Abstract
Chemical modifications of nociceptin/orphanin FQ (N/OFQ) peptide that result in increased potency and resistance to degradation have recently lead to the discovery of [(pF)Phe4Aib7Arg14Lys15]N/OFQ-NH2 (UFP-112), a novel N/OFQ peptide (NOP) receptor agonist. The aim of this study was to investigate the pharmacological profile of intrathecally administered UFP-112 in monkeys under different behavioral assays. Intrathecal UFP-112 (1–10 nmol) dose-dependently produced antinociception against an acute noxious stimulus (50 °C water) and capsaicin-induced thermal hyperalgesia. Intrathecal UFP-112-induced antinociception could be reversed by a NOP receptor antagonist, J-113397 (0.1 mg/kg), but not by a classic opioid receptor antagonist, naltrexone (0.03 mg/kg). Like intrathecal morphine, UFP-112 produced antinociception in two primate pain models with a similar magnitude of effectiveness and a similar duration of action that last for 4–5 h. Unlike intrathecal morphine, UFP-112 did not produce itch/scratching responses. In addition, intrathecal inactive doses of UFP-112 and morphine produced significant antinociceptive effects when given in combination without increasing scratching responses. These results demonstrated that intrathecal UFP-112 produced long-lasting morphine-comparable antinociceptive effects without potential itch side effect. This study is the first to provide functional evidence that selective NOP receptor agonists such as UFP-112 alone or in conjunction with morphine may improve the quality of spinal analgesia.
Keywords: Nociceptin/orphanin FQ, NOP receptors, UFP-112, Spinal cord, Capsaicin, Morphine
1. Introduction
Spinal administration of morphine is one of the most common clinical procedures for pain relief because of its long-lasting analgesic effects [7,8]. However, itch/pruritus is characteristic of intrathecal morphine treatment, with reported incidence rates ranging from 30% to 100% in humans [36]. Such unique physiological functions of intrathecal morphine can also be observed in non-human primates. For example, a single antinociceptive dose of intrathecal morphine elicited profound long-lasting itch/scratching responses in monkeys [17]. Importantly, pharmacological studies using monkeys have demonstrated that opioid analgesic-induced scratching response is selectively mediated by mu opioid receptors (MOP) [18] and that kappa opioid receptor (KOP) agonists have the therapeutic potential as antipruritics under this context [20,25]. These findings support that clinically used drugs with low or moderate intrinsic efficacy on MOP or/and KOP are effective in alleviating opioid-induced itch [9,12,38]. Nevertheless, identification of novel targets as spinal analgesics devoid of MOP-induced pruritic effects still remains a challenge to the drug development.
Since the identification of the nociceptin/orphanin FQ (N/OFQ) peptide as the endogenous ligand of the N/OFQ peptide (NOP) receptor [26,33], this novel peptide receptor system has been implicated in the modulation of pain [23,39]. A variety of pain assays in rodents have shown the effect of intrathecally administered N/OFQ to be antinociceptive [11,29]. Peculiarly, unlike dual actions of intrathecal N/OFQ in rodents [13,35], intrathecal N/OFQ over a wide dose range only produced antinociceptive effects in monkeys [21]. More interestingly, using an established itch behavioral assay in monkeys [17], intrathecal N/OFQ produced dose-dependent antinociception without eliciting scratching responses [19,21]. These findings indicate that NOP receptor agonists may represent a promising target as spinal analgesics.
[(pF)Phe4Aib7Arg14Lys15]N/OFQ-NH2 (UFP-112) is a recently designed NOP receptor agonist that results from chemical modifications to the N/OFQ peptide by increasing its agonist potency and decreasing its susceptibility to peptidase actions [1,34]. In rodent assays investigating a variety of physiological functions, UFP-112 consistently mimicked the effects of N/OFQ with markedly higher potency and longer duration of action [6]. Given that intrathecal morphine-induced antinociception only last for 1–2 h in rodents [4,27] and the duration of antinociception can be distinguished between intrathecal morphine (4–5 h) and N/OFQ (2–3 h) in monkeys [19], it is important to examine whether the high potency and long duration of action of UFP-112 can translate to non-human primates in the absence of an itch side effect.
Therefore, the aim of this study was to investigate the pharmacological profile of intrathecally administered UFP-112 in monkeys. Of chief concern were the two behavioral endpoints of antinociception and scratching. The potency and duration of intrathecal UFP-112- and morphine-induced antinociceptive effects were compared using both monkey models of acute nociception and capsaicin-induced hyperalgesia. Antagonist studies were conducted to determine the receptor mechanism underlying UFP-112-induced effects. In addition, the drug combination study was conducted to explore whether intrathecal UFP-112 in conjunction with morphine produced antinociception with less scratching responses.
