Abstract
Stem cells are critical for maintaining tissue homeostasis and are commonly governed by their niche microenvironment, although the intrinsic mechanisms controlling their multipotency are poorly understood. Polycomb group (PcG) genes are epigenetic silencers, and have emerged recently as important players in maintaining stem cell multipotency by preventing the initiation of differentiation programs. Here we describe an unexpected role of specific PcG genes in allowing adult stem cell differentiation and preventing stem cell-derived tumor development. We show that Posterior sex combs (Psc), which encodes a core Polycomb-repressive complex 1 (PRC1) component, functions redundantly with a similar gene, Suppressor of zeste two [Su(z)2], to restrict follicle stem cell (FSC) self-renewal in the Drosophila ovary. FSCs carrying deletion mutations of both genes extrude basally from the epithelium and continue to self-propagate at ectopic sites, leading to the development of FSC-like tumors. Furthermore, we show that the propagation of the mutant cells is driven by sustained activation of the canonical Wnt signaling pathway, which is essential for FSC self-renewal, whereas the epithelial extrusion is mediated through the planar cell polarity pathway. This study reveals a novel mechanism of epithelial extrusion, and indicates a novel role of polycomb function in allowing adult stem cell differentiation by antagonizing self-renewal programs. Given evolutionary conservation of PcG genes from Drosophila to mammals, they could have similar functions in mammalian stem cells and cancer.
Keywords: Follicle stem cell, Psc, Su(z)2, stem cell differentiation, cell extrusion, Wnt signaling
Stem cells reside in many adult tissues. They are distinguished by the ability to self-renew and generate differentiated cells throughout life to maintain tissue homeostasis. Adult stem cells usually reside in a special microenvironment or niche, and intercellular signals produced from the niche have critical roles in controlling stem cell behavior (Morrison and Spradling 2008). In addition, there are intrinsic mechanisms controlling stem cell maintenance and self-renewal. Increasing evidence indicates that stem cells have a unique chromatin state that allows them to execute appropriate differentiation programs when different developmental signals are received, and stabilizing the chromatin state within stem cells is critical for maintaining their self-renewal (Buszczak and Spradling 2006). Polycomb group (PcG) genes are epigenetic silencers, and are emerging candidates to maintain the epigenetic state of chromatin in stem cells.
PcG genes were initially characterized in Drosophila as the repressors of homeotic (Hox) gene expression, and function to maintain the repressive state of chromatin but not to initiate transcriptional repression. The products of PcG genes form evolutionarily conserved multiprotein complexes—called Polycomb-repressive complexes (PRCs)—to covalently modify histone tails and to repress transcription of their targets (Schwartz and Pirrotta 2007). The PRC2 complex catalyzes the di- and trimethylation of Lys 27 of histone H3, which is believed to serve as a docking site for recruitment of the PRC1 complex. The components of the PRC1 complex are more variable, but include core components Polycomb (Pc), Polyhomeotic (Ph), Posterior Sex Combs (Psc), and Sex Combs Extra (Sce) in Drosophila, and correspondingly PC1-3, PH1-3, BMI-1/Mel18, and RING1A/RING1B/RNF2 in mammals. In mammalian embryonic stem (ES) cells, PcG proteins bind to the promoters of many inactive developmental genes, indicating a role for PcG proteins in maintaining stem cell fate by preventing differentiation (Boyer et al. 2006; Lee et al. 2006). During mouse embryogenesis, the expression of Ezh2, a core PRC2 component, is down-regulated during satellite cell-to-myoblast differentiation, and in vitro studies suggest that Ezh2 is localized to the promoters of several inactive muscle-specific genes in the satellite cells (Caretti et al. 2004). A recent study also demonstrated specific requirements for Ezh2 in epidermal progenitor cells in maintaining their proliferative potential and preventing precocious differentiation (Ezhkova et al. 2009). Therefore, PcG genes are critical players in preventing the differentiation of both embryonic and adult stem cells. The above observations are consistent with a derepression model proposed for PcG gene function in cell fate specification in which cell fates are determined by the selective derepression of a subset of differentiation genes (Buszczak and Spradling 2006).
In the germarium at the tip of the Drosophila ovary, germline stem cells (GSCs) and follicle stem cells (FSCs) are attractive systems to study adult stem cell regulation in vivo, given that the stem cells have been well characterized and the derived daughters can be traced easily (Xie and Spradling 2001). In each germarium, there are two to three GSCs at the anterior tip directly contacting cap cells, and two FSCs located midway between the 2A and 2B regions (Fig. 1A; Margolis and Spradling 1995; Xie and Spradling 2000; Nystul and Spradling 2007). Interaction of FSCs with anterior escort cells through adherens junctions is required for the maintenance of FSCs within the niche (Song and Xie 2002). Hedgehog (Hh), Wingless (Wg), and BMP signals secreted from the stromal cells at the anterior tip directly regulate the maintenance of FSCs over a long distance. Disruption of each signaling cascade in FSCs results in their loss by differentiation. Conversely, overactivation of each pathway in stem cells causes altered life span or activity of stem cells, as well as the accumulation of follicular progenitors (Forbes et al. 1996a,b; Zhang and Kalderon 2000, 2001; Song and Xie 2003; Kirilly et al. 2005). In mammals, Shh, Wnt, and BMP have also been shown to regulate epithelial stem cell self-renewal, suggesting conserved mechanisms regulating epithelial stem cells from Drosophila to mammals (Blanpain et al. 2007). Despite the discovery of multiple extrinsic signals that regulate epithelial stem cell self-renewal, however, little is known about possible intrinsic mechanisms controlling stem cell self-renewal.
Figure 1.
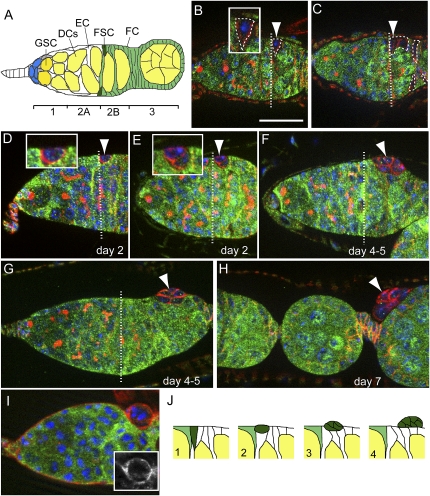
Psc Su(z)2 mutant FSC undergoes basal extrusion from the epithelium. The straight dashed lines in B–G denote the position of the 2A/2B boundary. (B–H) In all staining images, anti-LacZ staining is shown in green, anti-α-Spectrin is shown in red, and DAPI (DNA dye) is shown in blue. (A) A schematic diagram of a germarium. (DCs) Developing germline cysts; (EC) escort cell; (FC) follicle cell. The germarium is divided into four regions: 1, 2A, 2B, and 3; two FSCs are located between the 2A and 2B regions. (B) A newly marked wild-type FSC (dashed line, arrowhead, and inset) recognized by the lack of LacZ expression (green). Bar, 20 μm. (C) A wild-type FSC clone examined at 2 wk ACI. The marked FSC as well as its derived daughter follicle cells were all labeled by the absence of LacZ expression (dashed lines). (D) A newly generated mutant FSC (arrowhead) located at the boundary between the 2A and 2B regions (dashed line). (Inset) The mutant FSC had a retracted apical membrane, and became ball-like in shape. (E, inset) A mutant FSC began to move away from the niche location. (F, G) A mutant FSC clone that had left the niche location. It moved posteriorly and extruded gradually out of the epithelium. (H) Part of an ovariole showing that a 7-d-old FSC clone (arrowhead) had extruded completely out of the epithelium. (I) An extruding mutant FSC clone at day 4–5 ACI stained with anti-LanA (red). The basal membrane was continuous outside of the extruding clone, and there was a new basement membrane developing between the extruding clone and the epithelium (see also inset). (J) Schematic diagrams for the basal extrusion process of Su(z)21.b8 mutant FSC.
