Abstract
This study estimated differences by ethnicity in the diagnoses assigned prior to the diagnosis of autism. In this sample of 406 Medicaid-eligible children, African-Americans were 2.6 times less likely than white children to receive an autism diagnosis on their first specialty care visit. Among children who did not receive an autism diagnosis on their first visit, ADHD was the most common diagnosis. African-American children were 5.1 times more likely than white children to receive a diagnosis of adjustment disorder than of ADHD, and 2.4 times more likely to receive a diagnosis of conduct disorder than of ADHD. Differences in diagnostic patterns by ethnicity suggest possible variations in parents’ descriptions of symptoms, clinician interpretations and expectations, or symptom presentation.
Keywords: Autistic disorder, Autism spectrum disorder, African-Americans, Minorities, Child health services, Community mental health services
Despite increasing evidence that autism can be accurately identified in very young children (Baird et al., 2001; Bryson, Rogers, & Fombonne, 2003; Charman et al., 2005; Lord et al., 2006), diagnosis is often delayed until children are of school age (Howlin & Moore, 1997; Mandell, Listerud, Levy, & Pinto-Martin, 2002; Mandell, Novak, & Zubritsky, 2005a). This delay may be due to inadequate screening practices (Dearlove & Kearney, 1990; Dobos, Dworkin, & Bernstein, 1994; Sices, Feudtner, McLaughlin, Drotar, & Williams, 2003), pediatricians’ slow response to parental concerns (Glascoe, 1997; Shevell, Majnemer, Rosenbaum, & Abrahamowicz, 2001), the low sensitivity of screening instruments for autism (Dumont-Mathieu & Fein, 2005), and a general lack of awareness of symptoms (Shah, 2001). Some researchers have suggested that the similarity of the features of autism with other disorders can lead to misdiagnosis (Cuccaro et al., 1996; Noterdaeme, Amorosa, Mildenberger, Sitter, & Minow, 2001; Ohta, Nagai, Hara, & Sasaki, 1987). Symptoms common to autism, such as delayed speech, poor response to others and behavioral difficulties, can lead to misdiagnosis of language impairment or Attention Deficit/ Hyperactivity Disorder (ADHD). In older children, the presence of repetitive behaviors may steer clinicians toward a diagnosis of obsessive-compulsive disorder (OCD) and non-compliance related to resistance to change may lead clinicians to diagnose oppositional defiant disorder.
To complicate matters, autism often co-occurs with other behavioral or developmental symptoms. Mental retardation is associated with 70–80% of cases of children with autistic disorder (Bonde, 2000; Chakrabarti & Fombonne, 2001). Children with autism often exhibit attention and impulsivity problems (Aman & Langworthy, 2000; Goldstein & Schwebach, 2004) or severe behavior problems (Hatton et al., 2002; Remington, Sloman, Konstantareas, Parker, & Gow, 2002). Criteria from the Diagnostic and Statistical Manual of Mental Disorder IV-TR specify, however, that a diagnosis of autism must be ruled out before diagnosing ADHD or OCD (American Psychiatric Association, 2000). Even a co-occurring disorder exists, it is important that autism be diagnosed as well, since missed diagnosis may lead to misattribution of behavior and inappropriate treatment.
The probability of children receiving different psychiatric diagnoses differs by ethnicity (Cuffe, Waller, Cuccaro, Pumariega, & Garrison, 1996; Kilgus, Pumariega, & Cuffe, 1995; Wu et al., 1999). While there is no known difference by race or ethnicity in the epidemiology of autism (Fombonne, 2003; Yeargin-Allsopp et al., 2003), Cuccaro et al. (1996) suggest that clinicians may assign the diagnosis differentially by child ethnicity. Their vignette study found no effect of children's ethnicity or socio-economic status on the diagnosis of autism or ADHD; however, Mandell et al. (2002) found that African-American children with autism were diagnosed an average of 1.4 years later than white children and spent eight more months in mental health treatment before being diagnosed.