2. Materials and methods
2.1. Subjects
Eighteen adult female and male rhesus monkeys (Macaca mulatta) ranging in body weight (7.1–12.1 kg) were used. The monkeys were individually housed and their daily diet consisted of approximately 25–30 biscuits (Purina Monkey Chow; Ralston Purina Co., St. Louis, MO), fresh fruit, and free access to water. All monkeys used were previously trained in the warm water tail-withdrawal assay and acclimated to being video-recorded in-cage. For 1 month prior to the study, the monkeys did not have exposure to any opioid compound. Six monkeys (three males and three females) participated in the first two parts of the study (see details in Section 2.3). Another six monkeys (two males and four females) participated in the third part of the study. The remaining six monkeys (three males and three females) were used in the last part of the study. The monkeys were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies were conducted in accordance with the University Committee on the Use and Care of Animals in the University of Michigan (Ann Arbor, MI) and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health (Bethesda, MD).
2.2. Procedures
2.2.1. Nociceptive responses
2.2.1.1. Acute thermal nociception
The warm water tail-withdrawal assay was used to measure nociceptive responses to thermal stimuli and antinociceptive effects of test compounds [16]. Monkeys were seated in primate-restraining chairs, which allowed access to their shaved backs and exposure of their shaved tails (approximately 15 cm) to thermal flasks containing water maintained at 42, 46, or 50 °C. Forty-two and 46 °C water were used as normally non-noxious stimuli whereas 50 °C water was used as an acute noxious stimulus. If monkeys did not remove their tails within 20 s, the flask was removed and a maximum time of 20 s was recorded. Test session began with control determinations at each temperature. Then, tail-withdrawal latencies were determined at multiple time points after intrathecal administration of the test compound.
2.2.1.2. Capsaicin-induced hyperalgesia
Capsaicin 0.1 mg was administered subcutaneously in the terminal 5 cm of the unanesthetized monkey’s tail. The monkey’s tail waggled within the first 2 min after capsaicin administration. The 15 min after administration was the time of peak hyperalgesic effects of capsaicin and it was the time point to measure the tail-withdrawal latency in 46 °C water in order to evaluate antihyperalgesic effects of test compounds [16,22]. This hyperalgesic response was manifested as a reduced tail-withdrawal latency from a maximum value of 20 s to approximately 2–3 s in 46 °C water. The test compounds were administered at different time points before capsaicin administration. As noted, all behavioral responses were measured by individuals blinded to experimental conditions.
2.2.2. Itch/scratching responses
Monkeys were recorded in-cage for scratching behavior, which has been previously associated to an itch sensation [18]. Recording was done in 15-min intervals and scored by trained individuals blinded to experimental conditions. A scratch was counted and defined as a short (≤1 s) episode of scraping contact of the monkey’s forepaw or hind paw on the skin surface of other body parts. Scratches often occurred by a hind paw repetitively at the same location. In addition, monkeys were monitored for sedation and muscle relaxation while in their home cages as previous studies [19,21,25].
2.3. Experimental design
The first part of the study was to determine and compare the behavioral responses of intrathecally administered UFP-112 (1–10 nmol) and morphine (100 nmol) along with corresponding dose-dependent effects. The dose range was selected based on previous studies and our pilot study [19,34]. Tail-withdrawal latency measurements were made at hour intervals for the duration of a 5-h time course following the intrathecal injection. The tail-withdrawal latencies at 42 and 46 °C were used to detect potential hyperalgesic/pronociceptive effects whereas the 50 °C latencies were used to measure antinociceptive effects. Recording for scratching observation was also done at 1 h intervals for the duration of a 5-h time course after each session of tail-withdrawal latency measurements. For clarification, the antinociceptive effects were measured during the 25th to 40th min of each hour. Subsequently, monkeys were returned to their home cages and scratching responses were recorded during the 45th to 60th min of each hour. Monkeys were monitored for their general motor functions such as gait and balance during each transition between their home cages and the procedure room.
The second part of the study was to determine the receptor mechanism underlying intrathecal UFP-112-induced antinociception. A single dose of 0.03 mg/kg naltrexone and 0.1 mg/kg J-113 397 (1-[3R,4R/3S,4S)-1-(Cyclooctylmethyl)-3-(hydroxymethyl)-4-piperidinyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one) was used to compare their antagonist effects against both morphine (100 nmol)- and UFP-112 (10 nmol)-induced antinociception. The doses for both naltrexone and J-113397 were chosen based on a recent study [22] showing that both antagonists produced similar degrees of rightward shifts of dose–response curves of MOP and NOP agonists, respectively. The dose of J-113397 did not produce pronociceptive effects by itself [19,22].
The third part of the study was to assess the potency and duration of antihyperalgesia of intrathecally administered UFP-112 and morphine against capsaicin. Transient receptor potential vanilloid subfamily member 1 (TRPV1) is a transduction molecule for noxious stimuli. Capsaicin elicits pain sensation by activating TRPV1 and it has been used in monkeys [5,16] and humans [10,32] to study experimental analgesics in a broader therapeutic-relevant context. Given that TRPV1-containing nerve fibers are involved in various pain origins, it is essential to determine and compare the antinociceptive effectiveness of intrathecal UFP-112 and morphine in monkeys receiving capsaicin. The drug was administered intrathecally 1 h prior to administration of capsaicin. Then, tail-withdrawal latencies were measured 15 min following capsaicin administration. The dose–response curves of intrathecal UFP-112 (0.3–10 nmol) and morphine (3–100 nmol) were established by using a single dosing procedure. In addition, intrathecal UFP-112 (10 nmol) and morphine (100 nmol) were used to determine their durations of antinociception at different time points before capsaicin administration.
