Abstract
In order for osteocytes to perceive mechanical information and regulate bone remodeling accordingly they must be anchored to their extracellular matrix (ECM). To date the nature of this attachment is not understood. Osteocytes are embedded in mineralized bone matrix, but maintain a pericellular space (50–80nm) to facilitate fluid flow and transport of metabolites. This provides a spatial limit for their attachment to bone matrix. Integrins are cell adhesion proteins that may play a role in osteocyte attachment. However, integrin attachments require proximity between the ECM, cell membrane and cytoskeleton, which conflicts with the osteocytes requirement for a pericellular fluid space. In this study we hypothesize that the challenge for osteocytes to attach to surrounding bone matrix, while also maintaining fluid-filled pericellular space, requires different “engineering” solutions than in other tissues that are not similarly constrained. Using novel rapid fixation techniques, to improve cell membrane and matrix protein preservation, and Transmission Electron Microscopy, the attachment of osteocyte processes to their canalicular boundaries are quantified. We report that the canalicular wall is wave-like with periodic conical protrusions extending into the pericellular space. By immunohistochemistry we identify that the integrin αvβ3 may play a role in attachment at these complexes; a punctate pattern of staining of β3 along the canalicular wall was consistent with observations of periodic protrusions extending into the pericellular space. We propose that during osteocyte attachment the pericellular space is periodically interrupted by underlying collagen fibrils that attach directly to the cell process membrane via integrin-attachments.
Key words of the paper: Bone, mechanical loading, extracellular matrix protein, integrin, attachment, osteocyte, cell attachment, mechanotransduction
Introduction
Bone modeling and remodeling take place throughout life to continuously adapt and renew bone tissue. Osteocytes are thought to influence these processes in response to changes in tissue strain and fluid flow that result from physiological loading [Rubin & Lanyon, 1985; Lanyon, 1993; Klein-Nulend et al, 2003]; however, the mechanisms by which osteocytes perceive and respond to mechanical information are not well defined. Mechano-responsiveness in many cell types is mediated by integrins, heterodimeric, signal-transducing transmembrane glycoproteins that link extracellular matrix constituents and in some cases co-receptors on adjoining cell surfaces to intracellular elements of the actin cytoskeleton [Hynes, 1992, Yamada, 1997; Cary et al, 1999; Hynes, 2002]. All bone cell types express integrins [Horton and Davies, 1989; Albelda and Buck, 1990; Watt and Jones, 1993], and osteocytes in particular were reported to express integrins of both β1 and β3 families [Hughes et al, 1993]. Yet how osteocyte integrins might participate in the response to mechanical signaling remains an open question. Integrin-mediated adhesion (and subsequent signal transduction) requires direct contact between the cell plasma membrane and extracellular integrin ligands [Hynes, 1992, Yamada, 1997; Cary et al, 1999; Hynes, 2002]; however, osteocytes in vivo typically maintain a distance of 50–80 nm from the lacunar and canalicular walls [You et al, 2004]. This space, which is essential to allow the fluid flow and metabolite transport needed for cell viability [Cooper et al, 1966; Piekarski and Munro, 1977; Ayasaka et al, 1992; Knothe-Tate et al, 1998], contains a loose pericellular matrix that includes fibers bridging the gap between osteocyte processes and canalicular walls [You et al, 2004; Han et al, 2004]. Such tethering fibers have been proposed to participate in osteocyte mechanotransduction by amplifying fluid flow-induced stresses on the osteocyte processes [You et al, 2001; Han et al, 2004]; however, glycosaminoglycans are not considered major ligands for any integrin class, and so are unlikely to participate in integrin-based mechanotransduction. Direct osteocyte-bone matrix contacts of the type needed for integrin-mediated mechanotransduction have not been clearly demonstrated.
