Abstract
Genetic mutations associated with α-synuclein (α-Syn) are implicated in the pathogenesis of Parkinson’s disease (PD). PD is primarily a movement disorder, but patients are known to experience anxiety and other mood disorders. In this study, we examined the effect of the hA53T mutation during development by analyzing the protein expression of norepinephrine (NET), serotonin (SERT), and dopamine (DAT) transporters in addition to assessing locomotor and anxiety-like behavior. We observed significant decreases in DAT expression at 8 months in transgenic animals compared with normal and younger mice. We used the elevated plus maze, open-field test, and rotarod apparatus to evaluate wild-type and hA53T hemizygous mice at 2, 8, and 12 months of age. Our results showed that 12-month-old transgenic mice spend more time in the open arms and display a greater number of open entries of the elevated plus maze compared with wild-type controls and younger mice. Open-field test results showed that 12-month-old mice travel a greater distance overall and travel more in the inner zone than either wild-type or younger mice. Rotarod testing showed that 8- and 12-month-old transgenic mice perform better than either wild-type controls or younger mice. Overall, 8–12-month-old transgenic mice showed a trend toward reduced anxiety-like behavior and increased hyperactivity. These results indicate a possible role of the A53T α-Syn mutation in anxiety-like and hyperactive behaviors in a PD mouse model, suggesting that these behaviors might be comorbid with this disease.
Keywords: behavior, dopamine transporter, anxiety disorders, Parkinson’s disease
Parkinson’s disease (PD) is a common neurodegenerative movement disorder affecting approximately 1% of the elderly population. Clinical manifestations reflect a loss of dopaminergic (DA) neurons in the substania nigra pars compacta and include bradykinesia, resting tremor, stiffness, postural instability, and periods of freezing (Simuni and Hurtig, 2000). One of the most common histopathological features of PD is the Lewy body (LB), a proteinaceous fibrillar cytoplasmic inclusion present in the neuronal perikarya. Recent evidence shows that the main components of LB’s are amyloid-like fibrils composed of αsynuclein (α-Syn) protein. α-Syn is a small acidic protein (~14 kD) composed of 140 amino acids and is expressed predominantly in the presynpatic terminals, particularly in the neocortex, hippocampus, striatum, thalamus, and cerebellum (Iwai et al., 1995). The amino acid sequence of α-Syn can be subdivided into three domains: the highly conserved lipid binding amino-terminal domain (amphipathic region: 1–65 aa), the central NAC domain known as the non-α β amyloid component of Alzheimer’s disease senile plaques (hydrophobic region: 66–95 aa), and the acidic carboxyl-terminal domain (hydrophilic region: 96–140 aa). Recent biochemical studies show functional interactions between α-Syn and dopaminergic neurotransmitter system, including the regulation of dopamine synaptic availability and homeostasis, modulation of dopamine release, and synthesis and targeting of the dopamine transporter to the plasma membrane (Davidson et al., 1998; Jensen et al., 1998; Murphy et al., 2000; Abeliovich et al., 2000; Stefanis et al., 2001; Lee at al, 2001; Perez et al., 2002; Cabin et al., 2002; Wersinger et al., 2003a,b). In addition, α-Syn is involved in modulating serotonergic and noradrenergic neurotransmission through trafficking of the serotonin and norepinephrine transporter to and from the plasma membrane, thereby regulating cell surface expression of the transporters, as well as through direct binding to the transporters decreasing transporter activity (Wersinger et al., 2006a,b; Jeannotte and Sidhu, 2007).
Although the exact physiological function of α-Syn is still being investigated, biochemical and genetic studies have provided valuable insight into the functional role of wild-type and mutant forms of α-Syn. The importance of α-Syn in PD is evident from genetic and biochemical studies that have identified disease-causing missense mutations (A53T and A30P) in the α-Syn gene that are associated with cases of early-onset PD in families of European origin (Recchia et al., 2004). The A53T mutation (Kotzbauer, 2004) has been especially important in the Contrusi kindred of PD (Duda, 2002) and in diffuse Lewy body disease with the A53T mutation (Yamaguchi et al., 2005). In addition to PD, aberrant aggregation of the α-Syn protein has been detected in a large number of other neurodegenerative diseases, classified as synucleopathies (Hamilton, 2000; Simuni and Hurtig, 2000; Arai et al., 2001; Jellinger, 2004; Nemes et al., 2004; Galpren and Lang, 2006; Griffin et al., 2006).