The last part of the study was to investigate whether combination of inactive doses of intrathecal UFP-112 (1 nmol) and morphine (3 nmol) produced increased antihyperalgesia with less scratching responses. UFP-112, morphine, or the combination of UFP-112 and morphine was administered intrathecally 1 h before capsaicin administration. For scratching measurement, the same dosing conditions were conducted separately in the same subjects without capsaicin administration. In addition, antagonist studies were conducted to investigate the receptor mechanism underlying antihyperalgesia by the mixture of intrathecal UFP-112 (1 nmol) and morphine (3 nmol). A single dose of 0.03 mg/kg naltrexone or/and 0.1 mg/kg J-113397 were administered 30 min after administration of the mixture to determine the degree of antagonist effects.
2.4. Data analysis
Mean values (mean ± SEM) were calculated from individual values for all behavioral endpoints. As noted, we did not find a significant difference in nociceptive responses or effects of drugs between male and female monkeys, so mean values for all monkeys in the same dosing condition were used for data analysis. Measurement differences were compared across all tests sessions in the same experiment. Data were analyzed by using two-way analysis of variance followed by the Newman–Keuls test for multiple comparisons. Comparisons of data at a single time point were conducted by using one-way analysis of variance followed by the Dunnett test for multiple comparisons. The criterion for significance for all tests was set at p < 0.05.
2.5. Drugs
UFP-112 (synthesized and purified as described by Arduin et al. [1] at the University of Ferrara, Ferrara, Italy), morphine sulfate (Mallinckrodt, Hazelwood, MO), (±)J-113397 (1-[3R,4R/3S,4S)-1-(Cyclooctylmethyl)-3-(hydroxymethyl)-4-piperidinyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one) (Tocris Bioscience, Ellisville, MO), and naltrexone HCl (National Institute on Drug Abuse, Bethesda, MD) were dissolved in sterile water. Capsaicin (Sigma, St. Louis, MO) was dissolved in a solution of ethanol/Tween 80/saline in a ratio of 1:1:8. Intrathecal doses were administered in a random order at a total volume of 1 mL. A detailed description of the intrathecal drug delivery procedure has been previously described [18]. All experiments (i.e., all four parts of the study) using intrathecal administration were conducted with a 10-day inter-injection interval as previous studies did [18–21].
3. Results
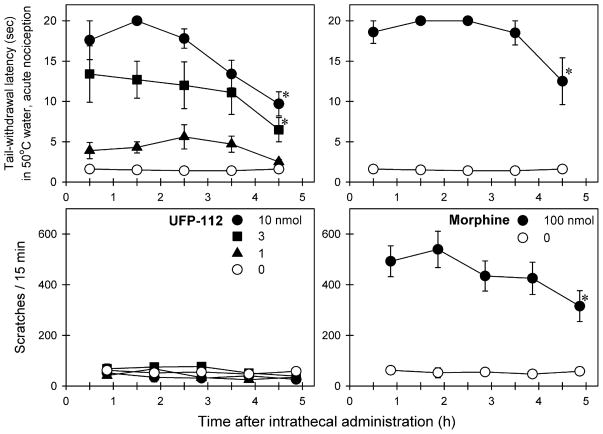
Fig. 1 compares the behavioral responses of intrathecally administered UFP-112 and morphine in monkeys. Intrathecal UFP-112 produced antinociception against acute nociceptive stimulus, 50 °C water, in both dose-[F(3, 15) = 33.7; p < 0.05] and time [F(4, 20) = 7.8; p < 0.05]-dependent manners. Post hoc comparisons indicated that both 3 and 10 nmol of intrathecal UFP-112 produced significant antinociception between 0.5- and 4.5-h time points. Although intrathecal UFP-112 produced long-lasting antinociception, it did not elicit significant scratching responses [F(3, 15) = 2.3; p = 0.1] at any dose or time point. In contrast, 100 nmol of intrathecal morphine produced both antinociception and scratching during the entire 5-h session (p < 0.05). Administration of either UFP-112 or morphine at these doses did not cause any overt side effects including sedation and muscle relaxation. In addition, there was no motor dysfunction detected during monkeys’ transition between their home cages and the procedure room.
Fig. 1.
Comparison of behavioral effects produced by intrathecal administration of UFP-112 and morphine. Abscissas: time in hours after intrathecal administration. Ordinates: latency to withdraw the tail in 50 °C water (top panels) and scratches per 15 min (bottom panels). Each value represents mean ± SEM (n = 6). Symbols represent different dosing conditions in the same monkeys. The asterisk represents a significant difference from the vehicle condition at corresponding time points (*p < 0.05).
