Abstract
Objectives
Liver disease in Alagille syndrome is highly variable ranging from biochemical abnormalities only to end-stage disease. It is not possible to predict whether a child with cholestasis will have improvement or progression of liver disease. This poses a challenge to the clinician in terms of timing therapies. The study aim was to identify laboratory markers present under the age of 5 years that could predict the ultimate outcome of liver disease in Alagille syndrome.
Methods
A retrospective review of laboratory data from 33 Alagille syndrome subjects was performed. Patients greater than 10 years of age were stratified into mild (22) and severe (11) hepatic outcome groups. Non-parametric analysis was performed on longitudinal data from birth-5years to determine association with hepatic outcome. JAGGED1 mutational analysis was performed on available samples.
Results
The following variables were statistically different between severe and mild outcome groups; total bilirubin (p= 0.0001), conjugated bilirubin (p =0.0066), and cholesterol (p =0.0022). Further analysis revealed cutoff values that differentiated between severe and mild outcomes; total bilirubin 6.5mg/dL(111micromol/L), conjugated bilirubin 4.5mg/dL(77micromol/L) and cholesterol 520mg/dL(13.5mmol/L). Genetic analysis of JAGGED1 mutations did not reveal genotype-phenotype correlation.
Conclusions
Total bilirubin above 6.5mg/dL, conjugated bilirubin above 4.5mg/dL and cholesterol above 520mg/dL under the age of 5 years are likely to be associated with severe liver disease in later life. These data represent cutoff values below which a child is likely to have a benign outcome and above which more aggressive therapy may be warranted, and can thus be used to guide management.
Keywords: Alagille, liver, cholestasis, transplantation
Introduction
Alagille syndrome (AGS), an autosomal dominant genetic disorder, is one of the major forms of chronic liver disease in childhood, with a minimal estimated frequency of 1/50,000 individuals (1). AGS is a significant cause of liver transplantation. Traditionally the clinical diagnosis of AGS has been based on the criteria established by Alagille et al. (2) and includes the histological finding of paucity of the interlobular bile ducts on liver biopsy in association with a minimum of three of five major clinical features (chronic cholestasis, cardiac disease, skeletal abnormalities, ocular abnormalities and characteristic facial features). Several other organs and structures have been noted to be involved to a lesser degree (kidney, pancreas, cerebrovasculature, and the extremities) (3-7). Within families, the clinical manifestations range from minimal features (sub-clinical) to individuals with end-stage liver and heart disease (1). Twenty to 30% of patients will require liver transplantation (5, 8-10).
In the majority of infants with AGS, conjugated hyperbilirubinemia is noted in the first weeks or months of life. Of patients who present with liver disease in infancy, 20-30% will eventually go on to develop intractable portal hypertension, cirrhosis or synthetic liver failure. At present there are no known predictors of progression to end-stage liver disease. In the early reports of AGS, the frequency of liver disease was thought to be close to 100%, as the diagnosis was made based on clinical criteria that included cholestasis. However, with the demonstration that AGS is caused by mutations in the JAGGED1 (JAG1) gene, it became possible to identify a wider spectrum of mutation carriers, and these studies demonstrated that JAG1 mutations can be associated with a wider range of liver phenotypes (1). A cohort of JAG1 mutation-positive relatives was ascertained in order to describe the milder end of the AGS phenotype. Whereas the mutation-positive proband's in this group were universally affected by liver disease, 45% of the mutation-positive relatives had no biochemical liver abnormalities at all.
