Abstract
Background
Generalized granuloma annulare (GGA) is a benign skin disorder of an unknown etiology. Though some cases of GGA have been reported, few systemic reviews of the clinical and pathological features of GGA have been performed.
Objective
The purpose of this study is to analyze and correlate the clinical and pathological characteristics of GGA in Korean patients.
Methods
We conducted a retrospective study that included 54 biopsy specimens of Korean GGA patients, and the clinical and pathological features of GGA were reviewed and analyzed for their correlation.
Results
The cutaneous lesions could be divided into the annular (24, 44%) and nonannular types (30, 56%), and the lesions were more common in males than in females (29 males and 25 females). The incidence of GGA showed a bimodal age distribution. The number of patients who presented within the first decade was 24 cases (44%), and 24 cases (44%) were over the fifth decade. Eight patients (15%) had systemic diseases. Especially, diabetes mellitus (DM) occurred only in the adult GGA patients over forty years old. The pathological findings showed dermal granulomatous lesions that consisted of either a palisading pattern (28, 52%) or an interstitial pattern (26, 48%).
Conclusion
In contrast to the previously reported studies, the age of GGA onset showed a bimodal distribution, and GGA was observed more often in males. The prevalence of DM in the GGA affected individuals was higher than that found in the general Korean population. Therefore, it is recommended to perform a work-up for DM in the GGA affected patients who are over forty years old.
Keywords: Clinical, Generalized granuloma annulare, Pathological
INTRODUCTION
Granuloma annulare (GA) is a transient, benign, cutaneous inflammatory disorder that's pathologically characterized by necrobiotic collagen associated with an infiltration of histiocytes and lymphocytes. The lesions of classical GA consist of localized, small, firm, asymptomatic papules that are flesh-colored or pale red, and they are grouped in a ring-like or circinate fashion. The unusual variants of GA include generalized, perforating, patch and subcutaneous GA1. These various types of GA share similar pathological findings2. There are four pathological structural patterns of GA: palisading, interstitial, perforating and subcutaneous.
Generalized granuloma annulare (GGA) has been defined as lesions that occur on the trunk and on both sets of extremities or on either the upper or lower extremities3. GGA was reported to be differentiated from the localized form (classical GA) by a later age of onset, a wide distribution of lesions, a protracted course with only rare spontaneous resolution and a poor response with administering therapy4. In addition, the interstitial pattern was reported to be predominant in the annular type of GGA3. Because it is a rare variant of GA, the clinicopathological correlations of GGA have not yet been clearly established.
The purpose of the present study is to review the Korean cases of GGA in order to evaluate and correlate the clinical and pathological features and the relationship of GGA with systemic diseases or its precipitating factors.
MATERIALS AND METHODS
We retrospectively studied 54 Korean patients who were diagnosed with GGA between January 1995 and March 2007 and who were seen at 10 dermatology tertiary referral centers and hospitals that were representative of each province and city in Korea. GGA was defined as affecting the trunk and both or either of the upper or lower extremities. The following clinical data was collected: age of onset, gender, the appearance of the lesions, the distribution of the lesions, the precipitating factors, the associated diseases and the treatment.
All the cutaneous samples were fixed in formalin, processed and embedded in paraffin. Sections of 4-µm thickness were stained with hematoxylin and eosin and with Alcian blue (pH 2.5), and they were analyzed for the pathological features, including the structural patterns of an inflammatory cell infiltrate, mucin deposition and infiltration of eosinophils, nuclear dusts and neutrophils.
Statistical analysis was performed using the χ2 test where appropriate. p values ≤0.05 were considered statistically significant. All data was computed using the SPSS 12.0 statistical package (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical findings
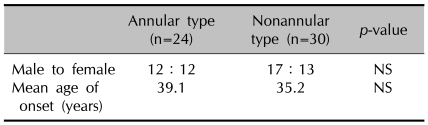
1) The gender ratio and age of onset (Table 1)
Table 1.
