Abstract
There is a pressing need for new and more efficient boron delivery agents to tumor cells for use in boron neutron capture therapy (BNCT). A class of boronated unnatural cyclic amino acids has demonstrated a remarkable selectivity toward tumors in animal and cell culture models, far superior to currently used agents in clinical BNCT. One of these amino acids, 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC), has shown a tumor to blood ratio of 8 and a tumor to normal brain ratio of nearly 21 in a melanoma bearing mouse model. This work represents further biological characterization of this compound for tumor targeting in an EMT6 murine mammary carcinoma mouse model and a T98G human glioblastoma cell line. Female BALB/c mice bearing EMT6 tumors were injected with the fructose complex form of racemic mixtures of cis- and trans isomers of ABCPC in identical concentrations. Boron concentrations were measured in the tumor, blood, brain, skin, and liver tissues at 1, 3, and 5 hr post injection. These observations revealed a remarkable difference in racemic mixtures of cis and trans isomers in tumor targeting by boron. This implies that further separation of the L and D forms of this compound may enhance tumor targeting to an even higher degree than that provided by the racemic mixtures. Since the uptake measurements were made in homogenized tumor and normal tissues, little is known about the subcellular location of the boron arising from the various isomeric forms of the amino acid. To study subcellular delivery of boron from ABCPC in T98G human glioblastoma cells, we employed secondary ion mass spectrometry (SIMS) based technique of ion microscopy, which is capable of quantitatively imaging isotopic (elemental) gradients in cells and tissues at 500 nm spatial resolution. The T98G cells were exposed to the nutrient medium containing 100 ppm boron equivalent of a mixture of both L and D isomers of ABCPC in the form of a fructose complex for 1 hr. Following this treatment, the cells were fast frozen, freeze-fractured, and freeze-dried for SIMS analysis. Within an hour of exposure, ABCPC provided partitioning of intracellular to extracellular boron of 3/1. SIMS imaging revealed that boron from ABCPC was distributed throughout the cell, including the nucleus. This level of boron delivery within an hour of exposure is superior to p-boronophenylalanine (BPA) and sodium borocaptate (BSH), which have been previously studied by SIMS in the same cell line. These encouraging observations provide compelling support for further isomeric separations of ABCPC into the D and L forms for enhanced tumor targeting and continued testing of these compounds as new boron carriers in BNCT.
Keywords: Amino acids, BNCT, Boron biodistribution, SIMS
1. Introduction
Boron neutron capture therapy (BNCT) of cancer depends on the selective targeting of sufficient quantities of boron-10 (approximately 30 µg/g of tumor) to the tumor cells for effective killing via the boron neutron capture fission reaction (Sweet 1997; Godwin et al., 1955). Since the lethal reaction produced in BNCT is limited to a range of less than a single cell diameter, BNCT is potentially capable of killing individual cancer cells while sparing the neighboring normal cells. Extensive international efforts have been made by physicists, chemists, clinicians, and biologists for over 50 years focused on the development and testing of BNCT in the treatment of cancer (Barth et al., 2005; Coderre and Morris, 1999; Hatanaka, 1991; Kabalka et al., 2003; Kabalka and Yao, 2006; Li et al., 2006; Pellettieri et al., 2008). Clinical interest in BNCT has focused primarily on the treatment of high-grade gliomas, either cutaneous primaries or cerebral metastases of melanoma, and, more recently, head and neck and liver cancer (Barth et al., 2005; Pellettieri et al., 2008; Cordoso et al., 2007; Dagrosa et al., 2007; Kankaaranta et al., 2007; Wittig et al., 2008).
The clinical success of BNCT depends on two factors: (i) selective targeting and delivery of sufficient quantities of 10B atoms to tumor cells and (ii) a neutron flux sufficient to achieve the required nuclear reaction while minimizing damage to healthy tissue. Early BNCT clinical trials were disappointing since they failed to achieve either of these goals (Sweet 1997). However, significant advances in the modification of nuclear reactors and in selectively tumor-seeking boron-containing agents have been made in recent years (Barth et al., 2005; Soloway et al., 1998). To minimize damage to normal tissues, the quantity of boron in tumor cells must exceed that found in surrounding normal cells by at least a factor of three (Fairchild and Bond, 1985; Zamenhof et al., 1992). In BNCT, the cell killing efficiency is enhanced by intranuclear localization of 10B, where the resulting fission products have a greater probability of damaging the DNA (Gabel et al., 1987). Consequently, the development of microanalytical techniques capable of accurately analyzing subcellular concentrations at the parts-per-million (ppm) level of boron is critically needed in BNCT. Single cell and subcellular measurements are also necessary for understanding mechanistic aspects of boron delivery, as well as for identifying the partitioning of boron between the blood and the tumor cells.
