Abstract
Benzyl isothiocyanate (BITC), a constituent of cruciferous vegetables such as gardencress, inhibits growth of human breast cancer cell lines in culture. The present study was undertaken to determine in vivo efficacy of BITC against MDA-MB-231 human breast cancer xenografts. The BITC administration retarded growth of MDA-MB-231 cells subcutaneously implanted in female nude mice without causing weight loss or any other side effects. The BITC-mediated suppression of MDA-MB-231 xenograft growth correlated with reduced cell proliferation as revealed by immunohistochemical analysis for Ki-67 expression. Analysis of the vasculature in the tumors from BITC-treated mice indicated smaller vessel area compared with control tumors based on immunohistochemistry for angiogenesis marker CD31. The BITC-mediated inhibition of angiogenesis in vivo correlated with down-regulation of vascular endothelial growth factor (VEGF) receptor 2 protein levels in the tumor. Consistent with these results, BITC treatment suppressed VEGF secretion and VEGF receptor 2 protein levels in cultured MDA-MB-231 cells. Moreover, the BITC-treated MDA-MB-231 cells exhibited reduced capacity for migration compared with vehicle-treated control cells. In contrast to cellular data, BITC administration failed to elicit apoptotic response as judged by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay. In conclusion, the present study demonstrates in vivo anti-cancer efficacy of BITC against MDA-MB-231 xenografts in association with reduced cell proliferation and suppression of neovascularization. These preclinical observations merit clinical investigation to determine efficacy of BITC against human breast cancers.
Keywords: breast cancer, isothiocyanate, VEGF receptor, angiogenesis
Introduction
Breast cancer remains a leading cause of cancer-related deaths among women worldwide despite significant advances towards targeted therapy as well as screening techniques leading to early detection of the disease [1-4]. The known risk factors for breast cancer include family history, Li-Fraumeni syndrome, atypical hyperplasia of the breast, late-age at first full-term pregnancy, early menarche, and late menopause [5-7]. Because some of these risk factors are not easily modifiable, other strategies for reduction of the breast cancer risk are desirable. Even though selective estrogen-receptor (ER) modulators (e.g., tamoxifen) appear promising for prevention of breast cancer, this strategy is largely ineffective against ER-negative breast cancers [8,9]. Moreover, selective ER modulators have serious side effects including increased risk of uterine cancer, thromboembolism, cataracts, and perimenopausal symptoms [8,9]. Therefore, novel agents for prevention and treatment of human breast cancers, especially hormone-independent breast cancers, are highly desirable. Natural products have received increasing attention in recent years for the discovery of novel anti-cancer agents [10].
Epidemiological studies have suggested that dietary intake of cruciferous vegetables may lower the risk of breast cancer [11,12]. For example, a case-control study involving >300 breast cancer cases and matched controls (matched by age and menopausal status, date of urine collection, and day of laboratory test) documented an inverse correlation between urinary levels of isothiocyanates (ITCs) as a biological measure of cruciferous vegetable intake and the risk of breast cancer [11]. Similarly, Ambrosone et al. [12] have reported that consumption of cruciferous vegetables is inversely associated with the risk of breast cancer in premenopausal women.
Anticarcinogenic effect of cruciferous vegetables is attributed to ITCs, which are generated upon hydrolysis of corresponding glucosinolates through catalytic mediation of myrosinase [13,14]. Benzyl-ITC (BITC) has attracted a great deal of research interest because of its ability to inhibit chemically-induced cancer in animal models [13,14]. For example, BITC is a potent inhibitor of mouse lung carcinogenesis induced by polycyclic hydrocarbons and hepatocarcinogenesis in rats induced by diethylnitrosamine [15,16]. Inhibition of benzo[a]pyrene-induced lung tumorigenesis in A/J mice by dietary administration of N-acetylcysteine conjugate of BITC has also been documented [17]. We have shown previously that BITC treatment inhibits proliferation of cultured human breast cancer cells by causing apoptosis [18]. Interestingly, a spontaneously immortalized non-tumorigenic normal human mammary epithelial cell line (MCF-10A) is significantly more resistant to BITC-mediated apoptosis compared with breast cancer cells [18] demonstrating selectivity of BITC towards malignant breast cells. More recent studies from our laboratory have revealed that the proapoptotic response to BITC in human breast cancer cells is intimately linked to mitochondria-mediated generation of reactive oxygen species due to inhibition of mitochondrial electron transport chain [19]. The BITC-mediated generation of reactive oxygen species in human breast cancer cells serves to trigger activation of c-Jun N-terminal kinase leading to mitochondrial translocation (activation) of multidomain proapoptotic protein Bax [19].