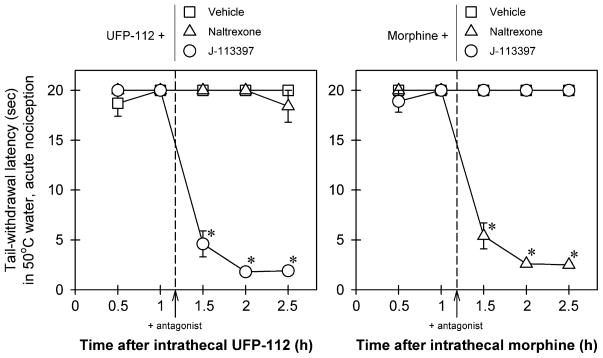
Fig. 2 illustrates the effects of antagonists on intrathecal UFP-112 (10 nmol)- and morphine (100 nmol)-induced antinociception in monkeys. For antagonist effects on UFP-112 antinociception (left panel), there were significant differences among the dosing conditions [F(2, 10) = 458.4; p < 0.05]. Post hoc comparisons indicated that a post-injection with a NOP receptor antagonist, J-113397 (0.1 mg/kg), significantly blocked intrathecal UFP-112-induced antinociception (p < 0.05). However, naltrexone 0.03 mg/kg failed to block intrathecal UFP-112-induced antinociception. For antagonist effects on morphine antinociception (right panel), there were significant differences among the dosing conditions [F(2, 10) = 673.1; p < 0.05]. Post hoc comparisons indicated that a post-injection with the same dose of an opioid receptor antagonist, naltrexone (0.03 mg/kg), significantly blocked intrathecal morphine-induced antinociception (p < 0.05). However, J-113397 (0.1 mg/kg) failed to block intrathecal morphine-induced antinociception.
Fig. 2.
Effects of antagonists on intrathecal UFP-112 and morphine-induced antinociception in monkeys. The antagonist, either naltrexone (0.03 mg/kg) or J-113397 (0.1 mg/kg), was given subcutaneously 10 min after the 1-h time point. Abscissas: time in hours after intrathecal administration. Ordinates: latency to withdraw the tail in 50 °C water. Each value represents mean ± SEM (n = 6). Symbols represent effects with different post-injection conditions for the same monkeys. The asterisk represents a significant difference from the vehicle post-injection condition between time points 1.5 and 2.5 h (*p < 0.05).
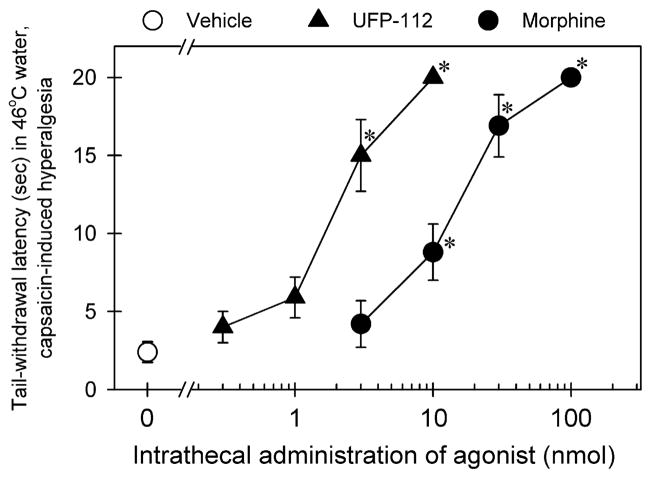
Fig. 3 compares the effectiveness and potency of intrathecal UFP-112- and morphine-induced antihyperalgesia. Pretreatment with UFP-112 [F(4, 25) = 36.5; p < 0.05] or morphine [F(4, 25) = 31.4; p < 0.05] dose-dependently attenuated capsaicin-induced hyperalgesia in 46 °C water. The ED50 (95% confidence limit) values of intrathecal UFP-112 and morphine were 2.0 (1.2–3.4) and 11.8 (4.7–29.5) nmol, respectively. Post hoc comparisons indicated that 3–10 nmol of UFP-112 or 10–100 nmol of morphine significantly produced antihyperalgesia as compared with the vehicle condition under this context (p < 0.05).
Fig. 3.
Antinociceptive effects of intrathecally administered UFP-112 and morphine against capsaicin-induced hyperalgesia in 46 °C water. The agonist was given intrathecally 1 h before administration of capsaicin (0.1 mg/tail). Each data point was determined 15 min after capsaicin administration and represents mean ± SEM (n = 6). Symbols represent effects with different dosing conditions in the same monkeys. The asterisk represents a significant difference from the vehicle condition (*p < 0.05).