Although two BNCT agents, BPA and sodium borocaptate (BSH), are being used in clinical trials in Europe and Japan focused on brain tumors, melanomas, head and neck tumors, and liver metastases, there is increasing emphasis on the need of better tumor-targeting BNCT agents (Barth et al., 2005; Kabalka and Yao, 2006; Li et al., 2006). It is believed that the amino acids are preferentially taken up by growing tumor cells (Wittig et al., 2000). Efforts at the University of Tennessee have focused on the synthesis of boronated unnatural amino acids as potential boron carriers to tumor cells (Kabalka and Yao, 2006; Kabalka et al., 2004). The logic for the synthesis of these boron carriers came from positron emission tomography (PET) studies of glioblastoma multiforme (GBM) and metastatic malignant melanoma patients using fluorine-18 labeled BPA and carbon-11 labeled 1-aminocyclobutanecarboxylic acid (Hubner et al., 1988; Nichols et al., 2002). These studies revealed that cyclic amino acids localize in GBM and metastatic malignant melanoma tumors more avidly than BPA. The fact that 1-aminocycloalkanecarboxylic acids cross the blood brain barrier (Aoyagi et al., 1988), provided further impetus to us for focusing on the synthesis of boronated unnatural cyclic amino acids as boron carriers in BNCT with potential for targeting the infiltrating glioblastoma cells in the normal brain.
In initial biodistribution studies in mice tumor models, three unnatural boronated amino acids have shown significantly improved selectivity for targeting the tumor cells when compared to BPA (Kabalka et al., 2004). The boron analogue of one of these unnatural amino acids, 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC), has shown a tumor to blood ratio of 8 and a projected tumor/normal brain ratio of nearly 21 (Kabalka et al., 2004). The demonstrated boron delivery by this unnatural amino acid is far superior to that of BPA and BSH, the compounds currently used in clinical BNCT. The present work presents further isomeric separation and characterization of this compound for tumor targeting in an EMT-6 murine mammary carcinoma mouse model and the T98G human glioblastoma cell line.
2. Experimental section
Synthesis, separation, and biodistribution of 1-amino-3-boronocyclopentanecarboxylic acid (ABCPC) in EMT-6 murine mammary carcinoma mouse model
The structure of boronated amino acids under study are shown in Fig. 1. BPA is listed as Compound 1. Several boronated amino acids, Compounds 2–7 (Fig. 1), have been prepared and their in vivo biodistribution determined in previous studies (Kabalka et al., 2004, Kablka et al., 2001). The data for the cyclic five membered ring analogue, 5 (ABCPC), was striking, exhibiting a nearly 22:1 ratio of boron concentration for tumor to brain at the two hour time point, dropping to 7.3 after six hours (Kabalka et al., 2004). It is important to note that all of the amino acids were synthesized as racemic and diastereomeric mixtures. For compounds 3, 4 and 5, four isomers were present in the injectate (cis and trans isomers along with each of their enantiomers.) (Two stereoisomers (enantiomers) were present in each of the injectates of 2, 6 and 7.) Thus, we reasoned that a single enantiomer of 4 and 5, as well as a single isomer of 7 might exhibit enhanced selectivity and elevated concentrations in the tumor. To test the hypothesis, we carried out preliminary studies in which compound 5 was separated into two pairs of racemates, 8a, 8b and 9a, 9b (Fig. 2).
Fig. 1.
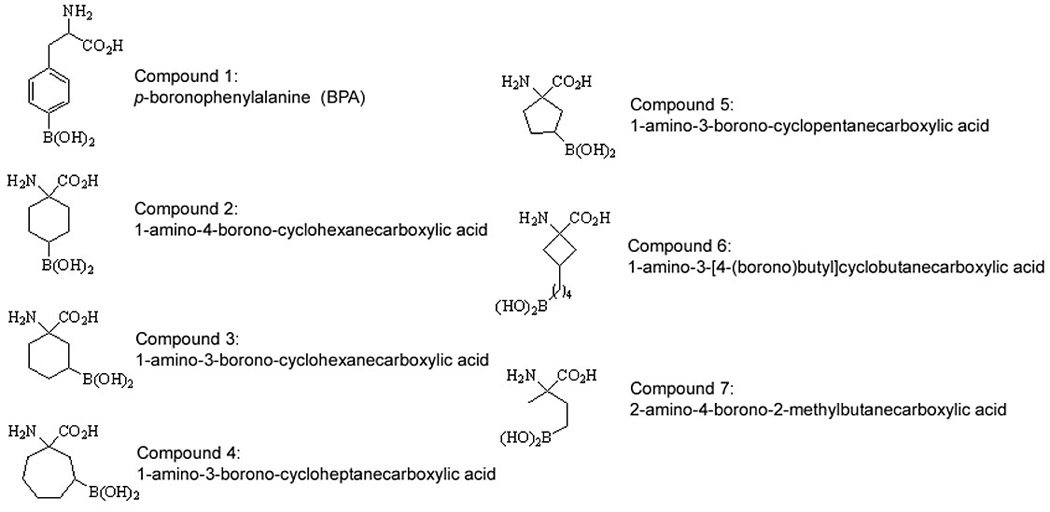
Structures of BPA (1) and six boronated unnatural amino acids (2–7).
Fig. 2.
