Abstract
Refractory celiac disease (RCD) is defined by persistent or recurrent malabsorptive symptoms and villous atrophy despite strict adherence to a gluten-free diet (GFD) for at least 6–12 months in the absence of other causes of non-responsive treated celiac disease (CD) and overt malignancy. Symptoms are often severe and require additional therapeutic intervention besides GFD. RCD can be classified as type 1 (normal intraepithelial lymphocyte phenotype), or type 2 (defined by the presence of abnormal [clonal] intraepithelial lymphocyte phenotype). Some patients with RCD may never have responded to a GFD or may have relapsed despite adherence and initial response to the GFD. RCD type 1 usually improves after treatment with a combination of aggressive nutritional support, adherence to GFD, and alternative pharmacologic therapies. By contrast, clinical response to alternative therapies in RCD type 2 is less certain and the prognosis is poor. Severe complications such as ulcerative jejunitis and enteropathy-associated T-cell lymphoma (EATL) may occur in a subgroup of patients with RCD. The aims of this article are (1) to review recent advances in the diagnosis and management of patients with RCD and (2) to describe current and novel methods for classification of patients with RCD into categories that are useful to predict outcome and direct treatment.
Keywords: lymphoma, sprue, clone, serology, intraepithelial lymphocytes
Material and Methods (Review Criteria)
PubMed was searched in July 2009 for full articles published in English-language journals with the following keywords alone or in combination: “celiac disease” “aberrant lymphocytes” “refractory sprue” “clonality” “refractory celiac disease” “unresponsive celiac disease” “gluten-free diet”, “intractable diarrhea”, “malignancy” and “lymphoma”. In this literature search, several points became obvious: 1) the prevalence of RCD in the community is unknown; 2) a precise definition of refractory celiac disease is lacking and the diagnostic criteria for RCD vary from center to center; 3) the mechanisms underlying RCD are poorly understood; 4) the natural history, clinical evolution, and prognostic factors in RCD require further study; 5) prospective multicenter clinical trials to test novel therapies are needed; 6) most recommendations for evaluation and treatment are based on expert opinion, not evidence-based reasoning; 7) there are well-described, relatively large case series from referral centers; 8) there are emerging data that might improve current classification and clinical staging of RCD; and 9) novel therapies for patients with RCD type 2 are needed to not only effectively control symptoms but also reduce complications particularly progression to lymphoma. Citations were chosen on the basis of their relevance to the text.
Background
Celiac disease (CD) is an immune-mediated disorder affecting genetically predisposed subjects, caused by the ingestion of gluten present in cereals such as wheat, barley and rye.1 CD affects around 1% of the general population in developed and developing countries, with increasing prevalence over time reported in the United States and Europe.2–4 Lifelong gluten-free diet (GFD) is the only effective treatment to alleviate the symptoms, normalize antibodies and the intestinal mucosa in patients with CD.5
Clinical response is observed in most patients with CD after only few weeks on a GFD.6 However, complete clinical response and mucosal recovery does not occur in all patients with treated CD. 7 Indeed, a subgroup of patients with CD may have persistent or recurrent symptoms (e.g., diarrhea, abdominal pain, and weight loss), inflammation of the intestine, and villous atrophy despite strict adherence to a GFD.8, 9 Symptoms are often severe and require additional therapeutic intervention besides GFD.5, 8 Refractory celiac disease (RCD) is defined by persistent or recurrent malabsorptive symptoms and villous atrophy despite strict adherence to a gluten-free diet (GFD) for at least 6–12 months in the absence of other causes of non-responsive treated celiac disease (CD) and overt malignancy.10–12 The aims of this article are (1) to review recent advances in the diagnosis and management of patients with RCD and (2) to describe current and novel methods for classification of patients with RCD into categories that are useful to predict outcome and direct treatment.
Epidemiology
The real prevalence of RCD is unknown but is probably rare. Evidence of the rarity of RCD is the low number of cases reported in the literature, most often from major CD referral centers.13–18 However, RCD may be the cause underlying persistent or recurrent symptoms in treated CD in just 10 to 18% of the patients evaluated in referral centers.10, 11
Estimates of the occurrence of RCD in non-referral, population-based cohorts are very scarce. RCD was diagnosed in only 5 (0.7%) of 713 patients with CD from the Derby cohort (United Kingdom) from 1978 to 2005.19 From 204 biopsy-confirmed CD residents of Olmsted County (Minnesota, United States) identified from 1950 to 2006, only 3 (1.47%, 95% CI: 0.3%–4.2%) had a subsequent diagnosis of RCD type 1 (n=2) or type 2 (n=1). The incidence per 100,000 person-years was 0.06 (95% CI: 0.0–0.12) adjusted for age and gender to the 2000 US white population. (A.R-T, unpublished data 2009) Thus, RCD appears to be an uncommon condition but with a poor outcome.1
RCD affects two to three times as many women than men,13, 15, 17 consistent with the predominance of diagnosed CD in adult women.1 The predominance of disease in women diminishes somewhat in those patients with both RCD and EATL.13, 17 RCD diagnosis is exceptional before the age of 30 years and most cases are diagnosed around the age of 50 years or thereafter.15, 17
Clinical manifestations
Persistent diarrhea, abdominal pain, and involuntary loss of weight are the most common symptoms in RCD.20 Multiple vitamin deficiencies, anemia, fatigue, and malaise are also frequent.8, 20 Thromboembolic events and coexisting autoimmune disorders are frequent in RCD.14 The majority of patients with RCD are diagnosed because of the development of new symptoms or recurrence of diarrhea after initial clinical response to GFD for years (“secondary” RCD).15, 17 However, a subgroup of patients is diagnosed because of the necessity of early intervention to control their symptoms due to lack of response after 6–12 months of GFD (“primary” RCD).15, 17
Laboratory Findings
Low hemoglobin and hypoalbuminemia are frequent findings and may indicate a poor prognosis.13, 17 Chronic elevated levels of transaminases were detected in one half of patients in one series.15 High stool output (median of 1L/day) often associated with significant steatorrhea is common.17 HLA-DQ2 is present in up to 98% of the cases, with HLA-DQ8 present in almost all others.15, 17
While CD-specific serology (either endomysial or tissue transglutaminase antibodies) is usually positive at the initial diagnosis of CD,1, 7 most patients had negative CD-specific antibodies at the time of RCD diagnosis reflecting strict adherence to GFD.13, 15, 17 Positive CD-specific serology can be present in 19%–30% of patients with RCD despite good compliance to GFD as assessed by dietitian interview, thus, positive CD-specific serology does not necessarily exclude the diagnosis of RCD.15, 17 Although low-grade gluten contamination by a hidden source could be an issue,21, 22 other non-dietary reasons for persistence of positive CD-specific serology in RCD could be: 1) CD-specific antibodies kinetics, 2) induction of tissue transglutaminase up-regulation by severe inflammation or destructive lesions, and 3) coexistent autoimmune disorders associated with false-positive CD serology. 15, 23–25 A short period of close dietary surveillance is usually useful to clarify the origin of persistence of positive CD-specific serology but additional therapies or nutritional support should not be withheld if the clinical condition of the patient deteriorates.16, 17
Endoscopic and Imaging Findings
Standard upper GI endoscopy and capsule endoscopy frequently show macroscopic features of villous atrophy or ulcerations.15, 17, 26, 27 (Figure 1)
Figure 1. Endoscopic abnormalities in refractory celiac disease.