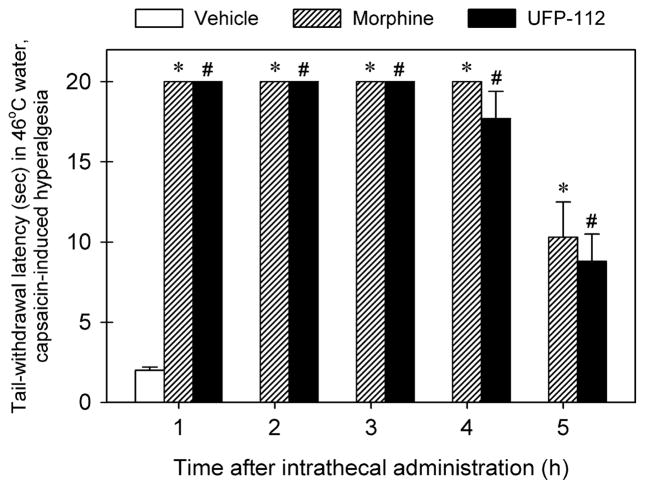
Fig. 4 illustrates the duration of antihyperalgesia of intrathecal UFP-112 and morphine. A single dose 10 nmol of intrathecal UFP-112 produced antihyperalgesia in a time-dependent manner [F(5, 30) = 59.7; p < 0.05]. A single dose 100 nmol of intrathecal morphine also produced antihyperalgesia time-dependently [F(5, 30) = 71.4; p < 0.05]. Both intrathecal UFP-112 and morphine had similar durations of antihyperalgesia, i.e., full antihyperalgesia at the 4-h time point followed by partial antihyperalgesia at the 5-h time point (p < 0.05).
Fig. 4.
Comparison of durations of intrathecal UFP-112- and morphine-induced antihyperalgesia in 46 °C water. The antihyperalgesic effect of the agonist, either morphine (100 nmol) or UFP-112 (10 nmol), was determined by using a single dosing procedure. The agonist was given intrathecally at different time points before administration of capsaicin (0.1 mg/tail). Each data point was determined 15 min after capsaicin administration and represents mean ± SEM (n = 6). Symbols represent effects with different dosing conditions for the same monkeys. The asterisk represents a significant difference between morphine-treated condition and the vehicle condition (*p < 0.05). The symbol # represents a significant difference between UFP-112-treated condition and the vehicle condition (*p < 0.05).
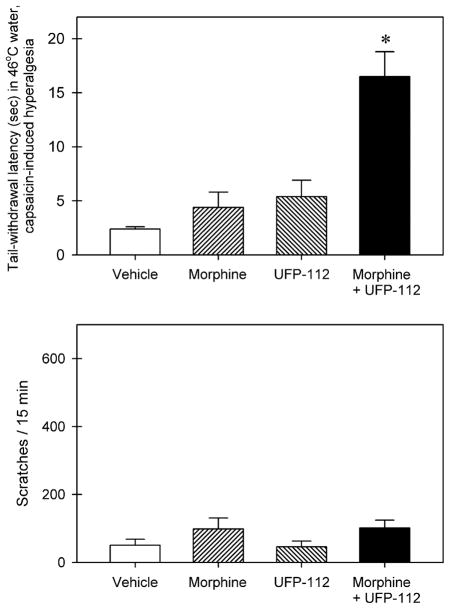
Fig. 5 illustrates behavioral responses of intrathecal UFP-112 in combination with morphine. The top panel shows the antihyperalgesic effects of intrathecal UFP-112 (1 nmol), morphine (3 nmol), and the combination of UFP-112 and morphine against capsaicin. There were significant differences among the dosing conditions [F(3, 20) = 16.9; p < 0.05]. Intrathecal UFP-112 or morphine alone did not produce statistically significant effects. In contrast, intrathecal UFP-112 in combination with morphine significantly produced antihyperalgesia (p < 0.05). The bottom panel shows the scratching–eliciting effects of intrathecal UFP-112 (1 nmol), morphine (3 nmol), and the combination of UFP-112 and morphine. There was no significant difference among the dosing conditions [F(3, 20) = 1.6; p = 0.2]. Intrathecal UFP-112 in combination with morphine did not increase scratching responses.
Fig. 5.
Behavioral responses of intrathecally administered UFP-112 in combination with morphine. Effects of intrathecal morphine (3 nmol) or UFP-112 (1 nmol) alone were determined separately. Then effects of intrathecal administration of a mixture, i.e., 3 nmol of morphine combined with 1 nmol of UFP-112, were tested in the same monkeys. Each value represents mean ± SEM (n = 6). The asterisk represents a significant difference from the vehicle condition (*p < 0.05).
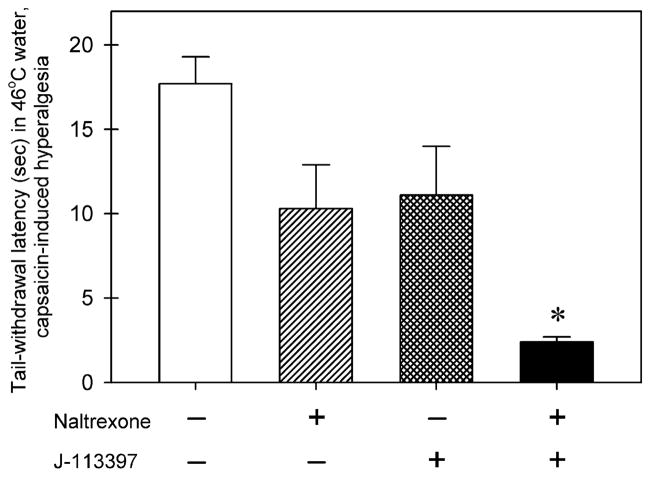
Fig. 6 compares the effects of antagonists on antihyperalgesia produced by intrathecal combination of UFP-112 (1 nmol) and morphine (3 nmol) (i.e., a mixture). There were significant differences among the dosing conditions [F(3, 20) = 8.8; p < 0.05]. Post-injection with naltrexone (0.03 mg/kg) or J-113397 (0.1 mg/kg) alone did not produce a significant blockade of intrathecal mixture-induced antihyperalgesia. However, combined systemic administration of naltrexone and J-113397 significantly blocked intrathecal mixture-induced antihyperalgesia (p < 0.05).