Previous studies have implicated a possible epigenetic mechanism in the regulation of stem cells in the Drosophila ovary, as specific chromatin remodeling factors have been implicated in regulating stem cell maintenance (Xi and Xie 2005). Chromatin remodeling factors are known to mediate both transcriptional activation and repression in response to extrinsic signals, and can interact with trithorax activators and PcG repressors. In addition, Posterior sex combs (Psc), a polycomb gene encoding a core component of the PRC1 complex, has enriched expression in both germline and somatic cells in the Drosophila ovary (Kai et al. 2005), but its function in stem cells is not yet known. In this study, we report that Psc functions redundantly with a similar gene, Suppressor of zeste two [Su(z)2], to specifically regulate FSC self-renewal. Unexpectedly, contrary to the known differentiation-preventing activity of PcG genes in stem cells, Psc and Su(z)2 as reported here function to allow FSC differentiation by inhibiting self-renewal programs, and this function is independent of the PRC1 complex function. In addition, this study also reveals a novel role of noncanonical Wnt signaling—referred to as the planar cell polarity (PCP) pathway—in mediating an epithelial extrusion process in the follicular epithelium.
Results
Psc and Su(z)2 function redundantly and specifically in FSC maintenance
Because Psc is an essential gene for adult viability, we used the FLP/FRT system to induce mitotic clones in both germline and somatic lineages (Xu and Rubin 1993) in order to address its potential function in GSCs and FSCs. GSCs can be identified reliably by location and spherical fusome, a germline-specific cytoplasmic organelle (Fig. 1A). FSCs can be identified by location and the ability to generate daughter follicle cells covering the germline cysts (Fig. 1B,C). Females of the appropriate genotypes were subjected to heat-shock treatments to induce mitotic clones, and the marked clones were identified by the absence of arm-lacZ expression. The percentage of marked GSC- or FSC-containing germaria was then determined at 4 and 20 d after clone induction (ACI). Changes in this percentage with time could reflect a gene requirement for stem cell maintenance. The wild-type GSC clones and FSC clones were maintained properly within their niche at day 20 ACI. A slight decline of stem cell clones over time indicates slow stem cell turnover, which is consistent with previous observations (Xie and Spradling 1998; Zhang and Kalderon 2001; Song and Xie 2003; Kirilly et al. 2005). Psce24 and Psch27 are either protein-null or genetic-null alleles, and the mutant GSC and FSC clones behaved similarly to wild-type clones (Table 1; Supplemental Figs. S1, S2), since the majority of mutant GSCs and FSCs were maintained at day 20 ACI. In addition, the marked GSC- and FSC-derived daughters could normally differentiate into germline cysts and follicular epithelia, respectively (Supplemental Figs. S1, S2), suggesting that Psc is dispensable for the maintenance and differentiation of both GSCs and FSCs.
Table 1.
Psc and Su(z)2 function redundantly for the maintenance of FSCs, but are dispensable for the maintenance of GSCs
(ND) Not determined.
aThe percentage of germaria carrying marked stem cell clones for a given genotype is determined by the number of germaria carrying one or more marked GSCs or FSCs divided by the number of total germaria examined (shown in parentheses).
bAt day 14 ACI.
cModified heat-shock regimes used for efficient induction of transgenes (see the Materials and Methods).
Su(z)2, which is adjacent to Psc, encodes another PcG protein similar to Psc. The protein shares similarity with Psc not only in the N-terminal region that contains a RING finger motif, but also in the overall protein sequence (Supplemental Fig. S3). Both proteins colocalize on the polytene chromosomes, and also show functional redundancy in repressing homeotic genes in embryos and imaginal discs (van Lohuizen et al. 1991; Rastelli et al. 1993; Soto et al. 1995; Beuchle et al. 2001). Biochemical studies also reveal their functional similarities in compacting chromatin and inhibiting chromatin remodeling, and the ability to form functional complexes with other polycomb proteins (Lo et al. 2009). Thus, it is possible that Su(z)2 can compensate for Psc function. Su(z)21.b7 is a genetic-null allele of Su(z)2, and clonal analysis showed that the majority of Su(z)21.b7 mutant FSCs and GSCs present at day 4 ACI were maintained at day 20 ACI (Table 1). In addition, their derived daughters were able to form largely normal egg chambers (Supplemental Figs. S1, S2), suggesting that Su(z)2 is also dispensable for the maintenance and differentiation of both GSCs and FSCs. To test whether Psc and Su(z)2 function redundantly, we used the Su(z)21.b8 allele, a chromosomal-deficient allele in which the coding regions of both Psc and Su(z)2 are deleted (Adler et al. 1989). Clonal analysis showed that Su(z)21.b8 mutant GSCs were maintained properly (Table 1; Supplemental Fig. S1). In addition, they were able to differentiate into germline cysts and egg chambers without any obvious defect (Supplemental Fig. S1), suggesting that Psc and Su(z)2 are not required for the maintenance and differentiation of GSCs. All Su(z)21.b8 mutant FSCs were not observed at the expected location at 2 wk ACI, however, as the percentage of germaria with marked FSCs was 23.7% at day 4 ACI and 0.0% at day 14 ACI (Table 1). Importantly, this phenotype could be rescued efficiently by either the hs-Psc transgene or the hs-Su(z)2 transgene, or both (Table 1; Fig. 3G,H). With the same heat-shock regime, coexpressing both transgenes showed better rescue efficiency than expressing either transgene alone, presumably due to additive gene expression levels (Table 1; data not shown). In addition, using two FRT-carrying transposable element insertions that flank the Psc and Su(z)2 genes (Parks et al. 2004), we generated a precise deletion allele of Psc and Su(z)2, named Su(z)2XL26 (Supplemental Fig. S4). These mutant FSC clones also showed extrusion and tumorous growth phenotypes indistinguishable from the Su(z)21.b8 clones (Supplemental Fig. S4), further suggesting that the loss of Psc and Su(z)2 is solely responsible for the observed phenotypes in Su(z)21.b8 clones. Taken together, we conclude that Psc and Su(z)2 function specifically and redundantly to maintain FSCs, and are dispensable for the maintenance and differentiation of GSCs.
Figure 3.
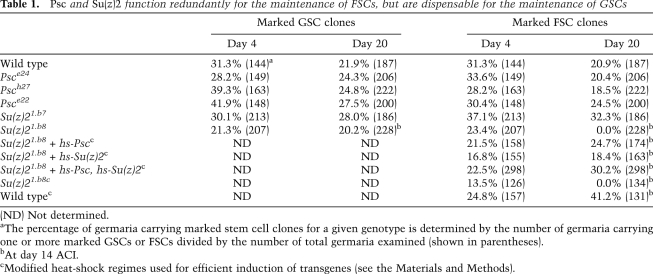
Psc Su(z)2 mutant FSCs are incapable of further differentiation. In all images, DAPI staining is in blue. Bars, 20 μm. (A,A′) A wild-type FSC (arrowhead) and its derived follicle cells are marked by the absence of LacZ expression (green) (dashed lines). Fas III expression (red) was observed in the differentiating follicle cells, but it was not detected in FSCs. (B,B′) Cut (red) had weak expression level in FSCs (arrowhead), and its expression was increased in the differentiating follicle cells. (C,D) In both early (C) and developing (D) Su(z)21.b8 mutant FSC-derived clones (dashed lines), Fas III expression (red) was not detected. (E,F) In both early (E) and developing (F) mutant FSC-derived clones (dashed lines), Cut (red) was expressed weakly, and the expression level was comparable with that in wild-type FSCs. (G,H) Induction of transgenes of Psc and Su(z)2 rescued both FSC loss and follicle cell differentiation defects, as the mutant FSCs were maintained in the niche (arrowhead) and their derived follicle cells (dashed lines) had normal levels of Fas III (G, red) and Cut (H, red) expression, and formed largely normal epithelia.