Demographic data of patients
NS: not significant
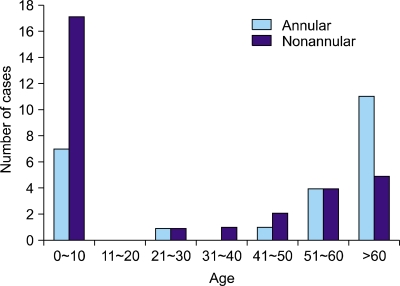
Among the 54 patients we studied, GGA was more common in males than in females (29 to 25, respectively, male:female ratio, 1.16:1). There were 12 males and 12 females (1:1) identified with the annular type compared to 17 males and 13 females (1.3:1) with the nonannular type GGA. The mean age of onset was 37.1 years for the entire group of patients (range: 3 months~84 years), and no statistically significant difference was observed between the annular (39.1 years) and nonannular type (35.2 years). The number of patients who presented within the first decade of life was 24 cases (44%), and the number presenting at ages over the fifth decade was 24 (44%). This data shows a bimodal distribution pattern. In particular, the nonannular type was predominant within the first decade of life (17, 31%), and the annular type predominated at ages over the fifth decade (15, 28%) (Fig. 1).
Fig. 1.
Distribution of the cases according to the age of onset.
2) Clinical appearance of the lesions
Based on the clinical appearance of lesions, the patients could be divided into two groups. The annular type was observed in 24 patients (44%), with the individual coalescing papules arranged in a ring-like configuration (Fig. 2). The nonannular type was observed in 30 patients (56%), with predominantly nonannular lesions where the eruption consisted of symmetrically scattered, often coalescing papules (Fig. 3). There were no perforating, patch or subcutaneous types observed.
Fig. 2.
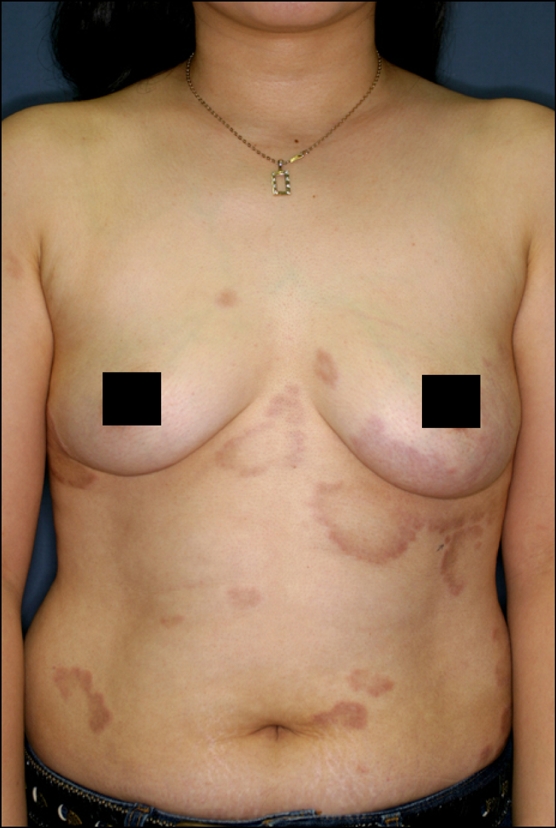
Widespread annular polycyclic erythematous plaques on the trunk.
Fig. 3.
