Abstract
There is a direct relationship between the magnitude of sham rage produced by brainstem transection in cat and the decrease of brainstem norepinephrine. The attacks of rage are augmented by protriptyline and inhibited by haloperidol, drugs respectively facilitating or depressing the action of norepinephrine centrally. Release of norepinephrine by brainstem neurons appears essential for appearance of this behavior.
Full text
PDF
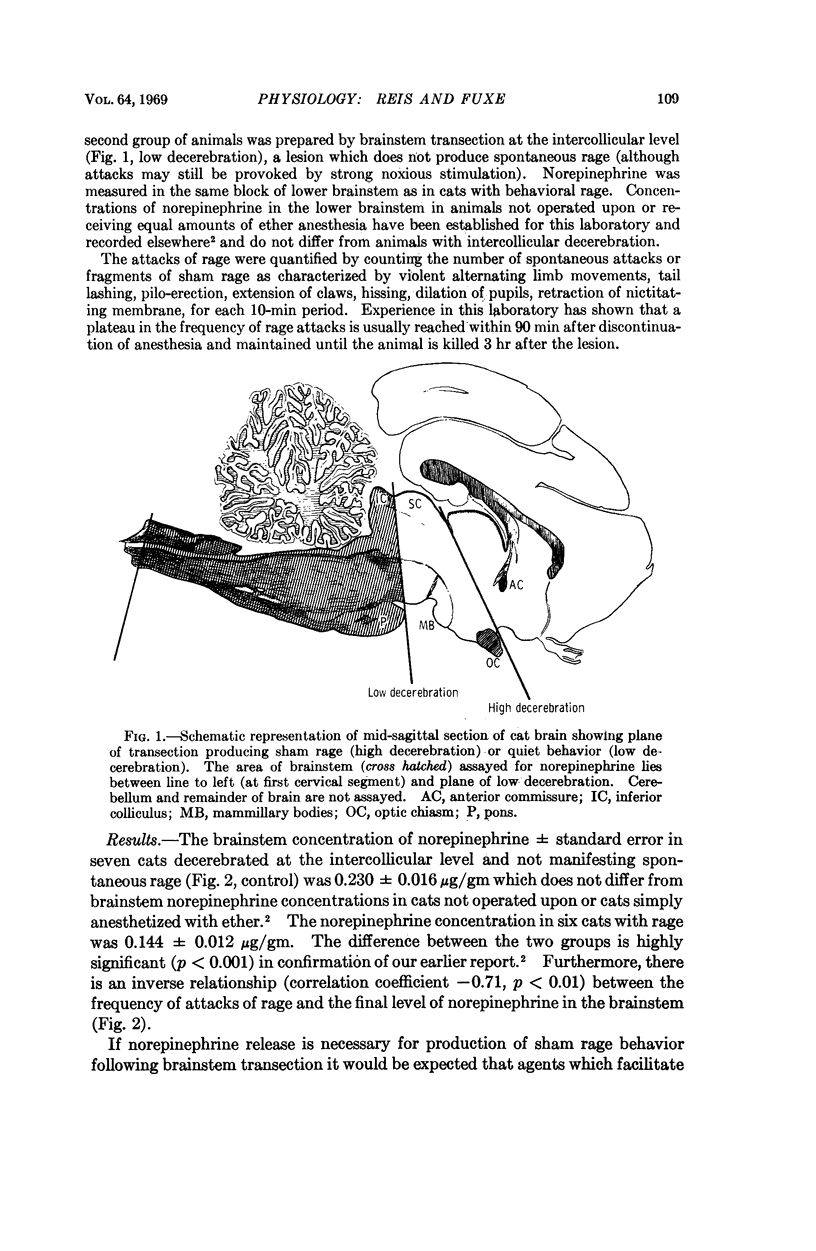
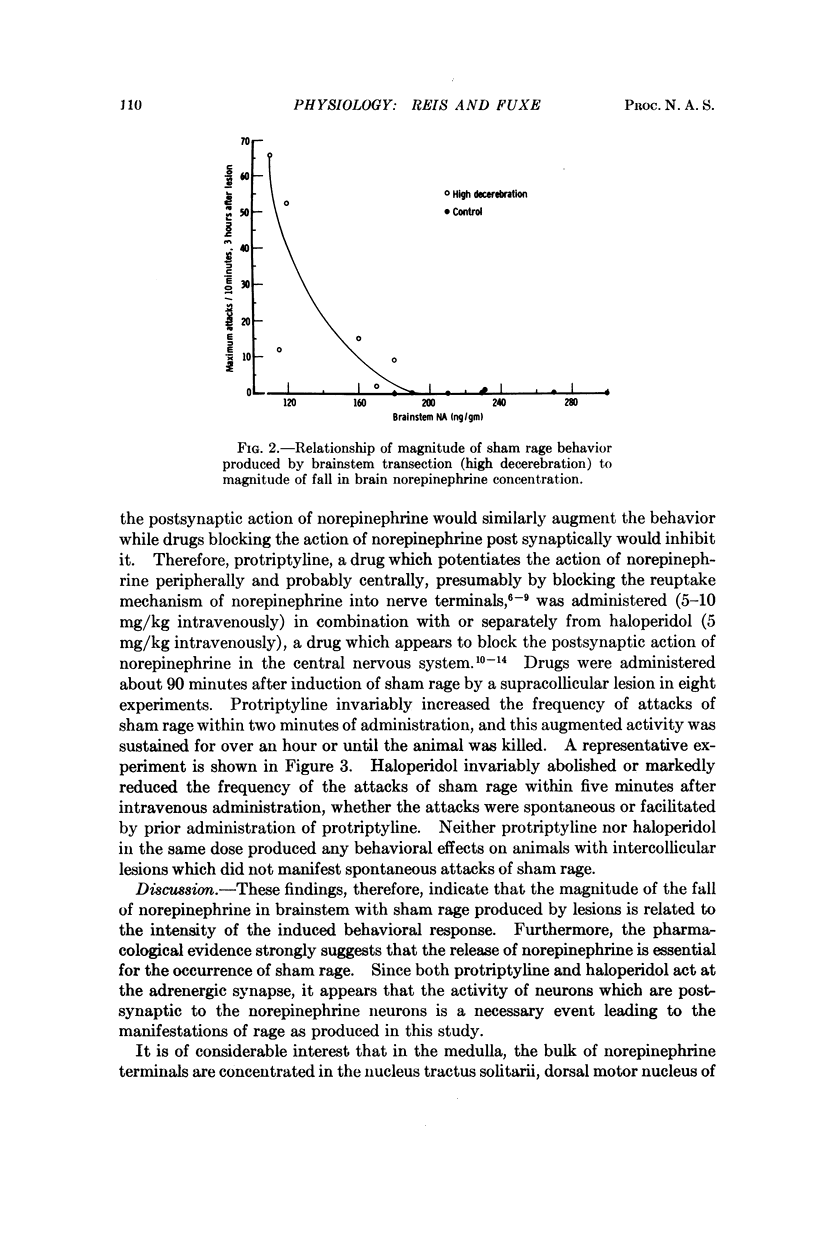
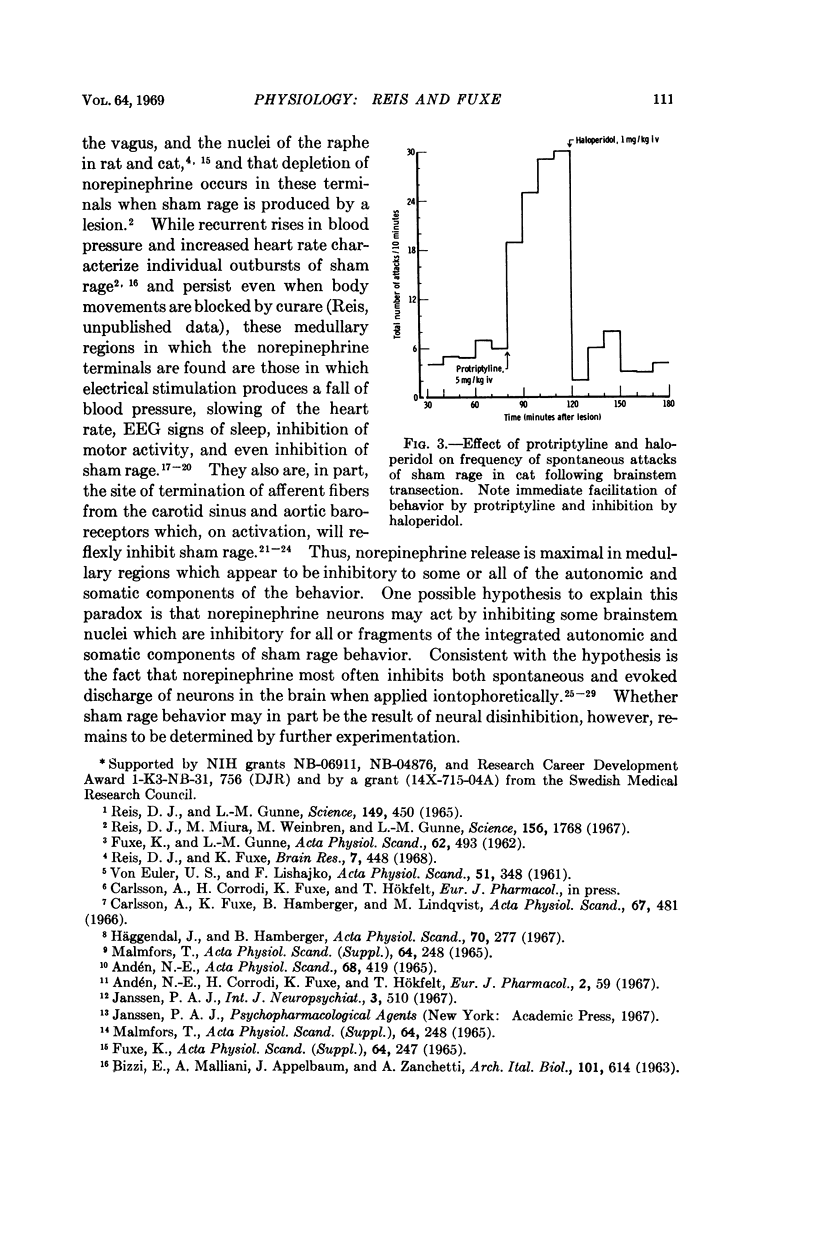

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACCELLI G., GUAZZI M., LIBRETTI A., ZANCHETTI A. PRESSOCEPTIVE AND CHEMOCEPTIVE AORTIC REFLEXES IN DECORTICATE AND IN DECEREBRATE CATS. Am J Physiol. 1965 Apr;208:708–714. doi: 10.1152/ajplegacy.1965.208.4.708. [DOI] [PubMed] [Google Scholar]
- BIZZI E., MALLIANI A., APELBAUM J., ZANCHETTI A. EXCITATION AND INHIBITION OF SHAM RAGE BEHAVIOR BY LOWER BRAIN STEM STIMULATION. Arch Ital Biol. 1963 Oct 5;101:614–631. [PubMed] [Google Scholar]
- Bloom F. E., Costa E., Salmoiraghi G. C. Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther. 1965 Nov;150(2):244–252. [PubMed] [Google Scholar]