Psc Su(z)2 mutant FSC undergoes basal cell extrusion from the epithelium
We noticed that, as early as 1 wk ACI, there were no Psc Su(z)2 mutant follicle cell clones observed in the epithelia of egg chambers outside the germanium. This raised the possibility that the mutant FSCs could be eliminated by cell death. Using TUNEL labeling to detect apoptosis, however, we found that, out of 36 mutant FSCs examined, none showed positive TUNEL labeling (Fig. 2F). Instead, the mutant FSCs, once generated, showed a series of morphological changes and quickly left their niche location. Normally, FSCs form columnar or cone-like shapes, with their basal surface contacting the basement of epithelium and the apical surface facing the developing germline cyst (Fig. 1A,B). The newly generated mutant FSCs, however, began to retract their apical surface to form ball-like shapes (Fig. 1D,E) and then moved outward and posteriorly from their niche location. Gradually and strikingly, the mutant FSCs, often together with their immediate daughter cells, formed spherical cell clusters, and extruded out from the basement membrane of epithelium (Fig. 1F–H,J). All FSC clones examined at days 5–7 ACI showed this basal extrusion phenotype (n = 41), which suggests that Psc and Su(z)2 play important roles in regulating the cell morphology of FSCs and maintaining FSCs within their niche. Staining with anti-Laminin A (LanA), a basement membrane marker, revealed that, during extrusion, the basement membrane was continuous in covering the surface of extruded clone. A new basement membrane was also seen in between the extruding clone and the epithelium at the extrusion site (Fig. 1I). Consequently, the resulting extruded clone was fully surrounded by a layer of basement membrane. These observations indicate that mutant FSCs do not necessarily degrade the basement membrane for basal extrusion.
Figure 2.
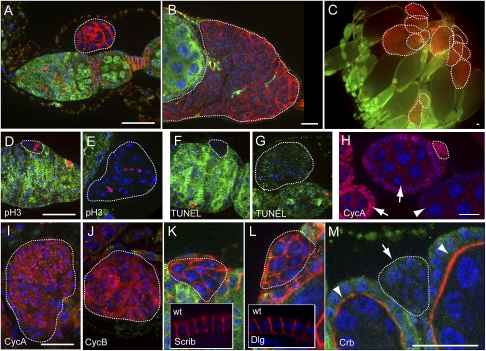
The mutant FSCs initiate tumorigenesis at ectopic sites. In all images, the mutant clones are marked by the absence of LacZ expression (green), and are highlighted by dashed lines. DAPI staining is in blue. For A–C, the red channel shows anti-α-Spectrin staining. Bars, 20 μm. (A) A germarium showing that a 7-d-old Su(z)21.b8 mutant FSC-derived clone had extruded out of the epithelium and sat in a space between the basement membrane of the epithelia and outer sheath cells. (B) A Su(z)21.b8 mutant FSC-derived clone at day 14 ACI had grown to a larger size. (C) A whole ovary at day 17 ACI contained multiple FSC-derived tumors (dashed lines), mostly accumulated at the posterior regions of the ovary. (D,E) pH3-positive cells (red) were detected in both a newly generated FSC clone (D) and a developing tumor next to a stage 7 egg chamber (E). (F, G) In a newly generated FSC clone (F) and a developing tumor above a stage 3 egg chamber (G), TUNEL labeling (red) was negative. (H) Part of an ovariole showing that the expression of CycA (red) was observed in the dividing follicle cells in early stage chambers (arrows), but was not observed in the follicle cells after stage 6 (arrowhead), and CycA expression was observed in a small FSC-derived clone (dashed line). The LacZ expression is not shown in this image. (I, J) A tumor next to a stage 6 egg chamber stained with CycA (I) or CycB (J). (K, L) Scrib (K, red) and Dlg (L, red) localized ubiquitously to the membrane of the cells in the mutant clones, while both localized to the lateral membrane of the wild-type follicle cells (insets). (M) A mutant FSC clone (arrow) showing that Crb was not associated with the membrane of mutant cells, while it localized to the apical membrane of wild-type follicle cells (arrowheads).
Psc Su(z)2-deficient FSC clones initiate neoplastic tumors at ectopic sites
Normally, the follicular precursors derived from FSCs form single layers of epithelia and continue to divide until stage 6 of egg chamber development in mid-oogenesis, when they stop dividing and undergo terminal differentiation into several follicle cell types (Fig. 6A, below; Spradling 1993). The mutant cell clusters that detached from the epithelium, however, seemed to divide continuously. The sizes of the mutant cell clones grew continuously with time (Fig. 2A,C), and mitotic cells were detected within the mutant cell clusters using a mitotic marker, phospho-Histone 3 (pH3) (Fig. 2D,E). The expression of two G2/M regulators, Cyclin A (CycA) and Cyclin B (CycB), which are present in the dividing follicle cells but not in follicle cells after stage 6 (Fig. 2H), were frequently observed in the tumor cells (Fig. 2I,J). TUNEL labeling assay showed that, with the increase in tumor mass over time, there was no obvious increase in the incidence of cell death (Fig. 2G). As a result, at 17 d ACI, large tumor masses full of mutant cells were found frequently and accumulated mostly at the posterior regions of ovarioles (Fig. 2C). These data demonstrate that Su(z)21.b8 mutant FSCs mimic tumor-initiating cells with the ability to extrude out from the epithelium, proliferate continuously, and form large tissue masses at ectopic sites.
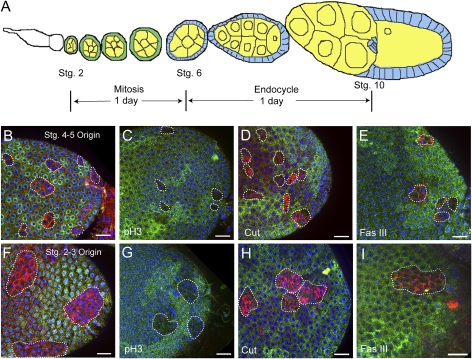
Figure 6.
Mutations in the differentiating follicle cells do not lead to tumorigenesis. (A) A diagram of an ovariole showing that follicle cells were mitotically active until stage 6, when they cease division and enter endocycle. It takes ∼1 d for an egg chamber to develop from stage 2 to stage 6, and another day to develop from stage 6 to stage 10. Therefore, a follicle cell clone generated in a stage 2 egg chamber will be in a stage 10 egg chamber after 2 d. (B–E) Su(z)21.b8 mutant follicle cells clones in stage 10 egg chambers originated from stage 4–5 egg chambers. (F–I) Mutant follicle clones originated from stage 2–3 egg chambers. In all images, clones were recognized by the absence of LacZ expression (green), and were costained with α-Spectrin (B,F), pH3 (C,G), Cut (D,H), and Fas III (E,I) (in red). DAPI is in blue. The mutant follicle cells showed mild morphological defects, negative pH3, and up-regulated Cut and Fas III expression. Bars, 20 μm.
The mutant cells in each extruded cluster showed irregular morphologies, without clear apicobasal polarity. We thus examined Crumbs (Crb), an apical marker, along with Scribble (Scrib) and Discs large (Dlg), two lateral markers, in the follicle cells. In the wild-type follicle cells, Scrib and Dlg localized to the lateral membrane; however, they localized around the whole membrane in the mutant cells (Fig. 2K,L). In the wild-type follicle cells, Crb localization was restricted to the apical membrane that contacts the germline cyst, while in the mutant cells, it did not localize to the membrane (Fig. 2M), hence demonstrating that the Psc Su(z)2 mutant tumor cells do not have apicobasal and lateral polarity.
Psc Su(z)2-deficient FSCs are blocked in differentiation
Continuous proliferation of tumor cells suggests that their differentiation may be arrested at stem cell or early progenitor stages. To determine the differentiation state of tumor cells, we examined the expression of several cell fate markers. Fasciclin III (Fas III) is normally expressed in all follicle cells in the germarium, while its expression in FSCs is low or undetectable (Fig. 3A,A′; Zhang and Kalderon 2001). We found that Fas III expression was undetectable in both newly generated mutant FSCs and developing tumor cells (Fig. 3C,D). Cut is a transcription factor expressed specifically in somatic follicle cells, which we found to have weak expression in FSCs, while its expression level was increased in the differentiating follicle cells (Fig. 3B,B′). In both newly generated mutant FSCs and developing tumor cells, it had weak expression levels, similar to those in FSCs (Fig. 3E,F). The above results suggest that Su(z)21.b8 mutant FSCs still maintain specific gene expression patterns after multiple rounds of cell division, indicating that the mutant FSCs are stuck at the stem cell-like stage, self-propagate continuously, and eventually develop into large tumors. By expressing Psc and Su(z)2 with the hs-Psc and hs-Su(z)2 transgenes, we were able to rescue both FSC maintenance and epithelial differentiation defects caused by the Su(z)21.b8 mutation (Fig. 3G,H), which demonstrates that mutations in Psc and Su(z)2 were responsible for the tumor phenotype, and both genes are critically required for FSC differentiation.