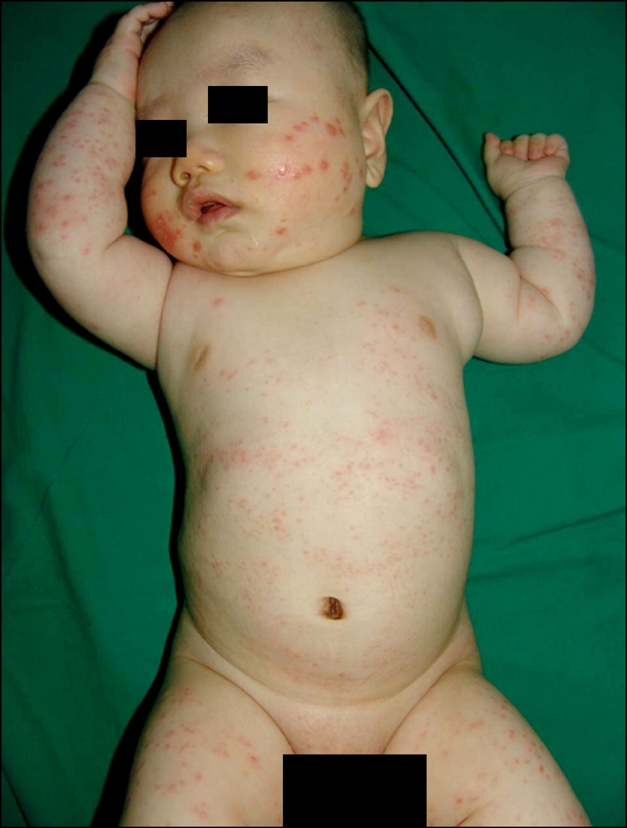
Numerous match head-sized erythematous papules over the entire body.
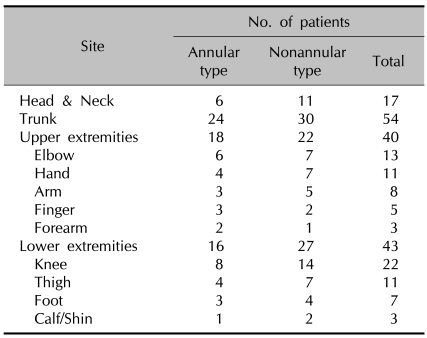
3) Distribution of lesions (Table 2)
Table 2.
Distribution of lesions
Consistent with the definition of GGA, the trunk was affected in all cases (54, 100%). The extremities were the next most commonly affected areas, and in particular the lower extremities were more commonly involved in the nonannular type (27, 50%) than in the annular type (16, 30%).
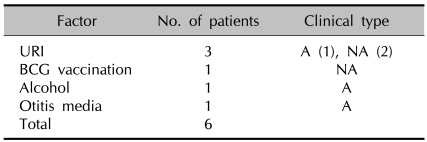
4) Precipitating factors (Table 3)
Table 3.
Precipitating factors
A: annular type, NA: nonannular type, URI: upper respiratory tract infection, BCG: Bacillus Calmette-Guerin
All the patients were evaluated for possible precipitating factors. Among them, upper respiratory tract infection (URI) was the most common (1 case of the annular type and 2 cases of the nonannular type). Bacillus Calmette-Guerin (BCG) vaccination (1 case of the nonannular type), excessive alcohol intake (1 case of the annular type), and otitis media (1 case of the annular type) were also noted.
5) Associated diseases (Table 4)
Table 4.
Associated diseases
A: annular type, NA: nonannular type, Tbc: tuberculous, SLE: systemic lupus erythematosus
Eight patients had systemic diseases, including diabetes mellitus (DM) (3 cases of the annular type and 1 case of the nonannular type), allergic contact dermatitis (1 case of the nonannular type), atopic dermatitis (1 case of the nonannular type), tuberculous lymphadenopathy (1 case of the nonannular type) and systemic lupus erythematosus (1 case of the nonannular type). Especially, DM occurred only in the adults over forty years old.
6) Treatments (Table 5)
Table 5.
Treatments
PUVA: psoralen and ultraviolet A
The rapid regression of lesions was observed in 10 of 13 patients treated with a topical corticosteroid, and a significant improvement was observed in 6 of 8 patients treated with a systemic corticosteroid. Other therapeutic modalities such as dapsone, hydroxychloroquine, systemic psoralen and ultraviolet A (PUVA), cyclosporine and isotretinoine were effective in some, but not all patients.