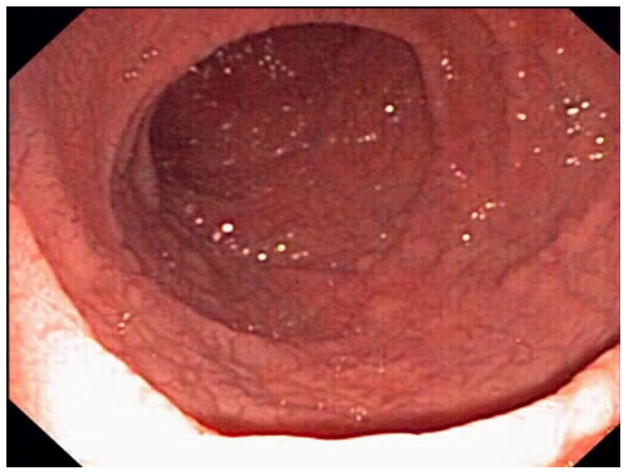
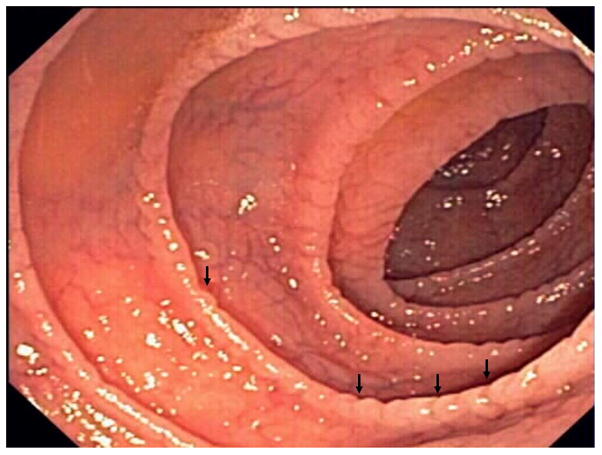
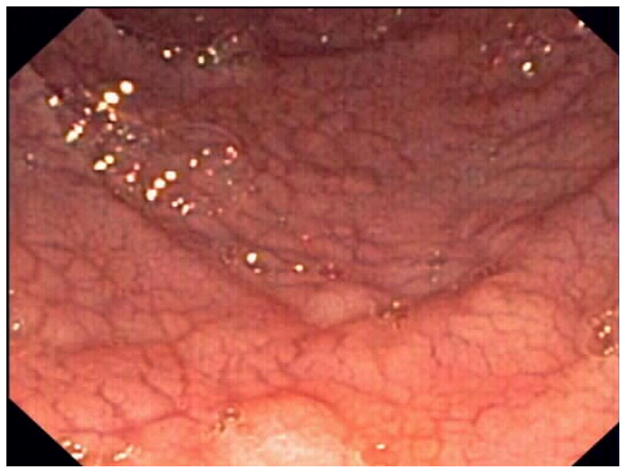
Note the presence of classic endoscopic signs of villous atrophy such as loss of Kerckring’s folds in the duodenum (Panel A), scalloping of circular folds - arrows (Panel B), and fissuring with a mosaic pattern (Panel C). These findings are not specific for refractory celiac disease but excellent predictors of mucosal disease. (Courtesy of Dr. Louis M. Wong Kee Song, Gastroenterology and Hepatology, Mayo Clinic, United States)
However, erosions or ulcerations detected by capsule endoscopy in some patients with symptomatic treated CD may not be always be related to RCD but to non-steroidal antiinflammatory drugs injury. Ulcerative jejunitis or large ulcerations (>1 cm) are common in patients with RCD type 2.15, 17 Ulcerated nodular mucosa, occluding mass, or stricture suggest malignant complications.26–28 Double-ballon enteroscopy can efficiently detect or exclude ulcerative jejunitis or EATL in patients with RCD, especially when suggested by other imaging modalities such as abdominal CT scan.29
Non-specific intestinal abnormalities (e.g., bowel-wall thickening or “malabsorption pattern”) or mesenteric lymphadenopathy are present in up to 50% of patients with RCD.17 Cavitating mesenteric lymph node syndrome characterized by cystic change in mesenteric lymph nodes with or without spleen atrophy is rare but characteristic of CD, often associated with RCD type 2. 17, 30 Small splenic volume (<122 cm3), intussusception, bowel wall thickening, and lymphadenopathy were more commonly detected by abdominal CT in patients with RCD type 2 or EATL as compared to uncomplicated CD or RCD type 1.31, 18 F-fluorodeoxyglucose PET was more sensitive and specific than conventional CT for detection of EATL in patients with RCD, although the overall number of patients included in this prospective comparative study was small.32
Diagnosis
General diagnostic approach and differential diagnosis
The diagnosis of RCD requires a combination of clinical and pathologic findings. Indeed, the diagnosis is made on the basis of strong evidence of CD, supplemented with systematic exclusion of both other causes of non-responsive CD or villous atrophy and malignancy. Although RCD is a diagnosis of exclusion, it is supported by objective findings in laboratory and histological studies. The availability of novel tests for detection of abnormal (clonal) intraepithelial lymphocytes in the intestine has facilitated the confirmation of RCD type 2. (Figure 2)
Figure 2.
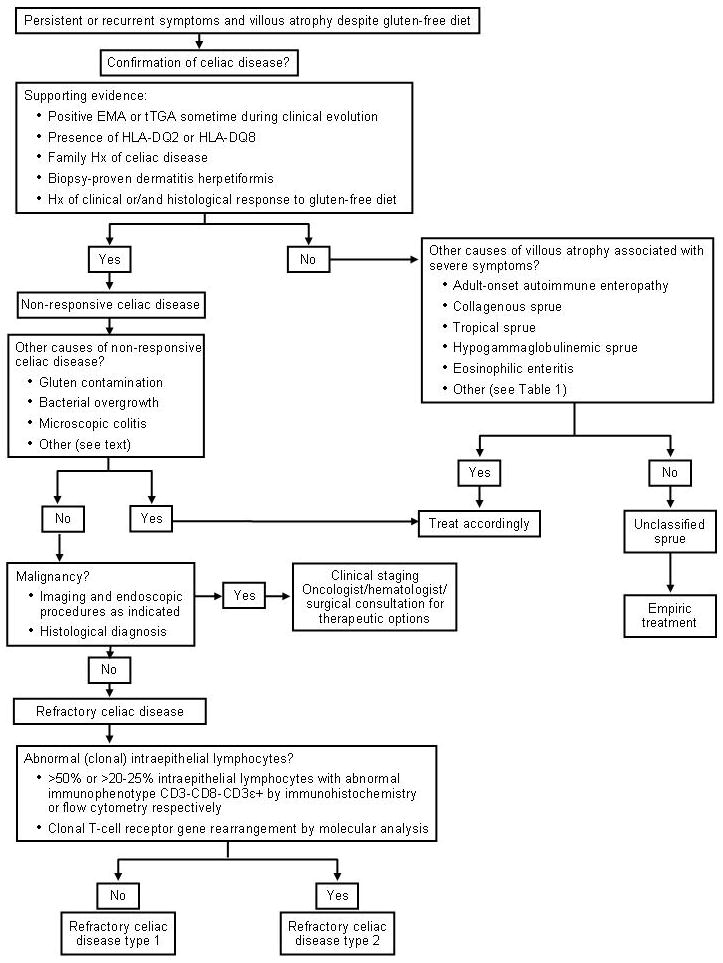
Diagnostic Approach in Refractory Celiac Disease
The differential diagnosis of RCD includes other causes of villous atrophy associated with severe symptoms and/or refractory diarrhea such as (not limited to) tropical sprue, collagenous sprue, adult-onset autoimmune enteropathy, hypogammaglobulinemia, and Crohn’s disease.8, 12 (Table 1) Folic acid deficiency, travel history to endemic areas, and clinical response to combination of folic acid and antibiotics supports tropical sprue as an alternate diagnosis.33 The presence of a subepithelial collagen deposition band together with clinical criteria of refractory diarrhea is characteristic of collagenous sprue.34 Gut epithelial cell antibodies (either anti-enterocyte or anti-goblet cell) are important supportive evidence for the diagnosis of adult-onset autoimmune enteropathy.35
Table 1.
Major Causes of Villous Atrophy in Adults
| Pathologic findings characteristic but not diagnostic |
| Celiac Disease* |
| Tropical Sprue* |
| Adult-onset autoimmune enteropathy |
| Hypogammaglobulinemia |
| Idiopathic AIDS enteropathy |
| Pathologic findings could be diagnostic |
| Eosinophilic gastroenteritis |
| Whipple disease |
| Abetalipoproteinemia |
| Intestinal lymphoma |
| Collagenous sprue |
| Tuberculosis |
| Giardiasis* |
| Crohn’s disease |
| Pathologic findings are non-specific |
| Small-bowel bacterial overgrowth* |
| Infectious enteritis* |
| Parasitic infestation* |
| Severe malnutrition |
| Small-bowel ischemia |
AIDS, acquired immune deficiency syndrome
More frequent causes
Specific diagnostic approach
Confirming the Diagnosis of Celiac Disease
The first step in the evaluation of a potential case of RCD is to confirm whether the initial diagnosis of CD was correct.8, 12 This requirement is easy to meet when patients had a combination of positive CD-specific serologic tests, compatible histologic findings in the intestinal biopsy, CD-permissive genes pairs encoding the human leukocyte antigens (HLA) DQ2 or DQ8, family history of CD, and past medical history of unequivocal clinical or histological response to GFD.1, 7 However, confirmation or exclusion of CD diagnosis can be challenging in some patients, especially those with a primary non-response to GFD.9 Practically all CD patients carry DQA1*05.DQB1*02 encoding HLA-DQ2 or DQA1*03.DQB1*0302 encoding HLA-DQ8, thus, the absence of either of these gene pairs has a very high negative predictive value for CD and should prompt consideration of other causes of refractory sprue.36, 37, 38 The presence of biopsy-proven dermatitis herpetiformis confirms the diagnosis of CD.39 Positive tissue transglutaminase (tTGA) or endomysial antibodies (EMA) at initial diagnosis of CD or at any time in clinical course of the disease helps to confirm the diagnosis of CD because of their excellent specificities >99% when villous atrophy is present, 40 however, most patients with developed RCD are likely to have negative tTGA and EMA.15, 17 Accordingly, negative CD-specific serology does not exclude the diagnosis of CD once the refractory state is fully developed. Family history of CD in first degree relatives (especially siblings) further supports the diagnosis of CD in patients with compatible histology.37 The diagnosis of CD relying only on histological findings or clinical improvement after GFD in the absence of other diagnostic criteria41 is not reliable because CD is just one of many causes of villous atrophy and either clinical response to GFD or exacerbation after gluten re-introduction may have unacceptable low positive predictive value.34, 42, 43 Thus, a critical review of prior tests and especially histology slides is crucial to determine the accuracy of a prior diagnosis of CD. A history of travel to or residence in a location at risk for tropical sprue is also important to identify this readily treatable disorder.44 A well-defined diagnosis of CD is clinically relevant to differentiate RCD from other more heterogeneous causes of non-CD associated refractory diarrhea or enteropathy histologically indistinguishable from CD.12, 35 The term “unclassified sprue” has been used to designate individuals in whom the underlying malabsorptive disorder could not be adequately defined using all current diagnostic methods.9, 12, 28(Box 1)
Box 1.
