Abstract
Vitamin D has received increased attention recently for its pleiotropic actions on many chronic diseases. The importance of vitamin D on the regulation of cells of the immune system has gained increased appreciation over the past decade with the discovery of the vitamin D receptor (VDR) and key vitamin D metabolizing enzymes expressed by cells of the immune system. Animal studies, early epidemiologic and clinical studies have supported a potential role for vitamin D in maintaining immune system balance. The hormonal form of vitamin D up-regulates anti-microbial peptides, namely cathelicidin, to enhance clearance of bacteria at various barrier sites and in immune cells. Vitamin D modulates the adaptive immune system by direct effects on T cell activation and on the phenotype and function of antigen-presenting cells (APCs), particularly of DCs. The purpose of this manuscript is to review the molecular and clinical evidence for vitamin D as a modulator of the innate and adaptive immune system.
Keywords: Vitamins, Innate immunity, Immunology
Introduction
Vitamin D has received increased attention recently for its pleiotropic actions on many chronic diseases including cancer, cardiovascular disease, autoimmune disease, diabetes, and neurologic disease [1]. It has been reported that vitamin D regulates over 900 genes [2]. The importance of vitamin D on the regulation of cells of the immune system has gained increased appreciation over the past decade with the discovery of the vitamin D receptor (VDR) and key vitamin D metabolizing enzymes expressed by cells of the immune system. Animal studies, early epidemiologic and clinical studies have supported a potential role for vitamin D in maintaining immune system balance.
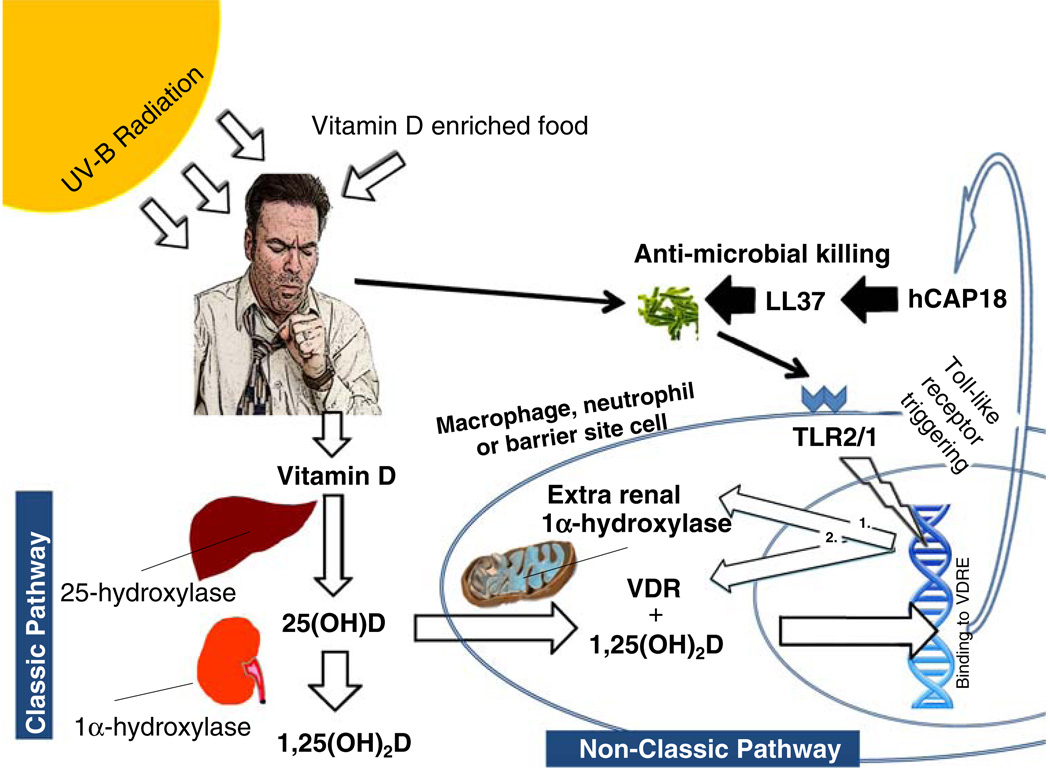
Vitamin D belongs to the family of steroid hormones and has its nuclear hormone receptor. Vitamin D has two major forms, cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Both forms of vitamin D (D2 and D3) can be found in foods or supplements; however, only vitamin D3 is produced in skin. Pre-vitamin D3 is formed from 7-dehydrocholesterol (also known as pro-vitamin D3) in skin upon exposure to ultraviolet B (UVB) radiation between the wavelengths of 290–315 nm. Pre-vitamin D3 rapidly undergoes a thermally induced isomerization to form vitamin D3. Further exposure of pre-vitamin D3 to UVB results in the formation of inactive vitamin D compounds which serves as a protective mechanism against vitamin D toxicity. Vitamin D3, that is formed in the skin, then enters the circulation bound to vitamin D binding protein where it undergoes a hydroxylation in the 25-position by the liver vitamin D-25-hydroxylase and in the 1-position by the kidney 25-hydroxyvitamin D-1-alpha-hydroxylase (1α-OHase) to form 1,25(OH)2D (Fig. 1). The hormonal form of vitamin D enters the target cell from the circulation and binds to the vitamin D receptor (VDR) in the cytoplasm which then enters the nucleus and heterodimerizes with the retinoid X receptor (RXR). The 1,25(OH)2D-RXR-VDR complex then binds to vitamin D response elements (VDRE) located on DNA.
Fig. 1.