Characterization of osteocytes in situ, especially their spatial relationships with the bone matrix, has been particularly difficult because the mineralized matrix of bone slows fixation using traditional approaches, often resulting in poor preservation of tissue structure. This problem is further exacerbated in older, adult bone tissue, which has high density. We developed a novel, rapid penetrating fixative to optimize preservation of osteocyte cell membranes and surrounding bone matrix architecture and proteins for study using transmission electron microscopy. This approach was designed to overcome many of the well-known difficulties in fixation and ultrastructural studies of adult osteocytes. These cells reside within the solid matrix of bone, are often located distant from the vasculature, and exchange to and from osteocytes occurs via the narrow, proteoglycan-filled, pericellular space separating the cells and their processes from the bony lacunar and canalicular walls. For mature bone and osteocytes within, this space is only on the order of about 0.5–1 μm surrounding the cell body and 75 nm in annular width surrounding the osteocyte process [You et al, 2004]. In this study, we tested an acrolein-based perfusion fixation method to improve tissue preservation in order to examine whether osteocytes in cortical bone exhibited direct connections with their extracellular matrix that would be consistent with integrin-mediated attachment. We related these findings to immunohistochemically determined distributions of the two major integrin classes (β1 and β3) reportedly expressed by osteocytes [Hughes et al, 1993].
Methods
Transmission Electron Microscopy
In this study we compare two methods of bone tissue fixation, with the intention of optimizing the fixation of bones cells and membranes to allow characterization of the attachment of osteocytes to the surrounding bone matrix. Both fixatives were delivered by perfusion as described below.
First a traditional Karnovsky’s fixative (2% Paraformaldehyde, 2.5% Glutaraldehyde) was prepared in 0.12M sodium phosphate buffer; this fixative is typically used to take advantage of the combination of rapid penetration of paraformaldhyde and the high efficiency of the glutaraldehyde. C57Bl/6 mice (10–20 weeks old, N=5) were anaesthetized using Avertin and were perfused via the ascending aorta first with heparinized phosphate buffered saline (PBS) at 37°C followed by the Karnovsky’s fixative for 2 hours (pH 7.4, 37°C). Perfusion was performed at a pressure of 120mm Hg. All animal procedures were performed under IACUC approval. Tibiae and femora were excised and immersed in fixative for 48 hours. Mid-diaphyseal cross-sectional ring slices (~1mm thick) of cortical bone were then cut using a low speed diamond blade saw. Bone slices were decalcified in EDTA in 0.1M TRIS-HCl buffer for 6 weeks and processed for TEM analysis as described below.
Second we used an acrolein-based rapid penetrating fixative to optimize preservation of osteocyte cell membranes, surrounding bone matrix architecture and proteins for study using transmission electron microscopy. This approach was designed to overcome difficulties in fixation and ultrastructural studies of adult osteocytes; they reside within the solid matrix of bone, distant from the vasculature, and exchange occurs via the proteoglycan-filled pericellular space, which is only 0.5–1 μm surrounding cell bodies and 75 nm surrounding osteocyte processes [You et al, 2004]. To improve penetration and fixation efficiency, we used an acrolein-based fixation approach. Acrolein, a very small aldehyde (CH2=CH-CHO), was introduced as a fixative in the early 1960’s and has been demonstrated to penetrate tissues and react several times faster than either formaldehyde or glutaraldehyde [Hayat, 1989]. As a result, there is very little cell shrinkage. Because of its extreme reactivity, acrolein cannot be used alone. In the current studies, we found that combination of acrolein-paraformaldehyde-glutaraldehyde (1% each in primary fixative (in 0.12M phosphate buffer) and 2% each in secondary fixative (same buffer)) provided excellent preservation of osteocyte structure. Accordingly, 10–20 week old C57Bl/6 mice (N=5) were anaesthetized as described above and were perfused via the ascending aorta first with heparinized PBS followed by the primary fixative (1% Acrolein, 1% Glutaraldehyde and 1% Paraformaldehyde in 0.12M phosphate buffer (pH 7.4, 37°C) for 15 minutes and the secondary fixative (2% Acrolein, 2% Glutaraldehyde and 2% Paraformaldehyde in the same buffer) for 2 hours as described above. Tibiae and femora were excised and immersed in secondary fixative for 48 hours. Mid-diaphyseal cross-sectional ring slices (~1mm thick) of cortical bone were then cut as described above and decalcified in EDTA.
After decalcification, specimens were washed in a 1:1 solution of EDTA and 0.12M Sodium Cacodylate Buffer (pH 7.4) for 30 mins, followed by a 30 min wash in the buffer alone. Samples were then post-fixed for 3 hours each in 1% Osmium Tetroxide and 1% Tannic Acid and then overnight in 1% Uranyl Acetate. Each post-fixing solution was prepared in the same buffer of 0.12M Sodium Cacodylate. Samples were washed by agitation in cold deionized water between post-fixing solutions. Samples (from each fixation technique) were dehydrated in graded ethanol and propylene oxide. Samples were infiltrated with propylene oxide, embedded in Epon 812 and polymerized at 60°C for 5 days. Ultrathin sections (20nm) were cut using a Reichert Ultramicrotome equipped with a diamond knife and mounted on Formvar grids. Sections were stained with 8% uranyl acetate solution in 50% alcohol and lead citrate. Sections were viewed using a Philips 300 transmission electron microscope. Images of osteocytes were acquired at 80,000–100,000× magnifications.