We now report that BITC administration retards growth of MDA-MB-231 cells subcutaneously implanted in female nude mice without causing any side effects. The BITC-mediated inhibition of MDA-MB-231 xenograft growth correlates with reduced cell proliferation and suppression of neoangiogenesis.
Materials and Methods
Reagents
BITC (purity ∼98%) was purchased from LKT Laboratories. The cell culture media, antibiotic mixture, and fetal bovine serum were purchased from Invitrogen. The anti-actin antibody was from Sigma; the antibody against Ki-67 was from DakoCytomation; and the antibodies against CD31 and vascular endothelial growth factor (VEGF) receptor R2 (VEGF-R2) were from Santa Cruz Biotechnology. Apoptosis in tumor sections was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay using ApopTag Plus Peroxidase In situ Apoptosis detection kit (Chemicon International-Millipore). The MDA-MB-231 cell line was purchased from American Type Culture Collection. Monolayer cultures of MDA-MB-231 cells were maintained in RPMI1640 medium supplemented with 10% fetal bovine serum and antibiotics at 37°C in an atmosphere of 5% CO2 and 95% air.
Subcutaneous Xenograft Study
Female nude (nu/nu) mice (6-7 weeks old) were purchased from Harlan Sprague-Dawley and acclimated for 1 week prior to start of the experiment. The mice were administered intraperitoneally (i.p.) with either 100 μL phosphate-buffered saline (PBS) or 100 μL vehicle containing 2.5-, 5.0, and 7.5 μmol BITC (n= 5-6) three times/week (Monday, Wednesday, and Friday) for 2 weeks prior to the subcutaneous tumor cell injection to mimic a prevention protocol. In another experiment, the mice were orally gavaged with either PBS (100 μL) or BITC (6 and 9 μmol) five times/week for 2 weeks prior to the subcutaneous tumor cell injection. The BITC administration in both experiments continued for the duration of the experiment. Exponentially growing MDA-MB-231 cells were suspended in PBS and mixed in a 1:1 ratio with Matrigel. A 0.1 mL suspension containing 2.5-5×106 cells was injected subcutaneously on both left and right flank of each mouse above the hind limb. Tumor volume and body weights were recorded as described by us previously [20].
Immunohistochemical Analysis of Ki-67 and CD31
A portion of the tumor tissue was fixed in 10% neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at 4-5 μm thickness. Representative tumor sections from control and BITC-treated mice were processed for immunohistochemical analysis of (a) Ki-67 expression to assess cell proliferation, and (b) CD31 to visualize blood vessels as described by us previously [21-23]. The vessel area/high power field was calculated with the use of a grid attached to the microscope lens, and expressed as % of total area evaluated in a particular field. Branching structures were counted as a single vessel. Red blood cells were not used to categorize a structure as a blood vessel. Images were analyzed using Image ProPlus 5.0 software (Media Cybernetics). At least five randomly selected non-overlapping high-power (400× magnification) fields were examined on each section.
Detection of Apoptotic Bodies by TUNEL Assay
The paraffin-embedded tissue sections were deparaffinized, rehydrated, and then used to visualize apoptotic bodies by TUNEL staining employing the ApopTag Plus Peroxidase In Situ Apoptosis kit and by following the manufacturer's protocol. Apoptosis was quantified by counting the number of TUNEL-positive cells in at least five non-overlapping high-power fields on each section as described by us previously [21,22].
In Vitro Migration Assay
The effect of BITC treatment on in vitro migration of MDA-MB-231 cells was determined using Transwell Boyden Chamber (Corning) containing a polycarbonate filter with a pore size of 8 μm. Briefly 0.2 ml of MDA-MB-231 cell suspension containing 4×104 cells in complete medium was mixed with desired concentrations of BITC or DMSO (control) and the suspension was added to the upper compartment of the chamber. The lower compartment of the chamber was filled with 0.6 ml of complete medium containing the same concentrations of BITC or DMSO. Following incubation at 37°C for 24 h, the migrated cells on the bottom face of the membrane were fixed with 90% ethanol and stained with eosin. Three randomly selected fields (200× magnification) were scored for migrated cells.