Variability of hepatic involvement is a hallmark characteristic of AGS. Hepatic disease in AGS ranges from asymptomatic elevation of hepatic transaminases to end stage liver disease, which occurs in approximately 20-30% of cases (5, 8-10). Most infants have cholestasis; however, this may resolve in certain patients by school-age and progress to end-stage liver disease in others. Children with mild liver disease in early childhood rarely have disease progression in later life. Furthermore there is no late-onset of cholestasis outside childhood. It is known that young children with significant hepatic involvement may demonstrate resolution of their cholestasis and this is a unique feature of AGS (5, 9). However, it is not possible to predict whether a child with cholestasis in early childhood will have improvement of their liver disease, or be in the group that progresses to persistent cholestasis and/or end-stage liver disease in later life. To date there have been no laboratory, radiologic, histologic or genotype predictors of this outcome (5, 9). A toddler with significant pruritus and xanthomas may still have resolution of liver disease and might benefit from aggressive medical management, but avoidance of liver transplantation. However, if this child falls into the group that will have progressive liver disease, additional time waiting for the outcome to be clinically apparent may worsen a child's nutritional status and overall health going into transplant. Thus the inability to predict hepatic outcome of a young child with AGS poses a challenge to the clinician in terms of timing therapies, in particular liver transplantation. We hypothesized that the extreme variability in liver disease outcome in AGS can be predicted using laboratory parameters identifiable in early childhood.
Materials and Methods
A cohort of individuals who met defining clinical criteria for AGS were ascertained from databases at The Children's Hospital of Philadelphia (CHOP) and King's College Hospital (KCH). These criteria were at least 3 of the 5 following features; cholestatic liver disease, congenital heart disease, skeletal anomalies, ocular abnormalities and characteristic facies. All patients had been enrolled under Institutional Review Board-approved protocols of consent. Subjects were ascertained based on age greater than 10 years and the availability of detailed laboratory and clinical information from birth to 5 years. Twenty-five individuals were identified from CHOP and 8 from KCH.
A retrospective review of longitudinal laboratory data was collected from this cohort of 33 AGS patients. Serial measurements of white cell count, hemoglobin, platelets, alanine aminotransferase, aspartate aminotransferase, total bilirubin (TB), conjugated bilirubin (CB), gamma-glutamyl transpeptidase, alkaline phophatase, cholesterol, triglycerides, albumin, and INR. Patients were stratified according to severity of liver disease (mild or severe) based on the most recent clinical data obtained at or above the age of 10 years. The cut-off of 10 years was selected as an appropriate age at or above which an individual could be confidently assigned to an outcome group.
Mild patients included those with biochemical abnormalities and mild cholestasis in whom symptoms were well controlled with medical therapies. This group included patients with mild to moderate pruritus and xanthomas but without cutaneous mutilation, significant growth impairment or disruption of daily functioning due to their disease. Individuals with severe disease comprised those with complications of cholestasis manifested by intractable pruritus or growth impairment requiring supplemental feeds. In addition the severe group included those patients who had had biliary diversion or liver transplantation. This severe group encompassed those AGS patients with unremitting cholestasis and a subset who developed fibrosis and portal hypertension following years of profound cholestasis. The clinical characteristics of the cohort are outlined in Table 1.
Table 1.
Clinical Characteristics of Study Participants at Time of Hepatic Classification
| Clinical Data Relevant for Hepatic Stratification | Mild Patients |
| Biochemical abnormalities without cholestasis | 2 |
| Cholestasis but no pruritus (no medications) | 5 |
| Cholestasis and pruritus responsive to medications | 15 |
| Severe Patients | |
| Severe cholestasis with pruritus, unresponsive to medication | 1 |
| Severe cholestasis with cirrhosis and portal hypertension | 2 |
| Biliary diversion for severe cholestasis and intractable pruritus | 1 |
| Transplantation for profound cholestasis | 5 |
| Transplantation for complications of portal hypertension | 2 |
Cholestasis defined by
• Conjugated bilirubin > 2mg/dL, or
• GGT > 3x upper limit of normal, or
For every independent variable the mean and median values of the serial laboratory measurements from birth to age 5 years was calculated for each patient. These means were calculated based on a range of 5-19 values of the laboratory parameter over 5 years per patient. For example, for total bilirubin the mean for the cohort was derived from 561 values which is an average of 17 (561/33) values per patient. Non-parametric analysis using the Wilcoxon test was then performed to identify statistical association between these means and the 2 hepatic outcome groups (mild/severe). Threshold values were calculated based on an average of the means of all the patients in each outcome group and p-values were obtained by using Fisher's exact test. An assessment of the relationship between any statistically significant variables was then made.