Immunohistochemistry
Immunohistochemistry (IHC) was used to test for the presence of specific integrin staining along osteocyte process and cell bodies. Bones for IHC were fixed in 4% paraformaldehyde, decalcified in EDTA, paraffin embedded and sectioned serially at 5 μm. We focussed on the β1 and β3 integrin subunits that have previously been shown in bone cells [Hughes et al, 1993; Aarden et al, 1996; Bennett et al, 2001; Miyauchi et al, 2006]. Glass-mounted sections were deparaffinized, re-hydrated and treated with 3% hydrogen peroxide for 20 minutes to inhibit endogenous peroxidase activity. A methanol-NaOH-based antigen retrieval system (DeCal, Biogenex, San Ramon, CA) was applied for 30 minutes at room temperature, after which samples were blocked using a universal protein block (Dako Cytomation, Carpinteria, CA) for a further 30 minutes. Sections were incubated overnight at room temperature with the anti-β1 integrin monoclonal antibody [1:100] or anti-β3 polyclonal antibody [1:50] (AB1 and AB2, respectively, Santa Cruz Biotechnology, San Ramon, CA). Detection was performed using an Alexa-Fluor 488 labelled rabbit anti-goat IgG (1:100, Sigma) according to the manufacturer’s protocol. Normal goat IgG was used as a negative control. Fibroblasts and osteoblasts within the same sections provided a positive control for β1 integrin staining, while osteoclasts at the growth plates of the same bones were used as positive control for β3 integrin staining. Immunostained sections were examined using fluorescence and confocal microscopy.
Results
Transmission Electron Microscopy
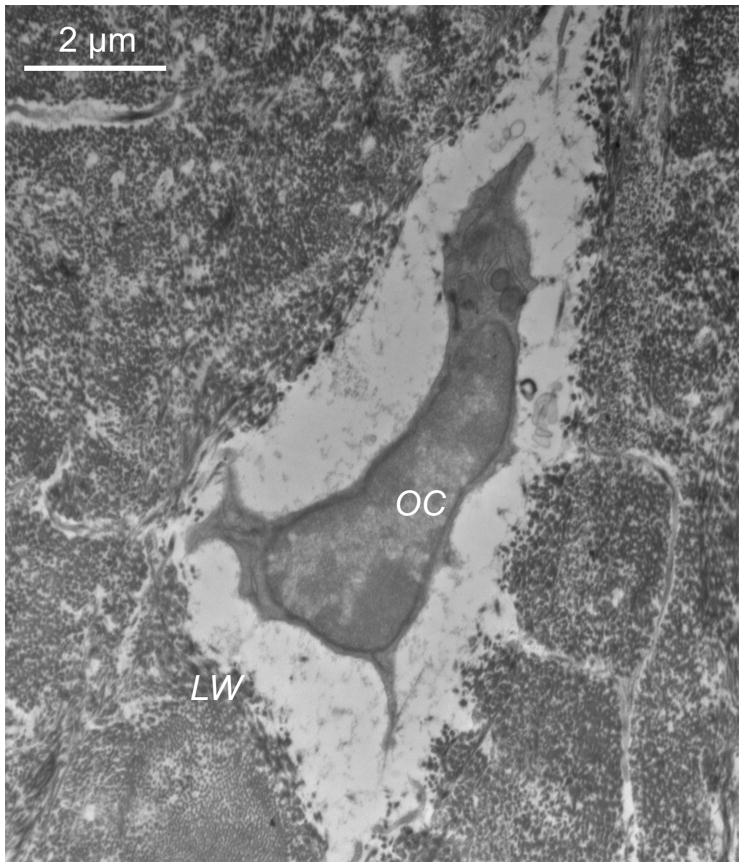
TEM studies carried out according to the traditional Karnovsky’s fixation approach revealed that osteocyte cell membranes and the surrounding bone matrix architecture were less well preserved than in the Acrolein-based method. Cell shrinkage was strongly suggested by the large pericellular space surrounding osteocyte processes and cell bodies (Fig 1).