Pathological findings
1) Structural patterns
Two structural patterns were identified:
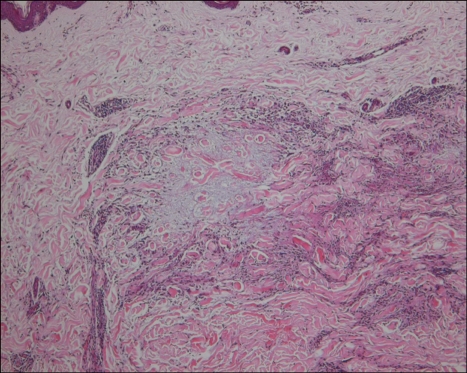
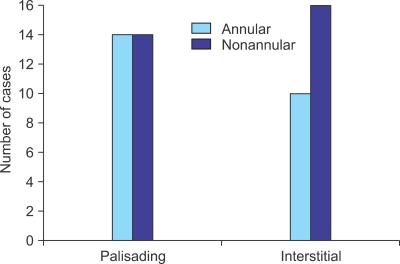
a) The palisading pattern, which showed a central zone of necrobiotic collagen surrounded by a palisade of histiocytes and varying number of lymphocytes (Fig. 4), was found in 28 cases (52%) (14 annular and 14 nonannular cases).
Fig. 4.
A palisading pattern granuloma with a central zone of necrobiotic collagen surrounded by a palisade of histiocytes and some lymphocytes (H&E, ×100).
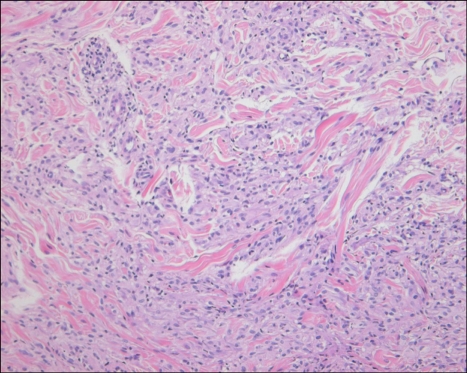
b) The interstitial pattern, which consisted of collections of histiocytes scattered between and around collagen bundles and around blood vessels in the upper and middle dermis (Fig. 5), was found in 26 cases (48%). This pattern was present in 16 of the 30 (53%) nonannular type cases and in 10 of the 24 (41%) annular type cases. It seemed that the interstitial pattern was more common in the nonannular type, although no statistical significance was observed (p=0.425) (Fig. 6).
Fig. 5.
An interstitial pattern granuloma with an infiltrate of histiocytes between collagen bundles (H&E, ×200).
Fig. 6.
Structural patterns of the annular and nonannular types.
2) Mucin deposition and inflammatory cell infiltrations (Table 6)
Table 6.
Mucin deposition and inflammatory cell infiltrations
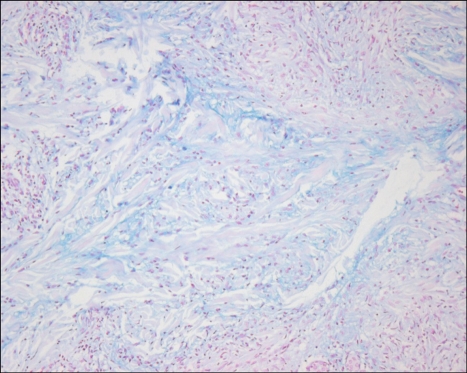
Alcian blue pH 2.5 staining showed mucin deposition in 51 cases (94%) (Fig. 7). An eosinophilic infiltrate was observed in 24 cases (44%). Nuclear dusts were found in 18 (33%), and a neutrophilic infiltrate was found in 12 cases (22%). Frank vasculitis was not observed.
Fig. 7.
Alcian blue pH 2.5 staining showed the deposition of increased bluish material among the degenerated collagen fibers (×200).