Measurement of VEGF Secretion
The MDA-MB-231 cells were seeded in 12-well plates at a density of 2×105 cells/well and allowed to attach by overnight incubation. Cells were exposed to DMSO or specified concentrations of BITC. After 24 h, culture medium was collected and used for measurement of VEGF secretion using a kit from R&D Systems according to the supplier's instructions.
Immunoblotting
Control and BITC-treated cells and tumor tissues from control and BITC-treated mice were processed for immunoblotting as described by us previously [18,23]. Supernatant proteins were resolved by sodium-dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membrane. Immunoblotting was performed as described by us previously [18,23]. Change in the level of desired protein was determined by densitometric scanning of the immunoreactive bands.
Results
BITC Administration Inhibited Growth of MDA-MB-231 Xenografts in Female Nude Mice
We have shown previously that BITC inhibits growth of cultured MDA-MB-231 cells by causing apoptosis induction [18,19]. To test in vivo relevance of these cellular findings, we determined the effect of i.p BITC administration on growth of MDA-MB-231 cells subcutaneously implanted in female nude mice. The BITC concentrations used in the present study are within the range utilized in previous animal studies [24,25]. The average tumor volume in mice treated with 2.5 and 7.5 μmol BITC was significantly lower compared with vehicle-treated control mice (Figure 1A). For example, 50 days after tumor cell injection the average tumor volume in control mice (1581 ± 240 mm3) was about 2.5- to 3-fold higher compared with BITC-treated mice (P<0.05; Figure 1A). The average wet weight of the tumor was also lower in the BITC treatment groups compared with control mice (Figure 1B). The initial and the final body weights of the control and BITC-treated mice did not differ significantly (Figure 1C). In addition, the BITC-treated mice did not exhibit any signs of stress such as impaired movement or posture, indigestion, and areas of redness or swelling.
Figure 1.
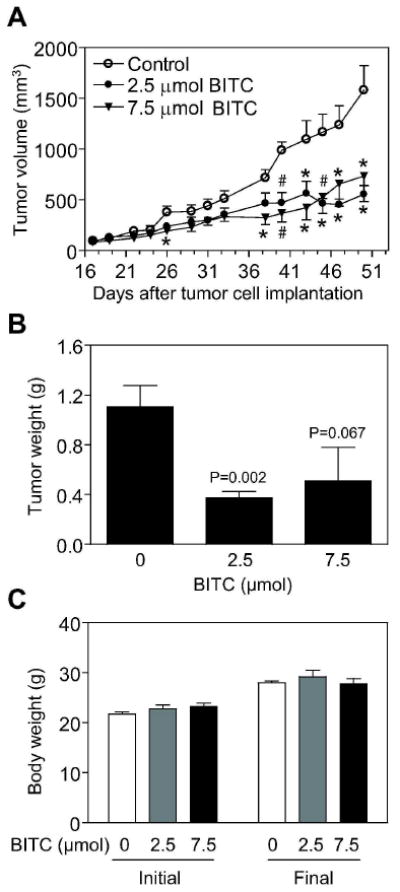
Intraperitoneally administered benzyl isothiocyanate (BITC) inhibited growth of MDA-MB-231 cells subcutaneously implanted in female nude mice. A, average tumor volume as a function of time in vehicle-treated control mice and BITC-treated mice (i.p. administration, three times per week). Points, mean (n= 8-9 for the control group, n= 8-10 for the 2.5 μmol BITC group, and n= 9-12 for the 7.5 μmol BITC group); bars, SE. There were 5 mice in the control and 2.5 μmol BITC group and 6 mice in the 7.5 μmol BITC group with tumor cells implanted on both left and right flank of each mouse. The tumor did not grow on one side in one mouse of the control group. One mouse in the 2.5 μmol BITC group was sacrificed on day 43 after tumor cell injection due to large tumor burden on one side. In one mouse of the 7.5 μmol BITC group, the tumor regressed on both sides by the day 31 and in another mouse of this group complete tumor regression was evident on one side on day 45 after tumor cell injection. Significantly different, *P<0.05 and #P<0.01, compared with control by one-way ANOVA followed by Dunnett's test. B, average wet weight of the tumor in vehicle-treated control and BITC-treated mice. Columns, mean (n= 8-10); bars, SE. Statistical significance was determined by Student's t-test. C, average initial and final body weights of the control and BITC-treated mice. Columns, mean; bars, SE.