Figure 1.
TEM images showing the results of the fixation using the traditional Karnovsky’s approach. The cell (OC) has significantly shrunken back from the lacunar wall (LW), cell membranes and pericellular matrix organization are poorly preserved.
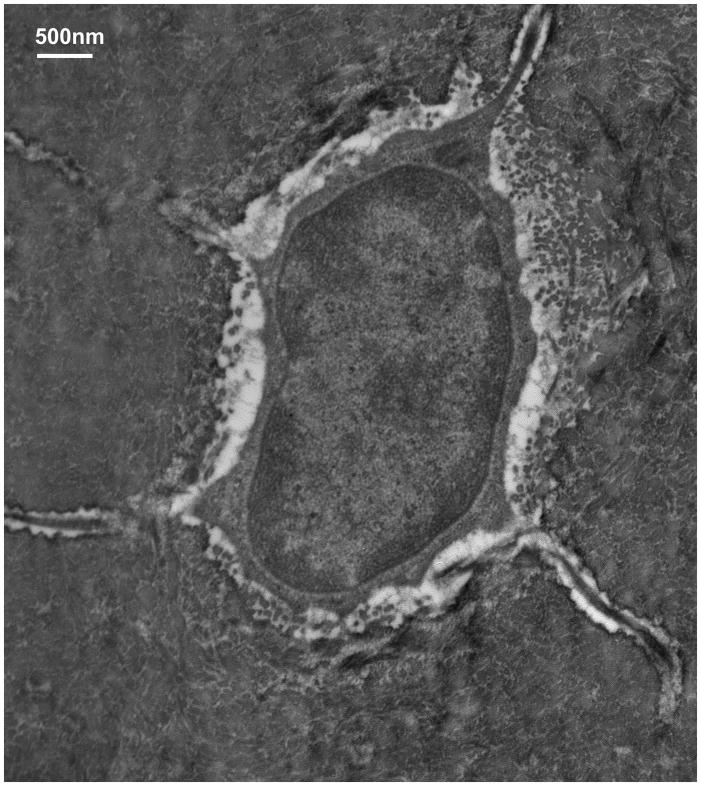
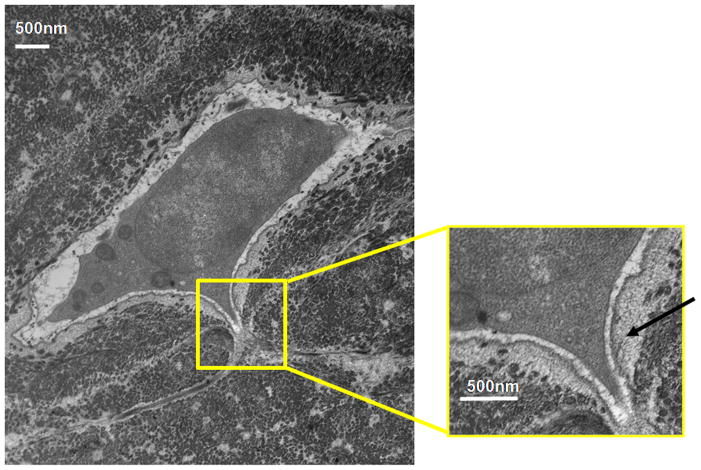
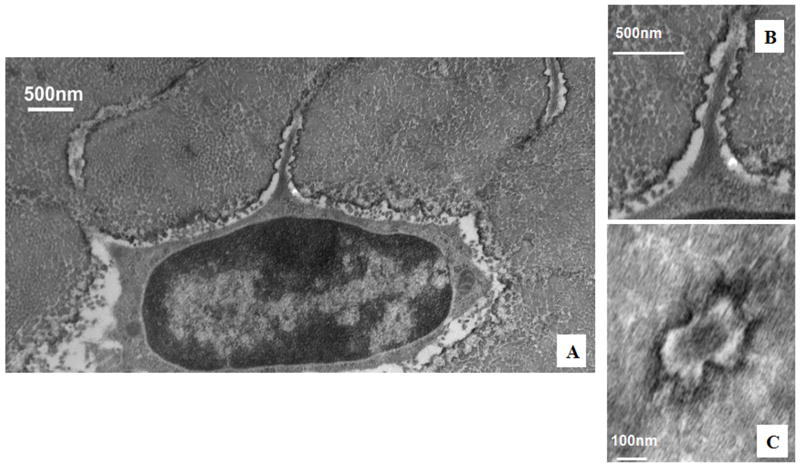
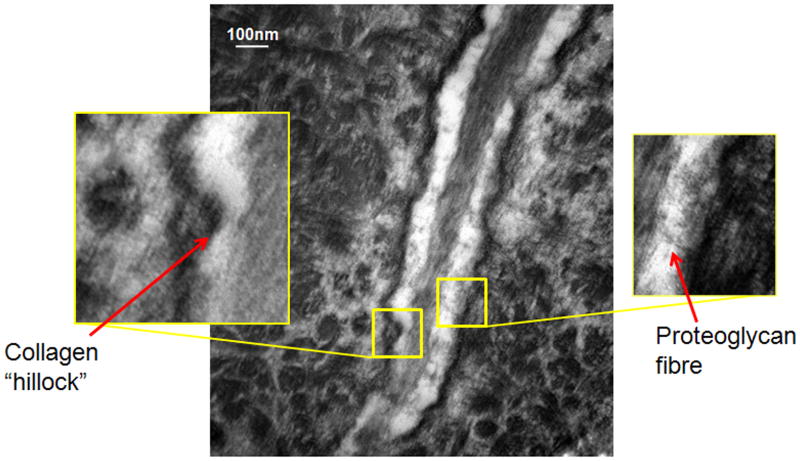
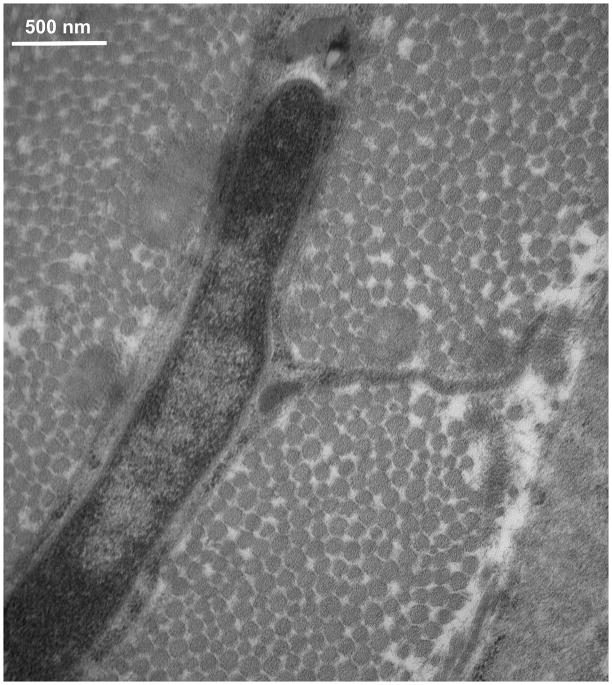
TEM studies with the Acrolein-based fixation approach revealed that osteocyte cell membranes and surrounding bone matrix architecture were very well preserved (Fig 2), and that the pericellular space surrounding osteocyte processes and cell bodies was consistently well-defined (Fig 3). Moreover, the protrusions from canalicular walls completely crossed the pericellular space to contact the cell membrane of the osteocyte process (Fig 4). These contact points were asymmetrically distributed, appearing on one side of the cell process but not the other (Fig 4A). These projections were themselves axisymmetric, showing the same profile geometry in transverse (Fig. 4C) as well as longitudinal sections (Fig. 4B), consistent with a cone-like or hillock-like structure. Internally, these protrusions were composed of matrix fibrils, identical in size and appearance to the collagen fibrils observed in the rest of the bone matrix (Fig 4, Fig 7). Based on longitudinal sections along osteocyte processes (N=100 processes), the apex to apex distance between neighboring protrusions was 131+/−40 nm.
Figure 2.
TEM images showing the results of osteocyte fixation using the Acrolein-based fixative, demonstrating markedly improved osteocyte cell membrane and bone matrix protein preservation.
Figure 3.
TEM images showing the results of osteocyte fixation using the Acrolein-based fixative. Arrow in the enlargement at right shows the pericellular matrix that occupies the space between the cell body and the lacunar wall
Figure 4.
TEM photomicrograph of osteoctye (A) shows osteocyte and enlarged longitudinal (B) and cross-sections (C) of cell process showing that the bony wall of the canaliculus has protrusions projecting from the wall completely across the pericellular space to contact the cell membrane of the osteocyte process. These protrusions were internally composed of collagen fibrils, identical in size and appearance to other collagen fibrils observed in the bone. That these protrusions have similar profiles in longitudinal and transverse orientations indicates a axisymmetrical structure like a small hillock.