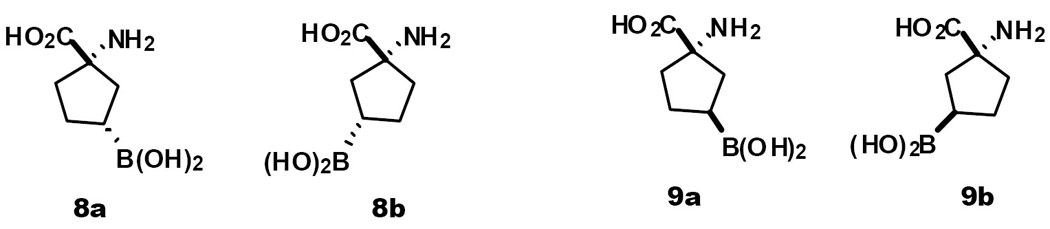
Target molecules; cis and trans isomers of the cyclopentanecarboxylic acids (parent compound 5)
We were able to prepare the hydantoin precursor 10 to amino acids 8a, 8b and 9a, 9b using their previously reported methodology (Aoyagi et al., 1988). Attempts to isolate the two racemates by column chromatography on alumina were unsuccessful due to decomposition of the borate ester. Fortunately, we discovered that the racemates (8a, 8b and 9a, 9b) could be readily separated by recrystallization of the corresponding hydantoins using methanol as solvent prior to hydrolysis. The stereochemistry of the enantiomeric pair 11a, 11b was confirmed by x-ray crystallography. Hydrolysis of hydantoins, 11a and 11b, in the presence of hydrochloric acid (12 M), gave amino acids 8 (a and b) and 9 (a and b), respectively (see Fig. 3 for a synthesis scheme. For clarity, only one isomer of each racemic pair is shown.)
Fig. 3.

Syntheses and separation of 8 and 9 (as racemates). Reagents and conditions: (a) CuCl, Bu3P, DMF, rt; (b) (NH4)2CO3, KCN, EtOH/H2O (1:1), 60 °C; (c) separation of diastereoisomers 8a and 8b in methanol; (d) HCl (12 M), 150 °C
The racemic amino acids (8 and 9) were solubilized as the fructose complex following procedures previously described for the amino acid BPA (Hubner et al., 1988). Briefly, 1 millimole of 8 or 9 was combined with 1.1 millimoles of fructose in ~1 ml of water. The pH was adjusted by addition of 10 M NaOH, added dropwise with stirring, until the pH reached 9.5–10.0 and all solids dissolved. The pH was then adjusted to 7.4 with aqueous HCl. The final volume was adjusted to 4 ml by addition of water and the solution was sterilized by passage through a 0.22 µm syringe filter. The boron concentration in the injection solutions was 1.87 mg B/ml for isomer 8 and 2.12 mg B/ml for isomer 9. Both compounds contained natural abundance boron. All boron concentrations quoted below represent the total boron concentration.
The biodistribution of racemates 8 and 9 was evaluated in the EMT-6 murine mammary carcinoma in collaboration with Dr. Jeffrey Coderre, Dr. Y. Chung, and Dr. K. Riley of the Massachussetts Institute of Technology for carrying out the in vivo biodistribution studies on compounds 8 and 9. Female BALB/c mice were injected subcutaneously on the flank with 1 × 106 EMT-6 tumor cells in a volume of 0.1 ml. After 8–10 days the tumors had reached a diameter of ~5 mm and were used for the biodistribution study. Five tumor-bearing mice were used at each of the following time points: 1, 3, and 5 hours post injection. Each mouse received an injection of 0.2 mL into the retro-orbital sinus while under brief isofluorane anesthesia. At the indicated time points, the mice were euthanized by isofluorane overdose, their blood removed directly from the heart, and tumor and normal tissues collected for boron analysis.
Subcellular SIMS studies of ABCPC in T98G human glioblastoma cells
The T98G human glioblastoma cells were exposed for 1 hr to a nutrient medium containing 100 ppm boron equivalent of compound ABCPC as a mixture of both L and D-isomers in the form of a fructose complex. Following this treatment, the cells were fast frozen, freeze-fractured using our sandwich method, and freeze-dried prior to SIMS analysis (Chandra et al., 1986; Chandra et al., 2000).
3. RESULTS & DISCUSSION
The biodistribution data for racemate 8 show no difference between tumor and blood at any of the time points (Fig. 4). The boron concentration in liver is higher than that in tumor or blood at 1 hour (tumor/liver boron concentration ratio ~0.4:1) and decreases to nearly the blood level by 5 hours post-injection.
Fig. 4.