The first step in the evaluation of a potential case of RCD is to confirm whether the initial diagnosis of CD was correct
Negative CD-specific serology does not exclude the diagnosis of CD once the refractory state is fully developed
HLA-DQ status may be clinically relevant to exclude the diagnosis of CD
Critical review of prior laboratories studies including serology, pathology, and HLA-DQ status are crucial to confirm a prior diagnosis of CD
Etiologies and diagnostic approach in “Non-responsive” CD
“Non-responsive CD” is a relatively common clinical scenario characterized by a lack of initial response to a GFD, or recurrence of symptoms or laboratory abnormalities typical of untreated CD while on a GFD in a patient who responded initially to GFD.10 The etiologies of non-responsive CD vary from center to center.10, 11, 45 Overt or inadvertent gluten contamination appears to be the most common cause of non-responsive CD seen in 36% to 51% of referral patients.10, 11 Other etiologies include microscopic colitis, small-bowel bacterial overgrowth, lactose intolerance, and functional bowel disorders.8, 10, 11, 45 (Table 2) RCD is a diagnosis of exclusion that requires the elimination and/or treatment of other causes of non-responsive CD.
Table 2.
Etiologies of non-responsive celiac disease
| Etiology | Representative Case Series | Diagnostic approach | ||
|---|---|---|---|---|
| Abdulkarim A10 (n=49) | Leffler D11 (n=99*) | Fine K45 (n=11**) | ||
| Gluten contamination | 25 | 36 | 1 | Dietary review, celiac serology |
| Microscopic colitis | 9 | 6 | 3 | Colonic biopsies |
| Bacterial overgrowth | 7 | 6 | 0 | Breath tests, culture of small bowel aspirate, antibiotics trial |
| Pancreatic insufficiency | 6 | 0 | 2 | Pancreatic testing, enzymes trial |
| Fructose/lactose intolerance | 1 | 7 | 2 | Breath tests, exclusion trial |
| Irritable bowel syndrome | 4 | 22 | 2 | Clinical criteria |
| Refractory Celiac Disease | 4 | 10 | 0 | Discussed in this review |
| Other | 4 | 12 | 1 | Miscellaneous tests |
| Total | 60*** | 99 | 11 | |
99 of 113 with confirmed etiologies
11 of 13 with persistent diarrhea after GFD
some patients had more than one condition likely associated with persistent symptoms
While complete histological recovery after gluten exclusion may take several years in adults, clinical and serological responses are usually attainable after just few weeks or months of gluten withdrawal, respectively.6, 40, 46 Thus, persistent or recurrent symptoms, positive CD-specific serology, and/or villous atrophy after 6 to 12 months on a GFD are atypical for uncomplicated CD and may require further evaluation. Persistently positive tTGA or EMA are suggestive of gluten contamination.11 Detailed evaluation of strictness of the GFD by a skilled dietitian with especial emphasis in reveal potential hidden sources of gluten is required.21, 46 Other causes of symptoms on a GFD such as microscopic colitis, pancreatic insufficiency, small-intestine bacterial overgrowth, and irritable bowel syndrome require careful consideration prior to diagnosing RCD.8 Repeat intestinal biopsy may help to differentiate causes of non-responsive CD associated with ongoing villous atrophy (e.g., gluten contamination, small-bowel bacterial overgrowth, RCD) from those associated with normal duodenal biopsy (e.g., microscopic colitis, irritable bowel syndrome). Additionally, upper endoscopy permits the collection of duodenal fluid for culture of bacteria and extensive mucosal sampling to detect the presence of abnormal (clonal) T-cells in the intestine. Persistent inflammation and villous atrophy after GFD without symptoms, while not traditionally considered as part of RCD spectrum, may represent in a minority of patients a “latent” form of RCD with a higher risk of development of symptomatic RCD or lymphoma over time.47 Finally, multiple conditions associated with persistent symptoms may occur together in the same patient.10, 48 Thus, before a diagnosis of RCD could be taken as certain, a systematic investigation and exclusion of all other etiologies of non-responsive CD is advisable. 10, 17 (Box 2)
Box 2.
Overt or inadvertent gluten contamination appears to be the most common cause of non-responsive CD
Persistent or recurrent symptoms, positive CD-specific serology, and/or villous atrophy after 6 to 12 months on a GFD are atypical and may require further evaluation
RCD is a diagnosis of exclusion that requires the elimination of other causes of non-responsive CD associated with villous atrophy
Exclusion of Malignancy
The presence of fever, nocturnal diaphoresis, pruritus, significant unexplained weight loss, anorexia, overt or occult gastrointestinal bleeding, abdominal pain, and bowel obstruction do not occur in treated CD and suggest an underlying complication, especially ulcerative jejunitis or malignancies such as EATL and small-bowel adenocarcinoma.9, 13, 28, 49 Subjects with EATL diagnosed before CD has been diagnosed should not be considered as affected by RCD because the outcome is determined by the neoplasm.49 This group has been arbitrarily classified as “primary” EATL.13 Alternatively, “secondary” EATL is used to describe the development of EATL after long-lasting well-controlled CD for several months or years before the diagnosis of RCD and subsequent EATL.13, 15, 17, 50 Recently, two distinct groups of EATL were delineated morphologically and genetically, type 1 EATL, observed in 80–90% of cases, appears to be linked pathogenetically to CD and RCD, this subtype is characterized by non-monomorphic cytomorphology, CD56 negativity, and chromosomal gains of 1q and 5q.51 EATL is rare in the general population (incidence of 0.10 per 100,000 inhabitants per year in The Netherlands)52 but may occur in 60–80% of patients with RCD type 2 within 5 years and also has been occasionally described in patients with RCD type 1.13–15, 17 The presence of intraepithelial γ/δ T-lymphocytes is inversely correlated with lymphomagenesis in RCD.53 Extensive discussion of EATL treatment is beyond the scope of this review but regardless of EATL subtype, the prognosis is poor despite aggressive therapies with a 5-year survival rate of 8–20%.13, 49
CD patients have a higher risk of small bowel adenocarcinoma that may be a rare cause of deterioration of well-controlled treated CD.8, 54, 55 Small bowel adenocarcinoma in the context of CD is associated with better survival than the sporadic counterpart.56
Novel endoscopic or imaging techniques are available to visualize the entire small-bowel mucosa such as wireless capsule endoscopy, computerized tomography (CT) or magnetic resonance imaging (MRI) enterography, double or single balloon enteroscopy, and positive emission tomography (PET) scan. These new techniques are of great utility to exclude malignancy in patients with possible RCD. 1, 8, 13, 15–17, 29, 42, 55 However, the most cost-effective diagnostic approach is unclear because sensitivity and specificity of individual or combined findings with each diagnostic modality are unknown and comparative studies between methods are lacking.20, 27 It is the authors practice to use a combination of wireless capsule endoscopy that provides excellent luminal detail and CT-enterography to evaluate the wall of the intestine and extraintestinal structures as initial screening tests to exclude malignancy in patients with RCD. Small-bowel enteroscopy is used to sampling areas beyond the reach of standard endoscopy when the suspicion of malignancy is high. PET scan may be useful in patients with RCD type 2. Other diagnostic modalities are selected in a case-by-case basis.