Figure 7.
TEM image of cell process in osteocyte canaliculus; enlargements demonstrate the collagen “hillocks” versus tethering fibers that occupy the space between the cell process and the lacunar wall
In contrast to osteocyte processes, osteocyte cell bodies did not appear to have comparable direct attachments to the bony lacunar wall. Instead, osteocyte cell bodies were surrounded by a pronounced (~0.5–1μm thick) layer of noncollagenous, glycocalyx-like pericellular matrix (PCM) (Fig 3). Osteocyte cell bodies were not observed to attach or contact directly to the bone lacunar wall. the cell membranes of osteocyte bodies were found to contact the thickened surface layer of the PCM (arrow, Fig 3, left). The nature of these cellular attachments to the pericellular matrix around osteocytes is currently obscure, but cell-to-glycocalyx attachments in other cells (e.g., endothelial cells, dendritic cells) can involve CD43, sialic acid, P-selectins and well as several other attachment molecules including α5β1 [Ingber, 2006]. The functional attributes of these attachments are not yet known, but attachments between the cell body and the PCM will clearly be much less rigid and the local forces much lower than for the attachments between the cell processes and their bony walls. This has important implications for the transmission of hydrodynamic and strain induced forces as discussed in the next section.
Finally, in osteoid the osteocytes cell bodies and processes were observed to be in intimate contact with the collagen fibrils of the recently deposited bone surrounding them (Fig 5). The pericellular space and PCM around the cell body and process of mature osteocytes was not present in these osteoid osteocytes, indicating that this space develops after the cell is embedded in the tissue, perhaps in association with the mineralization.
Figure 5.
TEM image of osteocyte in osteoid (Acrolein-based fixation) showing that in newly formed bone matrix, collagen fibrils are in intimate contact with the cell membrane along the entire osteocyte cell body and process.
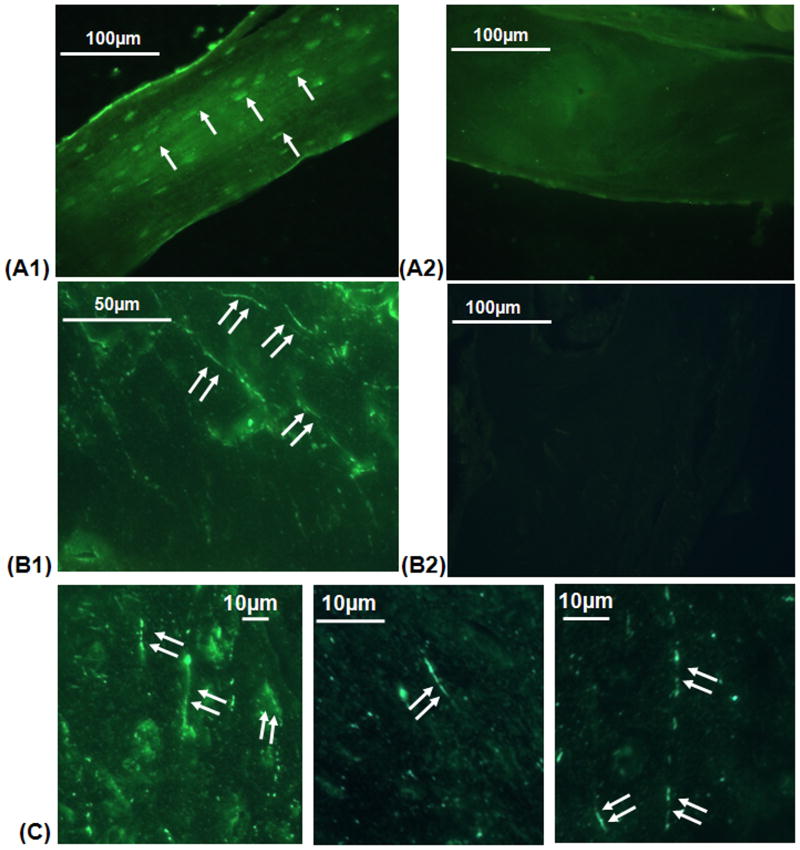
Immunohistochemistry studies reveal a differential pattern of the β1 and β3 integrins in osteocytes. Strong β1 integrin staining was found at osteocyte cell bodies, lining cells along the bone lining surface and only rarely and weakly along processes (Fig 6A). In contrast, intense β3 integrin staining was observed only along osteocyte cell processes (Fig 6B and C). Moreover, β3 integrin expression was not continuous along the process, but rather showed punctate distributions along the canalicular wall; the mean distance between these staining foci in confocal microscopy images was 180±42 nm.