Syntheses of health disparities research provide compelling evidence of race and ethnicity as social, rather than biological constructs, and the root of related health care disparities as being largely due to unequal social interactions (Institute of Medicine, 2002). Cooper, Beach, Johnson, and Inui, (2006) refer to observable constructs such as race, ethnicity and social class as the “tip of the iceberg” in understanding health disparities, with patient and clinician values, beliefs, attitudes and understanding playing critical but understudied roles. It is in this context that the current study attempts to build on our previous research showing that African-American children with autism are diagnosed at a later age than white children (Mandell et al., 2002). In our previous study, we observed that African-American children entered specialty care later and took more specialty visits to receive a diagnosis of autism than white children. Later entry into specialty care most likely represents disparities in access (Guevara, Mandell, Rostain, Zhao, & Hadley, 2006). The reason the diagnosis of autism was unequally delayed once children entered specialty care, however, was unclear. We hypothesized that the diagnoses children received prior to receiving a diagnosis of autism once they entered specialty care—especially given that parents of children with autism and those of children with other disorders often present for treatment with the same complaints (Mandell, Walrath, Manteuffel, Sgro, & Pinto-Martin, 2005b)—might provide insight into clinicians’ thought processes and family behavior that lead to delayed diagnosis. The goal of this study therefore was to determine, among a sample of Medicaid-eligible children, the extent to which children ultimately diagnosed with autism were first diagnosed with other disorders and whether those diagnoses differ by ethnicity.
Methods
Data for this study were obtained from the Medicaid-reimbursed mental health claims for Philadelphia, Pennsylvania from July 1993 through June 1999. In Pennsylvania, children with autism are often eligible for Medicaid-reimbursed services regardless of family income, and those services are delivered through the behavioral health system.
All subjects were Medicaid-eligible residents of Philadelphia County during fiscal year 1999, had birth dates between January 1983 and May 1996 and received, in FY 1999, at least $1000 of Medicaid-reimbursed services associated with a diagnosis of autistic disorder (International Classification of Diseases—9th Edition code 299.00) (Medicode, 1987). Autistic disorder was chosen rather than autistic spectrum disorders, since its features are more clear cut and therefore differential diagnosis should be clearer (Eisenmajer et al., 1996; Hill et al., 2001; Mahoney et al., 1998) and the diagnosis of autistic disorder more stable in young children than other autism spectrum diagnoses (Charman et al., 2005; Lord et al., 2006).
Since data on diagnoses and service utilization were available starting from July 1992 and it was important for the purposes of this study to have information from the date of entry into the specialty mental health system, children were enrolled in the study using the following criteria:
Among children and adolescents meeting the above criteria, those whose birth dates occurred after June 1989 were included in the sample, making these children no older than 3 years at the start of data collection.
Children and adolescents who had received at least two Medicaid claims in FY 1999 for services related to a diagnosis of autistic disorder and whose birth date occurred before June 1989 were included in the study if the Medicaid claims indicated that their first mental health service contact occurred after June 1993. Therefore, at least 1 year of Medicaid claims showed that they had no previous mental health service, which ensured that children in the sample did not receive an autistic disorder diagnosis before data were available.
Missed Diagnosis was considered to occur if children in the sample received any diagnosis other than autism on their first mental health visit.
Diagnoses Assigned Prior to the Autism Diagnosis were obtained from Medicaid claims prior to the receipt of an autism diagnosis. All ICD-9 diagnoses between 295 and 319, inclusive, were coded. These diagnoses were then grouped into five categories: adjustment disorder (309), ADHD (314), conduct-related disorders (312 and 313), cognitive disorders (315–319), and other disorders. Children who received more than one diagnosis were categorized by the most frequently occurring diagnosis. The first four categories accounted for 95% of prior diagnoses.
Demographic Characteristics such as age at first visit, sex and ethnicity were obtained from the Medicaid claims. During the study period, ethnicity was coded as American-Indian, Asian, Black, Latino, White and “Other.” Because of the small number of individuals in categories other than black or white, other groups were collapsed into one category.
Medicaid Eligibility was obtained from the Medicaid eligibility records from July 1993 through June 1999. The number of months families were eligible for Medicaid prior to diagnosis was included because children with autism are eligible for Medicaid services even if their families’ incomes are greater than that needed normally to qualify for Medicaid. Therefore, this variable classified children as eligible for Medicaid because of income versus disability.
The first phase of data analysis included simple descriptive statistics for important variables for the total sample and for subsamples organized by ethnicity (African-American, white, other). The model identification phase consisted of two separate logistic regression analyses. First, multiple logistic regression was used to examine differences by ethnicity with respect to the odds of receiving a diagnosis of autism on the child's first specialty mental health visit. The dependent variable was regressed onto ethnicity, sex, age of entry into mental health services and a variable indicating whether the child's family had been eligible for Medicaid for at least 1 year prior to receiving the autistic disorder diagnosis. Multinomial logistic regression was used to determine the relative odds of receiving various diagnoses prior to autism. In this analysis, the sample consisted only of children (n = 228) who had received other diagnoses prior to autistic disorder. Because ADHD was the most common diagnosis among this group, it was used as the reference diagnosis. Diagnostic categories were regressed onto ethnicity, sex, and age of entry into the mental health system. Previous time eligible for Medicaid was removed from the multinomial analysis because 97% of this subsample had been on Medicaid for more than 1 year, and the estimates associated with this variable were unstable. Alpha was adjusted using Tukey (1977) adjustment for multiple comparisons across both analyses.