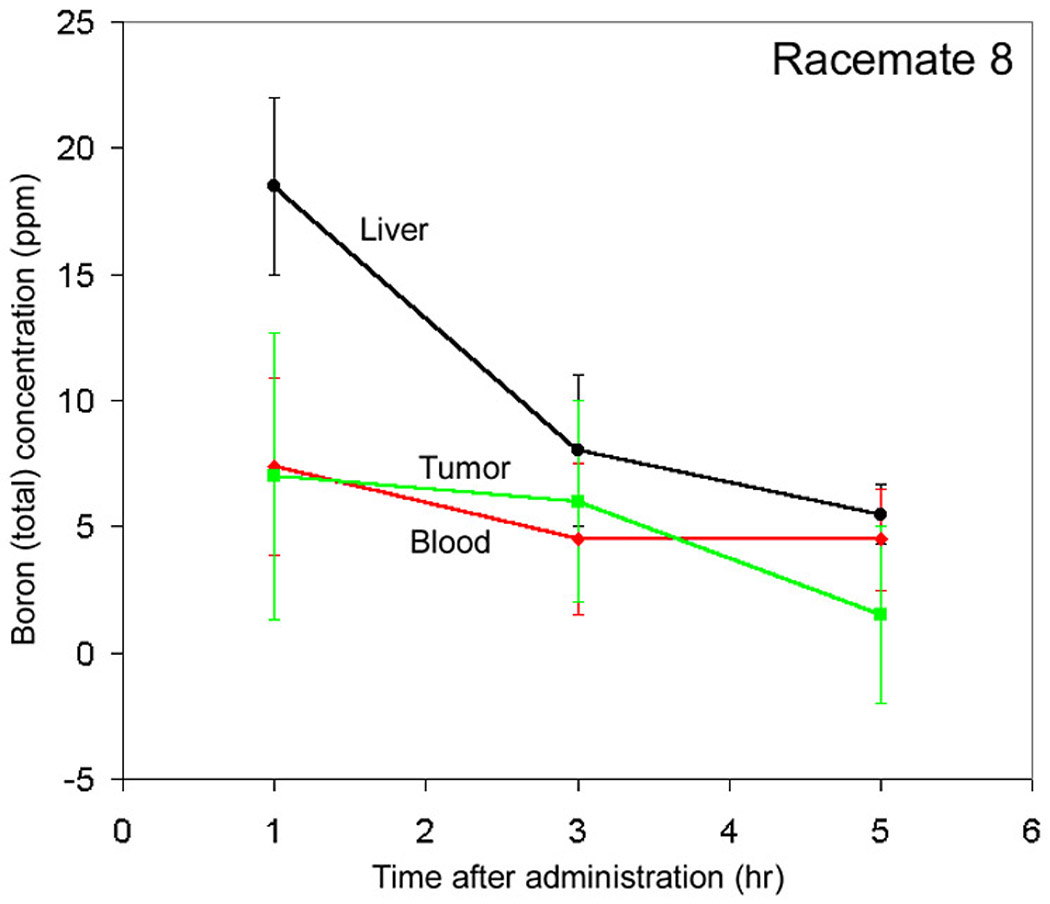
Biodistribution of Compound 8a (as a racemate).
Racemate 9, however, shows a tumor boron concentration that is significantly greater than blood at all time points (Fig. 5). At 1, 3 and 5 hours post-injection, the tumor/blood boron concentration ratios for racemate 9 are 2.8 ± 0.8, 2.4 ± 0.6 and 1.8 ± 0.5, respectively. With racemate 9, the normal tissues, skin and brain were similar to blood at 1 hour. The boron concentration in liver was higher than that in tumor at 1 hour.
Figure 5.
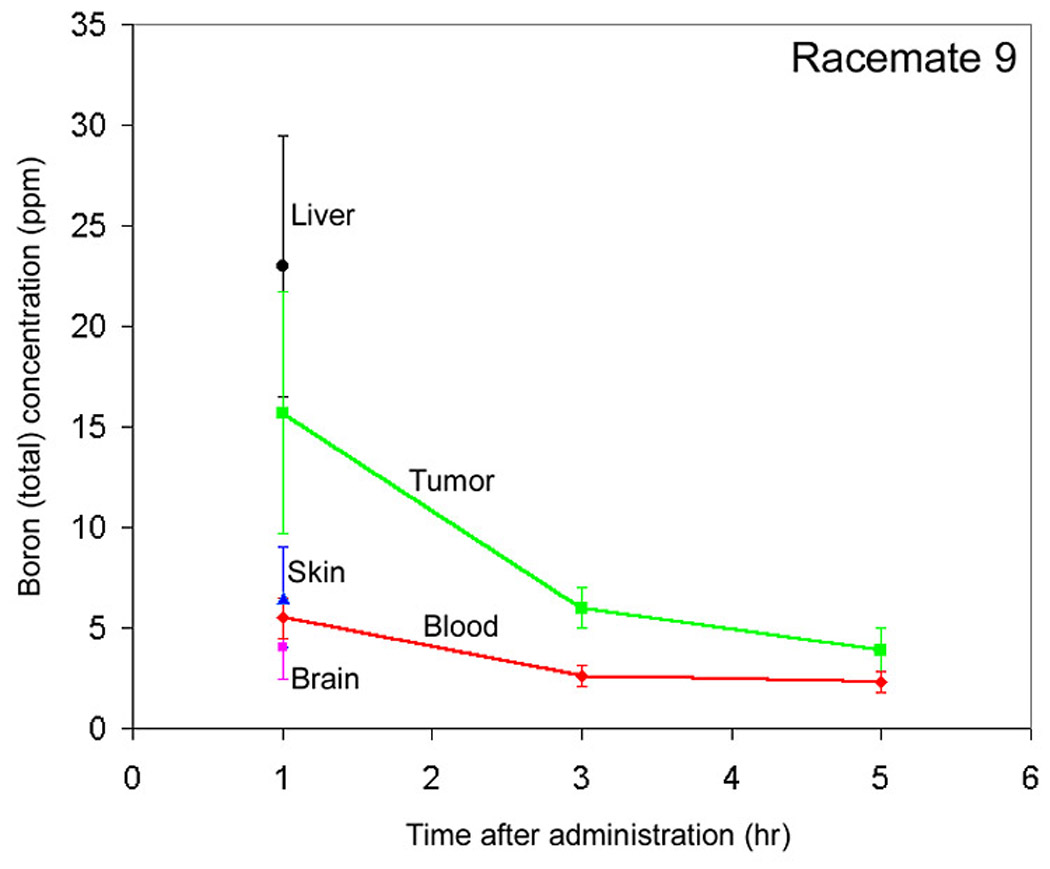
Biodistribution of Compound 9a (as a racemate).