Tumorous growth is driven by sustained activation of Wingless signaling
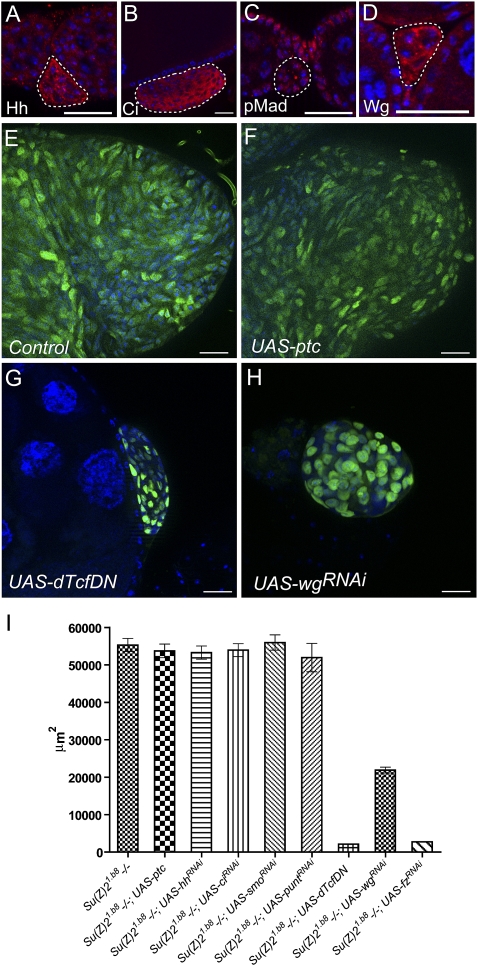
We next investigated the mechanisms underlying the tumor formation initiated from Psc Su(z)2-deficient FSCs. Because the mutant cells were arrested at stem cell-like stages, self-renewal signaling activities might be enhanced in the mutant cells. To test this possibility, we examined the activities of Hh, Wg, and BMP signaling, three known essential players in controlling FSC self-renewal. Immunostaining with specific antibodies showed that Hh signaling components—including Hh, Ci, and Wg, the Wnt singling ligand—were up-regulated (Fig. 4A,B,D) in Su(z)21.b8 FSC-derived clones compared with the wild-type differentiating follicle cells. Meanwhile, the expression of phospho-Mad (pMad, an active form of Mad) or Dad-lacZ (an enhancer trap of the Dad gene), both of which are reliable reporters for BMP signaling activity, was not altered (Fig. 4C; data not shown). These data indicate that sustained Hh and Wg self-renewal signaling in Su(z)21.b8 mutant cells might be responsible for the FSC-like tumor development. We next asked whether inhibition or elimination of BMP, Hh, or Wg signaling activity in Su(z)21.b8 mutant cells could prevent tumor development. We used the MARCM system to generate GFP-labeled Su(z)21.b8 homozygous FSC clones with or without the expression of specific transgenes of interest (Lee and Luo 1999). UAS-smo-RNAi, UAS-ci-RNAi, UAS-patched (ptc), and UAS-puntRNAi lines were used to inhibit Hh or BMP signaling activities, which are effective in inhibiting the relevant signaling pathway activities when expressed in follicle cells (Supplemental Fig. S5). Consistent with the expression pattern, inhibiting BMP signaling activity by expressing UAS-punt-RNAi had no apparent effect on the tumor growth (Fig. 4I). Surprisingly, inhibiting Hh signaling activity by expressing UAS-hh-RNAi, UAS-smo-RNAi, UAS-ci-RNAi, or UAS-ptc also had no obvious effect on the extrusion and growth of the mutant FSC clones (Fig. 4F,I). However, striking effects were observed with Wg signaling inhibition. In Drosophila, the Wg signal is transduced by the Frizzled (Fz), LRP families of receptors, and the scaffolding protein Dishevelled (Dsh), followed by the inhibition of Axin complexes and stabilization of Armadillo (Arm). Arm then translocates to the nucleus and forms a complex with dTCF transcription factor to regulate gene transcription (Moon et al. 2002). When the mutant clones had forced expression of a dominant-negative form of dTCF (UAS-dTcfDN), tumor growth was significantly inhibited, as the average area of tumors derived from single FSC clones at 3 wk ACI was significantly decreased (Fig. 4G,I). A similar, albeit weaker, inhibitory effect was also found with UAS-wg-RNAi expression (Fig. 4H,I). The tumor cells with UAS-dTcfDN or UAS-wg-RNAi expression showed reduced mitotic index (Supplemental Fig. S6), suggesting that Wg signaling inhibition reduces tumor cell proliferation. These data suggest that only Wg signaling, not Hh or BMP signaling, is primarily responsible for the tumorous growth of Psc Su(z)2-deficient FSCs. Inhibiting canonical Wnt signaling activity, however, could not prevent epithelial extrusion of the mutant FSC clones (Figs. 4G,H, 5E), indicating a separate mechanism mediating cell extrusion from the epithelium.
Figure 4.
Tumorous growth is driven by sustained activation of Wingless signaling. In all images, DAPI staining is in blue. Bars, 20 μm. (A–D) Su(z)21.b8 mutant FSC clones (dashed lines) stained with antibodies against Hh (A), Ci (B), pMad (C), and Wg (D). Hh, Ci and Wg, but not pMad, had higher expression levels compared with that in the wild-type follicle cells. (E–H) Images of typical tumor masses (GFP, green) from su(z)1.b8 mutant FSC-derived clones at 3 wk ACI, with or without transgene expression, as indicated: control, without transgene expression (E); with UAS-ptc (F); with UAS-dTcfDN (G);and with UAS-wgRNAi (H). (I) A plot to compare the area size of tumor masses at 3 wk ACI without or with various transgene expression as indicated. Error bars represent SEM. Number of tumors examined for each genotype, n = 15.
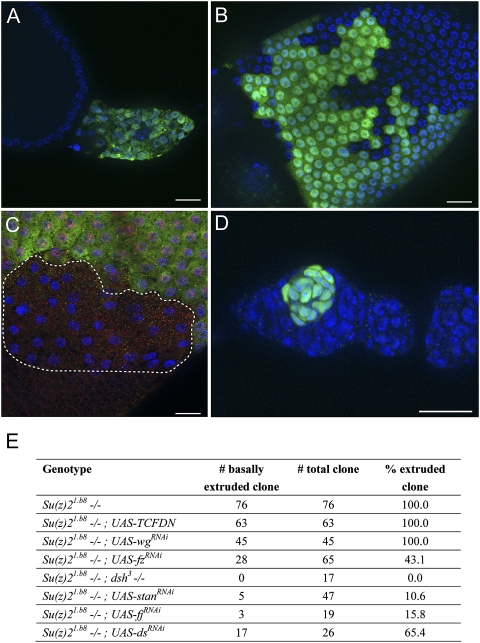
Figure 5.
Cell extrusion is mediated through the noncanonical Wnt signaling pathway. In all images, DAPI staining is in blue. (A) A Su(z)21.b8 mutant FSC-derived clone (GFP, green) with the expression of UAS-fz-RNAi at 3 wk ACI. Note that the tumor size was relatively small. (B) A Su(z)21.b8 mutant FSC-derived clone (GFP, green) with the expression of UAS-fz-RNAi. The clone was able to form largely normal epithelial cells. (C) A dsh3 Su(z)21.b8 mutant FSC-derived clone (dashed line) at day 8 ACI. The clone was recognized by the absence of both LacZ (red) and GFP (green) expression. Note that the mutant cells were able to form epithelial cells. (D) A Su(z)21.b8 mutant FSC-derived clone (GFP, green) with the expression of UAS-stanRNAi at 3 wk ACI. The clone did not undergo basal extrusion, but rather stayed within the germarium. (E) A table shows the numbers and percentages of extruded/nonextruded tumors derived from Su(z)21.b8 mutant FSCs with various transgene expression or mutation.