In another experiment, we evaluated the effect of BITC administration by oral gavage (6 and 9 μmol, five times per week) on growth of MDA-MB-231 cells subcutaneously implanted in female nude mice. The growth of MDA-MB-231 xenograft was inhibited significantly by oral gavage with 9 μmol BITC (Figure 2A), but not with 6 μmol (results not shown), compared with vehicle-treated control. The average initial body weight of the mice from BITC treatment group was modestly but significantly higher compared with control mice and this differential was maintained at the termination (final body weight) of the study (Figure 2B). Mice orally gavaged with BITC did not exhibit any signs of distress. These results indicated that BITC administration inhibited growth of MDA-MB-231 xenografts without causing any side effects.
Figure 2.
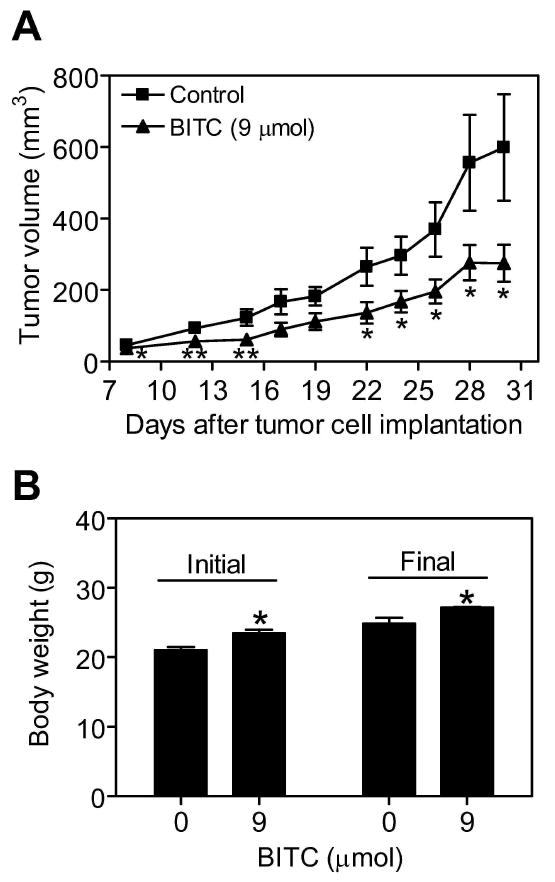
Oral gavage of benzyl isothiocyanate (BITC) inhibited MDA-MB-231 xenograft growth in female nude mice. A, average tumor volume over time in vehicle-treated control mice and mice orally gavaged with 9 μmol BITC (5 times/week). Points, mean (n= 7 for the control group, n= 9 for the 9 μmol BITC group); bars, SE. There were 5 mice in both the control and the 9 μmol BITC treatment groups with tumor cells implanted on both left and right flank of each mouse. Tumor did not grow on both sides of one mouse in the control group, and tumor size was very small on one side in one mouse of both the control and the BITC-treated groups. Significantly different (*P<0.05 and **P<0.02) compared with control by Students t-test. B, average initial and final body weights of the control and BITC-treated mice. Columns, mean; bars, SE.
BITC Administration Suppressed Cell Proliferation and Neoangiogenesis in the Tumor
Next, we proceeded to determine whether the BITC-mediated suppression of MDA-MB-231 xenograft growth in vivo was accompanied by inhibition of cell proliferation and/or apoptosis induction using tumor tissues collected from the first subcutaneous xenograft study. Figure 3A depicts immunohistochemical analysis for Ki-67 expression in representative tumor section from a mouse of both the control group and the 7.5 μmol BITC treatment group. The Ki-67 is a large nuclear protein expressed during all active phases of the cell cycle and a well-accepted marker of cellular proliferation [26]. As can be seen in Figure 3A, the BITC administration resulted in a dose-dependent and statistically significant decrease in Ki-67 expression relative to vehicle-treated control. For example, the number of Ki-67 positive cells was lower by about 38% (P= 0.04 compared with control) and 67% (P= 0.04 compared with control) in tumor sections from mice administered i.p. with 2.5 and 7.5 μmol BITC, respectively, compared with tumors from control mice (Figure 3A). Contrary to the results in MDA-MB-231 cells [18,19], the BITC administration failed to elicit apoptosis in the tumor as judged by TUNEL assay (Figure 3B).
Figure 3.