Mutational analysis of JAG1, was performed on all available samples using a genomic sequencing approach.
Results
The study cohort was stratified into 22 mild and 11 severe subjects. The biologic characteristics of the subjects on which their stratification was based are outlined in Table 1. Non-parametric analysis of the mean laboratory values in each outcome group revealed 8 variables that met statistical significance (p<0.05), and of these 3 were highly significant, namely TB, CB and cholesterol (Table 2). Albumin was one of the variables that met statistical significance but with a less dramatic p value (p=0.23). INR data was not included as there were missing values for each patient and the vitamin K status at the time of the blood draw was unknown. Similar results with statistical significance were obtained with the mean and medians and therefore mean data only is shown here.
Table 2.
Results of Non-parametric Testing Using the Mean of Each Variable for Each Patient Aged Birth to Five Years
| Independent Variable | Mild | Severe | Total | p-value* | |||
|---|---|---|---|---|---|---|---|
| N | mean | N | mean | N | mean | ||
| AST (SGOT) (U/L) | 21 | 176.5 | 11 | 241.3 | 32 | 198.8 | 0.018 |
| Total Bilirubin (mg/dL) | 22 | 2.8 | 11 | 10.5 | 33 | 5.3 | 0.0001 |
| Unconjugated Bilirubin (mg/dL) | 7 | 0.8 | 7 | 1.7 | 14 | 1.2 | 0.048 |
| Conjugated Bilirubin (mg/dL) | 6 | 1.6 | 7 | 7.4 | 13 | 4.7 | 0.0066 |
| Cholesterol (mg/dL) | 18 | 358.5 | 11 | 685 | 29 | 482.4 | 0.0022 |
| Triglycerides (mg/dL) | 16 | 171.5 | 10 | 249.7 | 26 | 201.5 | 0.015 |
| Albumin (g/dL) | 21 | 4.5 | 11 | 4.0 | 32 | 4.3 | 0.023 |
p-value is obtained from Wilcoxon two-sided
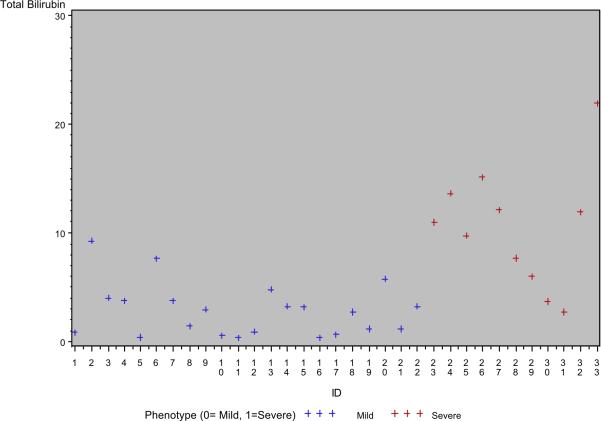
Scatter plots of the highly significant mean variables for each patient are displayed in Figure 1. There is clear clustering of the patients into mild and severe outcome groups based on mean laboratory values of interest. Non-parametric analysis identified threshold or cut-off values that differentiated between the 2 outcome groups (Table 3). A TB of 6.5 mg/dL (111micromol/L), CB of 4.5 mg/dL (77micromol/L) and cholesterol of 520 mg/dL (13.5mmol/L) represent threshold values that differentiate between mild and severe outcome groups with statistical significance.
Figure 1. Scatter Plots of Highly Significant Variables.
Each cross represents the mean of the serial laboratory values from birth to 5 years for an individual patient. Red crosses represent patients from the severe outcome group and black crosses represent patients from the mild outcome group. Total bilirubin, conjugated bilirubin and cholesterol are expressed as mg/dL along the y-axis of each plot.