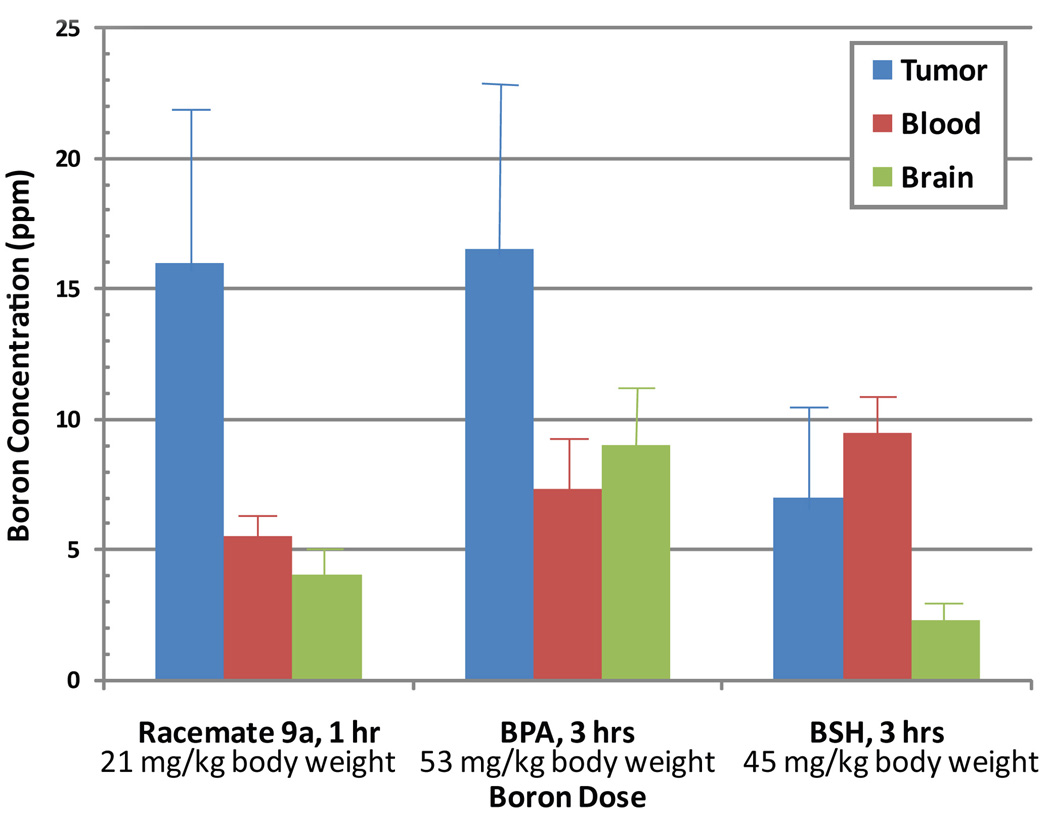
A direct comparison of the biodistribution of compound 9a (as a racemate) with published literature on BPA and BSH in Balb/c mice bearing implanted EMT-6 mammary carcinomas (Miura et al., 2001) is shown in Fig. 6 at the time of maximum concentration in the tumor. These observations unequivocally show that the compound 9a is at least as efficient as BPA in delivering boron to EMT-6 tumors but its time dependent uptake characteristics are quite different than BPA (Fig. 5–6). It should also be noted that compound 9a delivered approximately the same amount of boron to the tumor at the peak tumor concentration at 1 hr from less than one-half of the dose in comparison to BPA (Fig. 6). The compound 9a (as a racemate) is clearly superior to BSH in delivering boron to EMT-6 tumors in mice. The observations for boronated unnatural amino acids also indicate that these compounds, like any other BNCT agents, will differ in tumor targeting depending on the type of tumor and the ways of delivery (Barth et al., 2005; Miura et al., 2001).
Fig. 6.
A comparisons of boron concentrations in (mean ± SD) in tumor, blood, and brain from single injection of a boronated unnatural amino acid (compound 9a as a racemate), BPA, and BSH at the time of maximum concentration in the tumor. Five mice were used for observations on compound 9a.