DISCUSSION
GA is a chronic, benign inflammatory skin disease that classically presents as arciform to annular plaques that are located on the extremities of young people. As a clinical variant of GA, GGA has been defined as consisting of hundreds of papules that are either discrete or confluent, but they only rarely show an annular arrangement5. In accordance with Dabski and Winkelmann3, we defined GGA as affecting the trunk and both or either of the upper or lower extremities. Friedman-Birnbaum6 reported that in contrast to classical GA, the average age of onset for patients with GGA was 53.9 years, and 84.8% of the cases were in their sixth and seventh decades of life. He also reported that GGA occurred predominantly in females (male:female, 1:6.1). However, our study revealed a bimodal distribution in which the number of patients who presented within the first decade of life was 24 cases (44%), and the number of patients who presented over the fifth decade was 24 (44%) (Fig. 1). In addition, GGA occurred more commonly in males than in females by 29 to 25, respectively (male:female, 1.16:1). In particular, males outnumbered females (male: female, 1.3:1) in the group with the nonannular type of GGA (Table 1).
Although the pathogenesis of GGA has not been fully elucidated, the presence of activated T-cells in the lymphocytic infiltrate of GA has been demonstrated, suggesting there is a cell-mediated immune response to various precipitating factors7. It has been reported that GA occurred following an insect bite8, sun exposure9, BCG vaccination7,10 and viral infection11,12. In our study, URI was the most common precipitating factor (1 case of the annular type and 2 of the nonannular type). In addition, BCG vaccination, excessive alcohol intake and otitis media were also revealed to be predisposing factors.
Several investigators have attempted to demonstrate a relationship between GGA and other systemic diseases, most notably DM. The association of GGA with DM is not yet clearly established. Studer et al13 noted that 10 of 84 adult patients (12%) with GA (localized or generalized) had DM, as opposed to the 5% prevalence of DM among the regional population. However, other studies that looked for carbohydrate intolerance by performing glucose tolerance testing4 or assessing the hemoglobin A1c values14 failed to find an increased prevalence of altered carbohydrate metabolism in the patients with GA. In our study, DM was diagnosed in 4 patients (7%) (3 cases of the annular type and 1 case of the nonannular type) who were all older than forty years. This prevalence is higher than that found in the general Korean population (5.42%)15. Therefore, a DM workup is recommended for the GGA affected patients who are over forty years old.
Generally, treatment for classical GA is unnecessary because spontaneous resolution is expected1. However, GGA was reported to be different from classical GA by exhibiting a protracted course with only rare spontaneous resolution and a poor response to therapy4. A variety of treatment modalities for GGA have been used, but they have shown only partial responses. These include topical application of vitamin E, cryotherapy, laser destruction and topical or intralesional injection of corticosteroids16. Systemic trials of PUVA17, dapsone18, hydroxychloroquine19, niacinamide20, chlorambucil21, isotretinoine22 and potassium iodide23 have been reported, but the observed efficacy was limited and the side effects most often outweighed the benefits. In our study, systemic or topical corticosteroid application showed more effective results than did dapsone, hydroxychloroquine, PUVA, cyclosporine or isotretinoine.
Pathologically, GGA shows an infiltrate of histiocytes and a perivascular infiltrate of lymphocytes that is usually sparse. The histiocytes may be present in an interstitial pattern without apparent organization, or they may be in a palisading pattern, surrounding areas with prominent mucin. Some cases may display a mixture of patterns and considerable variability exists between consecutive sections. In these cases, Friedman-Birnbaum et al2 made another diagnostic category such as the 'mixed histologic pattern'. We classified such lesions into an interstitial or a palisading pattern according to the prominent pathological features.