Homozygous mice expressing the A53T mutant human α-Syn under the control of the mouse prion promoter develop and display severe motor impairments with accumulation of α-Syn in the brain, similar to patients with the A53T mutation (Giasson et al., 2002). Homozygous A53T mutant mice have also been used to show decreased anxiety in 2-month-old animals (George, 2008) and increased locomotor activity and altered dopaminergic neurotransmission in 7–19-month-old mice (Unger et al., 2006). We decided to investigate whether mood disorders could also occur in the hemizygous strain of the A53T mouse. In this study, we show that late adult transgenic mice exhibit reduced anxiety-like behaviors on the elevated plus maze and increased hyperactivity on the open-field test. Moreover, we report a behavior phenotype of the A53T mutant human α-Syn mice characterized by both reduced anxiety-like behavior and locomotor hyperactivity.
MATERIALS AND METHODS
Animals
Mice used in accordance with the Georgetown University Animal Care and Use Committee approval were bred and maintained in University-approved animal facilities and handled by trained personnel. Mice were group housed (2–5 animals/cage) in temperature- and humidity-controlled rooms under a 12-hr light/dark cycle and fed an ad libitum diet of standard mouse chow. Mice were originally obtained in breeding pairs from Jackson Laboratories (Bar Harbor, ME) to generate a stable breeding colony. A breeding colony of transgenic mice that carry the hA53T mutation driven by the mouse prion promoter was established according to previously characterized mice (Giasson et al., 2002). Mice hemizygous for the hA53T mutation were bred on a mixed C57B1/6 × C3H background to produce transgenic and nontransgenic littermates. In all cases, 2-, 8-, and 12-month-old transgenic mice were directly compared with age-matched nontransgenic (wild-type) littermates. To identify transgenic mice, PCR amplifications were performed on 1 µl of proteinase K-digested (Promega, Madison, WI) tail DNA samples using a sense (5′-TGTAGGCTCCAAAACCAAGG-3′) and an antisense (5′-TGTCAGGATCCACAGGCATA-3′) primer. PCRs (30 µl) consisted of 10× NH4 buffer (Bioline, London, United Kingdom), 1.5 mM MgCl2, 0.05 mM each dNTP, 0.4 pM of each primer, and 0.1 units of Taq DNA polymerase (Bioline). Reactions were denatured at 95°C for 5 min and then subjected to 35 cycles of 95°C for 45 sec, 61°C for 1 min, 72°C for 1 min, and 72°C for 2 min. A 30-µl aliquot from each reaction was analyzed by gel electrophoresis in a 1% agarose gel for the presence of a 248-base-pair band. To confirm further the genotype of transgenic and nontransgenic littermates, tail DNA samples were processed by Charles River Genetic Testing Services (Troy, NY).
Western Blot
Striatum tissues from 2- and 8-month-old wild-type and transgenic mice were removed and homogenized with lysis buffer (10 mM Tris-HCl, pH 7.4,, 100 mM NaCl, 1 mM EDTA, pH 8.0, 1 mM EGTA, pH 8.0) containing 1 mM PMSF, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, 10% glycerol, phosphatase inhibitor cocktail (Halt Phosphatase Inhibitor Cocktail; Thermo Scientific, Rockford, IL), and protease inhibitor cocktail tablets (Complete Mini, EDTA-free; Roche Diagnostics, Mannheim, Germany). Lysates were left on ice for 15 min and sonicated with a Branson Sonifer 250. Protein concentrations were determined with a Bradford assay kit (Bio-Rad, Hercules, CA). Lysates containing 25 µg of proteins were separated on 4–12% gradient Bis-Tris gels (Invitrogen, Carlsbad, CA) and then transferred onto PVDF membranes (Bio-Rad; 162-0177). The blots were probed with either monoclonal anti-NET (1:500 dilution; Mab Technologies, Inc.; NET05-2), monoclonal anti-SERT (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA; sc-4678), and monoclonal anti-DAT (1:500 dilution; MAB369; Millipore, Billerica, MA). Secondary antibodies used were goat anti-mouse (1:3,000 dilution) and goat anti-rat (1:3,000 dilution; Bio-Rad). Membranes were then washed with stripping solution (Restore Western Blot Stripping Buffer). Blots were next probed with a polyclonal β-actin antibody (1:500 dilution; Santa Cruz Biotechnology; catalog No. sc-1616), and the resulting signal was used to normalize for protein loading. Membranes were detected with enhanced chemiluminescence (Perkin Elmer) and exposed to film. Protein bands were scanned with an EPSON Perfection 4870 Photo Scanner and quantitatively analyzed in Scion Image.