Results
Table 1 provides descriptive information on the total sample. The mean age of first specialty mental health visit for the sample was 6.7 years; 78.6% were male; 59.6% were African-American and 29.1% were white. The majority of children (93.4%) had been Medicaid-eligible for more than 1 year prior to receiving a diagnosis of autism.
Table 1.
Sample characteristics
| Black (n = 242) | White (n = 118) | Other (n = 46) | Total (n = 406) | |
|---|---|---|---|---|
| Male (%) | 77.3 | 85.6 | 67.4 | 78.6 |
| Age at first visit (SD) | 7.1 (2.9) | 6.3 (2.9) | 6.8 (2.5) | 6.7 (2.8) |
| Diagnosed with autism at first visit (%) | 34.7 | 60.2 | 50.0 | 43.8 |
| On welfare for more than 1 year (%) | 95.9 | 89.8 | 89.1 | 93.4 |
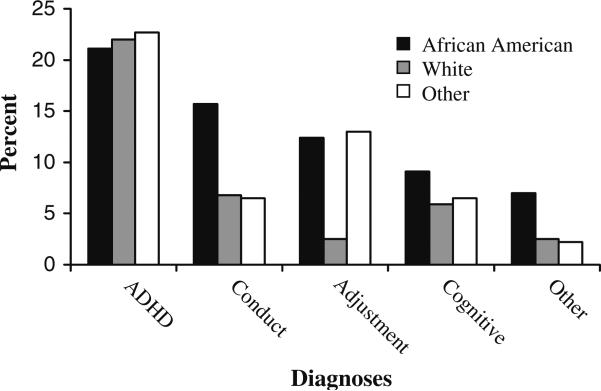
The figure shows the distribution of diagnoses prior to autism by ethnicity. Of the 406 children in the sample, 178 (43.8%) received an autism diagnosis on their first mental health visit. The most common diagnoses other than autism were ADHD (21.4%), followed by conduct-related disorders (12.1%), adjustment disorder (9.6%), and cognitive disorders (7.9%). Percentages of children diagnosed with ADHD or cognitive disorders were similar across ethnicity. In contrast, African-American children were approximately twice as likely as other children to receive a diagnosis of conduct disorder (15.7% vs. 6.7%). White children were approximately 5 times less likely than other children to receive a diagnosis of adjustment disorder (2.5% vs. 12.8%).
Table 2 provides the results of the logistic regression with a diagnosis other than autism on the first specialty mental health visit as the outcome. In this adjusted model, African-American children had 2.6 times the odds of receiving some other diagnosis compared with whites. Those who were Medicaid-eligible for more than 1 year had 3.4 times the odds of receiving some other diagnosis compared with those who were Medicaid-eligible for less than 1 year.
Table 2.
Odds of receiving a diagnosis other than autism prior to the autism diagnosis (n = 406)
| Odds ratio | P value | 95% CI | |
|---|---|---|---|
| Age at first specialty visit | 1.0 | 0.838 | 0.9, 1.1 |
| Other ethnicity | 1.4 | 0.320 | 0.7, 2.9 |
| Black | 2.6 | 0.000 | 1.7, 4.2 |
| Male | 1.5 | 0.105 | 0.9, 2.6 |
| Medicaid-eligible >1 year | 3.4 | 0.008 | 1.4, 8.4 |
Table 3 provides the results of the multinomial regression predicting presence of a particular diagnosis other than ADHD. The sample for this analysis consisted of the 228 children who received another diagnosis prior to autism. The results of this adjusted model were similar to the unadjusted results presented in Fig. 1. Compared with whites, black children were 5.1 times more likely to receive a diagnosis of adjustment disorder compared with ADHD, and 2.4 times more likely to receive a diagnosis of conduct disorder compared with a diagnosis of ADHD, when accounting for age, gender and time on welfare. Boys were 8.9 times more likely than girls to receive a diagnosis of adjustment disorder compared with ADHD, and 3.5 times more likely to receive a diagnosis of conduct disorder compared with ADHD when accounting for age, race and the time for which they were Medicaid-eligible prior to diagnosis. While not statistically significant at the P < 0.05 level, compared with whites, those in the other race/ethnicity category were 4.1 times more likely to receive a diagnosis of adjustment disorder compared with ADHD (P = 0.09).
