present at baseline) generally followed the same pattern as overall findings. The types of findings seen are shown in Table 2. The number of women with product-related findings and the overall number of findings reported was similar between the two centers (data not shown).
Table 2.
Colposcopic findings seen at any point in follow-up, categorized by type of disruption, deemed related or possibly related to product usea
| Epithelium intact? | Blood vessels intact? | Conceptrol (n = 12) |
PSS vehicle (n = 12) |
5% PSS (n = 13) |
10% PSS (n = 12) |
Total (N = 49) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Findings | Women | Findings | Women | Findings | Women | Findings | Women | Findings | ||
| Yes | Yes | 7 | 11 | 1 | 2 | 0 | 0 | 0 | 0 | 8 | 13 |
| Yes | No | 1 | 1 | 2 | 3 | 2 | 2 | 1 | 1 | 6 | 7 |
| No | Yes | 4 | 4 | 2 | 2 | 1 | 1 | 0 | 0 | 7 | 7 |
| No | No | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| No. of women with at least one findingb | 8 | 5 | – | 3 | – | 1 | – | 17 | – | ||
| No. of findings | – | 16 | – | 8 | – | 3 | – | 1 | – | 28 | |
Excludes findings seen at any time in follow-up that were also present at baseline. Findings that were present at more than one follow-up visit are presented only once.
A woman may have more than one finding.
Forty-three percent of the colposcopic findings seen for the first time after initiation of product use were seen after the first use, and 20% of these persisted later in follow-up. The proportion of findings that persisted from baseline to later points in follow-up and from after the first use to later points in follow-up was approximately the same across treatment groups.
3.4. Product leakage
The Conceptrol group appeared to have the lowest subjective ratings of leakage. The pad weight differences after first use for 5% PSS, 10% PSS, and the Conceptrol group were, respectively, 2.28 g, 3.15 g and 1.13 g (p = 0.004). There was poor agreement between these objective and subjective measurements (κ = −0.006).
Greater pad weight differences in the PSS groups raised the question of whether the better irritation profile observed with increasing PSS concentration was due to a protective effect of the product or to a smaller amount of the product remaining in the vagina. However, statistically holding leakage constant, there was still a trend toward less irritation among users of 10% PSS compared with 5% PSS, and 5% PSS compared with Conceptrol. Moreover, a subgroup analysis showed that when only the women in the four groups who had low leakage and presumably more product retained in the vagina were considered, there was significantly more irritation seen during follow-up among users of Conceptrol than with the other products (p = 0.023). These patterns held, although less strongly, when only product-related irritation was considered (p = 0.135).
3.5. Safety
There were no major changes in laboratory parameters between screening and the last product use. All individual results for APTT and platelet counts were within the normal range (±20%) at screening and follow-up in all treatment groups.
There were no AEs that were considered serious, unexpected and related to product use in any group. The number of women experiencing urogenital AEs in the Conceptrol, vehicle, 5% PSS and 10% PSS groups were 5, 3, 3 and 2, respectively.
3.6. Acceptability
The largest proportion of women willing to buy their assigned product was in the vehicle group (83%), followed by the Conceptrol group (66%), the 5% PSS group (61%) and the 10% PSS group (50%). Between one and three women in each group described problems using their assigned product, including leakage, irritation and nausea. The acceptability profiles of the products were similar in all other respects.
4. Discussion
The results of this study suggest that PSS is associated with less genital irritation than its vehicle or Conceptrol. In addition, there were no major changes in any of the indicators of systemic safety in any treatment group, including electrolyte levels and coagulation parameters. Although this study was not designed to look at the natural history of colposcopic findings, it is interesting to note that in this study, almost half the colposcopic findings seen after product use were seen after the first application. This suggests that the initial exposure to the product caused a proportionately greater reaction than each of the following 5 days of use. Furthermore, less than half of the baseline findings were observed again after the first use, and only a quarter of the findings seen for the first time after the first use were seen again at a point later in follow-up, with little difference between groups. This suggests that resolution of findings occurred rapidly in the presence of the products. This pattern of colposcopic findings was also seen in a previous safety study of similar design involving cellulose sulfate [18].
This was our second study to attempt to correlate subjective leakage of a vaginal product with an objective measure of it. As in the previous study [18], agreement between the objective and subjective measures of leakage was poor. This may stem from a true lack of agreement, from problems with the instruments used to obtain the data, or both. Poor agreement in the κ analysis may also be due to the difficulties in determining what range of pad weight differences logically corresponds to the various categories of subjective leakage.
Nevertheless, it may be useful to continue to measure both types of leakage. Pad weight differences may give an objective assessment of the amount of product exposure, while subjective reports may predict at least one aspect of acceptability, which affects the eventual level of use of a product.
There was a high degree of acceptability of all four products. However, women in the 10% PSS group were least likely to say they would buy their product, which seems to be in contradiction to the low level of irritation seen in that group. A review of the reasons given for buying or not buying the product as well as the problems experienced while using the product did not shed much light on the lower level of acceptability among 10% PSS users. The 10% PSS group was also the group noted to have the greatest objective measure of leakage. Follow-up analyses did not suggest that the greater objective measure of leakage in this group resulted in the decreased level of irritation observed but this may have somehow affected overall acceptability of the product. In retrospect, willingness to buy the product may not have been the best key acceptability parameter for this population, all of whom were either abstinent or protected against pregnancy by sterilization. Alternatively, it may have been better to have asked women if they would purchase the product if they intended to use a vaginal method for contraception. If the product were presented to women with more of a need for its contraceptive or antimicrobial characteristics, willingness to purchase it would probably be a better indicator of acceptability. These results suggest that PSS has a safety profile comparable to the marketed N-9 product, Conceptrol, and appears to be associated with less genital irritation. The 5% concentration of PSS may be preferable in terms of leakage and acceptability.
