Abstract
Background
Psoriasis is a chronic inflammatory skin disease that affects approximately 1~3% of the general population.
Objective
We performed cDNA microarray analysis with using the dendrimer labelling method to investigate the gene expression profile in the peripheral blood mononuclear cells (PBMCs) of psoriatic patients.
Methods
The peripheral blood mononuclear cells of 5 patients with psoriasis and 8 control subjects were used in the gene expression analyses of psoriasis.
Results
We identified 212 differentially expressed genes that showed at least a two-fold induction and/or reduction in psoriatic patients. Among those, 63 genes, including CD44, CD56 and IL7R, were induced, while 139 genes, including the sphingosine kinase 1 and p16-INK genes, were reduced in the psoriatic patients.
Conclusion
We can speculate that these genes may have a role for the pathogenesis of psoriasis via their affecting different cellular functions. Our results suggest a possible mechanism by which activated immune cells migrate from the blood to the skin in psoriatic patients, and we provide novel putative targets for developing drugs to treat psoriasis.
Keywords: Cell adhesion, Microarray, Peripheral blood mononuclear cells, Psoriasis
INTRODUCTION
Psoriasis is a common, hereditary and chronic inflammatory skin disease that affects approximately 1~3% of the general population. It is defined as a clinical entity that can affect the skin, nails, mucous membranes and joints, and it can be classified into several categories by the morphological appearance1. The most prominent histological features include epidermal hyperproliferation with abnormal keratinocyte differentiation, acanthosis with elongation of the rete ridges and the infiltration of inflammatory cells into the involved skin2,3. Although the precise etiology is largely unknown, it is strongly believed that psoriasis is caused by the combination of multiple factors, including the genetic background and environmental insults.
Until the introduction of cyclosporine A (CsA) as a treatment modality, psoriasis had primarily been regarded as a skin disease related with epidermal keratinocytes4-6. However, CsA has been proven to be highly efficacious for controlling psoriasis, and thereafter the role of T-lymphocytes has been recognized as an effector cells in the pathogenesis of psoriasis7. This notion is further supported by the evidence from an animal model in which human skin is engrafted on the back of SCID mouse8,9. As SCID mice have a congenital deficiency in producing either T cells or B cells, they do not show allograft rejection of the human skin. When the normal skin from the psoriatic patient is grafted on the SCID mouse, it can be transformed into the psoriasiform by an intradermal injection of autologous T cells. This result clearly shows that psoriasis is induced by a T-cell dependent mechanism. Over the past two decades, various transgenic mouse models have also been developed to demonstrate the pathologic features observed in psoriasis10-12.
Yet in spite of these efforts, little is known about the precise triggering factors that exist in the peripheral blood cells of psoriatic patients. Over the years, a variety of molecular techniques, such as subtractive hybridization, differential display and serial analysis of the gene expression, have allowed the generation of global expression profiles in many biological model systems. In addition, a microarray-based method has recently been introduced for high-throughput monitoring of the gene expression. This type of global gene expression study is very effective in identifying the diagnostic markers, as well as the intrinsic pathways, implicated in the pathogenesis of various diseases. In this study, we attempt to comprehensively characterize the gene expression signature in the peripheral blood cells of psoriatic patient with using cDNA microarray technology. Our data will provide important clues on which to base further investigations on the pathologic events in psoriasis.
MATERIALS AND METHODS
Blood sampling and RNA isolation
The blood of psoriatic patients was collected from 5 volunteers in accordance with a process approved by the Ethics Committee of Chungnam National University Hospital. All the patients were classified as having the plaque type psoriasis. Peripheral blood mononuclear cells (PBMCs) were isolated by removing the red blood cells (RBCs) with using RBC lysis buffer (Sigma-Aldrich). The PBMCs were resuspended in 500 µl of RNABee solution (Tel-Test, Friedswood, TX, USA) and then they homogenized directly in the 1.5 ml collection tube by pipetting. The total RNA was extracted by using the RNeasy kit (QIAGEN) according to the recommended protocol. For the comparative analysis, the reference RNA was prepared by mixing the total RNA isolated from normal volunteers (n=8). The quality of all the RNA samples was evaluated with agarose gel electrophoresis and using a spectrometer. The samples that correspond to the quality criteria for RNA integrity were used for the cDNA microarray experiment.