Table 3.
Multinomial regression predicting risk of receiving a particular diagnosis prior to autism compared with ADHD (n = 228)
| Odds ratio | P value | 95% CI | |
|---|---|---|---|
| Adjustment disorder | |||
| Age at first specialty visit | 1.0 | 0.76 | 0.8, 1.1 |
| Male | 8.9 | <0.01 | 3.5, 22.6 |
| Other ethnicity | 4.1 | 0.09 | 0.8, 21.7 |
| Black | 5.1 | 0.02 | 1.3, 19.1 |
| Cognitive disorders | |||
| Age at first specialty visit | 0.9 | 0.51 | 0.8, 1.1 |
| Male | 2.2 | 0.15 | 0.7, 6.4 |
| Other ethnicity | 1.1 | 0.92 | 0.2, 5.1 |
| Black | 1.7 | 0.31 | 0.6, 4.5 |
| Conduct disorders | |||
| Age at first specialty visit | 1.0 | 0.80 | 0.9, 1.2 |
| Male | 3.5 | 0.01 | 1.4, 8.8 |
| Other ethnicity | 0.9 | 0.86 | 0.2, 4.1 |
| Black | 2.4 | 0.05 | 1.1, 6.1 |
| Other disorders | |||
| Age at first specialty visit | 1.2 | 0.11 | 1.0, 1.4 |
| Male | 1.9 | 0.34 | 0.5, 6.8 |
| Other ethnicity | 0.8 | 0.83 | 0.1, 8.5 |
| Black | 2.6 | 0.16 | 0.7, 9.9 |
Fig. 1.
Percentage receiving diagnoses other than autism by ethnicity (n = 406)
Discussion
Previous research has found that even after entering specialty care, there is a continued delay in the diagnosis of autism among African-American children compared with white children (Mandell et al., 2002). The current study extended that research by examining diagnostic patterns after entry into specialty care. The main finding was that African-American children ultimately diagnosed with autism were nearly 3 times more likely than white children to receive another diagnosis first. Among children who received other diagnoses, African-American children were much more likely than white children to receive a diagnosis of conduct or adjustment disorder.
Receipt of diagnoses other than autism, especially ADHD, conduct and adjustment disorders, was common for the entire sample. It is possible that the sample represents a particularly diagnostically complex group of children, which would explain both their late diagnosis relative to other children (Mandell et al., 2005a) and the high proportion of children who received diagnoses other than autism. The role of economic disadvantage should not be discounted, however. Children with autism in families that had been Medicaid-eligible for more than 1 year were 3.4 times more likely to first receive some other diagnosis. It is possible that the presentation of autism differs among poorer children, perhaps because of environmental causes or mediators. It is also possible that wealthier families obtained Medicaid eligibility for their children only after confirming the diagnosis. Parent and clinician behavior, which are addressed within the context of ethnicity below, may also be applicable to families of lower socio-economic status.
A number of study limitations should be mentioned, primarily the unknown validity of diagnoses of autism in the Medicaid claims data. While its accuracy has not been specifically examined, Fombonne et al. (2004) found 97% positive predictive value for chart diagnoses and a research diagnosis of autism. Another important limitation is that there were no measures of symptoms or severity. It is likely that the presence of certain symptoms or the severity of the disorder is closely associated with accurate diagnosis; if, for example, higher functioning children are more likely to be misdiagnosed, it would explain the low prevalence of cognitive disorder diagnoses. There is no reason to suspect, however, that severity differs by race. Third, a number of important variables that may affect interpretation of symptoms and use of services, such as education and family structure, were not available. Similarly, the neighborhoods in which children live may confound the effects of race on diagnosis. Diagnostic services in some neighborhoods may be better than others, and those communities may differ by their racial and ethnic composition. Finally, this study is based on a Medicaid sample in one city and may not generalize to other populations.
Despite these limitations, there are important implications of these findings. Even if we accept the primary limitation that diagnosis of autism was not validated, it still begs the question of why diagnostic patterns differed by race. Possible causes include differences in (1) child presentation; (2) parent behavior in response to symptoms; and (3) clinician responses to child symptoms and parents’ complaints (Mandell & Novak, 2005).