Table 3.
Threshold Values based on the Average of the Means from Each Outcome Group
| Independent variable | # value <cutoff* | # value >cutoff* | Misclassification Rate*** | p-value** | |
|---|---|---|---|---|---|
| Total Bilirubin (cutoff=6.6) | Mild | 20 | 2 | 5/33 | 0.00043 |
| Severe | 3 | 8 | |||
| Conjugated Bilirubin (cutoff=4.5) | Mild | 6 | 1 | 1/13 | 0.0047 |
| Severe | 0 | 6 | |||
| Cholesterol (cutoff=520) | Mild | 15 | 3 | 7/29 | 0.017 |
| Severe | 4 | 7 | |||
These columns represent the numbers of patients in each classification group with a mean value of the variable above and below the cut-off thresholds.
p-value is two-sided and obtained from Fisher's Exact Test
The misclassification rate is a measure of how often a patient would be classified into the wrong outcome group based on the suggested threshold.
Further analysis of the relationship between these three statistically significant variables was performed. Multivariate analysis was not be done since the sample size is relatively small and therefore the power would be low and it may not have been possible to detect some important predictors. Therefore both Pearson's and Spearman's correlation coefficients were calculated. The total and conjugated bilirubin were clearly related (as expected) with high statistical significance (correlation coefficients from Pearson's 0.98 with p-value<0.0001 and Spearman's 0.95 with p-value<0.0001). In addition the correlation coefficients of cholesterol and the total bilirubin were 0.45 (p-value=0.015) and 0.74 (p-value<0.0001) from Pearson's and Spearman's methods, respectively, so both were statistically significant (at a level of <0.05).
Of the 33 study patients the 25 from CHOP had samples available for mutational analysis. All patients tested had JAG1 mutations, which were missense (14/25) or nonsense/frameshift mutations (11/25). There was no statistically significant genotypephenotype correlation between mutation type and hepatic outcome.
Discussion
It is well established that the liver disease of AGS can vary from biochemical abnormalities only to end-stage liver disease, in patients sharing the same JAG1 mutation (1). Furthermore, in any individual there is variability in the natural history of the disease. In a young child with mild liver disease, there is no disease progression outside childhood. However, in a young child with cholestasis, the liver disease may progress, resolve or remain static (9). The inability to predict the outcome in later childhood of the early presentation of cholestasis hampers the ability of clinicians to manage AGS liver disease optimally. This study demonstrates that early significant cholestasis can improve in AGS. In this cohort of 33 patients it can be seen that cholestasis under the age of 5 years commonly resolves and is associated with a good eventual hepatic outcome. A TB of up to 6.5 mg/dL, CB up to 4.5 mg/dL and cholesterol up to 520 mg/dL under the age of 5 years can still be associated with minimal liver disease in later life. More importantly, these data represent threshold values that differentiate between mild and severe hepatic outcomes.
There are several limitations of this study. The stratification of the individuals into mild and severe groups was based on a systematic but not validated approach. The patients were selected on the basis of availability of clinical and laboratory data and not on clinical picture so as to avoid any selection basis. The patients appear to reflect a representative AGS cohort without selection for extreme cases that may inflate the validity of statistical associations. Replication of this study in a larger cohort would address this issue. In addition, due to the small cohort it was necessary to take the laboratory values from 0-5 years and treat it as one dataset rather analyzing the data per patient year of age to identify cut-offs at different ages or even peak and trough levels in a given year. A larger dataset in a future study may allow this level of detailed analysis.
Although the cohort utilized in this study was small, the three variables identified as important predictors (TB, CB and cholesterol) were highly statistically significant in their association with outcome. These parameters also make biologic sense as predictors of outcome in AGS since the hepatic involvement is cholestatic and end-stage disease usually develops as a result of profound cholestasis, rather than progressive fibrosis and cirrhosis. This is also borne out by the lack of statistical association with albumin and the two outcome groups, which might have been expected given its value as a measure of synthetic function. Unfortunately the INR data was insufficient and not reported as the vitamin K status of the patients was unknown.