Detection of abnormal intraepithelial lymphocyte phenotype
The detection of abnormal intraepithelial lymphocyte phenotype is the basis for subclassification of RCD and may also have prognostic significance.8, 17, 57 The current methods can be done in fixed (double CD3/CD8 immunohistochemistry, T-cell receptor clonal rearrangement by polymerase chain reaction) or on fresh frozen intestinal tissue (flow cytometry).58–60, 57 The abnormal phenotype is supported by: 1) loss of normal surface markers CD3, CD4, and CD8 with preserved expression of intracytoplasmic CD3 (CD3ε) in >50% of intraepithelial lymphocytes as evaluated by immunohistochemistry or >20%–25% as determined by flow cytometry (e.g., CD103+, CD45+, CD7+, CD3−, CD8−) and 2) detection of T-cell receptor chains (γ or δ) clonal rearrangement by polymerase chain reaction.15, 57–59 (Figure 3) Defective synthesis or association of T-cell receptor chains may explain the loss of surface TCR-CD3 expression in patients with RCD type 2 and EATL.61 While immunohistochemistry and polymerase chain reaction methods have been widely accepted, application of flow cytometry for detection of abnormal intraepithelial lymphocyte phenotype is relatively novel with promising results in a single center.59 (Figure 4) Double CD3/CD8 immunohistochemistry have been proposed for initial evaluation of potential RCD because of the advantages of simplicity, accuracy in paraffin-embedded duodenal tissue, and low cost.58 T-cell receptor clonal rearrangement by polymerase chain reaction is required to confirm the presence of clonal intraepithelial lymphocyte phenotype.15, 17, 57, 58, 62 It is the authors practice to use both double CD3/CD8 immunohistochemistry and T-cell receptor clonal rearrangement by polymerase chain reaction for the clinical evaluation of patients with clinical criteria of RCD because of the imperfect correlation between the two techniques. 17 This approach is especially useful to detect RCD cases with a normal intraepithelial lymphocyte phenotype (CD3+CD8+) by immunohistochemistry but monoclonal T-cell receptor rearrangement. 63 Furthermore, the presence of concurrent persistent monoclonality and aberrant immunophenotype (especially if ≥80% CD3ε+CD8− intraepithelial lymphocytes) is a strong predictor of EATL development.64 Trisomy 1q22-44 has been observed in clonal intraepithelial lymphocytes characteristic of RCD type 2, thus, karyotypic studies may be helpful to categorize patients.15, 65 Abnormal (clonal) intraepithelial lymphocytes may also be detected in the subepithelial layer, lamina propria, or even extraintestinal locations such as skin, blood, bone marrow, liver, and bronchioloalveolar fluid in patients with RCD type 2, in the absence of overt EATL.15, 66
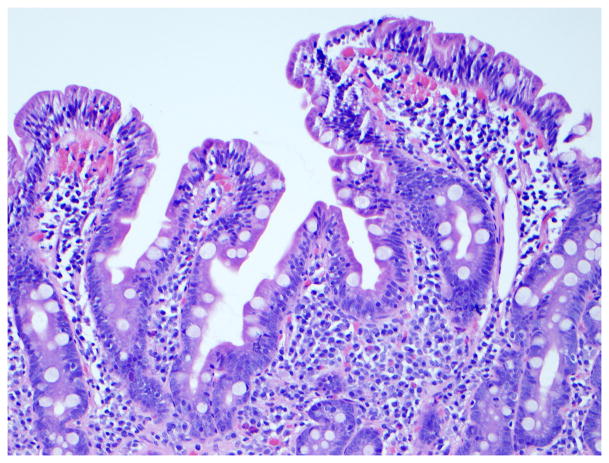
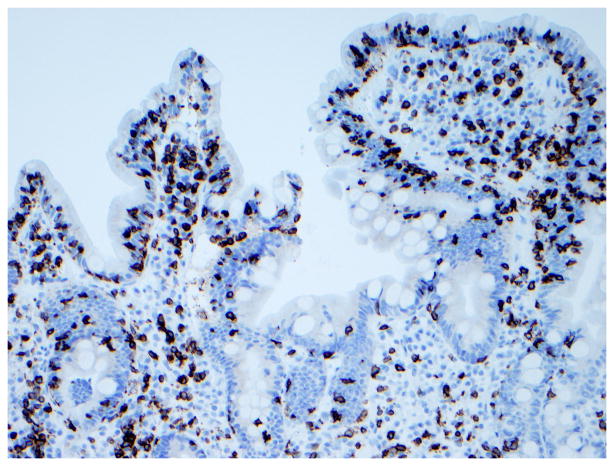
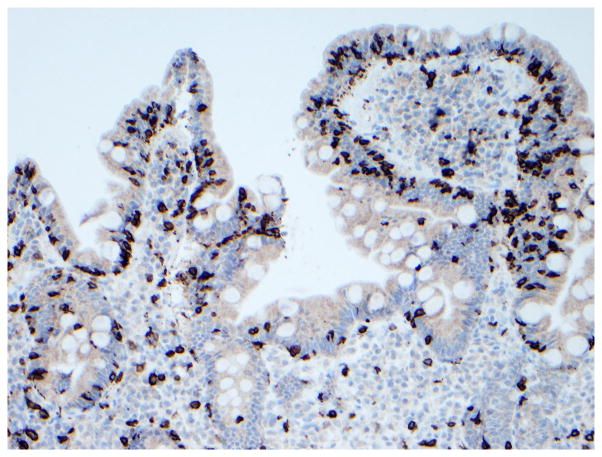
Figure 3. Immunophenotype of intraepithelial lymphocytes in RCD by CD3 and CD8 immunostaining.
Panel A, duodenal biopsy specimen from a patient with RCD type 1 (Panel A1, hematoxylin and eosin, 20x original magnification) showing partial villous atrophy and an increased number of intraepithelial lymphocytes with normal immunophenotype characterized by expression of CD3 (Panel A2) and CD8 (Panel A3). Panel B, duodenal biopsy specimen from a patient with type 2 RCD (Panel B1, hematoxylin and eosin, 20x original magnification) showing villous atrophy and abnormal intraepithelial lymphocytes characterized by expression of CD3 (Panel B2) but mostly CD8− (Panel B3). Brown color denotes positive immunostaining (20x original magnification). (Courtesy of Dr. Tsung-Teh Wu, Anatomic Pathology, Mayo Clinic, United States)
Figure 4. Example of flow cytometry analysis of intestinal lymphocytes isolated from duodenal biopsy specimens in a patient with RCD type 2.
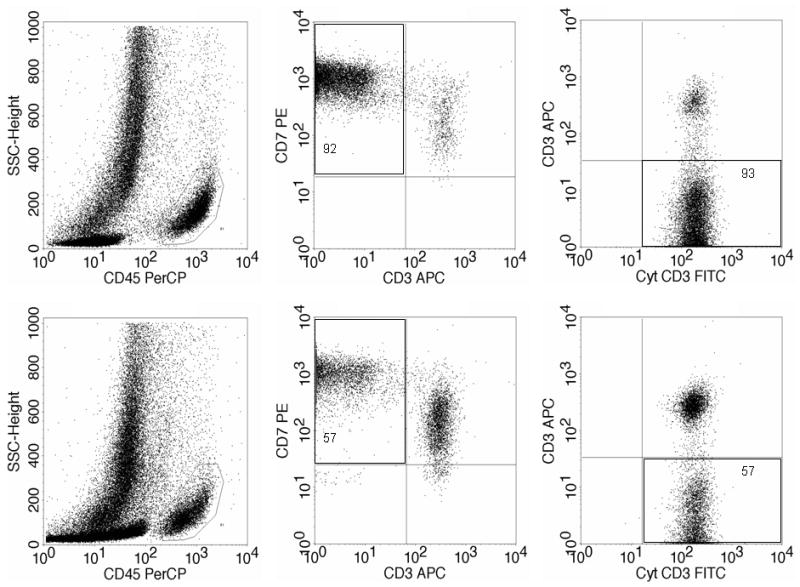
Upper half: abnormal intraepithelial lymphocytes (92–93%). Botton half: abnormal lamina propria lymphocytes (57%). Left: lymphocyte selection gate based on CD45 positivity and low side scatter. Middle: the abnormal T-cell population by double staining of surface CD3 and CD7 within the CD45+ cells. Right: the abnormal surface CD3−, cytoplasmic CD3+ cells by double staining shown within the CD7+ CD45+ cells. (Courtesy of Dr. Wieke HM Verbeek, VU University Medical Center, Amsterdam, Netherlands)
Clinical Classification and Prognosis
The clinical classification of RCD is based on the immunophenotype of intraepithelial lymphocytes and supported by clinical evolution. (Table 3) Abnormal (clonal) intraepithelial lymphocytes are the hallmark of RCD type 2. RCD type 2 is associated with poor prognosis despite conventional therapeutic intervention with 5-year survival rates of 40–58%.13, 15–17 Poor prognosis is largely explained by the much more frequent progression to overt EATL in patients with RCD type 2 (Figure 5).13, 15, 17, 57, 67, 50 Recent evidence suggests that continual monitoring of both immunophenotype and clonality of intraepithelial lymphocytes may be more accurate than snapshot analysis for both correct ascertainment of RCD subtype and prediction of risk of lymphomagenesis.64 The prognosis of RCD type 1 is much better as compared to RCD type 2 but the rates of complications and mortality appear to be much higher than those observed in non-complicated CD.14, 16, 17 Besides RCD subtype and lymphoma development, other prognostic factors appear to be important such as older age at RCD diagnosis (e.g., age ≥ 65 years old), albumin ≤ 3.2 g/dL, hemoglobin 11 ≤ g/dL, and total villous atrophy at RCD diagnosis.17 A new clinical staging model for RCD based on the cumulative effect of clinical prognostic factors has been proposed using single center data but requires further validation before being ready for clinical use.17
Table 3.