Volkmar and Pauls (2003) suggest conceptualizing autism as a set of behavioral phenotypes; while there is no published evidence regarding differences in presentation by race or ethnicity, their existence may differentially confound diagnosis. Evidence for underlying behavioral phenotypes has been mixed. Some studies have identified distinct clustering of symptoms (Cuccaro et al., 2003; Tadevosyan-Leyfer et al., 2003), while others have found one, continuously distributed underlying factor (Constantino et al., 2003; Spiker, Lotspeich, Dimiceli, Myers, & Risch, 2002). Cuccaro et al. (2005) report that African-American children with autism may have more impaired language development than white children, while oral communication with Lord (2005, oral communication) suggested no such differences. In their study of the involvement of GABA receptor subunit genes in autism, Collins et al. (2006) report invariance across white and a small group of African-American children, but note that different single nucleotide polymorphisms were associated in each ethnic group. Much further research is required to determine whether ethnic genotypic differences exist, and whether they are associated with different clinical presentation.
Some preliminary evidence suggests that cultural factors may affect parents’ recognition and interpretation of symptoms. Daley (2004) found that Asian Indian parents were more likely to first notice social difficulties rather than speech delays in their children with autism, while studies conducted in the United States have found that parents were more likely to detect general developmental delays or regression in language skills than social or communicative deficits (Coonrod & Stone, 2004). Daley postulates that these differences are due to the Indian culture, which values social conformity more than the United States. Coonrod and Stone suggest that American parents were less concerned about social milestones than about language development.
The presence of adjustment and conduct disorder diagnoses in the current study suggests the possibilities of ethnic differences in the exchange of information and description of symptoms. Ethnic differences in how parents describe symptoms may lead to an incomplete representation or misattribution of symptoms. The fact that African-American children with autism were more likely than children of other ethnicities to receive a diagnosis of conduct disorder suggests the possibility that African-American parents are more likely than others to describe their children's symptoms in ways that emphasize their children's disruptive behavior. The fact that African-American children and children of other ethnicities—which might include recent immigrants—were more likely than white children to receive a diagnosis of adjustment disorder suggests the possibility that these parents have difficulty communicating symptoms to clinicians in a manner that allows clinicians to translate parental descriptions into diagnostic categories. These speculations clearly require further exploration.
The misdiagnosing of autism may relate to lack of familiarity with the disorder or underutilization of standardized clinical measures. Clinicians may diagnose adjustment disorder when they require more time to evaluate a child. Assigning this diagnosis enables reimbursement and the opportunity for another visit. Conduct disorder may be diagnosed as a function of clinicians’ interpretation of children's disruptive or aggressive behaviors. Clinicians may also be concerned about the effects of labeling children as autistic, and therefore withhold the diagnosis as long as feasible.
The Institute of Medicine (2002) report, Unequal Treatment, suggests that the racial differences in diagnostic patterns observed in this and other studies may be attributable to general prejudices held by the clinician, specific stereotypes about health-related behaviors, and the application of rational (if erroneous) decision rules regarding health status. This last type of discrimination, referred to as statistical discrimination (Balsa & McGuire, 2001), occurs if clinicians have different expectations about the probability of autism occurring in children of different ethnicities. While this issue has not been specifically studied, Balsa, McGuire, & Meredith (2005) found that physicians had different expectations about the frequency of heart disease and diabetes in white and African-American adults, leading to different rates of diagnosis in the presence of similar symptoms. The authors point out that, alternatively, clinicians’ expectations can lead to disparities in health communication. For example, they found that ethnic disparities in the diagnosis of adults with depression were mediated in large part by clinicians’ failure to elicit or appropriately interpret African-Americans concerns. Similarly, a study of adult primary care practices found that African-Americans were much more likely than whites to say that their physician had not solicited or listened to their complaints and concerns, regardless of the ethnicity of the physician (Cooper-Patrick et al., 1999). Within this framework, one might hypothesize that the more frequent diagnosis of conduct disorder among African-Americans (but not other ethnic groups) may be associated with clinicians’ erroneous beliefs regarding the increased frequency of conduct disorder among African-American children, while the more frequent diagnosing of adjustment disorder among all groups other than whites may be the result of clinicians’ misinterpretation of parental concerns.