Elevated Plus-Maze Test
Tests were spaced at least 1 week apart, and the order of testing was chosen such that tests involving lower stress levels (elevated plus-maze and open-field) preceded those involving higher stress levels (rotarod). The elevated plus-maze was performed before all tests, because it is especially sensitive to previous testing history (Bailey et al., 2007). The elevated plus-maze test was assessed as described previously (Masood et al., 2003). The elevated plus-maze (Stoelting, Wood Dale, IL) apparatus was elevated 40 cm above the floor and consisted of four arms measuring 35 cm in length × 5 cm in width. Two of the arms (closed) were enclosed with 15-cm-high walls, and two arms had no walls (open). Testing was conducted in a quiet (50–55 dB ambient noise), dedicated room, which was dimmed to provide adequate testing. During the 5-min test session of free exploration, the time spent and the total number of entries into the open and closed arms for each animal when all four paws of the mouse moved into either the open or the closed arms was recorded by a blind observer not aware of genotype. The maze was sponged clean between each trial. Elevated plus maze behavior for each animal was analyzed using the ANY-maze video tracking system (Stoelting).
Open-Field Test
Normal (wild-type) and hA53T transgenic mouse locomotor activity was assessed using the open field test (Stoelting). The animal were placed in the center of the activity field arena, which is a transparent plexi cage (40 cm in width × 40 cm in diameter × 35 cm in height) equipped with a camera above to record activity. All animals were allowed 2 min of free exploration prior to testing. Testing lasted for 20 min per animal. The exploratory behavior for each animal was analyzed using the ANY-maze video tracking system (Stoelting). The analysis included distinguishing activity within an outer and inner zone of the open field to assess thigmotaxis.
Rotarod
The rotarod apparatus (IITC Life Sciences, Wood Hills, CA) was used to measure balance and motor coordination. During the training period, mice were allowed to explore the rotarod for 2 min without rotation. The drum was slowly accelerated to a speed of 4–40 rpm for a maximum of 300 sec. The latency to fall off the rotarod within this time period was recorded. Mice received three trials each day for 3 consecutive days. The mean latency to fall off the rotarod for the 3-day trial period was recorded and used for analysis. The cylinder of the rotarod was 71 cm long and its diameter was 3.2 cm. The mouse was placed on the rotarod with its head in the direction of rotation and so it had to turn to the opposite direction first. During the test the mice had to remain on the rod for as long as they could. The length of time for which the animal remained on the rod was recorded (a 300-sec maximal trial was used for each test).
Statistical Analysis
Behavior parameters were assessed by applying one-way independent parametric ANOVA, followed where appropriate by pairwise post hoc comparisons (Tukey HSD). All data are represented as mean ± SEM. Differences were regarded as statistically significant at P < 0.05. The number of animals (n) for each age group with respect to genotype was 25.
RESULTS
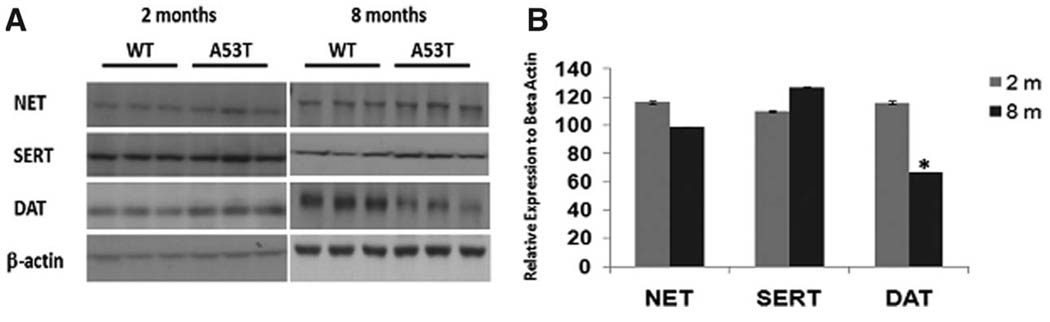
To assess whether monoamine transporter protein expression levels were altered in the A53T mice during development, striatal lysates were prepared and analyzed by Western blots (Fig. 1A). At 2 months, we noticed a 16% increase in norepinephrine transporter expression in transgenics compared with wild-type animals (Fig. 1B). However at 8 months, we did not detect changes in NET protein expression between transgenic and wild-type groups. We also noticed a modest increase in serotonin transporter expression in 2-month-old transgenics, and by 8 months a 27% increase in protein expression was observed in transgenic animals. Dopamine transporter protein expression levels increased by 15% in 2-month-old transgenic animals; however, for the 8-month-old transgenics, we noticed a significant decrease (34%) in total DAT expression (Fig. 1B).