Fig. 6.
Effects of antagonists on antihyperalgesia by intrathecal combination of UFP-112 (1 nmol) and morphine (3 nmol) (i.e., a mixture). The antagonist, either naltrexone (0.03 mg/kg) or J-113397 (0.1 mg/kg), was given subcutaneously 30 min after intrathecal administration of a mixture. The symbol “+” indicates the corresponding compound was given. The symbol “−” indicates the corresponding was not given. Each value represents mean ± SEM (n = 6). The asterisk represents a significant difference from the vehicle condition (*p < 0.05).
4. Discussion
This study demonstrated that intrathecal administration of UFP-112 produced long-lasting morphine-comparable antinociception against both acute pain and capsaicin-induced hyperalgesia. Intrathecal UFP-112-induced antinociception was not accompanied by itch/scratching responses and its action was exclusively mediated by NOP receptor activation. In addition, combination of inactive doses of intrathecal UFP-112 and morphine produced antihyperalgesia without scratching responses. This study is the first to provide direct functional evidence and translational value in primates that NOP receptor agonists such as UFP-112 alone or in conjunction with morphine will improve the quality of spinal analgesia.
As previously reported, intrathecal N/OFQ produced antinociception for 2–3 h in monkeys [19,21]. The duration of intrathecal N/OFQ-induced antinociception is significantly shorter than intrathecal morphine-induced antinociceptive effects that have at least 4–5 h in primates [17,19]. With improved resistance to enzymatic degradation, a novel NOP receptor agonist, UFP-112 [1,34], produced antinociception that last for 4–5 h in the same experimental context. Similar longer durations of actions were obtained in rodent studies while the same effects elicited by N/OFQ lasted only for approximately 1 h [6,34]. Furthermore, UFP-112 (10 nmol) is approximately 10-fold more potent than N/OFQ (i.e., 100 nmol) [19] in producing full antinociceptive effects in monkeys. This potency ratio between intrathecal UFP-112- versus N/OFQ-induced antinociception is consistent with rodent studies showing that UFP-112 displayed 10- to 100-fold higher potency than N/OFQ in a variety of rodent in vivo assays [6]. To our knowledge, UFP-112 is the first reported peptide that has such a long-lasting antinociceptive action comparable to morphine in a primate species. It will be interesting to further conduct pharmacokinetic studies comparing the CSF levels of UFP-112 and morphine following intrathecal administration.
The pharmacological profile of intrathecal morphine in producing antinociception with profound itch/pruritic effect is well known in the clinical setting [2,3,31]. Interestingly, such unique effects of intrathecal morphine in humans [2,31] can be modeled in monkeys, but not in rodents, as a single dose of morphine produced both antinociception and itch/scratching responses simultaneously in monkeys [17,20,24]. For studying opioid analgesics in vivo, the scratching response can be used as a selective behavioral endpoint corresponding to activation of MOP [18,25]. Given that pruritus is a long standing side effect associated with the use of intrathecal morphine [7,8], lack of scratching responses by intrathecal UFP-112 in monkeys strongly suggests that UPF-112 has the therapeutic potential as a spinal analgesic.
The results of the antagonist study on intrathecal UFP-112 mirrored those of antagonist studies on intrathecal N/OFQ [19]. Naltrexone, a classical opioid receptor antagonist, failed to block the antinociception produced by UFP-112, indicating that the actions of intrathecal UFP-112 are not mediated by classic opioid receptor subtypes. In contrast, J-113397, a selective NOP receptor antagonist [14,30], significantly blocked the antinociceptive effects of intrathecal UFP-112, but not morphine. The dose 0.1 mg/kg of J-113397 has been previously used to provide a large rightward shift (~10- to 30-fold) of the dose–response curve to the non-peptide NOP receptor agonist Ro 61–6198 in monkeys [22]. Intrathecal UFP-112-induced antinociception can be fully reversed by J-113397, demonstrating that the antinociceptive action of UFP-112 in monkeys is due to selective NOP receptor activation. These data confirm and extend to non-human primates the high selectivity of NOP action of UFP-112 that has been previously demonstrated in rodents. In fact, all the in vitro and in vivo actions of UFP-112 (and N/OFQ) in rats and mice are sensitive to the NOP selective antagonist UFP-101 and no longer present in the NOP receptor knockout mice [6].