Proposed mechanism for vitamin D’s action on the innate immune system. Vitamin D is produced in the skin upon exposure to UVB radiation from the sun or obtained from vitamin D containing foods. Vitamin D is converted to its major circulating form, 25-hydroxyvitamin D (25(OH)D), by the liver 25-hydroxylase and to 1,25-dihydroxyvitamin D (1,25(OH)2D) by the kidney 1-alpha-hydroxylase for optimal intestinal absorption of calcium in the classic vitamin D pathway. In the non-classic pathway of the immune system, the circulating 25(OH)D is taken up by macrophages, neutrophils or epithelial cells at locations exposed to the external environment. The 25(OH)D is converted to 1,25 (OH)2D in the target cell to act as an autocrine hormone. The locally produced 1,25(OH)2D binds to its nuclear receptor (VDR) and binds to the promoter of genes containing the vitamin D response element (VDRE). In neutrophils, macrophages and epithelial cells, this results in increased production of uncleaved cathelicidin (hCAP18 in humans) which undergoes further cleavage to the active cathelicidin (LL37 in humans) which results in killing of microorganisms. Of note, invading microorganisms that trigger specific toll-like receptors (in this example, TLR 2/1) result in increased production of the VDR and 1-alpha-hydroxylase which allows for vitamin D to enhance the production of cathelicidin only in the presence of adequate 25(OH)D substrate (adapted from Ref. [8])
The classic function of vitamin D is to enhance intestinal absorption of calcium by regulating several calcium transport proteins in the small intestine. However, other cells including cells of the immune system possess the 1α-OHase and VDR and thus are able to produce the hormonal form of vitamin D from circulating 25(OH)D and respond in an autocrine or paracrine fashion to form 1,25(OH)2D. It is important to note that the extra-renal 1α-hydroxylase is regulated differently in response to PTH, calcium and phosphorus than the renal 1α-hydroxylase [3]. In particular, the extra-renal 1α-hydroxylase is not up-regulated by PTH and thus production of 1,25(OH)2D is dependent on concentrations of the substrate 25(OH)D [4].
Recent evidence demonstrates that macrophages produce the anti-microbial peptide LL-37 in response to endogenously produced 1,25(OH)2D to enhance innate immunity. Additional evidence shows 1,25(OH)2D modulates the adaptive immune system as well through direct effects on T cell activation and on the phenotype and function of antigen-presenting cells (APCs), particularly of DCs. The purpose of this manuscript is to review the molecular actions of vitamin D in several aspects of the immune system with particular focus on the innate immune system and the adaptive immune system as it relates to autoimmune disease.
The role of vitamin D in the innate immune system
Historical background
The use of vitamin D as a treatment of infections has been practiced for over 150 years. In 1849, Williams reported favorable results with use of cod-liver oil, an excellent source of vitamin D3, in the treatment of over 400 patients with tuberculosis (TB) [5]. Williams commented that the cod-liver oil was a “highly nutrient material” and had properties superior to other oils. Fifty years later, Niels Finsen, received the third Nobel Prize in Medicine for his description of using UV light, an effective method to increase vitamin D status, to treat lupus vulgaris, a cutaneous form of TB, in over 800 patients [6]. A popular belief at the time was that UV light directly killed M. tuberculosis [7]. The use of cod-liver oil and UV light for the treatment of TB became widely used during the early 1900s. Following the discovery of the chemical structures of vitamin D2 and vitamin D3, found in cod-liver oil, by Nobel Laureate Alfred Windaus, several groups used vitamins D2 and D3 as a treatment for TB as reviewed by Martineau [8]. The introduction of more effective anti-TB therapy and the unidentified mechanism of vitamin D action led to decreased use and interest in vitamin D therapy against TB infection in the mid 1900s. In the 1980s, Rook et al. demonstrated that 1,25(OH)2D3 inhibited the proliferation of M. tuberculosis in culture; however, the mechanism still remained obscure [9]. As reviewed below, it is currently believed that vitamin D enhances innate immunity by up-regulating anti-microbial peptides such as cathelicidin in response to infection [10].
Vitamin D and anti-microbial peptides
The innate immune system serves as the first barrier of defense against invading microorganisms such as bacteria, viruses, protozoa, and fungi [11]. The first task of the innate immune system is to recognize foreign organisms and to trigger a cascade of events that ultimately result in the removal and/or destruction of the invading organism. Pattern recognition receptors are expressed by cells of the innate immune system to recognize molecular patterns that are conserved among different classes of pathogens. These conserved patterns are termed pathogen-associated molecular patterns (PAMPs) [12]. Examples of PAMPs include lipopolysaccharide (LPS), flagellin, viral proteins and single- and double-stranded RNA. Toll-like receptors (TLRs) are a sub-class of pattern recognition receptors that are expressed primarily on the cell membrane or on endosomes [11]. The innate immune system response depends on the specific TLR and/or combination of TLRs that are triggered by PAMPs. The response to TLR signaling includes the production of anti-microbial peptides and cytokines and apoptosis of the host cells among other responses [12]. There are three major families of anti-microbial peptides in humans: cathelicidin, and α- and β-defensins.
Humans have only one cathelicidin, hCAP18, which is cleaved to form LL-37 [13]. Cells of the immune system including neutrophils and macrophages and cells lining epithelial surfaces that are constantly exposed to potential pathogens such as the skin [14], respiratory tract [15], and gastrointestinal tract [16] produce cathelicidin. Cathelicidin has broad anti-microbial activity against gram-positive and -negative bacteria, as well as certain viruses and fungi [13]. The killing mechanism of cathelicidin involves bacterial lysis through membrane destabilization [17]. Humans with deficient cathelicidin production and cathelicidin knock-out mice are prone to infections of epithelial surfaces such as the skin and mucosal membranes. Therefore, anti-microbial peptides such as cathelicidin constitute an integral part of the innate immune response to a variety of infections especially at barrier sites.