Differential diagnosis of Refractory Celiac Disease Type 1 and Refractory Celiac Disease Type 2
| Clinical Criteria | Disease Category | |
|---|---|---|
| RCD type 1 | RCD type 2 | |
| Abnormal immunophenotype of IELs with loss of normal surface markers CD3, CD8, and T-cell receptor: either >50 % by immunohistochemistry or >20–25% by flow cytometry | No | Yes |
| T-cell receptor chains (γ or δ) clonal rearrangement by molecular methods | No | Yes |
| Clinical or histological response to steroids or other immunosuppressive drugs or biologics | Yes | Variable |
| Lymphomagenesis potential (especially EATL development) | Rare | Frequent |
RCD, refractory celiac disease; GFD, gluten-free diet; IELs, intraepithelial lymphocytes; EATL, enteropathy-type T-cell lymphoma
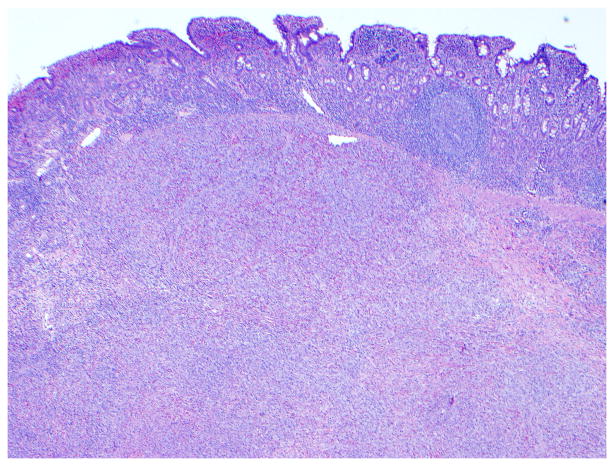
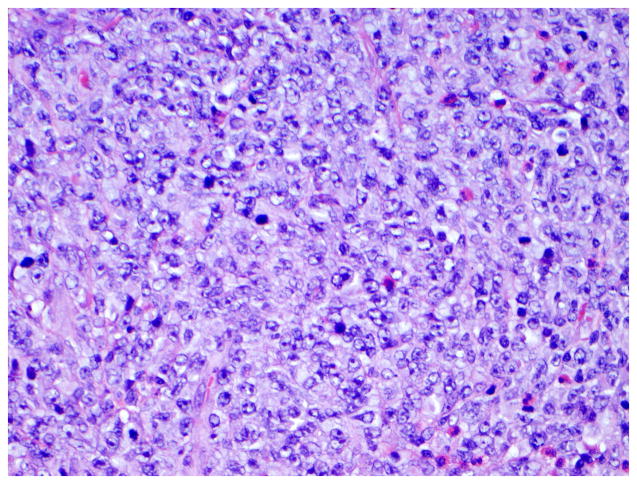
Figure 5. Histological features of enteropathy-type T-cell lymphoma.
Panel A, Low-power magnification of surgical specimen from the jejunum showing subtotal villous atrophy and diffuse infiltration by enteropathy-type T-cell lymphoma in a patient with long-lasting RCD type 2 (hematoxylin and eosin, 4x original magnification). Panel B, Detail of a monotonous tumor cell population with rounded vesicular nuclei, single nucleolus, and abundant cytoplasm (hematoxylin and eosin, 40x original magnification). (Courtesy of Dr. Tsung-Teh Wu, Anatomic Pathology, Mayo Clinic, United States)
Treatment
Evidence for treatment of RCD is based on case reports, open-label observational or prospective experiences, and expert opinion.8, 20, 68 There are no randomized clinical trials. The lack of data is partly explained by the rarity of RCD. The paucity of data should be recognized when making therapeutic decisions in patients with RCD.17 Furthermore, as the diagnostic criteria for RCD have changed over time, historical reports on treatment need to be interpreted cautiously because the absence of a clear distinction between RCD and other causes of refractory sprue as well separating RCD type 1 and type 2.
Dietary and Nutritional Assessment
Hospitalization is sometimes necessary to monitor adherence to GFD and for treatment of severe nutritional complications or dehydration. Total parenteral nutrition was necessary in 28%–60% of patients with RCD because of severe weight loss, malnutrition, multiple nutritional deficiencies, and severe hypoproteinemia and/or steatorrhea.15, 17, 16 Nutritional treatment should also include correction of trace element deficiencies including zinc and copper and addressing metabolic bone disease.46, 55 The GFD reduces overall morbidity and mortality in CD,69 thus, although the benefit of a GFD in RCD is unknown, strict adherence to GFD has been widely recommended.13, 15–17 GFD alone may be an effective maintenance therapy in exceptional cases. Elemental diet showed promising results in a small heterogeneous group of patients with refractory enteropathy without clonal intraepithelial lymphocyte phenotype.70
Alternative Therapies
Prednisone (0.5–1 mg/kg/day), budesonide (9 mg/day), or a combination of prednisone and azathioprine (2 mg/kg/day) are clinically effective to induce clinical remission and mucosal recovery in most patients with RCD type 1.13, 15–18, 57, 68, 71, 72 Clinical response to steroids is observed in the majority (~75%) of patients with RCD type 2, however mucosal recovery is infrequent and progression to EATL is not prevented.13, 15, 17 Steroid- dependence is observed in most patients with RCD type 1 or RCD type 2.15, 17 Clinical response to budesonide in RCD is clinically attractive because of its topical effect, extensive first-pass metabolism, good tolerability, and low frequency of serious adverse events after short-term use.17, 18, 73 Data on tolerability and safety after long-term use of budesonide in RCD are lacking but budesonide was well tolerated after 6 months of use for the maintenance of clinical remission in collagenous colitis. 74 Clinical and histological improvement was observed in up to 61% of patients with RCD after open-label treatment with oral cyclosporine (5 mg/kg/day).75 Other immunosuppressive drugs or biological modifiers have been used with some clinical benefit in steroid-dependent or steroid-refractory patients including azathioprine, cyclosporin, infliximab (5 mg/kg/day), and alemtuzumab (30 mg twice a week per 12 weeks), though publication of successfully treated cases rather than failures must be considered (publication bias).76–80 In a small open-label pilot study, recombinant human interleukin-10 administrated subcutaneously (8 mcg/kg three times a week per 3 months) was not effective to restore villi in the majority of patients with RCD.81 Although valuable for treatment of RCD type 1, azathioprine should be used with caution because of potential severe side effects especially myelosuppression, infections, and lymphomagenesis.82, 83 Lymphomagenesis is of special concern in patients with RCD type 2 because of the higher risk of EATL development in this subgroup and the fact that monoclonality persist after treatment with azathioprine.68, 71, 76 Intravenous cladribine (0.1 mg/kg/day for 5 days) was well tolerated in an open-label study in patients with RCD type 2 previously treated with prednisone and/or azathioprine and can induce clinical improvement (36%), histological improvement (59%), and significant decrease in the number of clonal intraepithelial lymphocytes (35%).84 However, up to 41% developed EATL and died despite cladribine therapy.84 Case reports showed that alemtuzumab (anti-CD52 monoclonal antibody) or a combination of pentostatin (4 mg/m2 every two weeks per 24 weeks) and budesonide could similarly induce clinical and histological response as well as a decrease but not disappearance of clonal intraepithelial lymphocytes.79, 80, 85 The effect of drug-induced T-cell clone suppression on lymphomagenesis is not completely understood but EATL may still occur over time in patients with RCD type 2 even after the T-cell clone was not further detected in the intestinal tissue by conventional methods.17 Accelerated lymphomagenesis is a concern with cladribine and alemtuzumab, although the number of cases with EATL progression was small.15, 84 Finally, high-dose chemotherapy followed by ASCT has been explored for RCD type 2 in a pilot study from a single center.86 The outcome of ASCT is disappointing for patients with overt EATL. 17, 87 For future studies, interleukin-15 blockade may represent one promising option for RCD type 2 because of this cytokine key role to disrupt intraepithelial lymphocyte homeostasis and lymphomagenesis.68, 88
Surgery
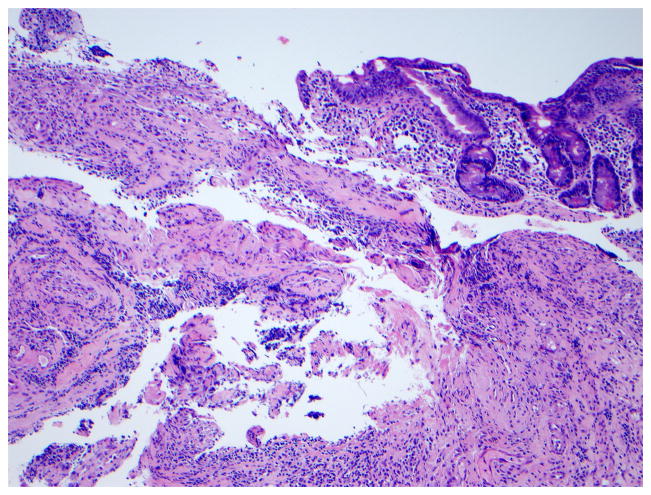
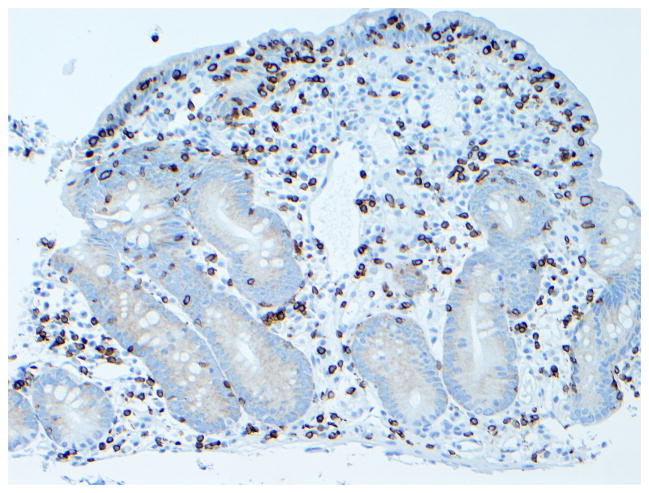
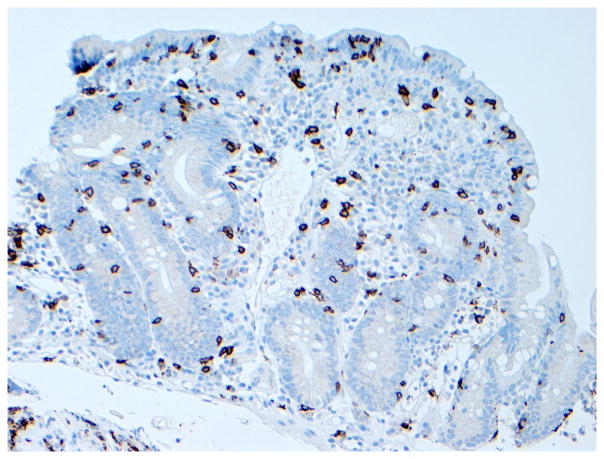
The role of surgery in RCD is limited to the management of complications such as perforation, massive hemorrhage, high-grade obstruction, and cancer.13, 15 Long-term clinical remission on GFD alone after complete resection of ulcerative jejunitis has been reported, particularly if ulceration is localized to one part of the intestine.16, 28 (Figure 6) The role of diagnostic laparotomy in RCD is now limited by the introduction of novel endoscopic modalities that permit visual evaluation and biopsy specimen sampling of the whole intestine such as the newer enteroscopy modalities.29
Figure 6. Histologic findings in ulcerative jejunitis.