To date, there has been only one comprehensive study of the clinical and pathological findings of GGA. Dabski and Winkelmann3 reported that an interstitial structural pattern was the most common pathological finding in GGA, and an interstitial pattern was predominant in the annular type. In contrast, our study showed that a palisading pattern was the most common pathological finding, and the interstitial pattern was more frequent in the nonannular type GGA (16 of 30, 53%) than in the annular type GGA (10 of 24, 41%). However the difference did not show statistical significance (p=0.425). These discrepant results suggest three possibilities: first, due to the limited number of cases we examined, the variations in the prevalence of the different pathological patterns may only indicate trends and they have no statistical significance; second, an interstitial pattern may be an intermediate process toward the palisading pattern; third, these two pathological patterns may originate from a different pathogenesis.
The role of eosinophils in GA remains unexplained. Localized to sites of lymphohistiocytic infiltration at the periphery of the granulomatous foci, eosinophils may be responding along with the other inflammatory cells to the chemotaxins and cytokines produced by this disease process24. In our study, an infiltrate of eosinophils located at the periphery of the necrobiotic foci was identified in 24 cases (44%). Therefore, it is unlikely that they play a principal role in the degeneration of collagen. Leukocytoclastic vasculitis has been previously identified in several cases25. In our study, we noted nuclear dusts in 18 cases (33%) and an infiltrate of neutrophils in 12 cases (22%). However, frank vasculitis was not observed.
In conclusion, GGA occurred in a bimodal distribution for the age of onset, and GGA was more common in males. The trunk was the most commonly affected region and the lower extremities were more commonly involved in the nonannular type than the annular type. URI was the most common precipitating factor. The prevalence of DM in GGA-affected individuals was higher than that found in the general Korean population. Therefore it is recommended to conduct a work-up for DM in GGA-affected patients who are over forty years old. Systemic or topical corticosteroid application was more effective than any other treatment modality. The interstitial structural pattern was more common in the nonannular type of GGA than in the annular type of GGA. It is uncertain whether these results that differed from the results of the previous reports reflect ethnic differences or other factors, and further investigations are needed to clarify this issue.
References
- 1.Muhlbauer JE. Granuloma annulare. J Am Acad Dermatol. 1980;3:217–230. doi: 10.1016/s0190-9622(80)80181-2. [DOI] [PubMed] [Google Scholar]
- 2.Friedman-Birnbaum R, Weltfriend S, Munichor M, Lichtig C. A comparative histopathologic study of generalized and localized granuloma annulare. Am J Dermatopathol. 1989;11:144–148. doi: 10.1097/00000372-198911020-00006. [DOI] [PubMed] [Google Scholar]
- 3.Dabski K, Winkelmann RK. Generalized granuloma annulare: histopathology and immunopathology. Systematic review of 100 cases and comparison with localized granuloma annulare. J Am Acad Dermatol. 1989;20:28–39. doi: 10.1016/s0190-9622(89)70004-9. [DOI] [PubMed] [Google Scholar]
- 4.Dicken CH, Carrington SG, Winkelmann RK. Generalized granuloma annulare. Arch Dermatol. 1969;99:556–563. [PubMed] [Google Scholar]
- 5.Glusac EJ, Shapiro PE. Noninfectious granuloma. In: Elder DE, Elenitsas R, Johnson BL Jr, Murphy GF, editors. Lever's histopathology of the skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 374–376. [Google Scholar]