There is significant value in having non-invasive parameters that can help predict outcome in such a variable disease. Liver histology in AGS does not appear to be associated with the degree of cholestasis in a given patient and the necessity of an invasive biopsy makes this an unhelpful tool in monitoring AGS liver disease and predicting outcome. Further studies are now warranted to replicate these threshold values and then potentially develop more sophisticated models of hepatic disease progression in AGS using this and other clinical data such as growth or bone fractures. Although a multivariate analysis was not possible here due to the small sample size, the significant correlation coefficients between the three variables of interest, suggests a relationship between the three, which may be of use for predictive modeling in a larger dataset.
Thus laboratory parameters in early childhood can predict the eventual outcome of liver disease in AGS, despite the variable natural history. This study suggests cutoff laboratory values below which a child is likely to have a benign outcome at a later age and above which more aggressive therapy may be warranted. These values may assist the clinician in guiding optimal management strategies. A child with laboratory values above these thresholds should be considered at greater probability of having a severe hepatic outcome in later life and therefore may benefit from earlier transplantation evaluation. More importantly, the presence of significant cholestasis in a young child should not automatically prompt a liver transplantation. These threshold values, in addition to clinical judgement, may be used as a guide to promote conservative measures and thereby possibly avoid unnecessary liver transplantation in young children with AGS. Replication of these findings in a larger cohort of AGS individuals is now warranted and would justify use of such parameters in the clinical setting.
Footnotes
Financial Disclosure: The authors have no financial relationships to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamath BM, Bason L, Piccoli DA, et al. Consequences of JAG1 mutations. J Med Genet. 2003;40:891–5. doi: 10.1136/jmg.40.12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alagille D, Estrada A, Hadchouel M, et al. Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr. 1987;110:195–200. doi: 10.1016/s0022-3476(87)80153-1. [DOI] [PubMed] [Google Scholar]
- 3.Berard E, Sarles J, Triolo V, et al. Renovascular hypertension and vascular anomalies in Alagille syndrome. Pediatr Nephrol. 1998;12:121–4. doi: 10.1007/s004670050418. [DOI] [PubMed] [Google Scholar]
- 4.Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr. 2005;41:99–107. doi: 10.1097/01.mpg.0000162776.67758.2f. [DOI] [PubMed] [Google Scholar]
- 5.Emerick KM, Rand EB, Goldmuntz E, et al. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29:822–9. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 6.Hyams JS, Berman MM, Davis BH. Tubulointerstitial nephropathy associated with arteriohepatic dysplasia. Gastroenterology. 1983;85:430–4. [PubMed] [Google Scholar]
- 7.Kamath BM, Spinner NB, Emerick KM, et al. Vascular anomalies in Alagille syndrome: a significant cause of morbidity and mortality. Circulation. 2004;109:1354–8. doi: 10.1161/01.CIR.0000121361.01862.A4. [DOI] [PubMed] [Google Scholar]
- 8.Hoffenberg EJ, Narkewicz MR, Sondheimer JM, et al. Outcome of syndromic paucity of interlobular bile ducts (Alagille syndrome) with onset of cholestasis in infancy. J Pediatr. 1995;127:220–4. doi: 10.1016/s0022-3476(95)70298-9. [DOI] [PubMed] [Google Scholar]
- 9.Lykavieris P, Hadchouel M, Chardot C, et al. Outcome of liver disease in children with Alagille syndrome: a study of 163 patients. Gut. 2001;49:431–5. doi: 10.1136/gut.49.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quiros-Tejeira RE, Ament ME, Heyman MB, et al. Variable morbidity in alagille syndrome: a review of 43 cases. J Pediatr Gastroenterol Nutr. 1999;29:431–7. doi: 10.1097/00005176-199910000-00011. [DOI] [PubMed] [Google Scholar]