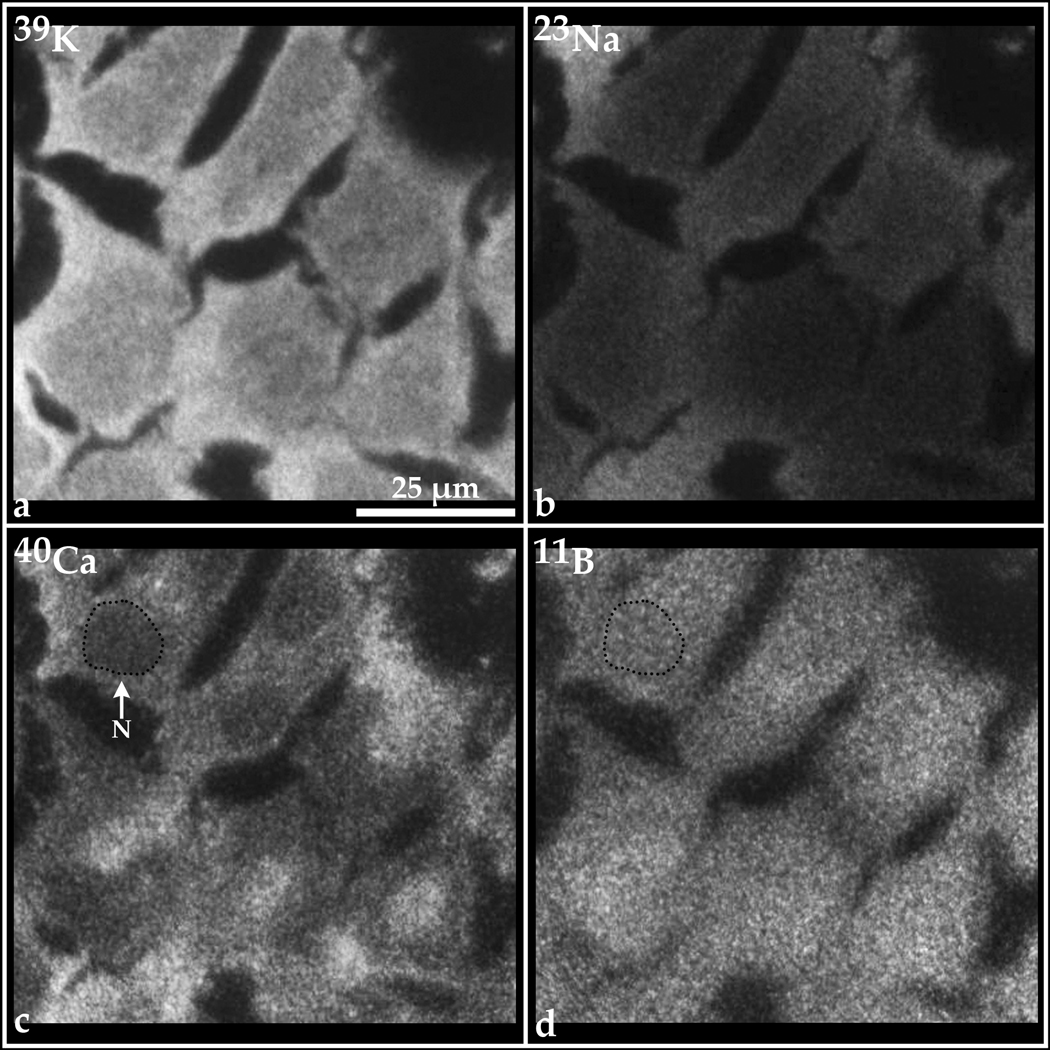
Figure 7 contains examples of SIMS analyses of 1-amino-3-boronocyclopentane carboxylic acid (ABCPC), using a mixture of both the L-and D-forms, in T98G human glioblastoma cells. The high 39K and low 23Na signatures in individual cells indicate the absence of toxicity to the cells and reveal healthy, well-preserved cells after treatment with ABCPC. The cell nucleus is identified in one cell by a dotted black line in the 40Ca and 11B images. The characteristic lower concentrations of total calcium (both free and bound forms) in nuclei of cells, as compared to the cytoplasm, clearly reveal the position of the nucleus in each cell in the field of view. SIMS analysis reveals that the boron-11 from ABCPC is present in all cells and is distributed with minor heterogeneity. The 11B is clearly present in the nucleus of each cell. It should be noted that this distribution is distinctly different than the boron distribution observed for BPA in this cell line (Chandra and Lorey, 2007). The perinuclear, mitochondrial-rich region that distinctly shows lower boron concentrations from BPA does not show this characteristic for ABCPC. The superiority of ABCPC for boron delivery to T98G human glioblastoma cells is evident from the data in Table 1, as ABCPC delivers at least twice as much boron to cell interiors when compared to BPA, within 1 hr of treatment. A similar comparison with BSH (not shown here, see data on BSH in Chandra et al., 2002) also indicates ABCPC to be far superior in boron delivery to T98G cells within an hour of exposure. Further isomeric separations of ABCPC may enhance boron delivery to even higher levels than observed for the isomeric mixture.
Fig. 7.
SIMS imaging of 39K, 23Na, 40Ca, and 11B subcellular distributions in T98G human glioblastoma cells treated with 100 ppm boron equivalent of 1-amino-3-boronocyclopentane carboxylic acid (ABCPC), a mixture of both L- and D- forms, as a fructose complex for 1 hr.
Table 1.