Fig 1.
Western blot. The protein expression levels of NET, SERT, and DAT were examined by Western blot using striatum lysates from 2- and 8-month-old wild-type and transgenic animals. A: Analysis of relative levels of protein expression was performed on samples with 25 µg of protein per lane. B: Means for three independent samples per age normalized to β-actin before net intensity levels of expression were compared. Data represent the means of actual values ± SE for mice of both genotypes. *P < 0.05 vs. the wild-type mice with ANOVA.
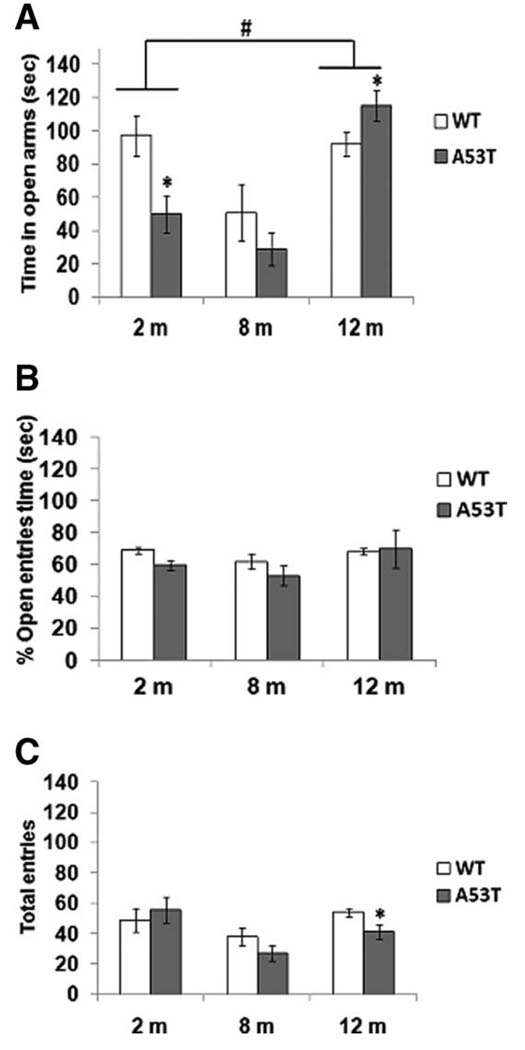
To examine anxiety-like behavior, 2-, 8-, and 12-month-old mice were observed in an elevated plus maze (Fig. 2A). At 2 and 8 months, the hA53T transgenic mice spent less time in the open arms compared with wild-type mice. Although both groups displayed similar behaviors on the elevated plus maze, values were significant only at 2 month of age. At 12 months, hA53T transgenic mice spent significantly more time in the open arms (Fig. 2A). Analysis of the percentage of number of open arm entries/total entries showed fewer open entries at 2 and 8 months in transgenic animals in comparison with wild-type mice (Fig. 2B). At 12 months, we observed no difference in the percentage of open arm entries/total entries among wild-type and transgenic groups. No differences in the total entries into the open arms between the transgenics and the wild-type mice were seen in the 2-month-old animals (Fig. 2C). For both the 8- and 12-month-old animals, by contrast, there was a decrease in total overall arm entries in the hA53T mice compared with wild-type animals, which reached statistical significance in the 12-month-old animals (Fig. 2C).
Fig 2.
Elevated plus-maze test. Time spent in the open (A), percentage of open entries (B), and total entries (C) are shown for wild-type (open column, n = 25) and hA53T transgenic mice (solid column, n = 25) in the elevated plus maze test during a 5-min testing period. Data represent the means of actual values ± SE for mice of both genotypes. *P < 0.05 vs. the wild-type mice with ANOVA. #P < 0.05 between groups.