Wang et al. provided one of the earliest studies that suggested that vitamin D could up-regulate the production of anti-microbial peptides [18]. They demonstrated that 1,25(OH)2D3 treatment up-regulated cathelicidin mRNA in several cell lines and primary cultures including keratinocytes, neutrophils, and macrophages [18]. Furthermore, they reported the presence of the vitamin D response element (VDRE) on the promoter of genes coding the anti-microbial peptides cathelicidin and β-defensin [18]. Active vitamin D was able to induce cathelicidin mRNA in all cell lines tested whereas in contrast β-defensin was inducible only in primary keratinocytes and in lung adenocarcinoma cells [18]. The presence of the VDRE in the cathelicidin gene promoter is highly conserved in humans and primates and not present in non-primate animals, suggesting an important recent adaptation in evolution [19]. Gombart et al. have also confirmed that 1,25(OH)2D3 up-regulates cathelicidin expression in several other human cell lines and primary cultures including those derived from skin, macrophages, neutrophils, lung, and colon [20, 21]. Taken together, these findings suggest that 1,25(OH)2D3 up-regulates anti-microbial peptide production, primarily cathelicidin, on a variety of different cells.
More recently, Liu et al. demonstrated that toll-like receptor stimulation with TLR2/1L of human macrophages resulted in up-regulation of the vitamin D receptor (VDR) and 25-hydroxyvitamin D-1-alpha-hydroxylase (1α-OHase or CYP27B1), the enzyme responsible for the conversion of 25(OH)D to 1,25(OH)2D [10]. Furthermore, 1,25(OH)2D3 up-regulated expression of cathelicidin mRNA in the presence of TLR2/1 L stimulation of human macrophage cultures. In cell viability assays of macrophages infected with M. tuberculosis, increasing concentrations of cathelicidin resulted in killing of intra-cellular M. tuberculosis [10]. When human sera from vitamin-D-deficient African-American subjects were added to human macrophage cultures stimulated by TLR2/1, no up-regulation of cathelicidin was detected; however, supplementation of the sera with 25(OH)D restored the cathelicidin response [10]. Therefore, the investigators propose a model in which M. tuberculosis triggering of toll-like receptors results in up-regulation of the vitamin D machinery, namely the VDR and the 1α-OHase, which leads to enhanced cathelicidin production only in the presence of adequate vitamin D status [10]. A similar reaction occurs in skin in response to injury. When skin is injured, there is up-regulation of CYP27B1 and cathelicidin [22]. In the presence of low 25(OH)D substrate or when either the VDR or CYP27B1 is inhibited in vitro, there is no longer up-regulation of cathelicidin. These studies indicate that 25(OH)D, the major form circulating form of vitamin D to determine vitamin D status, is important for local production of the hormonal form of vitamin D, 1,25(OH)2D, to up-regulate cathelicidin production in skin and in macrophages. Since keratinocytes also possess the 25-hydroxylase, UV light may directly stimulate cathelicidin production by providing the substrate 25(OH)D directly from cutaneously produced vitamin D3 [23].
Epidemiologic and clinical associations between vitamin D status and infection
A number of epidemiologic studies using vitamin D status or season as the exposure have found an inverse association between vitamin D and incidence of several infections, including influenza [24], upper respiratory tract infection [25–27], HIV infection [28], and bacterial vaginosis [29]. Recent cross-sectional studies have attempted to determine whether vitamin D status was associated with serum levels of cathelicidin. Jeng et al. examined a cohort of critically ill patients with and without sepsis and healthy controls and found a weak but statistically significant positive association between serum 25(OH)D and LL-37 [30]. Gombart et al. found that serum hCAP18 was associated with increased mortality from infection in patients with end-stage renal disease [31]. They did not find an association between 25(OH)D and hCAP18 likely due to the finding that 80% of the subjects had vitamin D insufficiency; however, there was a borderline (p=0.053) association between serum 1,25(OH)2D and hCAP18 [31].
Several randomized controlled trials have been conducted to examine whether vitamin D supplementation would reduce the risk of disease from viral, bacterial, fungal and protozoan infections [32]. However, given the heterogeneity in the dose, sample population, and duration of vitamin D therapy of the trials reviewed, there was insufficient data to conclusively state that vitamin D supplementation could result in lowered infection rate [32]. A meta-analysis comparing 25(OH)D concentrations in TB-infected subjects to healthy controls found a higher risk of vitamin D deficiency in TB-infected subjects [33]. Recent studies have been inadequate in dosing of vitamin D, including a large randomized controlled trial of TB-infected patients where both control and vitamin D treatment groups had similar 25(OH)D concentrations at the end of the study [34]. To determine whether vitamin D supplementation could raise cathelicidin levels in humans, Adams et al. gave osteoporotic women 50,000 IU of vitamin D2 twice weekly for 5 weeks and found no change in serum cathelicidin levels; however, cathelicidin mRNA expression in peripheral blood monocytes was increased after high-dose vitamin D supplementation [35]. Larger doses and more rapid dosing of vitamin D are likely required to up-regulate cathelicidin expression in response to infection in humans [36]. Furthermore, levels of cathelicidin are likely to be induced in barrier sites as opposed to in the systemic circulation for localized infections.
Vitamin D, innate immunity, and evolution
Several investigators have proposed that as early man migrated from the equator to higher latitudes, there was a selection pressure to de-pigment skin to maximize cutaneous production of vitamin D [37, 38]. Vitamin D deficiency leads to a rachitic pelvis increasing the risk of death in childbirth [39]. The discovery of a Homo erectus skull in Western Turkey with the earliest reported findings of TB infection in a hominin fossil lead the authors to speculate that reduced UV radiation leading to vitamin D deficiency resulted in increased susceptibility to TB infection, presenting another challenge to Northward migration [40]. Could vitamin D regulation of the innate immune system in primates be another adaptation to counteract susceptibility to infection? Because bacteria have increased mutagenesis in the presence of UV radiation, it may be that the regulation of anti-microbial peptides by vitamin D is a counter evolutionary response to increased bacterial resistance [41].