The DNA chip and probe labeling
The human 17K cDNA chip was purchased from GenomicTree Inc (Daejeon, Korea). A 3DNA Array Labeling and Detection Kit (Genesphere, PA, USA) was used for probe labeling according to the manufacture's protocol. The cDNA was synthesized with using 3 µg of the total RNA of reference and the psoriatic RNA with a capture sequence-conjugated random primer. Each cDNA was mixed in an identical tube and it was purified using a PCR purification column, and it was concentrated to 10 µl by using a microcon-YM30.
Microarray hybridization
The hybridization mixture (1X formamide hybridization buffer, human Cot DNA 10 µg and blocking reagent 1 µl) containing the psoriasis and reference cDNA was applied on the microarray slides and hybridization was performed in a humidified chamber at 42℃ for 12 h. The microarray slides chips were sequentially washed with primary washing buffer containing 2X SSC (0.6M NaCl, 0.06 M Sodium Citrate, pH 7.0) and 0.2% SDS, secondary washing buffer containing 2X SSC and 0.2% SDS at 42℃ for 15 min. The chips were finally washed with 2X SSC and 0.2X SSC at room temperature for 15 min, respectively. The microarry slides were dried by centrifuging them at 800 × g for 5 min. Secondary hybridization was carried out using the complimentary capture reagents provided in the 3DNA Array 50 kit (Genisphere). All the steps of secondary hybridization were performed in a light-protected condition. After secondary hybridization, washing procedures were performed as same to primary washing.
Image scanning and data analysis
The microarray slides were scanned using a GenePix 4000B axon scanner (Molecular Devices, Union City, CA, USA). The scanned data was analyzed using GeneSpring 4.0 software (Silicon Genetics, Inc., Redwood City, CA, USA) and it was normalized by Lowess's method. To select the differentially expressed genes from the microarray data, we set the cut-off limit as a two-fold change; a gene was regarded as significant if its expression was changed by at least two-fold in all the psoriatic patients' samples. Web-based databases such as Unigene (www.ncbi.nlm.nih.gov/UniGene) or Source search (http://source.stanford.edu/cgi-bin/source/sourceSearch) were used to predict the cellular functions of the selected genes.
RESULTS
cDNA microarray analysis
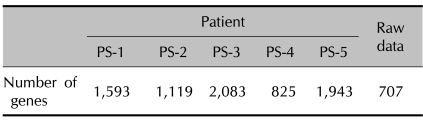
To investigate the global gene expression in the PBMCs of psoriatic patients, we performed cDNA microarray analysis with using a direct comparison method. This gene expression was compared with reference RNA that was prepared by mixing equal amounts of the total RNAs from 8 healthy individuals. In the cDNA microarray analysis, Cy3 dye (blue color) and Cy5 dye (red color) were used to label the reference RNA and the patients' RNA, respectively. We selected the differentially expressed genes by the cut-off limitation of two-fold changes. As the number of genes selected from each patient's samples was quite different, we filtered the genes whose expression was commonly induced and/or reduced in all the patients' samples for a further analysis (Table 1).
Table 1.
