Abstract
Hydroa vacciniforme (HV) is a photosensitivity disorder characterized by recurrent necrotic vesiculopapules on sun-exposed areas, which heal spontaneously during adolescence. Recently, an association has been reported between latent Epstein-Barr virus (EBV) infection and atypical HV-like eruption and malignant potential. However, latent EBV infection has also been reported in the setting of typical HV. An 11-year-old girl presented with recurrent, scattered, discrete vesicular eruptions with scarring on the face and the extensor surfaces of both forearms. In-situ hybridization was carried out to detect latent EBV infection. Based on the clinical and histopathological findings, typical EBV-associated HV was suspected.
Keywords: Epstein-Barr virus, Hydroa vacciniforme
INTRODUCTION
Hydroa vacciniforme (HV) is a rare photosensitivity disorder characterized by recurrent necrotic vesiculopapules on sun-exposed areas, which heal with vacciniform scarring. HV usually starts during childhood and resolves spontaneously during adolescence without systemic involvement.
Recently, there have been reports of patients with atypical HV-like eruptions in Asia and Mexico1-7. In contrast with the symptoms seen in typical HV, these patients showed facial swelling, indurated nodules on non-sun-exposed and sun-exposed areas, high-grade fever, wasting, and hepatosplenomegaly. Several of the reported patients progressed to hematological malignancies and death. Latent Epstein-Barr virus (EBV) infection was detected in most of the patients with this atypical HV-like eruption.
However, Iwatsuki et al8 reported that some patients with atypical HV-like eruptions did not progress to overt malignant hematological neoplasia. In addition, the investigators reported six patients with the typical manifestations of HV who had a number of EBV-encoded small nuclear RNA (EBER)+ cells in the cellular infiltrates in the dermis. These findings support the possibility that typical HV is also associated with latent EBV infection.
There have been several reports of HV in Korea1-3,9-12. However, to our knowledge, there has been no previous report of typical HV with confirmed latent EBV infection in Korea. We report a rare case of typical HV that occurred in association with latent EBV infection in an 11-year-old girl.
CASE REPORT
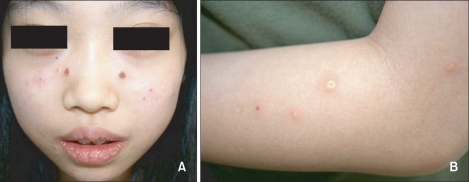
An 11-year-old Korean girl presented with a 5-year history of recurrent, scattered, discrete vesicular eruptions with scars on the face and the extensor surfaces of both forearms. Her skin lesions were aggravated by sun exposure and healed spontaneously with crusts and mild scars. There was no family or personal history of cutaneous photosensitivity. Physical examination showed multiple erythematous papules, vesicles, crusts, and shallow scars on the face, especially on the cheeks (Fig. 1). Except for the skin lesions, there were no remarkable findings on physical examination. Minimal erythemal doses (MEDs) of visible light and UVB were measured with Ektagraphic® (Eastman-Kodak, USA) and UV 800® (Waldmann, Germany) respectively. UVA MED measurements and photoprovocation tests were not performed due to patient rejection. The MED was 50 mJ/cm2 for UVB. No response was observed for a 30-minute exposure to visible light. Initial laboratory investigations showed that the erythrocyte sedimentation rate (27 mm/h) and the antistreptolysin O titer (433, positive) were slightly elevated, and the anti-SSB/La antibody was positive. Other laboratory studies were within normal limits, including complete blood cell count with differential cell count, platelet count, liver and renal function tests, antinuclear antibody concentration, lupus erythematosus cell preparation, anti-SSA/Ro antibody, and anti-Smith antibody.
Fig. 1.
The patient showed multiple, discrete vesicular eruptions with scarring on the face (A) and the extensor surfaces of the forearms (B).
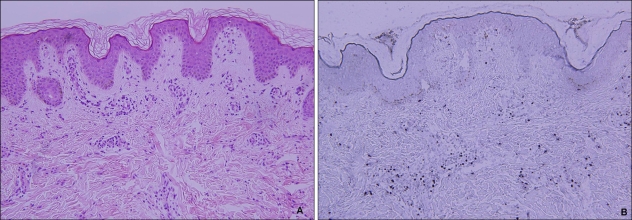
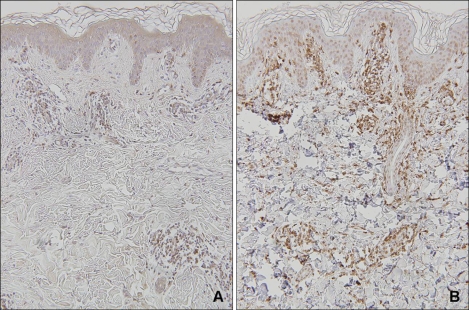
Histopathologic examination showed intraepidermal vesiculation with superficial and deep perivascular lymphocytic infiltrations in the dermis (Fig. 2A). In situ hybridization was carried out on paraffin-embedded skin biopsy sections, using fluorescein-conjugated oligonucleotide probes for EBV early RNAs (EBERs) (Dakopatts, Glostrup, Denmark) for the detection of latent EBV infection. Distinct, dark blue precipitates representing EBER transcripts were found in the nuclei of the lymphoid cells in the dermal infiltrate (Fig. 2B). Immunohistochemical staining was performed using monoclonal antibodies to CD3 (pan T-cell marker), CD45RO (memory T-cell marker), CD8 (helper T-cell marker), CD20 (B-cell marker), CD56 (NK-cell marker) (Dakopatts, Glostrup, Denmark), and latent membrane protein-1 (LMP-1). Most of the infiltrating cells in the dermis had a positive T-cell phenotype: CD3 and CD45RO positivity with some CD8 positivity. Only a few CD56-positive cells were present in the perivascular area. CD20-positive cells and LMP-1-positive cells were absent (Fig. 3).