The role of vitamin D in autoimmune disease
Background
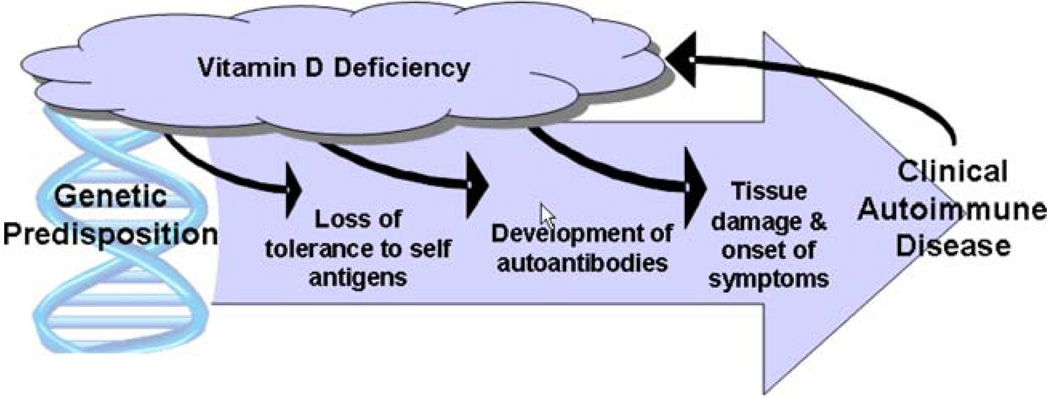
Although the natural history of autoimmunity remains largely unknown, the widespread theory is that both genetic susceptibility and environmental factors play a role in the development of clinical disease. Both experimental observations and clinical studies suggest a key role for vitamin D as a modifiable environmental factor in autoimmune disease (Fig. 2) [42]. Vitamin D has known immunomodulatory effects on a wide range of immune cells, including T lymphocytes, B lymphocytes, and dendritic cells [43, 44]. Each of these immune cell types express VDR and produce the enzymes 1α-OHase and 24-hydroxylase, and are therefore capable of locally producing active 1,25(OH)2D [45–49]. The autocrine and paracrine functions of 1,25(OH)2D are under tight immune system regulation and are dependent on an adequate supply of circulating 25(OH)D, making the epidemic of vitamin D deficiency critical to address for immune system health.
Fig. 2.
Proposed mechanism for vitamin D’s influence on the development and progression of autoimmunity. 1,25(OH)2D regulates DC maturation and the differentiation and activity of CD4+ T cells to prevent the loss of self-tolerance. In a genetically predisposed individual, it is more likely that autoantibodies will develop and proliferate in the setting of vitamin D deficiency. Ultimately, deficiency of vitamin D may act as an environmental trigger of clinical disease. Left untreated, the cycle of vitamin D deficiency will continue as many autoimmune diseases and several medications used to treat them lead to sun avoidance from photosensitivity. The role of vitamin D status in the natural history of autoimmunity warrants further investigation
Specific effects on T and B cells
Activation of CD4+ T cells results in a fivefold increase in VDR expression, enabling regulation of at least 102 identified genes responsive to 1,25(OH)2D [50]. 1,25(OH)2D suppresses T cell receptor induced T cell proliferation and alters their cytokine expression profile [46, 51]. The overall shift is away from a T helper (Th)1 phenotype toward a more tolerogenic Th2 phenotype [52, 53]. IFNγ and IL-2 production by T cells are diminished by exposure to 1,25(OH)2D while IL-5 and IL-10 are increased, consistent with a shift towards a Th2 response [54, 55]. The production of the Th2 cytokine IL-4 is up-regulated by 1,25(OH)2D in most, but not all, studies [54, 56]. Vitamin D appears to directly inhibit Th1 cells and may additionally modulate a skewing towards a Th2 response by its inhibitory effects upon IL-12 [57].
Th17 cells are a subset of CD4+ T cells involved in organ-specific autoimmunity, playing a role in maintaining inflammation which can lead to tissue damage [58]. In animal models of autoimmune uveitis [59] and inflammatory bowel disease [60], 1,25(OH)2D suppresses autoimmunity and tissue destruction by inhibiting the Th17 response at several levels, including the ability of dendritic cells to support priming of Th17 cells and the ability of Th17 cells to produce IL-17. Vitamin D inhibits the expression of IL-6 [61, 62], a cytokine which stimulates Th17 cell genesis, and suppresses IL-12p70, IL-23p19, and further IL-6 and IL-17 expression [60]. In addition to effects on CD4+ cells, vitamin D facilitates the induction of Foxp3+ T regulatory cells [57, 63] and there is a positive correlation between serum 25(OH)D levels and the ability of T regulatory cells to suppress T cell proliferation [64]. Altogether, the evidence supports an important role for vitamin D in influencing T cell responses and in tempering inflammation and tissue damage.
Vitamin D has a direct effect on B cells and inhibits immunoglobulin production [65]. Furthermore, when exposed in vitro to 1,25(OH)2D, differentiation of B lymphocytes is interrupted [47]. Peripheral blood mononuclear cells (PBMCs) from patients with SLE are sensitive to the effects of vitamin D; addition of 1,25(OH)2D to SLE PBMCs results in significant reduction of both spontaneous polyclonal antibody production and pathogenic anti-dsDNA autoantibody production by SLE B cells [66].
Effects on dendritic cells
Perhaps the most profound effects of 1,25(OH)2D on the immune system, and of high relevance to autoimmunity, are the effects on dendritic cells (DCs) [67, 68]. DCs have important functions in maintaining both protective immunity and self-tolerance [69, 70]: immature DCs promote T cell tolerance, whereas mature DCs activate naïve T cells. Mechanisms of action of 1,25(OH)2D on DCs include actions on the differentiation of monocytes into immature DCs, the maturation of DCs, and DC survival [67, 71–74]. Overall, 1,25(OH)2D leads to the development of DCs with tolerogenic properties. Expression of VDR increases rapidly as monocytes develop into monocyte-derived DCs (MDDCs) [68]. Physiologic levels of 1,25(OH)2D inhibit maturation of DCs, and maintain an immature and tolerogenic phenotype with inhibition of activation markers such as MHC class II, CD40, CD80, and CD86 and up-regulation of inhibitory molecules (ILT3) [57, 67]. Furthermore, 1,25(OH)2D down-regulates IL-12 and augments IL-10 production by DCs, promoting a shift from a Th1 to a Th2 phenotype [57]. MDDCs that have developed in the presence of 1,25(OH)2D exhibit less IL-12p40 in response to LPS (a maturation trigger for immature DCs) and are less responsive to inflammatory chemokines that regulate DC migration to lymph nodes [72]. Since the maturational state of DCs can be modulated by 1,25(OH)2D, the vitamin D status of an individual is likely to have important immunologic consequences.