Quantitative SIMS imaging of T98G human glioblastoma cells after 1 hr treatment with 100 µg/ml boron equivalent of the fructose complex form of 1-amino-3-boronocyclopentane carboxylic acid (ABCPC in both L- and D-forms). Concentrations of potassium and sodium are expressed as mean ± SD on a cellular scale. Concentrations of boron are expressed as mean ± SD in three subcellular compartments: nucleus, mitochondria-rich cytoplasm, and the remaining cytoplasm. SIMS observations were made in more than five imaging fields containing more than 30 cells. The dry weight concentrations obtained by SIMS measurements from individual freeze-dried cells were converted into wet weight concentrations by assuming 85% cell water content. A direct comparison of compound 5 with L-p-BPA treatment of 110 µg/ml boron equivalent of the fructose complex for 1 hr is also shown in this table for this cell line by listing the data on BPA from Chandra and Lorey (2007).
| µg/g Boron (wet weight) | |||||
|---|---|---|---|---|---|
| 1 hr. Exposure | Cellular Potassium (mM) |
Cellular Sodium (mM) |
Nucleus | Mitochondria-Rich Perinuclear Cytoplasm |
Remaining Cytoplasm |
| ABCPC (L + D) | 168 ± 15 | 18 ± 4 | 303 ± 69 | 370 ± 77 | 361 ± 55 |
| BPA | 171 ± 20 | 14 ± 3 | 136 ± 55 | 109 ± 15 | 176 ± 57 |
4. Conclusion
The present study further characterizes boronated unnatural amino acids as new and more efficient boron carriers to tumor cells. This preliminary study provides compelling support for further isomeric separations and testing of these compounds in both in vitro and animal tumor models at single cell resolution.
Acknowledgements
The authors wish to thank Drs. Jeffrey Coderre, Y. Chung, and K. Riley of the Massachussetts Institute of Technology for carrying out the in vivo biodistribution studies in BALB/c mice. This study was funded by the U.S. Department of Energy (GWK) and partial support from Cornell Core Facilities and NYSTAR program to Cornell SIMS Laboratory (S. Chandra). Partial support for this work was also provided by a NIH grant R01 CA129326-01A2.
References
- Aoyagi M, Agronoff GW, Washburn LC, Smith OR. Blood-brain-barrier transport of 1-aminocyclohexane carboxylic acid: a nonmetabolizable amino acid for in vivo studies of brain transport. J. Neurochem. 1988;50:1220–1226. doi: 10.1111/j.1471-4159.1988.tb10596.x. [DOI] [PubMed] [Google Scholar]
- Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin. Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- Chandra S, Morrison GH, Wolcott CC. Imaging intracellular elemental distribution and ion fluxes in cultured cells using ion microscopy: A freeze-fracture methodology. J. Microsc. 1986;144:15–37. doi: 10.1111/j.1365-2818.1986.tb04670.x. Oxford. [DOI] [PubMed] [Google Scholar]
- Chandra S, Smith DR, Morrison GH. Subcellular imaging by dynamic SIMS ion microscopy. Anal. Chem. 2000;72:104A–114A. doi: 10.1021/ac002716i. [DOI] [PubMed] [Google Scholar]
- Chandra S, Lorey DR, II, Smith DR. Quantitative subcellular secondary ion mass spectrometry (SIMS) imaging of boron-10 and boron-11 isotopes in the same cell delivered by two combined BNCT drugs: In vitro studies on human glioblastoma T98G cells. Radiat. Res. 2002;157:700–710. doi: 10.1667/0033-7587(2002)157[0700:qssims]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Chandra S, Lorey DR., II SIMS ion microscopy imaging of boronophenylalanine (BPA) and 13C15N-labeled phenylalanine in human glioblastoma cells: relevance of subcellular scale observations to BPA-mediated boron neutron capture therapy of cancer. Int. J. Mass Spect. 2007;260:90–101. [Google Scholar]
- Coderre JA, Morris GM. The radiation biology of boron neutron capture therapy. Radiat. Res. 1999;151:1–18. [PubMed] [Google Scholar]
- Cordoso JE, Trivillin VA, Heber EM, Nigg DW, Calzetta O, Blaumann H, Longhino J, Itoiz ME, Bumaschny E, Pozzi E, Schwint AE. Effect of boron neutron capture therapy (BNCT) on normal liver regeneration: towards a novel therapy for liver metastases. Int. J. Radiat. Biol. 2007;83:699–706. doi: 10.1080/09553000701570212. [DOI] [PubMed] [Google Scholar]
- Dagrosa MA, Thomacz L, Longhino J, Perona M, Calzetta O, Blaumann H, Rebagliati RJ, Cabrini R, Kahl S, Juvenal GJ, Pisarev MA. Optimization of boron neutron capture therapy for the treatment of undifferentiated thyroid cancer. Int. J. Radiat. Oncol. Biol. Phy. 2007;69:1054–1066. doi: 10.1016/j.ijrobp.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Fairchild RG, Bond BP. Current status of boron-10 neutron capture therapy: enhancement of tumor dose via beam filtration and dose rate, and the effects of these parameters on minimum boron content- A theoritical evaluation. Int. J. Radiat. Oncol. Biol. Phys. 1985;11:831–835. doi: 10.1016/0360-3016(85)90318-9. [DOI] [PubMed] [Google Scholar]