Acknowledgments
The authors wish to thank Therese Babb, LuAnn Gahagan, Alison Hart and Lynn Reid for coordinating the study at the two sites, Kim Linton for monitoring it for CONRAD, and Tina Habash, formerly of CONRAD, for her editorial assistance. Support for this subproject [CIG-99-42] was provided by the Consortium for Industrial Collaboration in Contraceptive Research (CICCR), a program of CONRAD and the National Institutes of Health [NIH grant #MO1RR00056]. The views expressed by the authors do not necessarily reflect the views of CONRAD, CICCR or NIH.
References
- 1.Anderson RA, Feathergill K, Diao X, et al. Evaluation of poly(styrene-4-sulfonate) as a preventive agent for conception and sexually transmitted diseases. J Androl. 2000;21:862–75. [PubMed] [Google Scholar]
- 2.Homm RE, Foldesy RG, Hahn DW. ORF 13904, a new long-acting vaginal contraceptive. Contraception. 1985;32:267–75. doi: 10.1016/0010-7824(85)90050-2. [DOI] [PubMed] [Google Scholar]
- 3.Zaneveld LJ, Waller DP, Anderson RA, et al. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate) Biol Reprod. 2002;66:886–94. doi: 10.1095/biolreprod66.4.886. [DOI] [PubMed] [Google Scholar]
- 4.Christensen ND, Reed CA, Culp TD, et al. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob Agents Chemother. 2001;45:3427–32. doi: 10.1128/AAC.45.12.3427-3432.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simoes JA, Citron DM, Aroutcheva A, et al. Two novel vaginal microbicides (polystyrene sulfonate and cellulose sulfate) inhibit Gardnerella vaginalis and anaerobes commonly associated with bacterial vaginosis. Antimicrob Agents Chemother. 2002;46:2692–5. doi: 10.1128/AAC.46.8.2692-2695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neurath AR, Strick N, Li YY. Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides. BMC Infect Dis. 2002;2:27. doi: 10.1186/1471-2334-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeitlin L, Whaley K, Hegarty TA, et al. Tests of vaginal microbicides in the mouse genital herpes model. Contraception. 1997;56:329–35. doi: 10.1016/s0010-7824(97)00154-6. [DOI] [PubMed] [Google Scholar]
- 8.Herold BC, Bourne N, Marcellino D, et al. Poly(sodium 4-styrene sulfonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J Infect Dis. 2000;181:770–3. doi: 10.1086/315228. [DOI] [PubMed] [Google Scholar]
- 9.Bourne N, Zaneveld LJ, Ward JA, Ireland JP, Stanberry LR. Poly-(sodium 4-styrene sulfonate): evaluation of a topical microbicide gel against herpes simplex virus type 2 and Chlamydia trachomatis infections in mice. Clin Microbiol Infect. 2003;9:816–22. doi: 10.1046/j.1469-0691.2003.00659.x. [DOI] [PubMed] [Google Scholar]
- 10.Boisson-Vidal C, Jozefonvicz J, Brash JL. Interactions of proteins in human plasma with modified polystyrene resins. J Biomed Mater Res. 1991;25:67–84. doi: 10.1002/jbm.820250106. [DOI] [PubMed] [Google Scholar]
- 11.Lonardo EC, Roy TA, Casalvieri JC, Rencher WF, Wearley LL. An in vitro percutaneous absorption procedure to assess the absorption potential of polystyrene sulfonate through human vaginal tissue. Toxicologist. 2001;60:126. [Google Scholar]
- 12.Inamori T, Ghanem A-H, Higuchi WI, Srinivasan V. Macromolecule transport in and effective pore size of ethanol pretreated human epidermal membrane. Int J Pharmaceutics. 1994;105:113–23. [Google Scholar]
- 13.Physicians’ Desk Reference 2003. Montvale (NJ): Thomson PDR; 2003. [Google Scholar]
- 14.Manual for the standardization of colposcopy for the evaluation of vaginal products: Update 2000, published jointly by CONRAD and the World Health Organization.
- 15.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS System. Cary (NC): SAS Institute; 1995. [Google Scholar]
- 16.Sellers GR, Vardeman SB, Hackert AF. A first course in statistics. 3. New York: Harper Collins; 1992. pp. 682–6. [Google Scholar]
- 17.Fleiss JL. Statistical methods for rates and proportions. 2. New York: Wiley and Sons; 1981. pp. 212–36. [Google Scholar]
- 18.Mauck C, Weiner DH, Ballagh S, et al. Single and multiple exposure tolerance study of cellulose sulfate gel: a phase I safety and colposcopy study. Contraception. 2001;64:383–91. doi: 10.1016/s0010-7824(01)00271-2. [DOI] [PubMed] [Google Scholar]