The number of reliable genes in each experiments and commonly 2-folds up or down regulated genes from the data of 5 independent microarrays
Gene clustering analysis
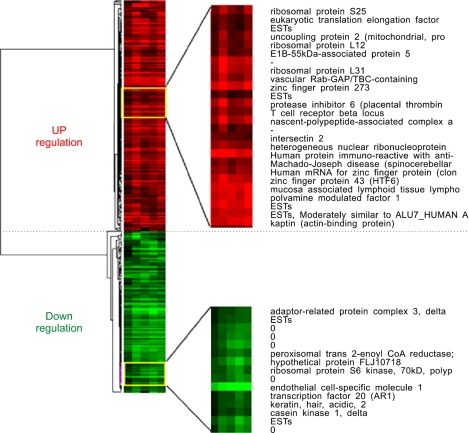
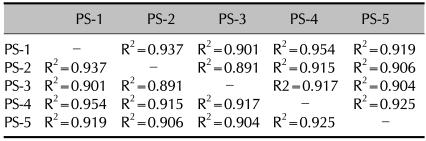
We clustered the genes based on their expression levels. We obtained 314 genes that showed a differential expression by at least two-fold in all the psoriatic patients. Among those, we excluded the expressed sequence tags (ESTs) because their function is not studied as the full length cDNA is not cloned yet. As a result, we obtained 212 differentially expressed genes. The genes were clustered and visualized in Fig. 1. For evaluate the sample variation, we compared the cDNA microarray data patient by patient. As shown in Table 2, almost all the regression coefficients were more than 0.9, indicating that the differentially expressed genes were successfully identified from the blood of the psoriatic patients.
Fig. 1.
The gene expression profile of the PBMCs from the 5 psoriatic patients. Each row represents a gene; each column shows the expression for the 212 genes expressed by each individual. The color red indicates the up-regulated genes in the psoriatic patients, while the color green indicates the down-regulated genes in the psoriatic patients.
Table 2.
Summary of the correlation coefficients between each patient
Increased genes in the psoriatic patients' blood
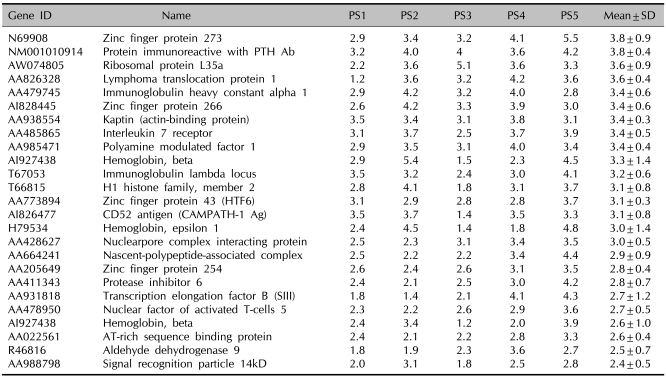
Sixty-three genes were identified as the increased genes in the psoriatic patients' PBMCs as compared with the normal controls (Table 3). Among those, zinc finger protein 273, which is a member of the krueppel C2H2-type zinc-finger family of proteins, showed the highest expression. In addition, several genes for coding zinc finger protein were also identified as up-regulated genes. Although this group of proteins generally exerts their roles as transcription factors, their precise function remains to be elucidated. Interestingly, the cell surface molecules CD44 and CD56 were identified as up-regulated genes in the psoriatic patients' PBMCs. These genes are known to regulate lymphocyte activities, and we can speculate that CD44 and CD56 may participate in the pathogenesis of psoriasis. Also, the increased genes in psoriasis include interleukin 7 receptor, nuclear factor of activated T-cells 5 (NF-AT 5) and signal recognition particle 14kD.
Table 3.