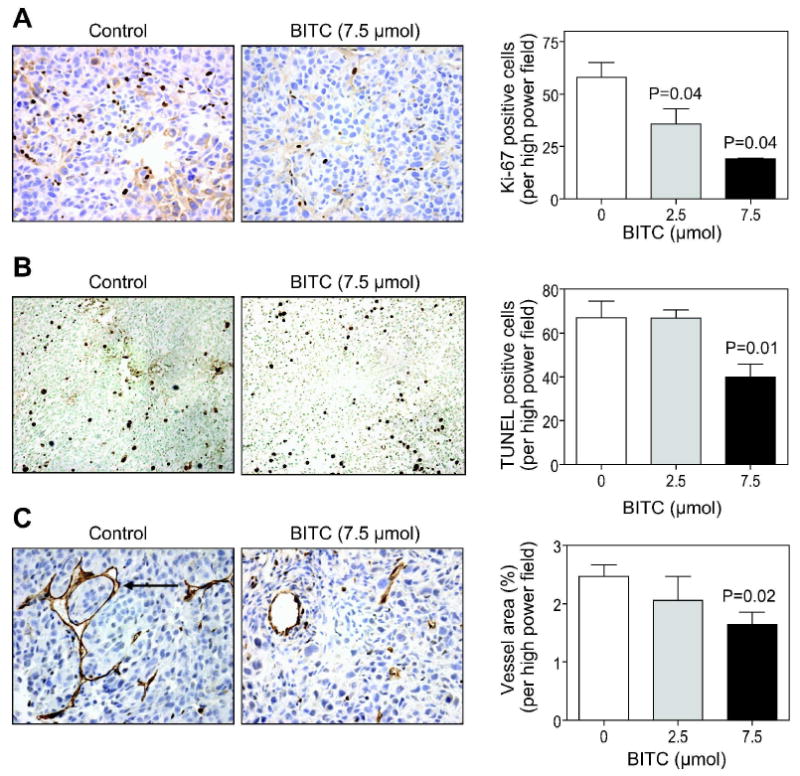
Benzyl isothiocyanate (BITC) administration reduced cell proliferation and suppressed angiogenesis in MDA-MB-231 tumors (subcutaneous study with i.p. BITC administration). (A) expression of Ki-67, (B) TUNEL-positive apoptotic bodies, and (C) CD31-positive blood vessels (identified by an arrow) in representative tumor section of a mouse of both the control group and the 7.5 μmol BITC treatment group (400× magnification). Columns, mean (n= 4-5 tumor sections from different mice); bars, SE. Statistical significance compared with control was determined by the Student's t-test.
Angiogenesis (formation of new blood vessels) is a highly complex and tightly regulated physiological process that is implicated in pathogenesis of many chronic diseases including cancer [27]. To test whether BITC administration resulted in suppression of angiogenesis, we performed immunohistochemistry for angiogenic marker CD31 to visualize blood vessels in tumor sections from control and BITC-treated mice. Staining for CD31 in tumor section from a representative mouse of both the control group and the 7.5 μmol BITC treatment group is shown in Figure 3C. The vessel area was significantly lower in the tumor sections from mice administered i.p. with 7.5 μmol BITC in comparison with control mice (P= 0.02). Together, these results indicated that BITC administration inhibited subcutaneous MDA-MB-231 xenograft growth in vivo by reducing cell proliferation and inhibiting angiogenesis.
BITC Treatment Inhibited MDA-MB-231 Cell Migration
Angiogenesis inhibition by BITC in vivo led us to investigate the effect of BITC treatment on migration potential of MDA-MB-231 cells by Boyden chamber assay. Figure 4A depicts eosin-stained images of migrating MDA-MB-231 cells after 24 h treatment with DMSO (control) or 5 μM BITC. As can be seen in Figure 4B, migration of MDA-MB-231 cells was inhibited significantly in a concentration-dependent manner. For example, compared to control the average number of migratory cells was decreased by ∼33% (P<0.001), 74% (P<0.001), and 87% (P<0.001), respectively, in the presence of 2.5, 5 and 7.5 μM BITC (Figure 4B). These results showed that migratory potential of MDA-MB-231 cells was significantly reduced in the presence of BITC.
Figure 4.