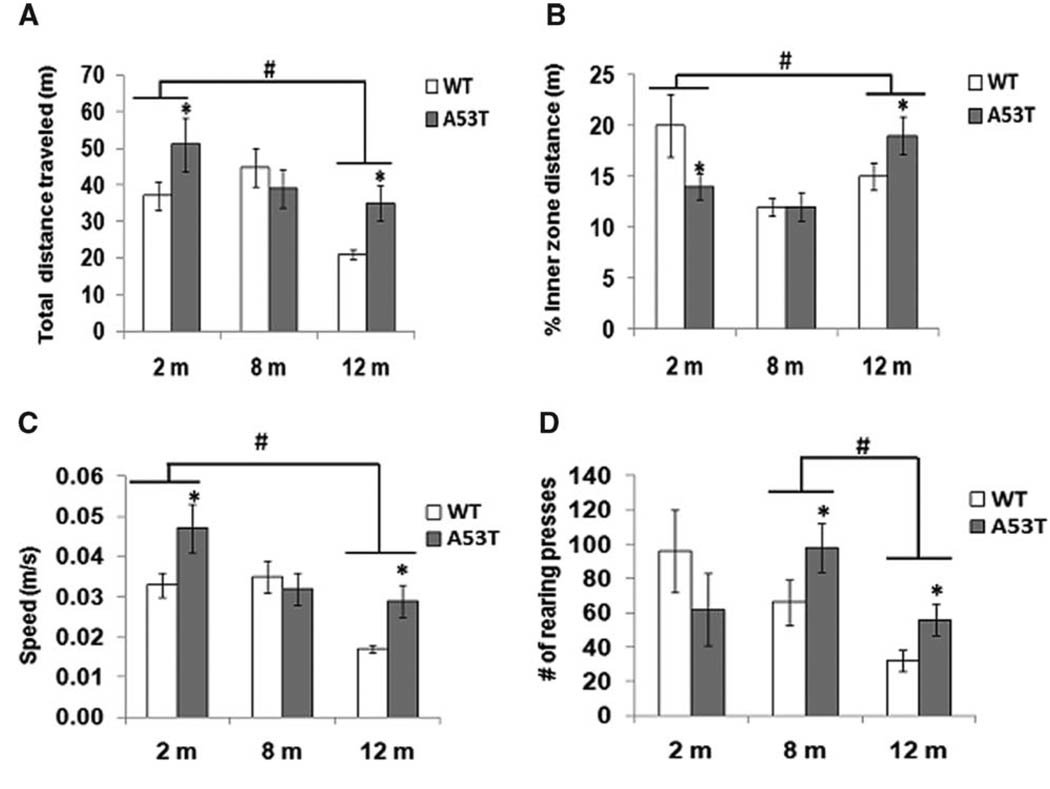
To determine changes in locomotor activity during development, the open field analysis was performed in 2-, 8-, and 12-month-old transgenic and age-matched wild-type animals. At 2 and 12 months, transgenic animals travel at significantly greater distances compared with wild-type animals (Fig. 3A). At 8 months, transgenic animals traveled the same distance as wild-type animals. During the 20 min of free exploratory activity, the 2-month-old hA53T animals had significantly fewer entries in the inner zone compared with normal age-matched control mice (Fig. 3B). At 8 months, the distance traveled in the inner zone was virtually identical in wild-type and transgenic mice. At 12 months, transgenic animals showed a significant increase in the distance traveled in the inner zone compared with normal mice (Fig. 3B). The increased time spent in the inner zone is a reversal of the behavior seen at 2 months of age, when the opposite effect was seen.
Fig 3.
Open-field test. Total distance traveled (A), percentage of inner zone distance traveled (B), average speed (C), and rearing (D) are shown for wild-type (open column, n = 25) and hA53T transgenic mice (solid column, n = 25) in the open-field test during a 20-min testing period. Data represent the means of actual values ± SE for mice of both genotypes. *P < 0.05 vs. the wild-type mice with ANOVA. #P < 0.05 between groups.
At all ages, we observed no difference between transgenic and wild-type animals in the distance traveled within the outer zone (data not shown). At 2 and 12 months, transgenic animals traveled at a significantly greater speed than wild-type controls (Fig. 3C). At 8 months, no difference was observed between transgenic and control groups. At 8 and 12 months of age, transgenic animals displayed significantly more rearing events compared with normal aged-matched controls (Fig. 3D). At 2 months, our results show less rearing in transgenic animals compared with wild-type controls.