Extensive ulceration (left) and villous atrophy (upper right) in the jejunum of a patient with refractory CD compatible with ulcerative jejunitis (Figure 6a, hematoxylin and eosin, 10x original magnification), most intraepithelial lymphocytes in the atrophic mucosa adjacent to ulceration express CD3 (Figure 6b) but not CD8 (Figure 6c). Brown color denotes positive immunostaining (20x original magnification). (Courtesy of Dr. Tsung-Teh Wu, Anatomic Pathology, Mayo Clinic, United States)
Summary
RCD is defined by persistent or recurrent malabsorptive symptoms and villous atrophy despite strict adherence to GFD for at least 6–12 months in the absence of other causes of non-responsive treated CD and overt malignancy
The real prevalence of RCD is unknown but is probably rare
RCD may be classified as type 1 (normal intraepithelial lymphocyte phenotype), or type 2 (defined by the presence of abnormal [clonal] intraepithelial lymphocyte phenotype)
Several alternative therapies are beneficial in RCD type 1 especially prednisone, budesonide or a combination of prednisone and azathioprine but there are no established treatments for RCD type 2
RCD is associated with higher risk of complications and mortality, especially RCD type 2 with a 5-year survival rate of 40%–58%
Chemotherapeutic drugs alone or high-dose chemotherapy followed by ASCT have been systematically explored for selected patients with RCD type 2 in a single center. Further studies in other centers or probably as a multicenter effort are needed
Advances in basic science may be translated into future novel therapies, such as interleukin-15 blockade either as a single-agent or combination therapy
Acknowledgments
This article was supported by the National Institutes of Health (NIH) under Ruth L. Kirschstein National Research Service Award/Training Grant in Gastrointestinal Allergy and Immunology (T32 AI-07047) (to ART) and the NIH grant DK-57892 (to JAM). The authors thank Deborah I. Frank for her assistance in the preparation of this manuscript.
Abbreviations
- ASCT
autologous hematopoietic stem cell transplantation
- CD
celiac disease
- CT
computed tomography
- EATL
enteropathy-associated T-cell lymphoma
- EMA
endomysial antibodies
- GFD
gluten-free diet
- HLA
human leukocyte antigens
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- RCD
refractory celiac disease
- tTGA
tissue transglutaminase antibodies
Footnotes
Copyright license statement: Per Mayo Clinic policy, please find attached as a separate PDF file a signed copyright release form.
Competing Interest: Dr. Murray is consultant for ActogeniX Inc., Flamentera Inc., Ironwood pharmaceuticals, and Alvine Inc. Dr Murray is also investigator for ALBA Therapeutics. Dr. Rubio-Tapia declares no competing interest.
References
- 1.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480–93. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 2.Catassi C. The world map of celiac disease. Acta Gastroenterol Latinoam. 2005;35:37–55. [PubMed] [Google Scholar]
- 3.Rubio-Tapia A, Kyle RA, Kaplan EL, Johnson DR, Page W, Erdtmann F, Brantner TL, Kim WR, Phelps TK, Lahr BD, Zinsmeister AR, Melton LJ, 3rd, Murray JA. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A, Maki M. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217–25. doi: 10.1111/j.1365-2036.2007.03502.x. [DOI] [PubMed] [Google Scholar]
- 5.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 6.Murray JA, Watson T, Clearman B, Mitros F. Effect of a gluten-free diet on gastrointestinal symptoms in celiac disease. Am J Clin Nutr. 2004;79:669–73. doi: 10.1093/ajcn/79.4.669. [DOI] [PubMed] [Google Scholar]
- 7.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Daum S, Cellier C, Mulder CJ. Refractory coeliac disease. Best Pract Res Clin Gastroenterol. 2005;19:413–24. doi: 10.1016/j.bpg.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Trier JS, Falchuk ZM, Carey MC, Schreiber DS. Celiac sprue and refractory sprue. Gastroenterology. 1978;75:307–16. [PubMed] [Google Scholar]
- 10.Abdulkarim AS, Burgart LJ, See J, Murray JA. Etiology of nonresponsive celiac disease: results of a systematic approach. Am J Gastroenterol. 2002;97:2016–21. doi: 10.1111/j.1572-0241.2002.05917.x. [DOI] [PubMed] [Google Scholar]
- 11.Leffler DA, Dennis M, Hyett B, Kelly E, Schuppan D, Kelly CP. Etiologies and predictors of diagnosis in nonresponsive celiac disease. Clin Gastroenterol Hepatol. 2007;5:445–50. doi: 10.1016/j.cgh.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Biagi F, Corazza GR. Defining gluten refractory enteropathy. Eur J Gastroenterol Hepatol. 2001;13:561–5. doi: 10.1097/00042737-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Al-Toma A, Verbeek WH, Hadithi M, von Blomberg BM, Mulder CJ. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut. 2007;56:1373–8. doi: 10.1136/gut.2006.114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daum S, Ipczynski R, Schumann M, Wahnschaffe U, Zeitz M, Ullrich R. High rates of complications and substantial mortality in both types of refractory sprue. Eur J Gastroenterol Hepatol. 2009;21:66–70. doi: 10.1097/MEG.0b013e328307c20c. [DOI] [PubMed] [Google Scholar]
- 15.Malamut G, Afchain P, Verkarre V, Lecomte T, Amiot A, Damotte D, Bouhnik Y, Colombel JF, Delchier JC, Allez M, Cosnes J, Lavergne-Slove A, Meresse B, Trinquart L, Macintyre E, Radford-Weiss I, Hermine O, Brousse N, Cerf-Bensussan N, Cellier C. Presentation and long-term follow-up of refractory celiac disease: comparison of type I with type II. Gastroenterology. 2009;136:81–90. doi: 10.1053/j.gastro.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 16.Maurino E, Niveloni S, Chernavsky AC, Sugai E, Vazquez H, Pedreira S, Periolo N, Mazure R, Smecuol E, Moreno ML, Litwin N, Nachman F, Kogan Z, Bai JC. Clinical characteristics and long-term outcome of patients with refractory sprue diagnosed at a single institution. Acta Gastroenterol Latinoam. 2006;36:10–22. [PubMed] [Google Scholar]
- 17.Rubio-Tapia A, Kelly DG, Lahr BD, Dogan A, Wu TT, Murray JA. Clinical staging and survival in refractory celiac disease: a single center experience. Gastroenterology. 2009;136:99–107. doi: 10.1053/j.gastro.2008.10.013. quiz 352–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brar P, Lee S, Lewis S, Egbuna I, Bhagat G, Green PH. Budesonide in the treatment of refractory celiac disease. Am J Gastroenterol. 2007;102:2265–9. doi: 10.1111/j.1572-0241.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 19.West J. Celiac disease and its complications: a time traveller’s perspective. Gastroenterology. 2009;136:32–4. doi: 10.1053/j.gastro.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Al-toma A, Verbeek WH, Mulder CJ. The management of complicated celiac disease. Dig Dis. 2007;25:230–6. doi: 10.1159/000103891. [DOI] [PubMed] [Google Scholar]
- 21.Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135–41. doi: 10.1053/j.gastro.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Biagi F, Campanella J, Martucci S, Pezzimenti D, Ciclitira PJ, Ellis HJ, Corazza GR. A milligram of gluten a day keeps the mucosal recovery away: a case report. Nutr Rev. 2004;62:360–3. doi: 10.1111/j.1753-4887.2004.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 23.Midhagen G, Aberg AK, Olcen P, Jarnerot G, Valdimarsson T, Dahlbom I, Hansson T, Strom M. Antibody levels in adult patients with coeliac disease during gluten-free diet: a rapid initial decrease of clinical importance. J Intern Med. 2004;256:519–24. doi: 10.1111/j.1365-2796.2004.01406.x. [DOI] [PubMed] [Google Scholar]
- 24.Ientile R, Caccamo D, Griffin M. Tissue transglutaminase and the stress response. Amino Acids. 2007;33:385–94. doi: 10.1007/s00726-007-0517-0. [DOI] [PubMed] [Google Scholar]