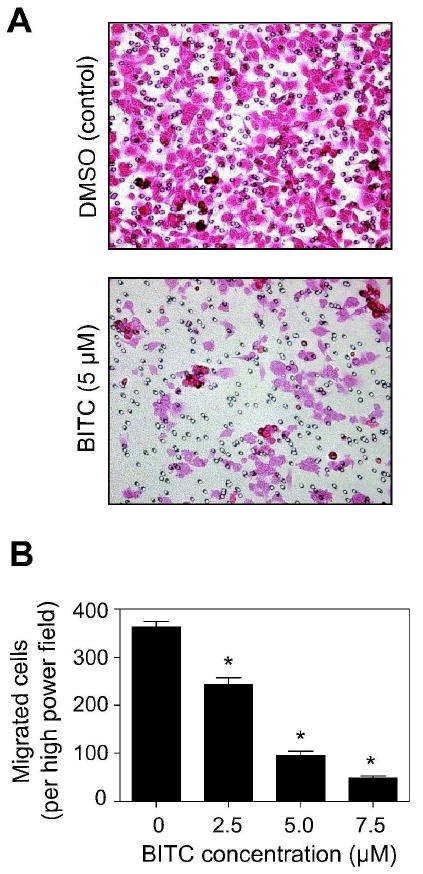
Benzyl isothiocyanate (BITC) treatment inhibited migration of cultured MDA-MB-231 cells. A, representative images depicting the effect of BITC treatment on MDA-MB-231 cell migration. B, quantitation of migrating cells per high power field. Columns, mean (n=3); bars, SE. Significantly different (*P<0.001) compared with control by Student's t-test.
BITC Treatment Suppressed VEGF Secretion and VEGF-R2 Protein Levels in MDA-MB-231 Cells In Vitro and In Vivo
VEGF plays an important role in angiogenesis by promoting endothelial cell proliferation, migration, and differentiation [28]. As shown in Figure 5A, BITC treatment modestly inhibited VEGF secretion into the medium especially at the 7.5 μM concentration (P=0.04 compared with DMSO-treated control). Next, we determined the effect of BITC treatment on protein level of VEGF-R2, a receptor involved in VEGF-mediated signal transduction [29,30], in cultured MDA-MB-231 cells. The protein lysates of MDA-MB-231 cells exposed to 2.5, 5.0, and 7.5 μM BITC for 24 h and 48 h revealed a 30-60% suppression in VEGF-R2 protein level compared to control (Figure 5B). We sought to confirm in vivo relevance of these observations by determining the expression of VEGF-R2 in the MDA-MB-231 tumors of mice that were injected i.p. with PBS or 7.5 μmol of BITC. Consistent with cellular results (Figure 5B), immunoblotting revealed significantly lower level of VEGF-R2 in the tumors from BITC-treated mice compared with control mice (P= 0.03 between BITC and control groups) (Figure 5C,D). Together these results indicated that BITC-mediated inhibition of MDA-MB-231 tumor xenograft growth was accompanied by suppression of VEGF-R2 expression.
Figure 5.
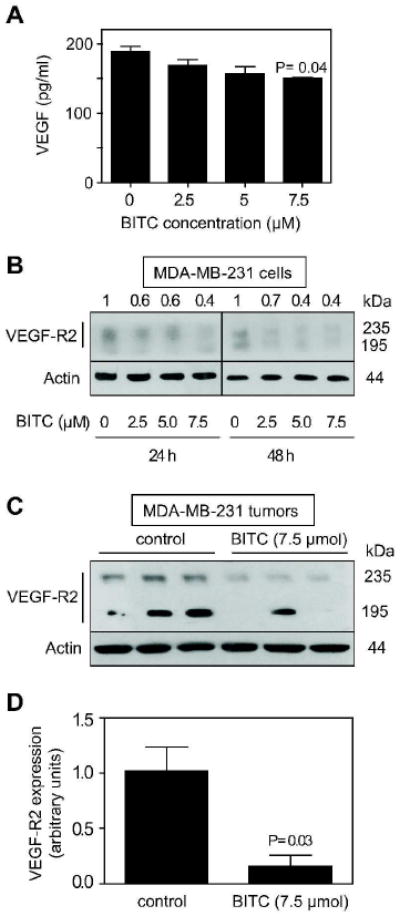
Benzyl isothiocyanate (BITC) reduced expression of VEGF-R2. A, VEGF concentration (pg/ml) in the media of MDA-MB-231 cells cultured for 24 h in the presence of DMSO (control) or 2.5-, 5-, and 7.5 μM BITC. Columns, mean (n=3); bars, SE. Statistical significance compared with control was determined by the Student's t-test. B, immunoblotting for VEGF-R2 using lysates from MDA-MB-231 cells exposed to DMSO (control) or 2.5-, 5-, and 7.5 μM BITC for 24 h or 48 h. The membrane was stripped and reprobed with anti-actin antibody to ensure equal protein loading. C, immunoblotting for VEGF-R2 using supernatants from tumors of mice injected i.p. with PBS or 7.5 μmol BITC. The membrane was stripped and reprobed with anti-actin antibody to ensure equal protein loading. D, quantification of VEGF-R2 expression in tumors from control and BITC-treated mice. Columns, mean (n=3); bars, SE. Statistical significance compared with control was determined by the Student's t-test.