Fig. 2.
(A) A skin biopsy obtained from the vesicular lesion on her forearm. Intraepidermal vesiculation with superficial and deep perivascular and periappendageal infiltrations in the upper dermis (H&E, ×200). (B) In situ hybridization. Many cells positive for EBV-encoded small nuclear RNA are present in the infiltrates (×200).
Fig. 3.
Immunohistochemical stains. The dermal infiltrates are composed mainly of lymphocytes expressing CD3-positive (A) and CD45RO-positive (B) cells (Immunoperoxidase stain, ×100).
Based on the clinical and histopathological findings, typical EBV-associated hydroa vacciniforme was suspected. Avoidance of sunlight and sunscreen application was recommended. After 1 year of follow-up, the patient has manifested no recurrence and shows no evidence of lymphoproliferative disorders.
DISCUSSION
Hydroa vacciniforme was first described by Bazin et al13 in 1862. It is clinically characterized by recurrent necrotic vesiculopapules on sun-exposed areas, which heal with vacciniform scarring. It is known to be a disease of early childhood that regresses spontaneously in adolescence and does not impair the general health of the patient14. Iwatsuki et al8 reported 6 patients with clinically and histologically typical HV who had EBV-encoded small nuclear RNA (EBER)+ cells in the dermal infiltrate. They suggested the possibility that typical HV and atypical HV are variants within the same disease spectrum of EBV-associated lymphoproliferative disorders8,15.
However, patients with EBV-associated HV-like eruptions, mainly reported in Asia1-3,16-18, present with atypical skin lesions in non-sun-exposed areas, as well as associated systemic symptoms. They occasionally progress to develop hematological malignancies. Such a clinical entity has been regarded as differing from typical HV, although it has several similar clinical and histopathological findings.
The EBV-associated HV-like eruptions reported in Korea1-3,19 have shown features more typical of EBV-associated lymphoproliferative disorders than of typical HV: skin lesions recurring continuously irrespective of sun exposure, associated systemic symptoms, with most patients progressing to develop hematological malignancies. Thus, in contrast to the preponderance of typical HV cases seen in Caucasians, most of the cases reported in Asians, including Koreans, show EBV-associated HV-like eruptions with malignant potential.
In the current case, clinical manifestations were similar to those seen in typical HV, with a benign course for the skin lesions, which cleared up after photoprotection was implemented. Associated latent EBV infection was confirmed by the presence of EBV-related RNAs, similar to those seen in the patients described by Iwatsuki et al8.
Immunohistochemical staining revealed that the EBER-ontaining cells predominantly had a T-cell phenotype (CD3, CD45RO). Only a few cells had an NK-cell (CD56) phenotype, and no cells had a B-cell (CD20) phenotype. No patients expressed LMP-1, similar to the patients described by Iwatsuki et al8. LMP-1 is a gene product of latent EBV infection and is known to have oncogenic activity18. However, detection of this gene product is not suitable for a screening test for EBV infection, since LMP-1 is not always expressed by EBV-infected cells20.
Previously, our group reported 6 patients with EBV-associated lymphoproliferative lesions presenting as HV-like eruption, with 3 different clinical courses19. The varying clinical courses in patients with EBV-associated HV-like eruptions are considered to be associated with the number of EBER+ cells in the skin lesions, subtype of EBV, and immune status of the patient16. Detailed pathophysiological mechanisms related to the various clinical manifestations occurring in the same disease spectrum remain to be determined.
Six cases of typical HV have been reported in Korea9-12, including a case of HV confirmed by repetitive UVA phototesting that showed spontaneous remission with the use of topical sunscreen9. To our knowledge, there has been no previous report of typical HV with confirmed latent EBV infection in Korea. Hence, we report this interesting case, which we believe to represent the first reported case of this entity in Korea.