Vitamin D and autoimmune disease
There have been several animal models of autoimmunity in which disease could either be prevented or ameliorated with the administration of either 1,25(OH)2D3 or one of its analogues. These animal models include autoimmune encephalomyelitis (EAE), collagen-induced arthritis, type-1 diabetes mellitus, inflammatory bowel disease, autoimmune uveitis, and lupus [43, 59, 75–86]. These studies demonstrate that treatment with hormonally active vitamin D is effective in modulating immune function and positively impacting autoimmune disease.
Vitamin D deficiency is a risk factor for development of a number of autoimmune diseases. Many of the clinical studies assess vitamin D status using dietary questionnaires, which is an inadequate surrogate without taking sun exposure and skin pigmentation into account [87]. This is especially true in later studies as increased awareness of skin cancer has resulted in greater general use of sunscreen and sun avoidance. These methodological limitations can explain some of the inconsistent results seen in large epidemiologic studies of vitamin D intake on the incidence of rheumatoid arthritis (RA). The Iowa Women’s Health Study, a population-based cohort of 41,837 post-menopausal women, found a significantly higher risk of developing RA in women reporting a lower intake of vitamin D at baseline [88]. These findings could not be replicated using similar methods in a different cohort (Nurses’ Health Study) [89]. Studies utilizing 25(OH)D levels include a report from Amsterdam that found no significant difference in 25(OH)D levels at three time points (1, 2, and ≥5 years prior to symptom onset) between 79 patients who subsequently developed RA and 79 age-, sex-, and season-matched controls [87]. However, other studies show that low levels of 25(OH)D contributes to disease activity and inflammation among those with established inflammatory arthritis [90] and RA [91].
The findings linking vitamin D deficiency to multiple sclerosis (MS), type 1 diabetes (T1DM), and systemic lupus erythematosus (SLE) are more consistent. A reduced risk of developing MS with vitamin D supplementation of ≥400 IU/day compared to no supplementation was documented in two large observational cohorts of women from the Nurses’ Health Study (RR 0.59, p for trend=0.006) [92]. Confirmation of these associations was made using the Department of Defense Serum Repository of 7 million US military personnel [93]. Among whites, the OR of developing MS was 0.59 (95% CI 0.36–0.97) with increasing levels of 25(OH)D; OR was 0.38 (95% CI 0.19–0.75) for the highest quintile of 25(OH)D [93]. Studies also suggest that disease activity measured in active MRI brain lesions and relapse rates in MS increase during seasonal periods of lower circulating 25(OH)D and decrease during periods of higher 25(OH)D [94–96].
Vitamin D status and type 1 diabetes mellitus
Type 1 diabetes mellitus (T1DM), one of the most prevalent chronic diseases with onset in childhood, results from an immune-mediated destruction of pancreatic insulin-producing β-cells. There is a marked geographic variation in incidence following a latitudinal gradient that is the inverse of the global distribution of ultraviolet B (UVB) irradiance [97].
One of the environmental factors thought to be protective against the development of T1DM is early supplementation with vitamin D. Four large case-control studies were included in a meta-analysis showing that the risk of T1DM was significantly reduced in infants who were supplemented with vitamin D compared to those who were not supplemented (pooled odds ratio 0.71, 95% CI 0.60 to 0.84) [97]. There also was evidence of a dose–response effect, with those using higher amounts of vitamin D being at lower risk of developing T1DM [97].
A birth cohort study in Finland, now more than 40 years ago, evaluated the effects of vitamin D supplementation on rickets and the subsequent development of T1DM [98]. All women due to give birth in 1966 were enrolled (12,058 live births). There were 10,366 children analyzed, of whom 81 developed T1DM. There was an approximately 80% reduction in the risk of T1DM in children receiving ≥ 2000 IU vitamin D/day, compared to those receiving less (adjusted RR 0.22; 95% CI 0.05, 0.89), which is in agreement with the meta-analysis of case-control studies [97]. Evidence from both human and animal studies show that vitamin D plays an important role in T1DM, and early intervention with supplemental vitamin D appears to offer protection from its development.
Vitamin D status and SLE
Type I interferons play an important role in SLE and the up-regulation of IFNα inducible genes, termed the “interferon signature,” is seen in approximately 50% of patients with SLE and correlates with disease activity [99, 100]. Plasmacytoid DCs, responsible for this signature, release IFNα after stimulation by nucleic acid-containing immune complexes. SLE plasma is capable of inducing/transferring the interferon signature to normal, non-autoimmune PBMCs. Observations that 1,25(OH)2D3 inhibits in vitro dendritic cell maturation/activation and type I interferon production are of interest and suggest that giving vitamin D as a therapeutic intervention may be beneficial in SLE [42, 73, 101].
Several methods have been used to examine potential links between vitamin D status and SLE, including case-control, cohort, and retrospective observational studies, using serum 25(OH)D levels and dietary intake of vitamin D as a surrogate marker of vitamin D status [102]. SLE cases have lower 25(OH)D levels compared to controls, suggesting that vitamin D deficiency may be a risk factor for SLE [103–105]. However, findings from the Nurse’s Health Study I and II prospective cohorts showed no association between vitamin D intake and development of SLE [106]. As mentioned previously, this study was limited by the use of intake questionnaires which are an inadequate surrogate for serum 25(OH)D levels. The majority of studies have also found higher SLE disease activity associated with lower levels of serum 25(OH)D [102]. Because patients with SLE often have photosensitivity and are advised to avoid direct sun exposure, detecting and replacing 25(OH)D deficiency with oral supplementation is even more critical.