- Carlsson A., Fuxe K., Hamberger B., Lindqvist M. Biochemical and histochemical studies on the effects of imipramine-like drugs and (+)-amphetamine on central and peripheral catecholamine neurons. Acta Physiol Scand. 1966 Jul-Aug;67(3):481–497. doi: 10.1111/j.1748-1716.1966.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Crill W. E., Reis D. J. Distribution of carotid sinus and depressor nerves in cat brain stem. Am J Physiol. 1968 Feb;214(2):269–276. doi: 10.1152/ajplegacy.1968.214.2.269. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Crawford J. M. Central synaptic transmission--microelectrophoretic studies. Annu Rev Pharmacol. 1969;9:209–240. doi: 10.1146/annurev.pa.09.040169.001233. [DOI] [PubMed] [Google Scholar]
- Engberg I., Ryall R. W. The inhibitory action of noradrenaline and other monoamines on spinal neurones. J Physiol. 1966 Jul;185(2):298–322. doi: 10.1113/jphysiol.1966.sp007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- FUXE K., GUNNE L. M. DEPLETION OF THE AMINE STORES IN BRAIN CATECHOLAMINE TERMINALS ON AMYGDALOID STIMULATION. Acta Physiol Scand. 1964 Dec;62:493–494. doi: 10.1111/j.1748-1716.1964.tb10450.x. [DOI] [PubMed] [Google Scholar]
- Häggendal J., Hamberger B. Quantitative in vitro studies on noradrenaline uptake and its inhibition by amphetamine, desipramine and chlorpromazine. Acta Physiol Scand. 1967 Jul-Aug;70(3):277–280. doi: 10.1111/j.1748-1716.1967.tb03626.x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Tebècis A. K., York D. H. The inhibitory action of monoamines on lateral geniculate neurones. J Physiol. 