Cell extrusion is mediated through a noncanonical Wnt signaling pathway
To further evaluate the contribution of Wg signaling to tumor development, we tested the effect of fz-RNAi in Su(z)21.b8 mutant clones. UAS-fz-RNAi expression also efficiently inhibited the growth of extruded mutant clones, as the sizes of tumor masses were significantly smaller compared with those without RNAi expression (Figs. 4I, 5A). Strikingly, more than half of the mutant FSC-derived clones did not extrude out from the epithelium; instead, they developed into single-layered epithelial cells with largely normal morphology (Fig. 5B,E). Staining with cell polarity markers also reveals that these cells have retained normal basal–lateral polarity (Supplemental Fig. S7). Thus, Su(z)21.b8 mutant FSC clones with inhibited fz function show rescue of both cell extrusion and cell polarity phenotype, in addition to the inhibition of tumor growth. Because fz functions not only in the canonical Wnt signaling pathway, but also in a noncanonical Wnt signaling pathway often referred as the PCP pathway (Seifert and Mlodzik 2007), the PCP pathway could mediate the epithelial extrusion of Psc Su(z)2-deficient FSC clones, and, presumably, fz knockdown in the mutant cells could bring both canonical and noncannonical Wnt signaling back to normal levels, resulting in rescue of both extrusion and proliferation phenotypes. To test this hypothesis, we asked whether inhibiting dishevelled (dsh), which has also been implicated in noncanonical Wnt signaling, could prevent the extrusion phenotype. We thus generated dsh3 Su(z)21.b8 double clones and examined their behavior at 1–2 wk ACI. The GFP− and LacZ+ clones were dsh3 homozygous mutant clones and showed no apparent morphological defects, consistent with previous observations (Song and Xie 2003). The GFP+ and LacZ− clones were Su(z)21.b8 homozygous FSC-derived clones, and these clones were consistently extruded from the epithelium and formed tumor masses (data not shown). However, the FSC-derived GFP− and LacZ− clones, which were dsh3 Su(z)21.b8 double mutants, showed distinct cellular behaviors. They could not extrude from the epithelium, but formed single-layered epithelial cells (Fig. 5C,E), demonstrating that dsh is also required for the extrusion of Psc Su(z)2-deficient FSC clones. These mutant FSC-derived clones were typically small in size (Fig. 5C; data not shown). Their underproliferation further suggests a critical requirement of Wg pathway activity in the proliferation of the tumor cells. To further confirm the requirement for the PCP pathway in the extrusion process, we asked whether inhibiting the functions of other PCP pathway components could prevent the extrusion phenotype. starry night (stan, also named flamingo [fmi]), four-jointed (fj), and dachsous (ds) are important PCP pathway components that do not have a role in Wg signaling (Fanto and McNeill 2004). Strikingly, UAS-stan-RNAi expression efficiently prevented basal extrusion of the majority of Psc Su(z)2-deficient FSCs (Fig. 5D,E). However, the mutant FSCs could not produce normal epithelium, but instead developed into small spherical tumor masses inside the germarium (Fig. 5D). Similar results were found with fj RNAi and ds RNAi (Fig. 5E). We therefore conclude that basal extrusion of Psc Su(z)2-deficient FSC clones is mediated through the PCP pathway.
These data indicate that the propagation and extrusion of Psc Su(z)2-deficient FSC clones are two genetically separable processes mediated by the canonical and noncanonical Wnt signaling pathways, respectively. Reduced function of fz or dsh could down-regulate both pathways such that both phenotypes could be suppressed and normal epithelium could form. On the other hand, inhibiting the PCP pathway alone could prevent basal extrusion, but could not prevent tumor development. Consequently, normal epithelium could not form. All extrusion-suppressed clones by fz or dsh inhibition, however, invariably showed single-layered epithelial morphology without any overgrowth phenotype. In addition, extrusion-suppressed clones by stan or fj RNAi did not develop into large tumors comparable with the clones extruded out of the epithelium (Fig. 5D). These observations suggest that cell extrusion, proliferation, and differentiation could be linked processes, as inhibition of extrusion from the epithelium could inhibit cell growth, possibly by providing contact inhibition or through unknown mechanisms. These data also indicate intriguing connections between the canonical and noncanonical Wnt signaling in coordinate cell morphogenesis, proliferation, and differentiation, while the underlying mechanisms await further investigation.
Deletion of Psc and Su(z)2 in the differentiating follicle cells does not lead to tumorigenesis
In the above analysis, we studied the behavior and the underlying mechanisms of Psc Su(z)2 mutant stem cell clones. We then asked whether tumors could also be generated by mutation in differentiating follicle cells. In ovarioles, follicle cells are proliferative until stage 6 of egg chamber development; therefore, mitotic follicle cell clones can be generated before this stage. It takes ∼1 d for an egg chamber to develop from stage 2 to stage 6, and another day from stage 6 to stage 10 (Fig. 6A; Spradling 1993). When a mutant follicle cell is generated in a stage 2 egg chamber, this mutant clone will be in a stage 6 egg chamber after 1 d and in a stage 10 egg chamber after 2 d. We found that, when Su(z)21.b8 mutant follicle cells were generated in stage 2–5 egg chambers, the mutant clones, although frequently showing mild morphological defects and smaller nuclear sizes, were able to form largely normal epithelium (Fig. 6B,F), and did not show uncontrolled proliferation phenotype. In addition, pH3-positive cells were rarely seen in the clones in stage 10 egg chambers (three positive cells out of 47 clones examined) (Fig. 6C,G). The incidence of mitotic cells in stage 10 egg chambers, although rare, suggests that the transition from mitosis to endocycle of the mutant follicle cells is delayed. Cut and Fas III, two follicle cell markers, are expressed dynamically during oogenesis. Both are expressed highly in differentiating follicle cells, but their expression is down-regulated after stage 6, when follicle cells undergo the transition from mitosis to endocycle. The mutant follicle cells originated from stages 2–5, however, still showed relatively high expression levels of Cut and Fas III expression when the egg chambers had reached stage 10 (Fig. 6D,E,H,I), suggesting that the mutant cells are defective or delayed in terminal differentiation. We conclude that the mutant follicle cells generated outside the germarium display defective or delayed terminal differentiation, but their mitotic activity largely ceased when egg chambers reached stage 10 and tumors could not be developed.
Mutations disrupting other PRC1 components do not lead to tumorigenesis
The Drosophila PRC1 complex includes four core PcG proteins: Pc, Ph, Sce (or dRing), and Psc (Shao et al. 1999). All of them are critically important for Hox gene repression. To test whether the tumor-suppressive activity of Psc in FSC was dependent on PRC1 complex function, we generated PcXT109, Sce1, and Sce33m2 mutant FSC clones by the FLP–FRT system. The clones were marked by the absence of either histone-GFP (for Pc clones) or arm-lacZ (for Sce clones) expression. One week or 2 wk ACI, none of the Pc or Sce mutant FSC clones showed the epithelial extrusion phenotype (>30 clones examined for each genotype) (Supplemental Fig. S8). In addition, the mutant FSC-derived daughters were able to differentiate into largely normal epithelial cells, and tumor development was not observed (Supplemental Fig. S8). Hence, the above results suggest that the tumor-suppressive activity of Psc and Su(z)2 in FSCs is independent of canonical PRC1 complex function. To test whether there was a difference in regulating Hox gene expression by the PRC1 components in follicle cells, we examined the expression of Abd-B and Ubx in Su(z)21.b8 or Pc mutant FSC-derived clones. Both markers were not expressed in wild-type follicle cells, but their expression could be detected in any Su(z)21.b8 or PcXT109 mutant clones (Supplemental Fig. S9; data not shown), indicating that the canonical PRC1 complex is critical for Hox gene suppression in follicle cells. These data also indicate that ectopic Hox gene expression does not seem to play a role in the tumorous growth of Psc Su(z)2-deficient FSC clones. Because Wg signaling activation is responsible primarily for tumor development from Psc Su(z)2-deficient FSC clones, we then asked whether differential requirement of individual PRC1 components for tumor suppression is due to their differential engagement in Wg signaling repression. Interestingly, expression of wg-lacZ, a wg enhancer trap line, was undetectable in either Pc or Sce mutant follicle cells, but was detected in many Su(z)21.b8 mutant follicle cells within the clones (Supplemental Fig. S10). These data suggest that only Psc and Su(z)2, not other core PRC1 components, are essential for wg suppression in FSC lineage. This observation explains, at least in part, why the tumor-suppressive role of Psc and Su(z)2 is independent of the canonical PRC1 function.