Discussion
The present study demonstrates that cruciferous vegetable constituent BITC significantly inhibits in vivo growth of an ER-independent human breast cancer cell line in female nude mice without causing weight loss or any other harmful side effects. The in vivo growth inhibitory effect of BITC is retained irrespective of the route of administration. A concentration of 2.5 μmol BITC by i.p. administration was significantly effective in retarding growth of MDA-MB-231 xenografts, whereas BITC-mediated growth inhibition by oral gavage was observed only at the higher dose (9 μmol). These observations suggest that oral bioavailability of BITC may be limited. In addition, a clear dose-response relationship was not evident in the experiment involving i.p. administration of BITC, which may partly be attributable to a relatively larger data scatter at the 7.5 μmol dose (Figure 1B). On the other hand, the possibility that higher i.p. dose of BITC triggers mechanisms that attenuate BITC-mediated growth inhibition can not be fully discarded. Future work may shed light into these possibilities. The concentrations of the BITC effective against MDA-MB-231 xenograft growth in vivo are within the range that can be generated through dietary intake of cruciferous vegetables [13,14]. Technical difficulties due to volatility prevented us from measuring the levels of BITC and its metabolites in the plasma and tumor tissues of mice. Nonetheless, the present study provides experimental evidence for in vivo efficacy of BITC against an estrogen-independent human breast cancer cell line.
Cellular systems are valuable in obtaining mechanistic insights not accessible otherwise. The observations made in cells, however, need to be confirmed in animal models to establish in vivo relevance of the cellular findings. In addition, an understanding of the mechanism by which BITC inhibits MDA-MB-231 cell growth in vivo is critical for its further development as a chemopreventive/therapeutic agent since this knowledge could lead to identification of biomarker(s) of BITC response potentially useful in future clinical trials. Identification of biomarker(s) is especially desirable for cancer chemoprevention trials because reduction of cancer incidence is too rigorous of an endpoint for malignancies with a long latency such as breast cancer. The present study reveals that, consistent with cellular data [18], the BITC-mediated suppression of MDA-MB-231 cell growth is accompanied by a marked reduction in cell proliferation. In contrast to the cellular findings [18,19], however, we were unable to see an increase in TUNEL-positive apoptotic bodies in the tumor sections from BITC-treated mice compared with control mice. Our previous work has demonstrated that BITC treatment causes apoptosis in both estrogen-independent (MDA-MB-231) and estrogen-responsive (MCF-7) human breast cancer cells [18,19]. However, it remains to be determined whether resistance of MDA-MB-231 xenografts to apoptosis induction by BITC administration in vivo is unique to this cell line or translates to the MCF-7 xenografts as well.
Angiogenesis in tumor permits the growth and invasiveness of cancer cells leading to metastasis to distant organs [30-33]. Angiogenesis is especially critical for growth and progression of solid tumors since growth in tumor mass beyond 2-3 mm is often preceded by an increase in formation of new blood vessels presumably essential for delivery of nutrients and oxygen to the tumor microenvironment [32,33]. Therefore, anti-angiogenic therapy represents one of the most promising approaches to control tumor growth and invasiveness [34]. The present study indicates that the BITC-mediated suppression of MDA-MB-231 xenograft growth is accompanied by inhibition of neovascularization in the tumor as evidenced by a marked reduction in the vessel area. It is reasonable to conclude that inhibition of angiogenesis is an important mechanism in anticancer effect of BITC.
VEGF is a pro-angiogenic growth factor associated with aggressive human cancer cells [35]. VEGF triggers pro-survival signals to normal and tumor-derived endothelial cells [28] by binding to VEGF-R2 receptor, also shown to be involved in angiogenesis [29,30]. We found that in vitro exposure of MDA-MB-231 cells to BITC results in the suppression of VEGF secretion and a reduction in VEGF-R2 protein level. The BITC-mediated suppression of VEGF-R2 expression is also observed in MDA-MB-231 tumors. Together, these results suggest that the inhibition of angiogenesis in MDA-MB-231 tumors is most likely due to the suppression of VEGF-R2 expression. However more work is needed to elucidate the mechanism by which BITC suppresses VEGF secretion and VEGF-R2 protein expression.