References
- 1.Cho KH, Kim CW, Lee DY, Sohn SJ, Kim DW, Chung JH. An Epstein-Barr virus-associated lymphoproliferative lesion of the skin presenting as recurrent necrotic papulovesicles of the face. Br J Dermatol. 1996;134:791–796. [PubMed] [Google Scholar]
- 2.Cho KH, Kim CW, Kwon OS, Yang SG, Park KC, Park MH, et al. Epstein-Barr virus-associated lymphoproliferative eruption with progression to large granular lymphocytic leukaemia. Br J Dermatol. 1997;137:426–430. [PubMed] [Google Scholar]
- 3.Cho KH, Kim CW, Heo DS, Lee DS, Choi WW, Rim JH, et al. Epstein-Barr virus-associated peripheral T-cell lymphoma in adults with hydroa vacciniforme-like lesions. Clin Exp Dermatol. 2001;26:242–247. doi: 10.1046/j.1365-2230.2001.00805.x. [DOI] [PubMed] [Google Scholar]
- 4.Magana M, Sangueza P, Gil-Beristain J, Sanchez-Sosa S, Salgado A, Ramon G, et al. Angiocentric cutaneous T-cell lymphoma of childhood (hydroa-like lymphoma): a distinctive type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 1998;38:574–579. doi: 10.1016/s0190-9622(98)70120-3. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Maldonado R, Parrilla FM, Orozco-Covarrubias ML, Ridaura C, Tamayo Sanchez L, Duran McKinster C. Edematous, scarring vasculitic panniculitis: a new multisystemic disease with malignant potential. J Am Acad Dermatol. 1995;32:37–44. doi: 10.1016/0190-9622(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 6.Oono T, Arata J, Masuda T, Ohtsuki Y. Coexistence of hydroa vacciniforme and malignant lymphoma. Arch Dermatol. 1986;122:1306–1309. [PubMed] [Google Scholar]
- 7.Asada H, Okada N, Tei H, Yamamura T, Hashimoto K, Kondo K, et al. Epstein-Barr virus-associated large granular lymphocyte leukemia with cutaneous infiltration. J Am Acad Dermatol. 1994;31:251–255. doi: 10.1016/s0190-9622(94)70157-1. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki K, Xu Z, Takata M, Iguchi M, Ohtsuka M, Akiba H, et al. The association of latent Epstein-Barr virus infection with hydroa vacciniforme. Br J Dermatol. 1999;140:715–721. doi: 10.1046/j.1365-2133.1999.02777.x. [DOI] [PubMed] [Google Scholar]
- 9.Hann SK, Im S, Park YK, Lee S. Hydroa vacciniforme with unusually severe scar formation: diagnosis by repetitive UVA phototesting. J Am Acad Dermatol. 1991;25:401–403. doi: 10.1016/0190-9622(91)70215-n. [DOI] [PubMed] [Google Scholar]
- 10.Paik SA, Hahm JH, Kook HI. Three cases of hydroa vacciniforme. Korean J Dermatol. 1977;15:341–345. [Google Scholar]
- 11.Lim SD. A case of hydroa vacciniforme. Korean J Dermatol. 1960;1:65–67. [Google Scholar]
- 12.Chang SH, Yoon TY. A case of hydroa vacciniforme with ocular involvment. Korean J Dermatol. 1993;31:612–615. [Google Scholar]
- 13.Bazin E, Baudot E, Guérard L. Leçons théoriques et cliniques sur les affections génériques de la peau. Vol. 1. Paris: Delahaye; 1862. p. 132. [Google Scholar]
- 14.Bickers DR, Demar LK, DeLeo V, Poh-Fitzpatrick MB, Aronberg JM, Harber LC. Hydroa vacciniforme. Arch Dermatol. 1978;114:1193–1196. [PubMed] [Google Scholar]
- 15.Iwatsuki K, Satoh M, Yamamoto T, Oono T, Morizane S, Ohtsuka M, et al. Pathogenic link between hydroa vacciniforme and Epstein-Barr virus-associated hematologic disorders. Arch Dermatol. 2006;142:587–595. doi: 10.1001/archderm.142.5.587. [DOI] [PubMed] [Google Scholar]
- 16.Iwatsuki K, Ohtsuka M, Harada H, Han G, Kaneko F. Clinicopathologic manifestations of Epstein-Barr virus-associated cutaneous lymphoproliferative disorders. Arch Dermatol. 1997;133:1081–1086. [PubMed] [Google Scholar]
- 17.Wu YH, Chen HC, Hsiao PF, Tu MI, Lin YC, Wang TY. Hydroa vacciniforme-like Epstein-Barr virus-associated monoclonal T-lymphoproliferative disorder in a child. Int J Dermatol. 2007;46:1081–1086. doi: 10.1111/j.1365-4632.2007.03102.x. [DOI] [PubMed] [Google Scholar]
- 18.Iwatsuki K, Xu Z, Ohtsuka M, Kaneko F. Cutaneous lymphoproliferative disorders associated with Epstein-Barr virus infection: a clinical overview. J Dermatol Sci. 2000;22:181–195. doi: 10.1016/s0923-1811(99)00084-5. [DOI] [PubMed] [Google Scholar]
- 19.Cho KH, Lee SH, Kim CW, Jeon YK, Kwon IH, Cho YJ, et al. Epstein-Barr virus-associated lymphoproliferative lesions presenting as a hydroa vacciniforme-like eruption: an analysis of six cases. Br J Dermatol. 2004;151:372–380. doi: 10.1111/j.1365-2133.2004.06038.x. [DOI] [PubMed] [Google Scholar]
- 20.Rowe M, Lear AL, Croom-Carter D, Davies AH, Rickinson AB. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66:122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]