Discussion
Niche signals as extrinsic factors play critical roles in the control of stem cell maintenance, differentiation, and division. Intrinsic mechanisms controlling stem cell behavior, however, are poorly understood. Identifying the genes involved and investigating how they function are important steps toward understanding the molecular mechanisms of stem cell self-renewal. Here we identified two PcG genes, Psc and Su(z)2, functioning redundantly and autonomously in FSCs for maintenance and differentiation. FSCs with deletion mutations of both genes were unable to differentiate further, and self-propagated continuously to develop into neoplastic tumors. In addition, we demonstrated that this tumor-suppressive function of Psc and Su(z)2 is not through PRC1 function. Our study also indicates that specific epigenetic silencers play critical roles in facilitating stem cell differentiation and suppressing tumor development by antagonizing self-renewal programs. Moreover, our study reveals a novel mechanism of epithelial extrusion for tumor cell migration at ectopic sites. Because many aspects of PcG function are conserved from Drosophila to mammals, their roles in adult stem cell maintenance and differentiation are likely to be conserved in mammals and humans as well.
In murine and human ES cells, PcG complexes bind to many genes that are involved in differentiation, indicating that they could be essential for maintaining ES cell pluripotency by preventing differentiation. Surprisingly, ES cells lacking PRC2 components such as Eed or SuZ12 can be maintained stably, although they simultaneously express key pluripotency factors as well as differentiation genes (Pasini et al. 2007; Chamberlain et al. 2008). However, when SuZ12 mutant ES cells are induced to differentiate, key pluripotency genes cannot be turned off, resulting in defective differentiation (Pasini et al. 2007). These observations indicate that PcG genes could serve as both positive and negative modulators of stem cell self-renewal. In this study, we found that specific PcG genes play critical roles in allowing adult stem cells to differentiate. Deletion mutations of Psc and Su(z)2 render FSCs incapable of further differentiation. Instead, they self-propagate continuously and develop into tumors. Thus, contrary to the proposed differentiation-preventing activity of PcG genes in stem cells, Psc and Su(z)2 have unexpected essential role for FSC differentiation in the Drosophila ovary. Our cellular and genetic analyses further demonstrate that derepression of Wg self-renewal signaling drives the stem cell-like tumor development in Psc Su(z)2-deficient FSCs, as inhibiting Wg signaling activity efficiently prevents tumorigenesis, suggesting that specific PcG genes are required for stem cell differentiation by inhibiting self-renewal programs. A critical role for Wnt signaling has been implicated in multiple types of stem cells and cancer (Klaus and Birchmeier 2008; Nusse 2008), and the latter one might be due to its ability to promote self-renewal of cancer stem cells. Previous genome-wide mapping of Polycomb targets in Drosophila demonstrates that PcG genes not only target genes that are important for cellular differentiation, but also target genes known as self-renewal signals for many types of adult stem cells, including hh and wg (Negre et al. 2006; Tolhuis et al. 2006; Schwartz and Pirrotta 2007). Therefore, it is possible that PcG proteins could target different sets of genes in different tissues or at different developmental stages, and, within a specific type of stem cell, they could control both directions of stem cell fate by regulating both self-renewal and differentiation programs.
An appealing explanation for the seemingly opposite stem cell functions of PcG genes is that different polycomb components or complexes may target different sets of genes that regulate either self-renewal or differentiation. Consistently, the differentiation-promoting activity of Psc and Su(z)2 in FSCs is independent of canonical PRC1 complex function, as mutations in other core PRC1 components, including Pc and Sce, do not lead to the same phenotype. We further demonstrate that the difference is due, at least in part, to the differential regulation of wg repression in the follicle cell lineage. Mutation in ph, which encodes another PRC component, causes underproliferation of follicle cells, yet produces a similar, but weak, follicle cell morphology phenotype (Narbonne et al. 2004), indicating that Ph might partner with Psc or Su(z)2 in regulating FSC differentiation and extrusion. Notably, although Psc is an essential component for PRC1 complex function, Su(z)2 has not been demonstrated to be associated with the PRC1 complex. Thus, it is also possible that Su(z)2 and Psc may form complexes with other unknown proteins for this novel function in FSCs. We propose that PcG genes may be central players in orchestrating both self-renewal and differentiation of stem cells, two opposite functions that could be achieved by different PcG protein components and/or complexes. To do so, they might function to modulate both self-renewal and differentiation programs and to maintain specific chromatin states in order to facilitate either self-renewal or differentiation. Differential requirements for epigenetic regulators in different types of stem cells have been demonstrated previously in the Drosophila ovary (Xi and Xie 2005). Here, we show that Psc and Su(z)2 are also specific for FSC maintenance, and are dispensable in GSCs. These observations further suggest that different epigenetic regulators may be used to maintain specific self-renewal programs and chromatin states in various tissue-specific stem cells.
In general, epithelial stem cell maintenance defects can be explained by two possible mechanisms: Cells could be eliminated by cell death, or they could undergo differentiation with or without transit amplification to form epithelium. The latter case is best seen in niche signaling pathway (Hh, Wg, or BMP)-compromised FSCs in the Drosophila ovary, in which the mutant FSCs move away from the niche and differentiate (Zhang and Kalderon 2001; Song and Xie 2003; Kirilly et al. 2005). The maintenance defects of Psc Su(z)2-deficient FSCs, however, cannot be explained by either mechanism. Instead, the mutant cells show a series of morphological changes that have not been observed previously, including apical membrane retraction and basal extrusion from the epithelium (Fig. 1J). Thus, our study reveals cell extrusion as a novel process of FSC loss from their normal location. We also demonstrate that the extrusion is mediated through the PCP pathway, which, to our knowledge, has not been implicated previously in the epithelial extrusion process. It is not clear how PCP controls epithelial extrusion, but because it has also been implicated in regulating directed cell movement (Seifert and Mlodzik 2007), a similar molecular machinery might be used in the epithelial extrusion process observed here. The PCP components include core Fz and Dsh as well as Fat-Dachsous (FT-DS) PCP factors, although whether those two pathways function linearly or in parallel is still not clear. Notably, we find that both Fz and Dsh and FT-DS PCP factors are required for tumor cell extrusion, indicating that the two pathways function nonredundantly in this process. Because tumor cells do not have apicobasal polarity, an intriguing question arises as to whether loss of apicobasal polarity could lead to basal extrusion. Disrupting the apicobasal polarity of FSCs, however, although disrupting the epithelial organization, does not cause cell extrusion. Instead, the mutant follicle cell clones usually invade apically into germline cysts (Bilder et al. 2000), suggesting that the extrusion phenotype cannot be reproduced by apicobasal polarity mutants. A similar epithelial cell extrusion process has been reported in Drosophila wing imaginal discs, where BMP pathway-compromised epithelial cells extrude from the monolayer epithelium (Gibson and Perrimon 2005; Shen and Dahmann 2005). BMP pathway cascade is also required for the maintenance of FSCs in the Drosophila ovary, but the mutant FSCs and their daughters do not display the extrusion phenotype (Kirilly et al. 2005). In addition, BMP pathway-compromised imaginal disc cells, although extruded from the epithelium, still maintain apicobasal polarity (Gibson and Perrimon 2005; Shen and Dahmann 2005), further suggesting that the process of epithelial extrusion of polycomb mutant FSCs occurs via a distinct mechanism. Because the mutant FSCs are able to initiate tumor formation, cell extrusion may be a novel mechanism for tumor cells to leave their original tissue context and migrate to ectopic sites. We propose that similar mechanisms could be used in mammalian cancer cells to promote their migration.
In this study, we show that, although Psc Su(z)2-deficient FSCs initiated tumor formation, Psc Su(z)2-deficient differentiating follicle cells outside the germarium could not initiate tumorigenesis, demonstrating that, in this case, the stem cells and possibly early progenitors were more prone to initiate tumorigenesis than the downstream differentiating cells upon oncogenic mutations. Increasing evidence supports the cancer stem cell theory, at least for certain types of cancers. But it is not clear what the origin of these cancer stem cells is. They could be derived from differentiated cells or stem cells. Although somewhat conflicting, studies of murine hematopoiesis showed that stem cells and multipotent progenitors harboring oncogenic mutations are generally more efficient in initiating leukemia (Wang and Dick 2005). The stem cell origin of tumors is also supported by a recent study showing that APC mutation in intestinal stem cells, not differentiating progenitors, initiates tumorigenesis (Barker et al. 2009). The establishment of this Drosophila model of stem cell-derived tumor formation may help to further understand the underlying mechanisms governing a cell's tumorigenic potential.