These and other strategies that more closely examine interactions between clinicians, patients and families (Roter et al., 1995, 1997; Wissow, Larson, Anderson, & Hadjisky, 2005) provide promising models for examining disparities in the diagnosis of children with psychiatric and developmental disorders such as autism. A statistical discrimination model suggests the need to better understand the heuristics clinicians use to make diagnostic decisions, while the miscommunication model suggests the need to more closely observe parent–clinician interactions around diagnosis, and how they differ by ethnicity. The results of this study also suggest the immediate need for further education among specialty practitioners in at least three areas: (1) the symptoms of autism and how they may be differentiated from those of other disorders; (2) greater sensitivity regarding ethnic differences in language used to describe child behavior, and; (3) education and values clarification regarding their own beliefs about the prevalence of autism and its presentation by ethnicity. They also suggest the urgent need to examine whether delivery of care subsequent to diagnosis differ as well.
Acknowledgments
This work was supported by a Career Development Award from the National Institute of Mental Health (MH067628) to Dr. Mandell. The authors are grateful to Nancy Minshew, MD, for her insightful comments on the text.
Contributor Information
David S. Mandell, Center for Autism and Developmental Disabilities Research and Epidemiology, University of Pennsylvania, Philadelphia, PA, USA Department of Psychiatry, Center for Mental Health Policy and Services Research, University of Pennsylvania School of Medicine, 3535 Market Street, 3rd Floor, Philadelphia, PA 19104, USA; Department of Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Richard F. Ittenbach, Center for Autism and Developmental Disabilities Research and Epidemiology, University of Pennsylvania, Philadelphia, PA, USA The Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Susan E. Levy, Center for Autism and Developmental Disabilities Research and Epidemiology, University of Pennsylvania, Philadelphia, PA, USA The Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Jennifer A. Pinto-Martin, Center for Autism and Developmental Disabilities Research and Epidemiology, University of Pennsylvania, Philadelphia, PA, USA University of Pennsylvania School of Nursing, Philadelphia, PA, USA.
References
- Aman M, Langworthy K. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. Journal of Autism & Developmental Disorders. 2000;30(5):451–459. doi: 10.1023/a:1005559725475. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders, fourth edition, text revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baird G, Charman T, Cox A, Baron-Cohen S, Swettenham J, Wheelwright S, et al. Screening and surveillance for autism and pervasive developmental disorders. Archives of Disease in Childhood. 2001;84(6):468–475. doi: 10.1136/adc.84.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsa A, McGuire T. Statistical discrimination in health care. Journal of Health Economics. 2001;20:881–907. doi: 10.1016/s0167-6296(01)00101-1. [DOI] [PubMed] [Google Scholar]
- Balsa A, McGuire T, Meredith L. Testing for statistical discrimination in health care. Health Services Research. 2005;40(1):227–252. doi: 10.1111/j.1475-6773.2005.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde E. Comorbidity and subgroups in childhood autism. European Child & Adolescent Psychiatry. 2000;9:7–10. doi: 10.1007/s007870050110. [DOI] [PubMed] [Google Scholar]
- Bryson S, Rogers S, Fombonne E. Autism spectrum disorders: Early detection, intervention, education, and psychopharmacological management. Canadian Journal of Psychiatry. 2003;48(8):506–516. doi: 10.1177/070674370304800802. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. Journal of the American Medical Association. 2001;285(24):3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cockerill H, Brown J, Baird G. Outcome at 7 years of children diagnosed with autism at age 2: Predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;46(5):500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Collins A, Ma D, Whitehead P, Martin E, Wright H, Abramson R, et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–174. doi: 10.1007/s10048-006-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J, Davis S, Todd R, Schindler M, Gross M, Brophy S, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Coonrod E, Stone W. Early concerns of parents of children with autistic and nonautistic disorders. Infants and Young Children. 2004;17(3):258–269. [Google Scholar]