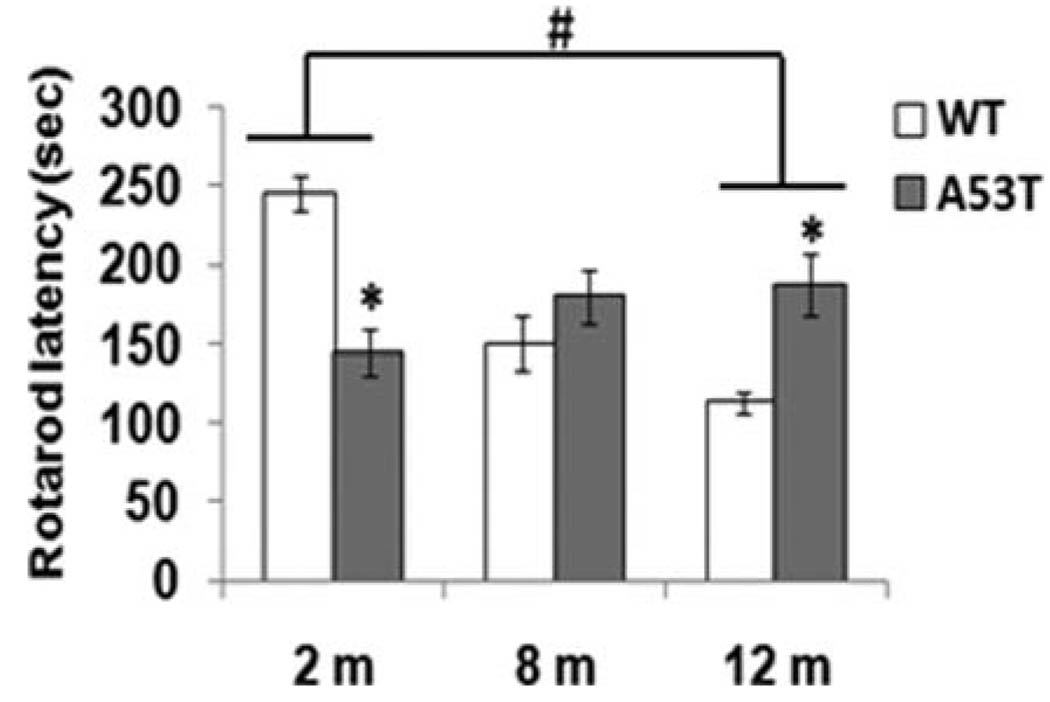
Because several animal models of PD are characterized by decreased locomotor activity, particularly at increased ages, it was essential to estimate whether these mice had reduced locomotion that might confound previous behaviors. We therefore measured locomotor activity in the 2-, 8-, and 12-month-old animals by rotarod, which measures balance and motor coordination. During rotarod tests, 2-month-old hA53T mice had significantly shorter latency to fall than normal mice (Fig. 4). By 8 months, transgenic animals showed slightly better motor coordination and balance than wild-type controls. At 12 months, transgenic mice showed better motor coordination performance than wild-type animals (Fig. 4); however, mean latencies of falls were significantly longer in 12 month A53T tg animals than in wild-type animals (P < 0.05).
Fig 4.
Rotarod test. Rotarod tests were performed at 4–40 rpm for 5 min. The length of time for which the animal remained on the rod was recorded. Bars are shown for wild-type (open column, n = 25) and hA53T transgenic mice (solid column, n = 25). Data represent the means of actual values ± SE for mice of both genotypes. *P < 0.05 vs. the wild-type mice with ANOVA. #P < 0.05 between groups.
DISCUSSION
In this study, we analyzed NET, SERT, and DAT protein expression during development in the A53T transgenic mice. We show that, at 2 and 8 months, NET and SERT protein expression levels are not significantly altered between transgenic and wild-type animals (Fig. 1). DAT, one of the most important proteins involved in the etiology of PD, was significantly decreased at 8 months in transgenic animals compared with normal and 2-month-old animals. We show that mice hemizygous for the A53T form of human ±-Syn develop an age-related increase in both locomotor activity and reduced anxiety-like phenotype. By 12 months of age, transgenic animals display decreased anxiety-like behavior on the elevated plus maze compared with either wild-type controls or younger transgenic animals (Fig. 2A). Previous studies have shown that homozygous A53T transgenic animals display a reduced anxiety-like phenotype at 2 months of age (George, 2008). In our study, we show that reduced anxiety is absent from young 2-month-old mice and is also not evident in the 8-month-old mice. Indeed, we do not observe any decreased anxiety-like behavior until the mice are 12 months of age.