Potential roles of PcG genes in tumorigenesis have been suggested in mammals and humans. PcG proteins such as EZH2 and Su(z)12 are frequently up-regulated in several types of human cancers. In addition, the mammalian bmi-1 gene, which is homologous to Psc and Su(z)2, is considered to be a proto-oncogene because up-regulated bmi-1 functions synergistically with c-myc to cause B or T lymphomas (Sparmann and van Lohuizen 2006). Furthermore, loss of bmi-1 function causes cell quiescence of both hematopoietic and neural stem cells, partially caused by the derepression of p16ink4 and p19ARF cell senescence genes (Molofsky et al. 2003, 2005; Park et al. 2003). Contrary to the oncogenic function of bmi-1, Psc and Su(z)2 have tumor-suppressive activity in the Drosophila ovary. It was also observed previously that Psc and Su(z)2-deficient epithelial cells in imaginal discs develop tumorous growths (Beuchle et al. 2001), which could be a consequence of cellular overgrowth caused by derepression of cell cycle genes (Oktaba et al. 2008), as well as the activation of JAK/STAT and Notch signaling pathways (Classen et al. 2009; Martinez et al. 2009). Interestingly, there is no obvious up-regulation of JAK/STAT or Notch signaling activities in Su(z)21.b8 mutant FSC clones, and inhibiting the activity of either pathway could not prevent tumor development from the mutant FSCs (data not shown), indicating that there are diverse mechanisms underlying the tumor-suppressive activity of Psc and Su(z)2 in different tissues. Further studies should reveal whether Psc and Su(z)2 have a common role in other epithelial stem cell types. Interestingly, mel-18, a mammalian gene closely related to bmi-1, has been reported to have tumor-suppressive activity in cultured breast cancer cells and in NIH 3T3 cells when injected subcutaneously into nude mice (Kanno et al. 1995; Guo et al. 2007). Thus, the tumor-suppressive activity of this RING finger family of PcG genes reported here might be conserved in mammals.
Taken together, this study reveals a novel mechanism of epithelial extrusion and a novel role of Drosophila PcG genes in suppressing self-renewal programs in epithelial stem cells to allow lineage differentiation. Dysfunction of these genes may lead to tumorigenesis in these tissues. Given evolutionary conservation of polycomb genes from Drosophila to mammals, it would be interesting to investigate whether these genes play a similar role in mammalian stem cells and cancer.
Materials and methods
Fly stocks
Information on the alleles used in this study can be found in either FlyBase (http://flybase.bio.indiana.edu) or as otherwise noted. The following mutant alleles and transgenes were used: Psce24, Psch27, and Su(z)21.b7 are either protein-null or genetic-null mutations (Adler et al. 1989; Wu and Howe 1995; King et al. 2005). Su(z)21.b8 is a deficiency that removes both Psc and Su(z)2 (Adler et al. 1989). Su(z)2XL26 is a deficiency that precisely removes both Psc and Su(z)2 (Supplemental Fig. S4). PcXT109 is a protein-null allele (Franke et al. 1995). Sce1 is a deletion mutation in which the C-terminal 113 amino acids are removed, and Sce33m2 is a hypomorphic allele (Fritsch et al. 2003). hs-Psc and hs-Su(z)2 are transgenes driven by the hsp70 promoter (Rastelli et al. 1993). dsh3 is a loss-of-function mutation (Wehrli and Tomlinson 1998). UAS-dTcfDN is a transgene expressing a dominant-negative form of dTCF (van de Wetering et al. 1997). UAS-ptc; UAS-RNAi lines for wg, smo, ci, punt, fz, fmi, and fj are from Vienna Drosophila RNAi Center. All fly crosses were reared at 25°C on standard corn meal food with wet yeast paste, unless otherwise noted.
The following FRT stocks were used: FRT42D arm-lacZ, FRT42D Psce22/SM6B, FRT42D Psce24/SM6B, FRT42D Psch27/CyO, FRT42D Su(z)21.b7/SM6B, FRT42D Su(z)21.b8/SM6B, FRT42B Su(z)2XL26/Cyo, hs-Psc; FRT42D Su(z)21.b8/TM3, hs-Su(z)2; FRT42D Su(z)21.b8/TM3, hs-Psc, hs-Su(z)2; FRT42D Su(z)21.b8/SM6B, tubGal4 UAS-GFP; FRT42D Tub-Gal80, ubi-GFP FRT19A; hs-flp, dsh3 FRT 19A, hs-flp; FRT82B arm-lacZ, FRT82B Sce1/TM3, FRT82B Sce33M2/TM3, Pc XT109 FRT2A/ TM3, and hs-flp; histone-GFP FRT2A.
Clonal analysis and heat-shock regimes
Female flies of appropriate genotypes were generated by crossing the appropriate stocks listed above. Induction of FLP expression was performed by heat-shocking females at 37°C in a running-water bath. For time-course clonal analysis to determine the requirements for stem cell maintenance, females were heat-shocked 1 h each time, twice per day for three consecutive days, and flies were dissected 4, 14, and 20 d after the last heat shock (or, as mentioned in the text, ACI).
In rescuing experiments using hs-Psc and/or hs-Su(z)2 transgenes, in order to sufficiently induce expression of transgenes, flies were heat-shock-treated continuously every day (twice each day, 1 h each time) ACI, until the last day before dissection, and flies were kept at 29°C in between treatments. To take into consideration the effects of different heat-shock regimes on clone induction rate, several controls for the experiments, including the wild-type control and Su(z)21.b8 mutant control without transgenes, were established and were heat-shock-treated and analyzed in the same way for appropriate comparison. In experiments for close trace of the behavior of FSC clones, flies were heat-shocked twice within a day, and were dissected every day ACI.
Antibody staining and imaging
Fly ovaries were dissected in Grace's insect medium, and were fixed in 4% paraformaldehyde for 10 min at room temperature. After washing with PBT (PBS containing 0.1% Triton X-100), samples were blocked for 1 h in 5% normal goat serum in PBT, and then incubated with primary antibodies overnight at 4°C. After washing with PBT, samples were incubated with secondary antibodies for 2 h at room temperature. For DAPI (4′, 6-diamidino-2-phenylindole) staining, samples were incubated with 1 μg/mL DAPI in PBT for 6 min, and the reaction was stopped by washing samples with PBT. Samples were mounted in the mounting media (70% glycerol, 2% DABCO in 1× PBS).
The following primary antibodies were used: rabbit anti-β-galactosidase (1:6000; Cappel), rabbit anti-pH3 (1:300; Upstate Biotechnologies), rabbit anti-GFP (1:400; Torrey Pines), mouse anti-β-galactosidase (1:50; Molecular Probes), rabbit anti-Hh (1:1000; a gift from T. Tetsuya), rat anti-Ci (1:10; a gift from A. Zhu), rabbit anti-pMad (1:100; a gift from E. Laufer), rabbit anti-Scrib (1:1000; a gift from C. Doe), mouse anti-GFP(1:500; Roche), and rabbit anti-LanA (1:1000; a gift from S. Baumgartner). The following monoclonal antibodies were from Developmental Studies Hybridoma Bank: mouse anti-α-Spectrin (1:50), mouse anti-Fas III (1:60), mouse anti-Cut (1:60), mouse anti-CycA (1:5), mouse anti-CycB (1:5), rat anti-DE-cadherin (1:60), mouse anti-Crb (1:10), mouse anti-Dlg (1:50), and mouse anti-Abd-B (1:50). Secondary antibodies—including goat anti-rabbit, anti-mouse, or anti-rat IgGs—conjugated to Alexa (488 or 568) (Molecular Probes) were used at a dilution of 1:300. Alexa fluor 568 Phalloidin was used at a dilution of 1:100.
All fluorescent images were collected using a Zeiss Imager microscope equipped with an ApoTome system. The area of the tumor mass shown in Figure 4I was measured using the Axiovision software measurement tool. The captured images were processed using Adobe Photoshop and Illustrator.
TUNEL labeling
Cell death stain was performed according to the manufacturer's protocol (In Situ Cell Death Detection Kit, catalog no.1684795, Roche). Briefly, samples were fixed as described above and were incubated in TUNEL reaction mix (enzyme solution: label solution = 1:9) for 1 h at 37°C. Reaction was stopped by washing samples with PBT three times.