While the acute noxious stimulus stands as a convenient pain model to test experimental analgesics, this study further demonstrated that intrathecal UFP-112 alleviated capsaicin-induced thermal hyperalgesia in monkeys. Capsaicin is a natural irritant found in hot-chili peppers that evokes pain sensation by activating at the TRPV1. TRPV1 and the up-regulation of its expression have been implicated in the transduction of a variety of noxious stimuli including tissue-injury induced thermal hyperalgesia, diabetic neuropathy, and neurogenic inflammatory response associated with many disease states [15,37]. Furthermore, capsaicin-induced hyperalgesia has been previously utilized as a pain model in both monkeys [5] and humans [10,32] to study experimental compounds as analgesics. Considering the variety of pain modalities capsaicin-sensitive fibers are linked to, the UFP-112’s ability to attenuate capsaicin-induced hyperalgesia would suggest a prominent clinical value.
The dose–response curves established under the capsaicin pain model illustrated that intrathecal UFP-112 was more potent than morphine. Importantly, the results illustrated equally maximum antihyperalgesic effect for both UFP-112 and morphine. A key characteristic of antinociceptive effects of NOP receptor agonists previously studied in both rodents and primates was a relatively short duration of action (2–3 h) compared to morphine (4–5 h) [19,21,28,34]. Then, it is clinically promising that, not only did UFP-112 produce long-lasting antinociception against acute thermal nociception, the time course of intrathecal UFP-112-induced antihyperalgesia essentially matched that of intrathecal morphine. Like intrathecal morphine, UFP-112 produced antinociception in two primate nociceptive models with a similar magnitude of effectiveness and a similar duration of action. Unlike intrathecal morphine, UFP-112 did not produce scratching responses. These findings together suggest that similar antinociceptive effects can be produced by two independent receptor mechanisms in the spinal cord of monkeys.
It is interesting to observe that when an inactive dose of intrathecal UFP-112 was combined with that of morphine, such a mixture produced significant antihyperalgesia. Such effects could be antagonized by combined administration of naltrexone and J-113397. Importantly, given its independent receptor mechanism for antinociception, UFP-112 was able to do so without increasing or decreasing the itch side effect. This finding is another supporting evidence to the therapeutic potential of UFP-112 as a spinal analgesic in both active and inactive doses as methods to alleviate morphine-induced itch while maintaining antinociception. It would be interesting to further investigate whether UFP-112 or other NOP agonist is able to additively or synergistically potentiate morphine-induced antinociception. Nevertheless, a recent study has demonstrated that N/OFQ enhanced intrathecal morphine-induced antinociception without producing motor-related side effects [21]. These findings along with this study suggest that intrathecal administration of a mixture of morphine with UFP-112 may produce antinociception with much less pruritic side effects.
In summary, this study reveals a promising functional profile of intrathecal UFP-112 in primates. Over the dose range of 1–10 nmol, intrathecal UFP-112 potently produced antinociceptive effects that were longer lasting than other NOP receptor agonists and comparable to those of intrathecal morphine. The antinociceptive effects were active against both acute nociception and capsaicin-induced hyperalgesia, providing support for its clinical value. Importantly, in all behavioral assays conducted, intrathecal UFP-112 produced antinociceptive effects without an itch side effect. Along with the finding that an inactive dose of UFP-112 in combined with morphine produced antihyperalgesia without itch/scratching, these results strongly suggest that UFP-112 has potential as a therapeutic spinal analgesic candidate for future clinical trials.
Acknowledgments
We thank Justin Knorr and Timothy Zdrodowski for technical assistance of data collection. This study was supported by U.S. Department of Defense, Peer Reviewed Medical Research Program, Grant W81XWH-07-1-0162 and Taiwan National Science Council Grant NSC-97-2628-H-004-089-MY2.
Footnotes
Conflicts of interest
All authors declare that they have no conflicts of interest.
References
- 1.Arduin M, Spagnolo B, Calo G, Guerrini R, Carra G, Fischetti C, Trapella C, Marzola E, McDonald J, Lambert DG, Regoli D, Salvadori S. Synthesis and biological activity of nociceptin/orphanin FQ analogues substituted in position 7 or 11 with Cα,α-dialkylated amino acids. Bioorg Med Chem. 2007;15:4434–43. doi: 10.1016/j.bmc.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 2.Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. Dose–response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. doi: 10.1097/00000542-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Baraka A, Noueihid R, Hajj S. Intrathecal injection of morphine for obstetric analgesia. Anesthesiology. 1981;54:136–40. doi: 10.1097/00000542-198102000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Buerkle H, Yaksh TL. Comparison of the spinal actions of the mu-opioid remifentanil with alfentanil and morphine in the rat. Anesthesiology. 1996;84:94–102. doi: 10.1097/00000542-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–63. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- 6.Calo’ G, Rizzi A, Cifani C, Micioni Di Bonaventura MV, Regoli D, Massi M, Salvadori S, Lambert DG, Guerrini R. UFP-112 a potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. CNS Neurosci Ther. doi: 10.1111/j.1755–5949.2009.00107.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- 8.DeBalli P, Breen TW. Intrathecal opioids for combined spinal–epidural analgesia during labour. CNS Drugs. 2003;17:889–904. doi: 10.2165/00023210-200317120-00003. [DOI] [PubMed] [Google Scholar]