- 25.Clemente MG, Musu MP, Frau F, Lucia C, De Virgiliis S. Antitissue transglutaminase antibodies outside celiac disease. J Pediatr Gastroenterol Nutr. 2002;34:31–4. doi: 10.1097/00005176-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Culliford A, Daly J, Diamond B, Rubin M, Green PH. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55–61. doi: 10.1016/s0016-5107(05)01566-x. [DOI] [PubMed] [Google Scholar]
- 27.Daum S, Wahnschaffe U, Glasenapp R, Borchert M, Ullrich R, Zeitz M, Faiss S. Capsule endoscopy in refractory celiac disease. Endoscopy. 2007;39:455–8. doi: 10.1055/s-2007-966239. [DOI] [PubMed] [Google Scholar]
- 28.Baer AN, Bayless TM, Yardley JH. Intestinal ulceration and malabsorption syndromes. Gastroenterology. 1980;79:754–65. [PubMed] [Google Scholar]
- 29.Hadithi M, Al-toma A, Oudejans J, van Bodegraven AA, Mulder CJ, Jacobs M. The value of double-balloon enteroscopy in patients with refractory celiac disease. Am J Gastroenterol. 2007;102:987–96. doi: 10.1111/j.1572-0241.2007.01122.x. [DOI] [PubMed] [Google Scholar]
- 30.Huppert BJ, Farrell MA, Kawashima A, Murray JA. Diagnosis of cavitating mesenteric lymph node syndrome in celiac disease using MRI. AJR Am J Roentgenol. 2004;183:1375–7. doi: 10.2214/ajr.183.5.1831375. [DOI] [PubMed] [Google Scholar]
- 31.Mallant M, Hadithi M, Al-Toma AB, Kater M, Jacobs M, Manoliu R, Mulder C, van Waesberghe JH. Abdominal computed tomography in refractory coeliac disease and enteropathy associated T-cell lymphoma. World J Gastroenterol. 2007;13:1696–700. doi: 10.3748/wjg.v13.i11.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadithi M, Mallant M, Oudejans J, van Waesberghe JH, Mulder CJ, Comans EF. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. J Nucl Med. 2006;47:1622–7. [PubMed] [Google Scholar]
- 33.Nath SK. Tropical sprue. Curr Gastroenterol Rep. 2005;7:343–9. doi: 10.1007/s11894-005-0002-4. [DOI] [PubMed] [Google Scholar]
- 34.Owens SR, Greenson JK. The pathology of malabsorption: current concepts. Histopathology. 2007;50:64–82. doi: 10.1111/j.1365-2559.2006.02547.x. [DOI] [PubMed] [Google Scholar]
- 35.Akram S, Murray JA, Pardi DS, Alexander GL, Schaffner JA, Russo PA, Abraham SC. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5:1282–90. doi: 10.1016/j.cgh.2007.05.013. quiz 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolters VM, Wijmenga C. Genetic background of celiac disease and its clinical implications. Am J Gastroenterol. 2008;103:190–5. doi: 10.1111/j.1572-0241.2007.01471.x. [DOI] [PubMed] [Google Scholar]
- 37.Rubio-Tapia A, Van Dyke CT, Lahr BD, Zinsmeister AR, El-Youssef M, Moore SB, Bowman M, Burgart LJ, Melton LJ, 3rd, Murray JA. Predictors of family risk for celiac disease: a population-based study. Clin Gastroenterol Hepatol. 2008;6:983–7. doi: 10.1016/j.cgh.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashtak S, Murray JA. Tailored testing for celiac disease. Ann Intern Med. 2007;147:339–41. doi: 10.7326/0003-4819-147-5-200709040-00009. [DOI] [PubMed] [Google Scholar]
- 39.Zone JJ. Skin manifestations of celiac disease. Gastroenterology. 2005;128:S87–91. doi: 10.1053/j.gastro.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests) Aliment Pharmacol Ther. 2006;24:47–54. doi: 10.1111/j.1365-2036.2006.02967.x. [DOI] [PubMed] [Google Scholar]
- 41.When is a coeliac a coeliac? Report of a working group of the United European Gastroenterology Week in Amsterdam, 2001. Eur J Gastroenterol Hepatol. 2001;13:1123–8. doi: 10.1097/00042737-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Rubio-Tapia A, Murray JA. Novel endoscopic methods for the evaluation of the small-bowel mucosa. Gastrointest Endosc. 2007;66:382–6. doi: 10.1016/j.gie.2007.03.1056. [DOI] [PubMed] [Google Scholar]
- 43.Campanella J, Biagi F, Ilaria Bianchi P, Zanellati G, Marchese A, Roberto Corazza G. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008:1–4. doi: 10.1080/00365520802200036. [DOI] [PubMed] [Google Scholar]
- 44.Westergaard H. Tropical Sprue. Curr Treat Options Gastroenterol. 2004;7:7–11. doi: 10.1007/s11938-004-0020-6. [DOI] [PubMed] [Google Scholar]
- 45.Fine KD, Meyer RL, Lee EL. The prevalence and causes of chronic diarrhea in patients with celiac sprue treated with a gluten-free diet. Gastroenterology. 1997;112:1830–8. doi: 10.1053/gast.1997.v112.pm9178673. [DOI] [PubMed] [Google Scholar]
- 46.See J, Murray JA. Gluten-free diet: the medical and nutrition management of celiac disease. Nutr Clin Pract. 2006;21:1–15. doi: 10.1177/011542650602100101. [DOI] [PubMed] [Google Scholar]
- 47.Kaukinen K, Peraaho M, Lindfors K, Partanen J, Woolley N, Pikkarainen P, Karvonen AL, Laasanen T, Sievanen H, Maki M, Collin P. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237–45. doi: 10.1111/j.1365-2036.2007.03311.x. [DOI] [PubMed] [Google Scholar]
- 48.Rubio-Tapia A, Barton SH, Rosenblatt JE, Murray JA. Prevalence of small intestine bacterial overgrowth diagnosed by quantitative culture of intestinal aspirate in celiac disease. J Clin Gastroenterol. 2009;43:157–61. doi: 10.1097/MCG.0b013e3181557e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795–803. doi: 10.1200/JCO.2000.18.4.795. [DOI] [PubMed] [Google Scholar]
- 50.Meresse B, Ripoche J, Heyman M, Cerf-Bensussan N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2009;2:8–23. doi: 10.1038/mi.2008.75. [DOI] [PubMed] [Google Scholar]
- 51.Deleeuw RJ, Zettl A, Klinker E, Haralambieva E, Trottier M, Chari R, Ge Y, Gascoyne RD, Chott A, Muller-Hermelink HK, Lam WL. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902–11. doi: 10.1053/j.gastro.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Verbeek WH, Van De Water JM, Al-Toma A, Oudejans JJ, Mulder CJ, Coupe VM. Incidence of enteropathy - associated T-cell lymphoma: A nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol. 2008:1–7. doi: 10.1080/00365520802240222. [DOI] [PubMed] [Google Scholar]
- 53.Verbeek WH, von Blomberg BM, Scholten PE, Kuik DJ, Mulder CJ, Schreurs MW. The presence of small intestinal intraepithelial gamma/delta T-lymphocytes is inversely correlated with lymphoma development in refractory celiac disease. Am J Gastroenterol. 2008;103:3152–8. doi: 10.1111/j.1572-0241.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 54.Swinson CM, Slavin G, Coles EC, Booth CC. Coeliac disease and malignancy. Lancet. 1983;1:111–5. doi: 10.1016/s0140-6736(83)91754-3. [DOI] [PubMed] [Google Scholar]
- 55.Ryan BM, Kelleher D. Refractory celiac disease. Gastroenterology. 2000;119:243–51. doi: 10.1053/gast.2000.8530. [DOI] [PubMed] [Google Scholar]
- 56.Potter DD, Murray JA, Donohue JH, Burgart LJ, Nagorney DM, van Heerden JA, Plevak MF, Zinsmeister AR, Thibodeau SN. The role of defective mismatch repair in small bowel adenocarcinoma in celiac disease. Cancer Res. 2004;64:7073–7. doi: 10.1158/0008-5472.CAN-04-1096. [DOI] [PubMed] [Google Scholar]
- 57.Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203–8. doi: 10.1016/s0140-6736(00)02481-8. [DOI] [PubMed] [Google Scholar]
- 58.Patey-Mariaud De Serre N, Cellier C, Jabri B, Delabesse E, Verkarre V, Roche B, Lavergne A, Briere J, Mauvieux L, Leborgne M, Barbier JP, Modigliani R, Matuchansky C, MacIntyre E, Cerf-Bensussan N, Brousse N. Distinction between coeliac disease and refractory sprue: a simple immunohistochemical method. Histopathology. 2000;37:70–7. doi: 10.1046/j.1365-2559.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- 59.Verbeek WH, Goerres MS, von Blomberg BM, Oudejans JJ, Scholten PE, Hadithi M, Al-Toma A, Schreurs MW, Mulder CJ. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in Refractory Celiac Disease. Clin Immunol. 2008;126:48–56. doi: 10.1016/j.clim.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Cellier C, Patey N, Mauvieux L, Jabri B, Delabesse E, Cervoni JP, Burtin ML, Guy-Grand D, Bouhnik Y, Modigliani R, Barbier JP, Macintyre E, Brousse N, Cerf-Bensussan N. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–81. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 61.Tjon JM, Verbeek WH, Kooy-Winkelaar YM, Nguyen BH, van der Slik AR, Thompson A, Heemskerk MH, Schreurs MW, Dekking LH, Mulder CJ, van Bergen J, Koning F. Defective synthesis or association of T-cell receptor chains underlies loss of surface T-cell receptor-CD3 expression in enteropathy-associated T-cell lymphoma. Blood. 2008;112:5103–10. doi: 10.1182/blood-2008-04-150748. [DOI] [PubMed] [Google Scholar]