The observation of increased exploratory and locomotor activity in the open field test and increased balance and motor coordination on the rotarod shows that these animals are hyperactive compared with either wild-type controls or younger mice. At 2 and 12 months, transgenic mice traveled a greater distance and faster than wild-type controls in the open-field test (Fig. 3). At 12 months, reduced anxiety-like phenotype of A53T mice is also apparent in the open-field test, which showed greater inner zone distance traveled (Fig. 3B) and significantly increased rearing in 12-month-old transgenics compared with wild-type controls (Fig. 3D). We found that the 2-month-old transgenic mice performed poorly on the rotarod compared with wild-type controls (Fig. 4). Rotarod performance improved by 8 months of age, and by 12 months transgenic mice showed better motor coordination than wild-type controls and younger animals. These data from hemizygous mice are consistent with previous reports showing that A53T transgenic mice are hyperactive only between 7 and 19 months of age, before the onset of motor dysfunction (Unger et al., 2006).
Numerous reports suggest that anxiety is comorbid with PD (Menza et al., 1993; Shiba et al., 2000; Marinus et al., 2002). Symptoms of anxiety are known to manifest before the onset of motor symptoms (Shiba et al., 2000). In our study, the hA53T transgenic mice show the phenotype of both reduced anxiety-like behavior and increased locomotor activity in the same animal, compared with other studies that have identified these behavioral phenotypes separately (Unger et al., 2006; George, 2008). Moreover, these previous studies were conducted on homozygous mice (George, 2008) in addition to various hA53T transgenic lines at different ages (Unger et al., 2006) compared with those used in our study. In our studies, it is interesting to note that several of the behaviors we studied in the 2-month-old transgenic mouse were opposite those seen in the 12-month-old animal, suggestive of a “switch” that may occur between these ages, reversing these behaviors. Thus, in general, results of the elevated plus maze test and the rotarod in the 2-month-old animals were opposite those of the 12-month-old animals. In the open field test, opposite results were also seen when measuring time spent in the inner zone (Fig. 3B), in which the 2-month-old animal spent less time in the inner zone, suggestive of increased anxiety-like behavior, consistent with our results obtained from the elevated plus maze.
The exact mechanisms involved in altered behavior in the elevated plus maze and increased locomotor activity in the open-field tests remains unclear. The neurotransmitter dopamine has been implicated in anxiety-like behaviors (Pogorelov et al., 2005). In our study, α-Syn-mediated effects on the dopaminergic system may be involved in the reduced anxiety-like phenotype observed in our mice. In the 7–19-month-old A53T transgenic mice, DAT expression is reduced in the nucleus accumbens and the striatum and was associated with reduced dopamine uptake in the striatum and increased hyperactivity in the A53T mice (Unger et al., 2006). In the 2-month-old homozygous A53T mutant animal, DAT expression was not measured (George, 2008), but our data suggest that reduced DAT expression and abnormalities in dopaminergic neurotransmission may also underlie the reduced anxiety-like behavior that we observe in our studies. In addition, dysregulated dopaminergic signaling may also occur through mechanisms independent of DAT expression levels, involving a dysregulation of DAT by the A53T mutant of α-Syn. Indeed, we have previously shown in cotransfected cells that the A53T mutant α-Syn is completely unable to traffick and regulate the reuptake functional activity of dopamine via DAT (Wersinger and Sidhu, 2005). Thus, the expression of the A53T mutant may lead to dysregulation of DAT, leading to hyperactivity in a mechanism reminiscent of that seen in the DAT knockout animal (Giros et al., 1996; Uhl et al., 1996). Although the effect of the A53T mutant on SERT trafficking and function is not known, it is likely that the A53T α-Syn mutation may cause a dysregulation of SERT. Although an overexpression of SERT may be predicted to lead to depressive behavior, due to reduced serotonin in the synapse and increased reuptake, we have not observed any depressive-like behavior in these mice at any age (data not shown).
In conclusion, late-adult hA53T transgenic mice show hypoanxiety-like behaviors on the elevated plus maze and increased locomotor activity on the open-field test and the rotarod. These mice will provide further valuable scientific information on the function of α-Syn-mediated effects on the brain with regard to PD and changes in behavior.
ACKNOWLEDGMENTS
We thank Bhudevi Bodreddigari from Charles River Testing Services for mouse genotyping and Patrick Forcelli for technical assistance with behavior studies.
Contract grant sponsor: National Institutes of Health; Contract grant number: R01MH075020; Contract grant number: R01AG028108; Contract grant number: R01NS060041.