- 6.Friedman-Birnbaum R. Generalized and localized granuloma annulare. Int J Dermatol. 1986;25:364–366. doi: 10.1111/j.1365-4362.1986.tb03420.x. [DOI] [PubMed] [Google Scholar]
- 7.Kakurai M, Kiyosawa T, Ohtsuki M, Nakagawa H. Multiple lesions of granuloma annulare following BCG vaccination: case report and review of the literature. Int J Dermatol. 2001;40:579–581. doi: 10.1046/j.1365-4362.2001.01248-2.x. [DOI] [PubMed] [Google Scholar]
- 8.Moyer DG. Papular granuloma annulare. Arch Dermatol. 1964;89:41–45. doi: 10.1001/archderm.1964.01590250047009. [DOI] [PubMed] [Google Scholar]
- 9.Stankler L, Leslie G. Generalized granuloma annulare. A report of a case and review of the literature. Arch Dermatol. 1967;95:509–513. doi: 10.1001/archderm.95.5.509. [DOI] [PubMed] [Google Scholar]
- 10.Houcke-Bruge C, Delaporte E, Catteau B, Martin De Lassalle E, Piette F. Granuloma annulare following BCG vaccination. Ann Dermatol Venereol. 2001;128:541–544. [PubMed] [Google Scholar]
- 11.Toro JR, Chu P, Yen TS, LeBoit PE. Granuloma annulare and human immunodeficiency virus infection. Arch Dermatol. 1999;135:1341–1346. doi: 10.1001/archderm.135.11.1341. [DOI] [PubMed] [Google Scholar]
- 12.Granel B, Serratrice J, Rey J, Bouvier C, Weiller-Merli C, Disdier P, et al. Chronic hepatitis C virus infection associated with a generalized granuloma annulare. J Am Acad Dermatol. 2000;43:918–919. [PubMed] [Google Scholar]
- 13.Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364–368. doi: 10.1159/000246297. [DOI] [PubMed] [Google Scholar]
- 14.Gannon TF, Lynch PJ. Absence of carbohydrate intolerance in granuloma annulare. J Am Acad Dermatol. 1994;30:662–663. doi: 10.1016/s0190-9622(09)80121-7. [DOI] [PubMed] [Google Scholar]
- 15.Park KS, Park YJ, Kim SW, Shin CS, Park DJ, Koh JJ, et al. Comparison of glucose tolerance categories in the Korean population according to World Health Organization and American Diabetes Association diagnostic criteria. Korean J Intern Med. 2000;15:37–41. doi: 10.3904/kjim.2000.15.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prendiville JS. Granuloma annulare. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 369–373. [Google Scholar]
- 17.Hindson TC, Spiro JG, Cochrane H. PUVA therapy of diffuse granuloma annulare. Clin Exp Dermatol. 1988;13:26–27. doi: 10.1111/j.1365-2230.1988.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 18.Steiner A, Pehamberger H, Wolff K. Sulfone treatment of granuloma annulare. J Am Acad Dermatol. 1985;13:1004–1008. doi: 10.1016/s0190-9622(85)70253-8. [DOI] [PubMed] [Google Scholar]
- 19.Carlin MC, Ratz JL. A case of generalized granuloma annulare responding to hydroxychloroquine. Cleve Clin J Med. 1987;54:229–232. doi: 10.3949/ccjm.54.3.229. [DOI] [PubMed] [Google Scholar]
- 20.Ma A, Medenica M. Response of generalized granuloma annulare to high-dose niacinamide. Arch Dermatol. 1983;119:836–839. [PubMed] [Google Scholar]
- 21.Rudolph RI. Disseminated granuloma annulare treated with low-dose chlorambucil. Arch Dermatol. 1979;115:1212–1213. [PubMed] [Google Scholar]
- 22.Schleicher SM, Milstein HJ. Resolution of disseminated granuloma annulare following isotretinoin therapy. Cutis. 1985;36:147–148. [PubMed] [Google Scholar]
- 23.Giessel M, Graves K, Kalivas J. Treatment of disseminated granuloma annulare with potassium iodide. Arch Dermatol. 1979;115:639–640. doi: 10.1001/archderm.1979.04010050063038. [DOI] [PubMed] [Google Scholar]
- 24.Silverman RA, Rabinowitz AD. Eosinophils in the cellular infiltrate of granuloma annulare. J Cutan Pathol. 1985;12:13–17. doi: 10.1111/j.1600-0560.1985.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 25.Dahl MV, Ullman S, Goltz RW. Vasculitis in granuloma annulare: histopathology and direct immunofluorescence. Arch Dermatol. 1977;113:463–467. [PubMed] [Google Scholar]