In conclusion, the present study indicates that BITC administration retards growth of MDA-MB-231 xenografts in female nude mice without causing weight loss or any other side effects. The BITC-mediated suppression of MDA-MB-231 xenograft growth in vivo correlates with inhibition of cell proliferation and suppression of neoangiogenesis.
Acknowledgments
This investigation was supported by the US PHS grant CA129347, awarded by the National Cancer Institute.
Abbreviations
- ITC
isothiocyanate
- BITC
benzyl isothiocyanate
- ER
estrogen receptor
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- i.p.
intraperitoneal
- VEGF
vascular endothelial growth factor
- VEGF-R2
VEGF receptor 2
- PBS
phosphate-buffered saline
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Jemal A, Ward E, Thun MJ. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 2008;19:537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 3.van de Ven SM, Elias SG, van den Bosch MA, Luijten P, Mali WP. Optical imaging of the breast. Cancer Imaging. 2008;8:206–215. doi: 10.1102/1470-7330.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz M, Estevez LG, Alvarez, et al. Evaluation of international treatment guidelines and prognostic tests for the treatment of early breast cancer. Cancer Treat Rev. 2008;34:701–709. doi: 10.1016/j.ctrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 6.Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–887. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1. Study J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 10.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 11.Fowke JH, Chung FL, Jin F, et al. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003;63:3980–3986. [PubMed] [Google Scholar]
- 12.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 13.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–255. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 14.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 15.Wattenberg LW. Inhibitory effects of benzyl isothiocyanate administered shortly before diethylnitrosamine or benzo[a]pyrene on pulmonary and forestomach neoplasia in A/J mice. Carcinogenesis. 1987;8:1971–1973. doi: 10.1093/carcin/8.12.1971. [DOI] [PubMed] [Google Scholar]
- 16.Sugie S, Okumura A, Tanaka T, Mori H. Inhibitory effects of benzyl isothiocyanate and benzyl thiocyanate on diethylnitrosamine-induced hepatocarcinogenesis in rats. Jpn J Cancer Res. 1993;84:865–870. doi: 10.1111/j.1349-7006.1993.tb02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YM, Conaway CC, Chiao JW, et al. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 18.Xiao D, Vogel V, Singh SV. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther. 2006;5:2931–2945. doi: 10.1158/1535-7163.MCT-06-0396. [DOI] [PubMed] [Google Scholar]
- 19.Xiao D, Powolny AA, Singh SV. Benzyl isothiocyanate targets mitochondrial respiratory chain to trigger ROS-dependent apoptosis in human breast cancer cells. J Biol Chem. 2008;283:30151–30163. doi: 10.1074/jbc.M802529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh SV, Mohan RR, Agarwal R, et al. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun. 1996;225:660–665. doi: 10.1006/bbrc.1996.1226. [DOI] [PubMed] [Google Scholar]
- 21.Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–9511. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SV, Warin R, Xiao D, et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009;69:2117–2125. doi: 10.1158/0008-5472.CAN-08-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao D, Lew KL, Kim Y, et al. Diallyl trisulfide suppresses growth of PC-3 human prostate cancer xenograft in vivo in association with Bax and Bak induction. Clin Cancer Res. 2006;15:6836–6843. doi: 10.1158/1078-0432.CCR-06-1273. [DOI] [PubMed] [Google Scholar]
- 24.Hecht SS, Kenney PM, Wang M, Trushin N, Upadhyaya P. Effects of phenethyl isothiocyanate and benzyl isothiocyanate, individually and in combination, on lung tumorigenesis induced in A/J mice by benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2000;150:49–56. doi: 10.1016/s0304-3835(99)00373-0. [DOI] [PubMed] [Google Scholar]
- 25.Boysen G, Kenney PM, Upadhyaya P, Wang M, Hecht SS. Effects of benzyl isothiocyanate and 2-phenethyl isothiocyanate on benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism in F-344 rats. Carcinogenesis. 2003;24:517–525. doi: 10.1093/carcin/24.3.517. [DOI] [PubMed] [Google Scholar]
- 26.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–156. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 29.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 32.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 34.Dhanabal M, Jeffers M, Larochelle WJ. Anti-angiogenic therapy as a cancer treatment paradigm. Curr Med Chem Anticancer Agents. 2005;5:115–130. doi: 10.2174/1568011053174882. [DOI] [PubMed] [Google Scholar]
- 35.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]