REFERENCES
- Abeliovich A, Schmitz T, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Arai Y, Mori O, Muramatsu H, Asano G, Katayama Y. Alpha-synuclein-positive structures in cases with sporadic Alzheimer’s disease: morphology and its relationship to tau aggregation. Brain Res. 2001;888:287–296. doi: 10.1016/s0006-8993(00)03082-1. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Duda JE. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- George S. α-Synuclein transgenic mice exhibit reduced anxiety-like behavior. Exp Neurol. 2008;210:788–792. doi: 10.1016/j.expneurol.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones S, Wightman R, Caron M. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jeanotte A, Sidhu A. Regulation of the norepinephrine transporter by alpha-synuclein-mediated interactions with microtubules. Eur J Neurosci. 2007;26:1509–1520. doi: 10.1111/j.1460-9568.2007.05757.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm. 2004;111:1219–1235. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- Kotzbauer PT. Fibrillization of alpha-synuclein and tau in familial Parkinson’s disease caused by the A53T alpha-synuclein mutation. Exp Neurol. 2004;187:279–288. doi: 10.1016/j.expneurol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Marinus J, Leentjens A, Visser M, Stiggelbout A, van Hilten J. Evaluation of the hospital anxiety and depression scale in patients with Parkinson’s disease. Clin Neuropharmocol. 2002;25:318–324. doi: 10.1097/00002826-200211000-00008. [DOI] [PubMed] [Google Scholar]
- Masood A, Banerjee B, Vijayan VK, Ray A. Modulation of stress-induced neurobehavioral changes by nitric oxide in rats. Eur J Pharmacol. 2003;458:135–139. doi: 10.1016/s0014-2999(02)02688-2. [DOI] [PubMed] [Google Scholar]
- Menza M, Robertson-Hoffman D, Bonapace A. Parkinson’s disease and anxiety: cormorbidity and depression. Biol Psychiatry. 1993;34:465–470. doi: 10.1016/0006-3223(93)90237-8. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes Z, et al. Cross-linking of ubiquitin, HSP27, parkin, and alpha-synuclein by gamma-glutamyl-epsilon-lysine bonds in Alzheimer’s neurofibrillary tangles. FASEB J. 2004;18:1135–1137. doi: 10.1096/fj.04-1493fje. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov V, Rodriguiz R, Insco M, Caron M, Wetsel W. Novelty seeking and stereotypic activation of behavior in mice with disruption of the Dat1 gene. Neuropsychopharmacology. 2005;30:1818–1831. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Recchia A, Debetto P, Negro A, Guidolin D, Skaper S, Giusti P. α-Synuclein and Parkinson’s disease. FASEB J. 2004;18:617–626. doi: 10.1096/fj.03-0338rev. [DOI] [PubMed] [Google Scholar]
- Shiba M, Bower J, Maraganore D, McDonald S, Peterson B, Ahlskog J, Schiad D, Rocca W. Anxiety and depressive disorders preceeding Parkinson’s disease: a case control study. Mov Disord. 2000;15:669–677. doi: 10.1002/1531-8257(200007)15:4<669::aid-mds1011>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Simuni T, Hurtig HI. Parkinson’s disease: the clinical picture. In: Clark CM, Trojanoswki JQ, editors. Neurodegenerative dementias. New York: McGraw-Hill; 2000. pp. 193–203. [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21:9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Vandenbergh DJ, Miner LL. Knockout mice and dirty drugs. Drug addiction. Curr Biol. 1996;6:935–936. doi: 10.1016/s0960-9822(02)00630-9. [DOI] [PubMed] [Google Scholar]
- Unger EL, Eve DJ, Perez XA, Reichenbach YX, Lee MK, Andrews AM. Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human alpha-synuclein in mice. Neurobiol Dis. 2006;21:431–443. doi: 10.1016/j.nbd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry. 2005;44:13612–13624. doi: 10.1021/bi050402p. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Niznik HB, Sidhu A. Mutations in the lipid-binding domain of alpha-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity. Mol Cell Neurosci. 2003a;24:91–105. doi: 10.1016/s1044-7431(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003b;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Jeanotte A, Sidhu A. Attenuation of the norephrine transporter acitivty and trafficking via interactions with alpha-synuclein. Eur J Neurosci. 2006a;24:3141–3152. doi: 10.1111/j.1460-9568.2006.05181.x. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Rusnak M, Sidhu A. Modulation of the trafficking of the human serotonin transporter by human alpha-synuclein. Eur J Neurosci. 2006b;24:55–64. doi: 10.1111/j.1460-9568.2006.04900.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, et al. Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha-synuclein gene. Acta Neuropathol. 2005;110:298–305. doi: 10.1007/s00401-005-1042-4. [DOI] [PubMed] [Google Scholar]