Acknowledgments
We thank S. Baumgartner, C. Doe, E. Laufer, K. Keleman, D. McKearin, J. Müller, T. Tetsuya, A. Zhu, the Bloomington Stock Center, Vienna Drosophila RNAi Center (VDRC), the Exelixis collection at the Harvard Medical School, and Developmental Studies Hybridoma Bank (DSHB) for fly stocks and reagents; and Y. Rao, X. Wang, and members of the Xi laboratory for comments on the manuscript. This work was supported by the Chinese Ministry of Science and Technology.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1901510.
Supplemental material is available at http://www.genesdev.org.
References
- Adler PN, Charlton J, Brunk B 1989. Genetic interactions of the suppressor 2 of zeste region genes. Dev Genet 10: 249–260 [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H 2009. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457: 608–611 [DOI] [PubMed] [Google Scholar]
- Beuchle D, Struhl G, Muller J 2001. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development 128: 993–1004 [DOI] [PubMed] [Google Scholar]
- Bilder D, Li M, Perrimon N 2000. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289: 113–116 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E 2007. Epithelial stem cells: Turning over new leaves. Cell 128: 445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, et al. 2006. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441: 349–353 [DOI] [PubMed] [Google Scholar]
- Buszczak M, Spradling AC 2006. Searching chromatin for stem cell identity. Cell 125: 233–236 [DOI] [PubMed] [Google Scholar]
- Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V 2004. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes & Dev 18: 2627–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SJ, Yee D, Magnuson T 2008. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells 26: 1496–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Bunker BD, Harvey KF, Vaccari T, Bilder D 2009. A tumor suppressor activity of Drosophila Polycomb genes mediated by JAK–STAT signaling. Nat Genet 41: 1150–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E 2009. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136: 1122–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanto M, McNeill H 2004. Planar polarity from flies to vertebrates. J Cell Sci 117: 527–533 [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC 1996a. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122: 1125–1135 [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Spradling AC, Ingham PW, Lin H 1996b. The role of segment polarity genes during early oogenesis in Drosophila. Development 122: 3283–3294 [DOI] [PubMed] [Google Scholar]
- Franke A, Messmer S, Paro R 1995. Mapping functional domains of the polycomb protein of Drosophila melanogaster. Chromosome Res 3: 351–360 [DOI] [PubMed] [Google Scholar]
- Fritsch C, Beuchle D, Muller J 2003. Molecular and genetic analysis of the Polycomb group gene Sex combs extra/Ring in Drosophila. Mech Dev 120: 949–954 [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N 2005. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science 307: 1785–1789 [DOI] [PubMed] [Google Scholar]
- Guo WJ, Zeng MS, Yadav A, Song LB, Guo BH, Band V, Dimri GP 2007. Mel-18 acts as a tumor suppressor by repressing Bmi-1 expression and down-regulating Akt activity in breast cancer cells. Cancer Res 67: 5083–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Williams D, Spradling AC 2005. The expression profile of purified Drosophila germline stem cells. Dev Biol 283: 486–502 [DOI] [PubMed] [Google Scholar]
- Kanno M, Hasegawa M, Ishida A, Isono K, Taniguchi M 1995. mel-18, a Polycomb group-related mammalian gene, encodes a transcriptional negative regulator with tumor suppressive activity. EMBO J 14: 5672–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IF, Emmons RB, Francis NJ, Wild B, Muller J, Kingston RE, Wu CT 2005. Analysis of a polycomb group protein defines regions that link repressive activity on nucleosomal templates to in vivo function. Mol Cell Biol 25: 6578–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly D, Spana EP, Perrimon N, Padgett RW, Xie T 2005. BMP signaling is required for controlling somatic stem cell self-renewal in the Drosophila ovary. Dev Cell 9: 651–662 [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W 2008. Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8: 387–398 [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L 1999. Mosaic analysis with a repressible neurotechnique cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461 [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, et al. 2006. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125: 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SM, Ahuja NK, Francis NJ 2009. Polycomb group protein Suppressor 2 of zeste is a functional homolog of Posterior Sex Combs. Mol Cell Biol 29: 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J, Spradling A 1995. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121: 3797–3807 [DOI] [PubMed] [Google Scholar]
- Martinez AM, Schuettengruber B, Sakr S, Janic A, Gonzalez C, Cavalli G 2009. Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling. Nat Genet 41: 1076–1082 [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ 2003. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425: 962–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R 2005. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes & Dev 19: 1432–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N 2002. The promise and perils of Wnt signaling through β-catenin. Science 296: 1644–1646 [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC 2008. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne K, Besse F, Brissard-Zahraoui J, Pret AM, Busson D 2004. polyhomeotic is required for somatic cell proliferation and differentiation during ovarian follicle formation in Drosophila. Development 131: 1389–1400 [DOI] [PubMed] [Google Scholar]
- Negre N, Hennetin J, Sun LV, Lavrov S, Bellis M, White KP, Cavalli G 2006. Chromosomal distribution of PcG proteins during Drosophila development. PLoS Biol 4: e170. doi: 10.1371/journal.pbio.0040170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R 2008. Wnt signaling and stem cell control. Cell Res 18: 523–527 [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A 2007. An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1: 277–285 [DOI] [PubMed] [Google Scholar]
- Oktaba K, Gutierrez L, Gagneur J, Girardot C, Sengupta AK, Furlong EE, Muller J 2008. Dynamic regulation by polycomb group protein complexes controls pattern formation and the cell cycle in Drosophila. Dev Cell 15: 877–889 [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF 2003. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423: 302–305 [DOI] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, et al. 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36: 288–292 [DOI] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K 2007. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol 27: 3769–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli L, Chan CS, Pirrotta V 1993. Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J 12: 1513–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V 2007. Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet 8: 9–22 [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M 2007. Frizzled/PCP signalling: A conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8: 126–138 [DOI] [PubMed] [Google Scholar]
- Shao Z, Raible F, Mollaaghababa R, Guyon JR, Wu CT, Bender W, Kingston RE 1999. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98: 37–46 [DOI] [PubMed] [Google Scholar]
- Shen J, Dahmann C 2005. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science 307: 1789–1790 [DOI] [PubMed] [Google Scholar]
- Song X, Xie T 2002. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci 99: 14813–14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xie T 2003. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development 130: 3259–3268 [DOI] [PubMed] [Google Scholar]
- Soto MC, Chou TB, Bender W 1995. Comparison of germline mosaics of genes in the Polycomb group of Drosophila melanogaster. Genetics 140: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M 2006. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6: 846–856 [DOI] [PubMed] [Google Scholar]
- Spradling AC 1993. Developmental genetics of oogenesis. In The development of Drosophila melanogaster (ed. Bate M, Martinez Arias A), pp. 1–71 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Tolhuis B, de Wit E, Muijrers I, Teunissen H, Talhout W, van Steensel B, van Lohuizen M 2006. Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat Genet 38: 694–699 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799 [DOI] [PubMed] [Google Scholar]
- van Lohuizen M, Frasch M, Wientjens E, Berns A 1991. Sequence similarity between the mammalian bmi-1 proto-oncogene and the Drosophila regulatory genes Psc and Su(z)2. Nature 353: 353–355 [DOI] [PubMed] [Google Scholar]
- Wang JC, Dick JE 2005. Cancer stem cells: Lessons from leukemia. Trends Cell Biol 15: 494–501 [DOI] [PubMed] [Google Scholar]
- Wehrli M, Tomlinson A 1998. Independent regulation of anterior/posterior and equatorial/polar polarity in the Drosophila eye; evidence for the involvement of Wnt signaling in the equatorial/polar axis. Development 125: 1421–1432 [DOI] [PubMed] [Google Scholar]
- Wu CT, Howe M 1995. A genetic analysis of the Suppressor 2 of zeste complex of Drosophila melanogaster. Genetics 140: 139–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi R, Xie T 2005. Stem cell self-renewal controlled by chromatin remodeling factors. Science 310: 1487–1489 [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94: 251–260 [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC 2000. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290: 328–330 [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling A 2001. The Drosophila ovary: An in vivo stem cell system. In Stem cell biology (ed. Marshak DR et al. ), pp. 129–148 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Xu T, Rubin GM 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kalderon D 2000. Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development 127: 2165–2176 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kalderon D 2001. Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410: 599–604 [DOI] [PubMed] [Google Scholar]