Optimal levels of 25-hydroxyvitamin D
It is clear that 1,25(OH)2D has physiologic effects beyond that of bone and mineral homeostasis and that the alarming prevalence of vitamin D deficiency seen worldwide may be contributing to immune-mediated diseases. Based on bone-related biomarkers such as intact parathyroid hormone, calcium absorption and bone mineral density, maintaining a 25(OH)D level of at least 32 ng/ml appears sufficient. The prospective studies needed to test whether similar levels are necessary for optimal immune health have not yet been completed. Potentially higher cutoffs of 25(OH)D will be needed and a better understanding will likely be available in the near future as research progresses.
Conclusions
Innate and adaptive immune balance
Potent immunomodulatory activities of vitamin D on both innate and adaptive immune responses have been recently discovered [12, 22, 25, 99, 107–114]. While innate immunity is enhanced against “high-affinity” foreign antigens, vitamin D sufficiency has a dampening effect on the processing of “low-affinity” self antigens. Although the precise mechanisms are still being discovered, the important role of vitamin D in maintaining immune homeostasis should not be overlooked. Interventional studies to further define the immunomodulatory effects of vitamin D in humans need to be done.
In summary, the effects of 1,25(OH)2D on the immune system include decreasing Th1/Th17 CD4+ T cells and cytokines, increasing regulatory T cells, downregulation of T cell-driven IgG production and inhibition of dendritic cell differentiation. While enhancing protective innate immune responses, 1,25(OH)2D helps maintain self-tolerance by dampening overly zealous adaptive immune responses [115].
Acknowledgments
Grant support: NIH K23-AR052364 (DK) “The Role of Vitamin D in Systemic Lupus Erythematosus”, NIH K23-AR054334 (VT) “Role of T-Cells in Post Menopausal Osteoporosis”, Emory University Research Committee (URC) Grant (VT)
Contributor Information
Diane L. Kamen, Division of Rheumatology, Department of Medicine, Medical University of South Carolina, Charleston, SC, USA
Vin Tangpricha, Division of Endocrinology, Metabolism and Lipids, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA; Nutrition Health Sciences Program, Graduate Division of Biomedical and Biological Sciences, Emory University, Atlanta, GA, USA; Center for Clinical and Molecular Nutrition, Emory University School of Medicine, Atlanta, GA, USA; 101 Woodruff Circle NE-WMRB 1301, Atlanta, GA 30322, USA vin.tangpricha@emory.edu.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al. Large-scale in silico and microarray-based identification of direct 1, 25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19(11):2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Ren S, Nguyen L, Adams JS, Hewison M. Splice variants of the CYP27b1 gene and the regulation of 1, 25-dihydroxyvitamin D3 production. Endocrinology. 2007;148(7):3410–3418. doi: 10.1210/en.2006-1388. [DOI] [PubMed] [Google Scholar]
- 4.Cross HS. Extrarenal vitamin D hydroxylase expression and activity in normal and malignant cells: modification of expression by epigenetic mechanisms and dietary substances. Nutr Rev. 2007;65(8 Pt 2):S108–S112. doi: 10.1111/j.1753-4887.2007.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Williams CJB. On the use and administration of cod-liver oil in pulmonary consumption. London Journal of Medicine. 1849;1:1–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Finsen NR. Nobel prize presentation speech by professor the count K.A.H. Morner, Rector of the Royal Caroline Institute on December 10, 1903. 1903 http://www.nobelprize.org.
- 7.Moller KI, Kongshoj B, Philipsen PA, Thomsen VO, Wulf HC. How Finsen's light cured lupus vulgaris. Photodermatol Photoimmunol Photomed. 2005;21(3):118–124. doi: 10.1111/j.1600-0781.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103(3–5):793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik S, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311 doi: 10.1126/science.1123933. 1773-3. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 13.Ramanathan B, Davis EG, Ross CR, Blecha F. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002;4(3):361–372. doi: 10.1016/s1286-4579(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 14.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 15.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95(16):9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272(20):13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 17.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- 18.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 19.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1, 25-dihydroxyvitamin D3. Faseb J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 21.Gombart AF, O'Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1, 25(OH)2D3 in various tissues. J Steroid Biochem Mol Biol. 2007;103(3–5):552–557. doi: 10.1016/j.jsbmb.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 22.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann B, Rudolph T, Pietzsch J, Meurer M. Conversion of vitamin D3 to 1alpha, 25-dihydroxyvitamin D3 in human skin equivalents. Exp Dermatol. 2000;9(2):97–103. doi: 10.1034/j.1600-0625.2000.009002097.x. [DOI] [PubMed] [Google Scholar]
- 24.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamaki H, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 26.Cannell JJ, Vieth R, Willett W, Zasloff M, Hathcock JN, White JH, et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann Otol Rhinol Laryngol. 2008;117(11):864–870. doi: 10.1177/000348940811701112. [DOI] [PubMed] [Google Scholar]
- 27.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64(5 Pt 1):226–233. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 29.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vitamin D status and antimicrobial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48(4):418–424. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15(5):438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 34.Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 35.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamshchikov AV, Oladele A, Leonard MK, Jr, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D as Adjunctive Therapy in Refractory Pulmonary Tuberculosis: A Case Report. South Med J. 2010 doi: 10.1097/SMJ.0b013e3181a5d37e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomis WF. Skin-pigment regulation of vitamin-D biosynthesis in man. Science. 1967;157(788):501–506. doi: 10.1126/science.157.3788.501. [DOI] [PubMed] [Google Scholar]
- 38.Chaplin G, Jablonski NG. Vitamin D and the evolution of human depigmentation. Am J Phys Anthropol. 2009;139(4):451–461. doi: 10.1002/ajpa.21079. [DOI] [PubMed] [Google Scholar]