The genes with an increased expression in the PBMCs of the psoriatic patients
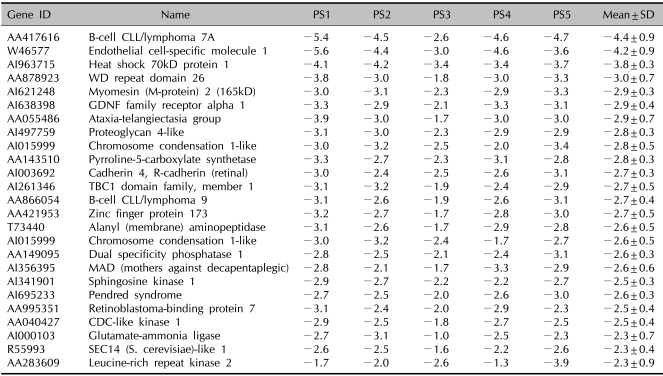
Decreased genes in the blood of psoriatic patients
The expression of many genes was reduced in the psoriatic patients' PBMCs as compared with that of the normal controls (Table 4). Examples include sphingosine kinase 1, CDC-like kinase 1 and cyclin-dependent kinase inhibitor 2A. As sphingosine kinase 1 attenuates NF-κB activity, there is a possibility that the NF-κB pathway would be more sensitive to inflammatory stimuli in psoriatic patients than that of normal individuals. The additional down-regulated genes included the kinase-related and/or phosphatase-related genes (CDC-like kinase 1, Cyclin-dependent kinase inhibitor 2A, SEC14-like 1, Leucinerich repeat kinase 2 and dual specificity phosphatase 1), genes that are involved in signal transduction (B-cell CLL/lymphoma 7A and 9 and heat shock 70kD protein 1), cell adhesion molecules such as cardherin 4 and some metabolic enzymes.
Table 4.
The genes with a decreased expression in the PBMCs of the psoriatic patients
DISCUSSION
Although its precise etiology is not fully understood, psoriasis has been established as a chronic inflammatory skin disorder that is mediated by the activation of abnormal lymphocytes. This notion is well supported by the fact that several immune modulators, including CsA, FK506 and methotrexate (MTX), are being successfully used to treat these patients. Since the evidence indicates that expressions of IL-2 and IFN-γ are increased while the expression of IL-4 is deceased in the involved skin of psoriasis patients, psoriasis is first considered to be a Th1 cell-mediated immune disease. However, the Th17 subset has recently been identified as an effector T cells that's involved in the pathogenesis of various inflammatory diseases such as psoriasis, systemic lupus erythematosus and inflammatory bowel diseases. Therefore, it is now recognized that both the Th1 and Th17 cell subsets are involved in the pathogenesis of psoriasis. In this study, we attempted to identify the differentially expressed genes in the PBMCs of psoriatic patients by using cDNA microarray analysis.
Although the results obtained in this study are not sufficient for dissecting the pathogenesis of psoriasis, our data provides important information on which to base further investigations on the pathologic events in psoriasis. For example, in this study, several up-regulated and/or down-regulated genes were identified, and their functional roles can be deduced from previous studies. One candidate is CD44, which may be involved in the homing process of activated lymphocytes from the blood to the inflamed skin. CD44 is a cell surface glycoprotein that plays important roles in cell-cell interaction and cell-matrix interaction through its affinity for hyaluronic acid (HA)13. CD44 is a typical receptor for HA and it can bind with osteoponin, collagens and matrix metalloproteinases (MMPs). The interaction of CD44 with its ligand regulates the lymphocyte activation and this leads to the migration of skin-homing T cells13,14. Another cell surface molecule CD56 was also identified as an up-regulated gene in the psoriatic PBMCs in this study. CD56 binds to fibroblast growth factor (FGF) and this leads to the stimulation of nerve cells growth. It is also involved in the skin-homing of natural killer (NK) cells15,16. Based on these observations, we speculate that the interactions of CD44/CD56 and their ligands may be important steps toward the lymphocytes homing from peripheral blood vessels to the inflamed skin. Moreover, in this study, the expression of NF-AT 5 and interleukin 7 receptor were highly increased in the psoriatic PBMCs. As noted, NF-AT 5 and IL-7 signaling through IL-7R regulates lymphocytes activation, proliferation and homing into the skin. Thus, our data strengthens the notion that up-regulation of these genes is related with the pathogenesis of psoriasis at the early stage.
Sphingosin kinase 1 and IκB kinase 2 were down-regulated in the psoriatic PBMCs as compared with that of the normal controls. As these kinases attenuate NF-κB activity, it could be speculated that the lymphocytes of psoriasis patient are more sensitive to inflammatory stimuli, and uncontrolled NF-κB activity prolongs the inflammatory reaction17. Additionally, several cell cycle regulators showed a lower expression in the PBMCs of the psoriasis patients. Therefore, it could be also assumed that the psoriasis patients' lymphocytes have different characteristics of cell cycle progression.