- Cooper-Patrick L, Gallo J, Gonzales J, Vu H, Powe N, Nelson C, et al. Race, gender, and partnership in the patient–physician relationship. Journal of the American Medical Association. 1999;282(6):583–589. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- Cooper L, Beach M, Johnson R, Inui T. Delving below the surface. Understanding how race and ethnicity influence relationships in health care. Journal of General Internal Medicine. 2006;21(Suppl 1):S21–S27. doi: 10.1111/j.1525-1497.2006.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccaro M, Donnelly S, Cope H, Wolpert C, Carney R, Abramson R, et al. Autism in african-american (aa) families: Phenotypic findings.. Paper presented at the American Society of Human Genetics 55th Annual Meeting; Salt Lake City, UT. 2005. [Google Scholar]
- Cuccaro M, Shao Y, Grubber J, Slifer M, Wolpert C, Donnelly S, et al. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry & Human Development. 2003;34(1):3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- Cuccaro M, Wright H, Rownd C, Abramson R, Waller J, Fender D. Professional perceptions of children with developmental difficulties: The influence of race and socioeconomic status. Journal of Autism & Developmental Disorders. 1996;26(4):461–469. doi: 10.1007/BF02172830. [DOI] [PubMed] [Google Scholar]
- Cuffe S, Waller J, Cuccaro M, Pumariega A, Garrison C. Race and gender differences in the treatment of psychiatric disorders in young adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;34(11):1536–1543. doi: 10.1097/00004583-199511000-00021. [DOI] [PubMed] [Google Scholar]
- Daley T. From symptom recognition to diagnosis: Children with autism in urban india. Social Science and Medicine. 2004;58:1323–1335. doi: 10.1016/S0277-9536(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Dearlove J, Kearney D. How good is general practice developmental screening. British Medical Journal. 1990;300:1177–1180. doi: 10.1136/bmj.300.6733.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos A, Dworkin P, Bernstein B. Pediatricians’ approaches to developmental problems: Has the gap been narrowed. Journal of Developmental and Behavioral Pediatrics. 1994;15:34–39. doi: 10.1097/00004703-199402000-00006. [DOI] [PubMed] [Google Scholar]
- Dumont-Mathieu T, Fein D. Screening for autism in young children: The modified checklist for autism in toddlers (m-chat) and other measures. Mental Retardation & Developmental Disabilities Research Reviews. 2005;11(3):253–262. doi: 10.1002/mrdd.20072. [DOI] [PubMed] [Google Scholar]
- Eisenmajer R, Prior M, Leekam S, Wing L, Gould J, Welham M, et al. Comparison of clinical symptoms in autism and asperger's disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(11):1523–1531. doi: 10.1097/00004583-199611000-00022. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. Journal of Autism and Developmental Disorders. 2003;33(4):365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Heavey L, Smeeth L, Rodrigues L, Cook C, Smith P, et al. Validation of the diagnosis of autism in general practitioner records. BMC Public Health. 2004;4 doi: 10.1186/1471-2458-4-5. Article 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascoe F. Parent's concerns about children's development: Prescreening technique or screening test? Pediatrics. 1997;99(4):522–528. doi: 10.1542/peds.99.4.522. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Schwebach A. The comorbidity of pervasive developmental disorder and attention deficit hyperactivity disorder: Results of a retrospective chart review. Journal of Autism & Developmental Disorders. 2004;34(3):329–339. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- Guevara J, Mandell D, Rostain A, Zhao H, Hadley T. Disparities in the reporting and treatment of health conditions in children: An analysis of the medical expenditure panel survey. Health Services Research. 2006;41(2):532–549. doi: 10.1111/j.1475-6773.2005.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton D, Hooper S, Bailey D, Skinner M, Sullivan K, Wheeler A. Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics. 2002;108(2):105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Hill A, Bolte S, Petrova G, Beltcheva D, Tacheva S, Poustka F. Stability and interpersonal agreement of the interview-based diagnosis of autism. Psychopathology. 2001;34(4):187–191. doi: 10.1159/000049305. [DOI] [PubMed] [Google Scholar]
- Howlin P, Moore A. Diagnosis of autism. A survey of over 1200 patients in the UK. Autism. 1997;1:135–162. [Google Scholar]
- Institute of Medicine . Unequal treatment. Institute of Medicine; Washington, DC: 2002. [Google Scholar]
- Kilgus M, Pumariega A, Cuffe S. Influence of race on diagnosis in adolescent psychiatric inpatients. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34(1):67–72. doi: 10.1097/00004583-199501000-00016. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore P, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):649–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Mahoney W, Szatmari P, MacLean J, Bryson S, Bartolucci G, Walter S, et al. Reliability and accuracy of differentiating pervasive developmental disorder subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(3):278–285. doi: 10.1097/00004583-199803000-00012. [DOI] [PubMed] [Google Scholar]