- 9.Dunteman E, Karanikolas M, Filos KS. Transnasal butorphanol for the treatment of opioid-induced pruritus unresponsive to antihistamines. J Pain Symptom Manage. 1996;12:255–60. doi: 10.1016/0885-3924(96)00154-6. [DOI] [PubMed] [Google Scholar]
- 10.Eisenach JC, Hood DD, Curry R, Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86:1279–87. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Erb K, Liebel JT, Tegeder I, Zeilhofer HU, Brune K, Geisslinger G. Spinally delivered nociceptin/orphanin FQ reduces flinching behaviour in the rat formalin test. Neuroreport. 1997;8:1967–70. doi: 10.1097/00001756-199705260-00034. [DOI] [PubMed] [Google Scholar]
- 12.Gunter JB, McAuliffe J, Gregg T, Weidner N, Varughese AM, Sweeney DM. Continuous epidural butorphanol relieves pruritus associated with epidural morphine infusions in children. Paediatr Anaesth. 2000;10:167–72. doi: 10.1046/j.1460-9592.2000.00447.x. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Okuda-Ashitaka E, Minami T. Central and peripheral roles of prostaglandins in pain and their interactions with novel neuropeptides nociceptin and nocistatin. Neurosci Res. 2001;41:299–332. doi: 10.1016/s0168-0102(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one (J-113397) J Med Chem. 1999;42:5061–3. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- 15.Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 Agonist) therapy for pain relief: farewell or revival? Clin J Pain. 2008;24:142–54. doi: 10.1097/AJP.0b013e318158ed9e. [DOI] [PubMed] [Google Scholar]
- 16.Ko MC, Butelman ER, Woods JH. The role of peripheral mu opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:150–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Ko MC, Naughton NN. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko MCH, Song MS, Edwards T, Lee H, Naughton NN. The role of central μ opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–76. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- 19.Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318:1257–64. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- 20.Ko MC, Husbands SM. Effects of atypical κ-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther. 2009;328:193–200. doi: 10.1124/jpet.108.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko MC, Naughton NN. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain. 2009;10:509–16. doi: 10.1016/j.jpain.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–96. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;8:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, Naughton NN, Woods JH, Ko MCH. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol. 2003;14:501–8. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Naughton NN, Woods JH, Ko MC. Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology. 2007;107:478–85. doi: 10.1097/01.anes.0000278876.20263.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Constantin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–5. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa T, Ikeda M, Kaneko T, Yamatsu K. Analgesic effects of dynorphin-A and morphine in mice. Peptides. 1985;6:75–8. doi: 10.1016/0196-9781(85)90079-8. [DOI] [PubMed] [Google Scholar]
- 28.Nazzaro C, Rizzi A, Salvadori S, Guerrini R, Regoli D, Zeilhofer HU, Calo G. UFP-101 antagonizes the spinal antinociceptive effects of nociceptin/orphanin FQ: behavioral and electrophysiological studies in mice. Peptides. 2007;28:663–9. doi: 10.1016/j.peptides.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Obara I, Przewlocki R, Przewlocka B. Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain. 2005;116:17–25. doi: 10.1016/j.pain.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H. In vitro and in vivo pharmacological characterization of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol. 2000;402:45–53. doi: 10.1016/s0014-2999(00)00520-3. [DOI] [PubMed] [Google Scholar]
- 31.Palmer CM, Emerson S, Volgoropolous D, Alves D. Dose–response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology. 1999;90:437–44. doi: 10.1097/00000542-199902000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Park KM, Max MB, Robinovitz E, Gracely RH, Bennett GJ. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–72. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- 33.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:92–4. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 34.Rizzi A, Spagnolo B, Wainford RD, Fischetti C, Guerrini R, Marzola G, Baldisserotto A, Salvadori S, Regoli D, Kapusta DR, Calo G. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides. 2007;28:1240–51. doi: 10.1016/j.peptides.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakurada C, Sakurada S, Katsuyama S, Sasaki J, Tan-No K, Sakurada T. Involvement of tachykinin NK1 receptors in nociceptin-induced hyperalgesia in mice. Brain Res. 1999;841:85–92. doi: 10.1016/s0006-8993(99)01800-4. [DOI] [PubMed] [Google Scholar]
- 36.Schug SA, Saunders D, Kurowski I, Paech MJ. Neuraxial drug administration: a review of treatment options for anaesthesia and analgesia. CNS Drugs. 2006;20:917–33. doi: 10.2165/00023210-200620110-00005. [DOI] [PubMed] [Google Scholar]
- 37.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1:10 years from channel cloning to antagonist proof of concept. Nat Rev Drug Discov. 2007;6:357–72. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama Y, Yokoyama T, Nagao Y, Nakagawa T, Magaribuchi T. Treatment of epidural morphine induced pruritus with butorphanol. Jpn J Anesth. 2009;58:178–82. [PubMed] [Google Scholar]
- 39.Zeilhofer HU, Calò G. Nociceptin/orphanin FQ and its receptor – potential targets for pain therapy? J Pharmacol Exp Ther. 2003;306:423–9. doi: 10.1124/jpet.102.046979. [DOI] [PubMed] [Google Scholar]