In summary, we investigated the gene expression profile in the PBMCs of psoriasis patients by conducting cDNA microarray analyses. Our results suggest a possible mechanism by which activated immune cells migrate from the blood to the skin in psoriatic patients. Accordingly, these findings extended our knowledge about the pathogenesis of psoriasis, and they may provide novel putative targets for developing drugs to treat the scourge of psoriasis.
References
- 1.Nickoloff BJ. Skin innate immune system in psoriasis: friend or foe? J Clin Invest. 1999;104:1161–1164. doi: 10.1172/JCI8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos JD, De Rie MA. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today. 1999;20:40–46. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 3.Werner S, Smola H. Paracrine regulation of keratinocyte proliferation and differentiation. Trends Cell Biol. 2001;11:143–146. doi: 10.1016/s0962-8924(01)01955-9. [DOI] [PubMed] [Google Scholar]
- 4.Jegasothy BV, Ackerman CD, Todo S, Fung JJ, Abu-Elmagd K, Starzl TE. Tacrolimus (FK 506)--a new therapeutic agent for severe recalcitrant psoriasis. Arch Dermatol. 1992;128:781–785. [PMC free article] [PubMed] [Google Scholar]
- 5.Takashima A, Morita A. Genomic, phenotypic, and functional analyses of T cells in patients with psoriasis undergoing systemic cyclosporin A treatment. J Invest Dermatol. 1991;96:376–382. doi: 10.1111/1523-1747.ep12466215. [DOI] [PubMed] [Google Scholar]
- 6.Asadullah K, Volk HD, Sterry W. Novel immunotherapies for psoriasis. Trends Immunol. 2002;23:47–53. doi: 10.1016/s1471-4906(01)02119-6. [DOI] [PubMed] [Google Scholar]
- 7.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H. Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol. 2004;135:1–8. doi: 10.1111/j.1365-2249.2004.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrone-Smith T, Nickoloff BJ. Dermal injection of immunocytes induces psoriasis. J Clin Invest. 1996;98:1878–1887. doi: 10.1172/JCI118989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehncke WH, Zollner TM, Dressel D, Kaufmann R. Induction of psoriasiform inflammation by a bacterial superantigen in the SCID-hu xenogeneic transplantation model. J Cutan Pathol. 1997;24:1–7. doi: 10.1111/j.1600-0560.1997.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 10.Li AG, Wang D, Feng XH, Wang XJ. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004;23:1770–1781. doi: 10.1038/sj.emboj.7600183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 12.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–168. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 13.Kugelman LC, Ganguly S, Haggerty JG, Weissman SM, Milstone LM. The core protein of epican, a heparan sulfate proteoglycan on keratinocytes, is an alternative form of CD44. J Invest Dermatol. 1992;99:886–891. doi: 10.1111/1523-1747.ep12614896. [DOI] [PubMed] [Google Scholar]
- 14.Golshani R, Lopez L, Estrella V, Kramer M, Iida N, Lokeshwar VB. Hyaluronic acid synthase-1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008;68:483–491. doi: 10.1158/0008-5472.CAN-07-2140. [DOI] [PubMed] [Google Scholar]
- 15.Carafoli F, Saffell JL, Hohenester E. Structure of the tandem fibronectin type 3 domains of neural cell adhesion molecule. J Mol Biol. 2008;377:524–534. doi: 10.1016/j.jmb.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottaviani C, Nasorri F, Bedini C, de Pita O, Girolomoni G, Cavani A. CD56brightCD16(-) NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. 2006;36:118–128. doi: 10.1002/eji.200535243. [DOI] [PubMed] [Google Scholar]
- 17.Nava VE, Lacana E, Poulton S, Liu H, Sugiura M, Kono K, et al. Functional characterization of human sphingosine kinase-1. FEBS Lett. 2000;473:81–84. doi: 10.1016/s0014-5793(00)01510-6. [DOI] [PubMed] [Google Scholar]