- 39.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kappelman J, Alcicek MC, Kazanci N, Schultz M, Ozkul M, Sen S. First Homo erectus from Turkey and implications for migrations into temperate Eurasia. Am J Phys Anthropol. 2008;135(1):110–116. doi: 10.1002/ajpa.20739. [DOI] [PubMed] [Google Scholar]
- 41.Dib J, Motok J, Zenoff VF, Ordonez O, Farias ME. Occurrence of resistance to antibiotics, UV-B, and arsenic in bacteria isolated from extreme environments in high-altitude (above 4400 m) Andean wetlands. Curr Microbiol. 2008;56(5):510–517. doi: 10.1007/s00284-008-9103-2. [DOI] [PubMed] [Google Scholar]
- 42.Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20(5):532–537. doi: 10.1097/BOR.0b013e32830a991b. [DOI] [PubMed] [Google Scholar]
- 43.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. Faseb J. 2001;15(14):2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 44.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147(12):5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 46.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1, 25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 48.van Etten E, Stoffels K, Gysemans C, Mathieu C, Overbergh L. Regulation of vitamin D homeostasis: implications for the immune system. Nutr Rev. 2008;66(10) Suppl 2:S125–S134. doi: 10.1111/j.1753-4887.2008.00096.x. [DOI] [PubMed] [Google Scholar]
- 49.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132(Pt 5):1146–1160. doi: 10.1093/brain/awp033. [DOI] [PubMed] [Google Scholar]
- 50.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89(5):922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 51.Bhalla AK, Amento EP, Serog B, Glimcher LH. 1, 25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133(4):1748–1754. [PubMed] [Google Scholar]
- 52.Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1, 25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30(2):498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 53.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1, 25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125(6 Suppl) doi: 10.1093/jn/125.suppl_6.1704S. 1704S–1708S. [DOI] [PubMed] [Google Scholar]
- 54.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha, 25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 55.Overbergh L, Decallonne B, Waer M, Rutgeerts O, Valckx D, Casteels KM, et al. 1alpha, 25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543) Diabetes. 2000;49(8):1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 56.Staeva-Vieira TP, Freedman LP. 1, 25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol. 2002;168(3):1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 57.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1, 25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 58.Eisenstein EM, Williams CB. The T(reg)/Th17 cell balance: a new paradigm for autoimmunity. Pediatr Res. 2009;65(5 Pt 2):26R–31R. doi: 10.1203/PDR.0b013e31819e76c7. [DOI] [PubMed] [Google Scholar]
- 59.Tang J, Zhou R, Luger D, Zhu W, Silver PB, Grajewski RS, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182(8):4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 61.Xue ML, Zhu H, Thakur A, Willcox M. 1 alpha, 25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol Cell Biol. 2002;80(4):340–345. doi: 10.1046/j.1440-1711.80.4august.1.x. [DOI] [PubMed] [Google Scholar]
- 62.van Etten E, Verlinden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, Ferreira GB, et al. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37(2):395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 63.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha, 25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167(4):1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 64.Smolders J, Thewissen M, Peelen E, Menheere P, Cohen Tervaert JW, Damoiseaux J, et al. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS ONE. 2009;4(8):e6635. doi: 10.1371/journal.pone.0006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemire JM, Adams JS, Sakai R, Jordan SC. 1 alpha, 25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74(2):657–661. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D(3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol. 2001;99(1):82–93. doi: 10.1006/clim.2000.4998. [DOI] [PubMed] [Google Scholar]
- 67.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98(12):6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szeles L, Keresztes G, Torocsik D, Balajthy Z, Krenacs L, Poliska S, et al. 1, 25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182(4):2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 69.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 70.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 71.van Halteren AG, Tysma OM, van Etten E, Mathieu C, Roep BO. 1alpha, 25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun. 2004;23(3):233–239. doi: 10.1016/j.jaut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 72.Gauzzi MC, Purificato C, Donato K, Jin Y, Wang L, Daniel KC, et al. Suppressive effect of 1alpha, 25-dihydroxyvitamin D3 on type I IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. J Immunol. 2005;174(1):270–276. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- 73.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164(9):4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 74.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha, 25-Dihydroxyvitamin D3 (1, 25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145(3):351–357. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 75.Van Amerongen BM, Dijkstra CD, Lips P, Polman CH. Multiple sclerosis and vitamin D: an update. Eur J Clin Nutr. 2004;58:1095–1109. doi: 10.1038/sj.ejcn.1601952. [DOI] [PubMed] [Google Scholar]
- 76.Cantorna MT, Hayes CE, DeLuca HF. 1, 25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93(15):7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Etten E, Branisteanu DD, Overbergh L, Bouillon R, Verstuyf A, Mathieu C. Combination of a 1, 25-dihydroxyvitamin D3 analog and a bisphosphonate prevents experimental autoimmune encephalomyelitis and preserves bone. Bone. 2003;32(4):397–404. doi: 10.1016/s8756-3282(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 78.Cantorna MT, Hayes CE, DeLuca HF. 1, 25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128(1):68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- 79.Larsson P, Mattsson L, Klareskog L, Johnsson C. A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin Exp Immunol. 1998;114(2):277–283. doi: 10.1046/j.1365-2249.1998.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zella JB, McCary LC, DeLuca HF. Oral administration of 1, 25-dihydroxyvitamin D3 completely protects NOD mice from insulin-dependent diabetes mellitus. Arch Biochem Biophys. 2003;417(1):77–80. doi: 10.1016/s0003-9861(03)00338-2. [DOI] [PubMed] [Google Scholar]