- Gabel D, Foster S, Fairchild RG. The Monte Carlo simulation of the biological effect of the 10B(n,α)7Li reaction in cells and tissue and its implications for boron neutron capture therapy. Radiat. Res. 1987;111:14–25. [PubMed] [Google Scholar]
- Godwin JT, Far LE, Sweet WH, Robertson JS. Pathological study of eight patients with glioblastoma multiforme treated by neutron-capture therapy using boron 10. Cancer. 1955;8:601–615. doi: 10.1002/1097-0142(1955)8:3<601::aid-cncr2820080326>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hatanaka H. In: Boron neutron capture therapy for tumors. Glioma AB, Karim MF, Lewis ER, editors. Berlin: Springer; 1991. pp. 233–249. [Google Scholar]
- Hubner KF, Thie JA, Smith GT, Kabalka GW, Keller IB, Cliefoth AB, Campbell SK, Buonocore E. Positron emission tomography (PET) with 1-aminocyclobutane-1-[11C]carboxylic acid (1-[11C]-ACBC) for detecting recurrent brain tumors. Clin. Positron Imag. 1998;1:165–169. doi: 10.1016/s1095-0397(98)00010-7. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Das BC, Das S. Synthesis of novel boron containing unnatural cyclic amino acids as potential therapeutic agents. Tetrahedron Letters. 2001;42:7145–7146. [Google Scholar]
- Kabalka GW, Nichols TL, Smith GT, Miller LF, Khan MK, Busse PM. The use of positron emission tomography to develop boron neutron capture therapy treatment plans for metastatic malignant melanoma. J. Neuro-oncol. 2003;62:187–195. doi: 10.1007/BF02699944. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Wu ZZ, Yao M-L, Natarajan N. The synthesis and in vivo biodistribution of novel boronated unnatural amino acids. Appl. Radiat. Isotopes. 2004;61:1111–1115. doi: 10.1016/j.apradiso.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Kabalka GW, Yao M-L. The synthesis and use of boronated amino acids for boron neutron capture therapy. Anti-cancer Agents Med. Chem. 2006;6:111–125. doi: 10.2174/187152006776119144. [DOI] [PubMed] [Google Scholar]
- Kankaaranta L, Seppala T, Kovinoro H, Saarilhti K, Atula T, Collan J, Salli E, Kortensniemi M, Uusi-Simola J, Makitie A, Seppanen M, Minn K, Kotilnoto P, Anterinen I, Savolainen S, Kouri M, Joensuu H. Boron neutron capture therapy in the treatment of locally recurred head and neck cancer. Int. J. Radiat. Oncol. Biol. Phy. 2007;69:475–482. doi: 10.1016/j.ijrobp.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Li T, Hamdi J, Hawthorne MF. Unilamellar liposomes with enhanced boron content. Bioconjugate Chem. 2006;17:15–20. doi: 10.1021/bc0501350. [DOI] [PubMed] [Google Scholar]
- Miura M, Morris GM, Micca PL, Lombardo DT, Youngs KM, Kalef-Ezra JA, Hoch DA, Slatkin DN, Ma R, Coderre JA. Boron neutron capture therapy of a murine mammary carcinoma using a lipophilic carboranyltetraphenylporphyrin. Radiat. Res. 2001;155:603–610. doi: 10.1667/0033-7587(2001)155[0603:bnctoa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Nichols TL, Kabalka GW, Miller LF, Khan MK, Smith GT. Improved treatment planning for boron neutron capture therapy for glioblastoma multiforme using fluorine-18 labeled boronophenylalanine and positron emission tomography. Med. Phys. 2002;29:2351–2358. doi: 10.1118/1.1507780. [DOI] [PubMed] [Google Scholar]
- Pellettieri L, H-Stenstam B, Rezaei A, Giusti V, Skold K. An investigation of boron neutron capture therapy for recurrent glioblastoma multiforme. Acta Neurol. Scand. 2008;117:191–197. doi: 10.1111/j.1600-0404.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- Soloway AH, Tjarks W, Barnum BA, Rong F-G, Barth RF, Codogni IM, Wilson JG. The chemistry of neutron capture therapy. Chem. Rev. 1998;98:1515–1562. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- Sweet WH. Early history of development of boron neutron capture therapy of tumors. J. Neuro-oncol. 1997;33:19–26. doi: 10.1023/a:1005752827194. [DOI] [PubMed] [Google Scholar]
- Wittig A, Malago M, Collette L, Huiskamp R, Buhrmann S, Nievaart V, Kaiser GM, Jockel KH, Schmid KW, Ortmann U, Sauerwein WA. Uptake of two 10B-compounds in liver metastases of colorectal adenocarcinoma for extracorporeal irradiation with boron neutron capture therapy (EORTC Trial 11001) Int. J. Cancer. 2008;122:1164–1171. doi: 10.1002/ijc.23224. [DOI] [PubMed] [Google Scholar]
- Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat. Res. 2000;153:173–180. doi: 10.1667/0033-7587(2000)153[0173:motopb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zamenhof RG, Kalend AM, Bloomer WD. BNCT: looking for a few good molecules. J. Natl. Cancer Inst. 1992;84:1290–1291. doi: 10.1093/jnci/84.16.1290-a. [DOI] [PubMed] [Google Scholar]