1967 Jun;190(3):563–581. doi: 10.1113/jphysiol.1967.sp008228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis D. J., Fuxe K. Depletion of noradrenaline in brainstem neurons during sham rage behaviour produced by acute brainstem transection in cat. Brain Res. 1968 Mar;7(3):448–451. doi: 10.1016/0006-8993(68)90010-3. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Gunne L. M. Brain Catecholamines: Relation to the Defense Reaction Evoked by Amygdaloid Stimulation in Cat. Science. 1965 Jul 23;149(3682):450–451. doi: 10.1126/science.149.3682.450. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Miura M., Weinbren M., Gunne L. M. Brain catecholamines: relation to defense reaction evoked by acute brainstem transection in cat. Science. 1967 Jun 30;156(3783):1768–1770. doi: 10.1126/science.156.3783.1768. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Salmoiraghi G. C. Responses of spinal cord interneurons to acetylcholine, norepinephrine and serotonin administered by microelectrophoresis. J Pharmacol Exp Ther. 1966 Sep;153(3):420–427. [PubMed] [Google Scholar]
- von EULER U., LISHAJKO F. Improved technique for the fluorimetric estimation of catecholamines. Acta Physiol Scand. 1961 Apr;51:348–355. doi: 10.1111/j.1748-1716.1961.tb02128.x. [DOI] [PubMed] [Google Scholar]