- Mandell D, Listerud J, Levy S, Pinto-Martin J. Race differences in the age at diagnosis among medicaid-eligible children with autism. Journal of the American Academy Of Child And Adolescent Psychiatry. 2002;41(12):1447–1453. doi: 10.1097/00004583-200212000-00016. [DOI] [PubMed] [Google Scholar]
- Mandell D, Novak M. The role of culture in families’ treatment decisions for children with autism spectrum disorders. Mental Retardation & Developmental Disabilities Research Reviews. 2005;11:110–115. doi: 10.1002/mrdd.20061. [DOI] [PubMed] [Google Scholar]
- Mandell D, Novak M, Zubritsky C. Factors associated with the age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005a;116(6):1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell D, Walrath C, Manteuffel B, Sgro G, Pinto-Martin J. Characteristics of children with autistic spectrum disorders served in comprehensive community-based mental health settings. Journal of Autism and Developmental Disorders. 2005b;35(3):113–121. doi: 10.1007/s10803-005-3296-z. [DOI] [PubMed] [Google Scholar]
- Medicode . International classification of diseases. 9th ed. Med-Index Publications; Salt Lake City, UT: 1987. [Google Scholar]
- Noterdaeme M, Amorosa H, Mildenberger K, Sitter S, Minow F. Evaluation of attention problems in children with autism and children with a specific language disorder. European Child & Adolescent Psychiatry. 2001;10(1):58–66. doi: 10.1007/s007870170048. [DOI] [PubMed] [Google Scholar]
- Ohta M, Nagai Y, Hara H, Sasaki M. Parental perception of behavioral symptoms in Japanese autistic children. Journal of Autism & Developmental Disorders. 1987;17(4):549–563. doi: 10.1007/BF01486970. [DOI] [PubMed] [Google Scholar]
- Remington G, Sloman L, Konstantareas M, Parker K, Gow R. Clomipramine versus haloperidol in the treatment of autistic disorder: A double-blind, placebo-controlled, crossover study. Journal of Clinical Psychopharmacology. 2002;21(4):440–444. doi: 10.1097/00004714-200108000-00012. [DOI] [PubMed] [Google Scholar]
- Roter D, Hall J, Kern D, Barker L, Cole K, Roca R. Improving physicians’ interviewing skills and reducing patients’ emotional distress: A randomized clinical trial. Archives of Internal Medicine. 1995;155:1877–1884. [PubMed] [Google Scholar]
- Roter D, Stewart M, Putnam S, Lipkin M, Jr, Stiles W, Inui T. Communication patterns of primary care physicians. Journal of the American Medical Association. 1997;277:350–356. [PubMed] [Google Scholar]
- Shah K. What do medical students know about autism. Autism. 2001;5(2):127–133. doi: 10.1177/1362361301005002003. [DOI] [PubMed] [Google Scholar]
- Shevell M, Majnemer A, Rosenbaum P, Abrahamowicz M. Profile of referrals for early childhood developmental delay to ambulatory subspecialty clinics. Journal of Child Neurology. 2001;16(9):645–650. doi: 10.1177/088307380101600904. [DOI] [PubMed] [Google Scholar]
- Sices L, Feudtner C, McLaughlin J, Drotar D, Williams M. How do primary care physicians identify young children with developmental delays? A national survey. Journal of Developmental and Behavioral Pediatrics. 2003;24(6):409–417. doi: 10.1097/00004703-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Spiker D, Lotspeich L, Dimiceli S, Myers R, Risch N. Behavioral phenotypic variation in autism multiplex families: Evidence for a continuous severity gradient. American Journal of Medical Genetics. 2002;114(2):129–136. doi: 10.1002/ajmg.10188. [DOI] [PubMed] [Google Scholar]
- Tadevosyan-Leyfer O, Dowd M, Mankoski R, Winklosky B, Putnam S, McGrath L, et al. A principal components analysis of the autism diagnostic interview-revised. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(7):864–872. doi: 10.1097/01.CHI.0000046870.56865.90. [DOI] [PubMed] [Google Scholar]
- Tukey J. Explanatory data analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]
- Volkmar F, Pauls D. Autism. Lancet. 2003;362(9390):1133–1141. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- Wissow L, Larson S, Anderson J, Hadjisky E. Pediatric residents’ responses that discourage discussion of psychosocial problems in primary care. Pediatrics. 2005;115(6):1569–1578. doi: 10.1542/peds.2004-1535. [DOI] [PubMed] [Google Scholar]
- Wu P, Hoven C, Bird H, Moore R, Cohen P, Alegria M, et al. Depressive and disruptive disorders and mental health service utilization in children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(9):1081–1090. doi: 10.1097/00004583-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. Journal of the American Medical Association. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]