- 81.Giulietti A, Gysemans C, Stoffels K, van Etten E, Decallonne B, Overbergh L, et al. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47(3):451–462. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 82.Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. Prevention of autoimmune diabetes in NOD mice by 1, 25 dihydroxyvitamin D3. Diabetologia. 1994;37(6):552–558. doi: 10.1007/BF00403372. [DOI] [PubMed] [Google Scholar]
- 83.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17(12):2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 84.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1, 25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130(11):2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 85.Koizumi T, Nakao Y, Matsui T, Nakagawa T, Matsuda S, Komoriya K, et al. Effects of corticosteroid and 1, 24R-dihydroxy-vitamin D3 administration on lymphoproliferation and autoimmune disease in MRL/MP-lpr/lpr mice. Int Arch Allergy Appl Immunol. 1985;77(4):396–404. doi: 10.1159/000233815. [DOI] [PubMed] [Google Scholar]
- 86.Lemire JM, Ince A, Takashima M. 1, 25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12(2):143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 87.Nielen MM, van Schaardenburg D, Lems WF, van de Stadt RJ, de Koning MH, Reesink HW, et al. Vitamin D deficiency does not increase the risk of rheumatoid arthritis: comment on the article by Merlino et al. Arthritis Rheum. 2006;54(11):3719–3720. doi: 10.1002/art.22191. [DOI] [PubMed] [Google Scholar]
- 88.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2004;50(1):72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 89.Costenbader KH, Feskanich D, Benito-Garcia E, Holmes M, Karlson E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2007;67:530–535. doi: 10.1136/ard.2007.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patel S, Farragher T, Berry J, Bunn D, Silman A, Symmons D. Association between serum vitamin D metabolite levels and disease activity in patients with early inflammatory polyarthritis. Arthritis Rheum. 2007;56(7):2143–2149. doi: 10.1002/art.22722. [DOI] [PubMed] [Google Scholar]
- 91.Cutolo M, Otsa K, Laas K, Yprus M, Lehtme R, Secchi ME, et al. Circannual vitamin d serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin Exp Rheumatol. 2006;24(6):702–704. [PubMed] [Google Scholar]
- 92.Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 93.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296(23):2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 94.Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;48(2):271–272. [PubMed] [Google Scholar]
- 95.Tremlett H, van der Mei IA, Pittas F, Blizzard L, Paley G, Mesaros D, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology. 2008;31(4):271–279. doi: 10.1159/000166602. [DOI] [PubMed] [Google Scholar]
- 96.van der Mei IA, Blizzard L, Ponsonby AL, Dwyer T. Validity and reliability of adult recall of past sun exposure in a case-control study of multiple sclerosis. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1538–1544. doi: 10.1158/1055-9965.EPI-05-0969. [DOI] [PubMed] [Google Scholar]
- 97.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93(6):512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 98.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358(9292):1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 99.Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–386. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 100.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54(2):408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 101.Dong X, Craig T, Xing N, Bachman LA, Paya CV, Weih F, et al. Direct transcriptional regulation of RelB by 1alpha, 25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function. J Biol Chem. 2003;278(49):49378–49385. doi: 10.1074/jbc.M308448200. [DOI] [PubMed] [Google Scholar]
- 102.Kamen DL, Aranow C. The link between vitamin D deficiency and systemic lupus erythematosus. Curr Rheumatol Rep. 2008;10(4):273–280. doi: 10.1007/s11926-008-0044-3. [DOI] [PubMed] [Google Scholar]
- 103.Kamen DL, Cooper GS, Bouali H, Shaftman SR, Hollis BW, Gilkeson GS. Vitamin D deficiency in systemic lupus erythematosus. Autoimmun Rev. 2006;5(2):114–117. doi: 10.1016/j.autrev.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 104.O'Regan S, Chesney RW, Hamstra A, Eisman JA, O'Gorman AM, Deluca HF. Reduced serum 1, 25-(OH)2 vitamin D3 levels in prednisone-treated adolescents with systemic lupus erythematosus. Acta Paediatr Scand. 1979;68(1):109–111. doi: 10.1111/j.1651-2227.1979.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 105.Muller K, Kriegbaum NJ, Baslund B, Sorensen OH, Thymann M, Bentzen K. Vitamin D3 metabolism in patients with rheumatic diseases: low serum levels of 25-hydroxyvitamin D3 in patients with systemic lupus erythematosus. Clin Rheumatol. 1995;14(4):397–400. doi: 10.1007/BF02207671. [DOI] [PubMed] [Google Scholar]
- 106.Costenbader KH, Feskanich D, Holmes M, Karlson EW, Benito-Garcia E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann Rheum Dis. 2008;67(4):530–535. doi: 10.1136/ard.2007.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niyonsaba F, Hirata M, Ogawa H, Nagaoka I. Epithelial cell-derived antibacterial peptides human beta-defensins and cathelicidin: multifunctional activities on mast cells. Curr Drug Targets Inflamm Allergy. 2003;2(3):224–231. doi: 10.2174/1568010033484115. [DOI] [PubMed] [Google Scholar]
- 108.Lazarus R, Klimecki WT, Raby BA, Vercelli D, Palmer LJ, Kwiatkowski DJ, et al. Single-nucleotide polymorphisms in the Toll-like receptor 9 gene (TLR9): frequencies, pairwise linkage disequilibrium, and haplotypes in three U.S. ethnic groups and exploratory case-control disease association studies. Genomics. 2003;81(1):85–91. doi: 10.1016/s0888-7543(02)00022-8. [DOI] [PubMed] [Google Scholar]
- 109.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111(3):273–281. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha, 25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106(13):4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 111.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175(3):1776–1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 112.Niyonsaba F, Nagaoka I, Ogawa H. Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties. Crit Rev Immunol. 2006;26(6):545–576. doi: 10.1615/critrevimmunol.v26.i6.60. [DOI] [PubMed] [Google Scholar]
- 113.Byers S, Shah S. Vitamin D and the regulation of Wnt/beta-catenin signaling and innate immunity in colorectal cancer. Nutr Rev. 2007;65(8 Pt 2):S118–S120. doi: 10.1111/j.1753-4887.2007.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 114.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, et al. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 2007;37(2):434–444. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 115.Van Etten E, Decallonne B, Verlinden L, Verstuyf A, Bouillon R, Mathieu C. Analogs of 1alpha, 25-dihydroxyvitamin D3 as pluripotent immunomodulators. J Cell Biochem. 2003;88(2):223–226. doi: 10.1002/jcb.10329. [DOI] [PubMed] [Google Scholar]