- 62.Ashton-Key M, Diss TC, Pan L, Du MQ, Isaacson PG. Molecular analysis of T-cell clonality in ulcerative jejunitis and enteropathy-associated T-cell lymphoma. Am J Pathol. 1997;151:493–8. [PMC free article] [PubMed] [Google Scholar]
- 63.de Mascarel A, Belleannee G, Stanislas S, Merlio C, Parrens M, Laharie D, Dubus P, Merlio JP. Mucosal intraepithelial T-lymphocytes in refractory celiac disease: a neoplastic population with a variable CD8 phenotype. Am J Surg Pathol. 2008;32:744–51. doi: 10.1097/PAS.0b013e318159b478. [DOI] [PubMed] [Google Scholar]
- 64.Liu H, Brais R, Lavergne-Slove A, Jeng Q, Payne K, Ye H, Liu Z, Carreras J, Huang Y, Bacon CM, Hamoudi RA, Save V, Venkatraman L, Isaacson PG, Woodward J, Du M-Q. Continual monitoring of intraepithelial lymphocyte immunophenotype and clonality is more important than snapshot analysis in the surveillance of refractory coeliac disease. Gut. 2009 doi: 10.1136/gut.2009.186007. (accepted for publication) [DOI] [PubMed] [Google Scholar]
- 65.Verkarre V, Romana SP, Cellier C, Asnafi V, Mention JJ, Barbe U, Nusbaum S, Hermine O, Macintyre E, Brousse N, Cerf-Bensussan N, Radford-Weiss I. Recurrent partial trisomy 1q22-q44 in clonal intraepithelial lymphocytes in refractory celiac sprue. Gastroenterology. 2003;125:40–6. doi: 10.1016/s0016-5085(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 66.Verbeek WH, von Blomberg BM, Coupe VM, Daum S, Mulder CJ, Schreurs MW. Aberrant T-lymphocytes in refractory coeliac disease are not strictly confined to a small intestinal intraepithelial localization. Cytometry B Clin Cytom. 2009 doi: 10.1002/cyto.b.20481. [DOI] [PubMed] [Google Scholar]
- 67.Daum S, Hummel M, Weiss D, Peters M, Wiedenmann B, Schaper F, Stein H, Riecken EO, Foss H. Refractory sprue syndrome with clonal intraepithelial lymphocytes evolving into overt enteropathy-type intestinal T-cell lymphoma. Digestion. 2000;62:60–5. doi: 10.1159/000007779. [DOI] [PubMed] [Google Scholar]
- 68.Cellier C, Cerf-Bensussan N. Treatment of clonal refractory celiac disease or cryptic intraepithelial lymphoma: A long road from bench to bedside. Clin Gastroenterol Hepatol. 2006;4:1320–1. doi: 10.1016/j.cgh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Haines ML, Anderson RP, Gibson PR. Systematic review: The evidence base for long-term management of coeliac disease. Aliment Pharmacol Ther. 2008;28:1042–66. doi: 10.1111/j.1365-2036.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- 70.Olaussen RW, Lovik A, Tollefsen S, Andresen PA, Vatn MH, De Lange T, Bratlie J, Brandtzaeg P, Farstad IN, Lundin KE. Effect of elemental diet on mucosal immunopathology and clinical symptoms in type 1 refractory celiac disease. Clin Gastroenterol Hepatol. 2005;3:875–85. doi: 10.1016/s1542-3565(05)00295-8. [DOI] [PubMed] [Google Scholar]
- 71.Goerres MS, Meijer JW, Wahab PJ, Kerckhaert JA, Groenen PJ, Van Krieken JH, Mulder CJ. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment Pharmacol Ther. 2003;18:487–94. doi: 10.1046/j.1365-2036.2003.01687.x. [DOI] [PubMed] [Google Scholar]
- 72.Daum S, Ipczynski R, Heine B, Schulzke JD, Zeitz M, Ullrich R. Therapy with budesonide in patients with refractory sprue. Digestion. 2006;73:60–8. doi: 10.1159/000092639. [DOI] [PubMed] [Google Scholar]
- 73.Miehlke S, Madisch A, Karimi D, Wonschik S, Kuhlisch E, Beckmann R, Morgner A, Mueller R, Greinwald R, Seitz G, Baretton G, Stolte M. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology. 2009;136:2092–100. doi: 10.1053/j.gastro.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 74.Bonderup OK, Hansen JB, Teglbjaerg PS, Christensen LA, Fallingborg JF. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009;58:68–72. doi: 10.1136/gut.2008.156513. [DOI] [PubMed] [Google Scholar]
- 75.Wahab PJ, Crusius JB, Meijer JW, Uil JJ, Mulder CJ. Cyclosporin in the treatment of adults with refractory coeliac disease--an open pilot study. Aliment Pharmacol Ther. 2000;14:767–74. doi: 10.1046/j.1365-2036.2000.00718.x. [DOI] [PubMed] [Google Scholar]
- 76.Maurino E, Niveloni S, Chernavsky A, Pedreira S, Mazure R, Vazquez H, Reyes H, Fiorini A, Smecuol E, Cabanne A, Capucchio M, Kogan Z, Bai JC. Azathioprine in refractory sprue: results from a prospective, open-label study. Am J Gastroenterol. 2002;97:2595–602. doi: 10.1111/j.1572-0241.2002.06029.x. [DOI] [PubMed] [Google Scholar]
- 77.Gillett HR, Arnott ID, McIntyre M, Campbell S, Dahele A, Priest M, Jackson R, Ghosh S. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology. 2002;122:800–5. doi: 10.1053/gast.2002.31874. [DOI] [PubMed] [Google Scholar]
- 78.Longstreth GF. Successful treatment of refractory sprue with cyclosporine. Ann Intern Med. 1993;119:1014–6. doi: 10.7326/0003-4819-119-10-199311150-00008. [DOI] [PubMed] [Google Scholar]
- 79.Vivas S, Ruiz de Morales JM, Ramos F, Suarez-Vilela D. Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T-cell lymphoma. N Engl J Med. 2006;354:2514–5. doi: 10.1056/NEJMc053129. [DOI] [PubMed] [Google Scholar]
- 80.Verbeek WH, Mulder CJ, Zweegman S. Alemtuzumab for refractory celiac disease. N Engl J Med. 2006;355:1396–7. doi: 10.1056/NEJMc061784. author reply 1397. [DOI] [PubMed] [Google Scholar]
- 81.Mulder CJ, Wahab PJ, Meijer JW, Metselaar E. A pilot study of recombinant human interleukin-10 in adults with refractory coeliac disease. Eur J Gastroenterol Hepatol. 2001;13:1183–8. doi: 10.1097/00042737-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731–43. doi: 10.1016/s1542-3565(04)00344-1. [DOI] [PubMed] [Google Scholar]
- 83.Connell WR, Kamm MA, Dickson M, Balkwill AM, Ritchie JK, Lennard-Jones JE. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet. 1994;343:1249–52. doi: 10.1016/s0140-6736(94)92150-4. [DOI] [PubMed] [Google Scholar]
- 84.Al-Toma A, Goerres MS, Meijer JW, von Blomberg BM, Wahab PJ, Kerckhaert JA, Mulder CJ. Cladribine therapy in refractory celiac disease with aberrant T cells. Clin Gastroenterol Hepatol. 2006;4:1322–7. doi: 10.1016/j.cgh.2006.07.007. quiz 1300. [DOI] [PubMed] [Google Scholar]
- 85.Dray X, Joly F, Lavergne-Slove A, Treton X, Bouhnik Y, Messing B. A severe but reversible refractory sprue. Gut. 2006;55:1210–1. doi: 10.1136/gut.2005.089987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-toma A, Visser OJ, van Roessel HM, von Blomberg BM, Verbeek WH, Scholten PE, Ossenkoppele GJ, Huijgens PC, Mulder CJ. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood. 2007;109:2243–9. doi: 10.1182/blood-2006-08-042820. [DOI] [PubMed] [Google Scholar]
- 87.Al-Toma A, Verbeek WH, Visser OJ, Kuijpers KC, Oudejans JJ, Kluin-Nelemans HC, Mulder CJ, Huijgens PC. Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig Liver Dis. 2007;39:634–41. doi: 10.1016/j.dld.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Mention JJ, Ben Ahmed M, Begue B, Barbe U, Verkarre V, Asnafi V, Colombel JF, Cugnenc PH, Ruemmele FM, McIntyre E, Brousse N, Cellier C, Cerf-Bensussan N. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–45. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]