Figure 6.
Fluorescence photomicrographs showing the different distributions of β1 and β3 integrin staining in osteocytes and canaliculi in cortical bone. Image A1 demonstrates staining for β1 integrin at osteocyte cell bodies and lacunae (arrows; A2 shows non-immune serum negative control). Images B1 and C show intense staining for β3 integrin along osteocyte processes throughout the bone (double arrows), with the staining in a punctate, “string of pearls” distribution (B2 shows non-immune serum negative control).
Discussion
In this study, we used a new TEM fixation approach that improves osteocyte cell membrane and bone matrix preservation. Using this approach we report that canalicular walls exhibit a wave-like structure with regular, though infrequent, protrusions (“hillocks”) that extend across the pericellular space to contact the plasma membrane of osteocyte processes. Though these hillocks are built upon underlying bone collagen fibrils, details of their nature and their precise origin remain obscure. The observation of close contact between the cell processes and recently deposited collagen fibrils in the osteoid (Fig. 5) suggests that these attachments may form before the tissue becomes mineralized, when the pericellular space and PCM around the cell body and process, seen in mature osteocytes, was absent. Recent data from Karsdal et al [2002] suggests that osteocytes employ proteolytic enzymes to create the lacunar and canalicular spaces in which the cell process resides. In this context it seems reasonable to speculate that the observed attachment foci are established before or during the remodeling process, and remain undegraded during canalicular formation.
The distribution of osteocyte attachment sites along canalicular walls resembled the punctate distribution of immunohistochemical staining for β3 integrins. The staining typically had cross sectional dimensions of approximately 1μm, indicating that the identified structures were bone canaliculi [Bell et al, 1999; Hirose et al, 2007; Hillier et al, 2007]. The punctate distribution of staining suggests that cell-matrix adhesion along the osteocyte processes is mediated, at least in part, by αVβ3 integrins (αV is the only partner for β3 except in platelets). αVβ3 integrins have been implicated in a variety of matrix-invasive or migratory processes including tumor cell invasion [Huang et al, 2000; Chatterjee and Chatterjee, 2001], vascular endothelial sprouting during angiogenesis [Liaw et al, 1995; Hotchkiss et al, 1998] and the formation of a sealing zone on bone surfaces by osteoclasts during bone resorption [Horton et al, 1991; Clover et al, 1992; Engleman et al, 1997]. In this context, αVβ3 integrins may play a role in establishing connections with neighboring cells when the osteocyte processes extend through bone matrix. However, our finding that β3 integrins are present in “mature” osteocyte processes demonstrates that this integrin can also function for longer-term cell-substrate interactions such as adhesion of osteocyte processes to bone matrix. However, αVβ3 integrins do not bind directly to collagen but rather recognize proteins such as fibronectin, vitronectin, osteopontin, von Willebrand factor, thrombospondins and bone sialprotein [Horton et al, 1991; Ross et al, 1993]. Of these, osteopontin is found in abundance along the canalilcular wall [McKee and Nancy, 1996; Devoll et al, 1997; Sodek and McKee, 2000; Noda et al, 2003] and therefore seems a likely ligand for the αvβ3-based adhesion. Furthermore, osteopontin-deficient mice are resistant to bone loss during disuse [Yoshitake et al, 1999], suggests that such an αVβ3-osteopontin attachment may play a role osteocyte mechanical sensing and osteocyte function, as is discussed further below.
While β3 integrin staining was localized exclusively along osteocyte processes, we found that β1 staining was overwhelmingly concentrated at osteocyte cell bodies. The β1 integrin subunit is found ubiquitously in osteoblast lineage bone cells (osteoblasts, osteocytes, bone lining cells) [Bennett et al, 2001] and is typically paired with the α5 subunit, though combination with α1 and α2 have also been reported [Brighton and Albeda, 1992; Adams and Watt, 1993; Grzeisk and Robey, 1994; Saito et al, 1994; Sinha and Tuan, 1996; Horton and Davies, 1997; Gronthos et al, 1997]. β1 family integrins recognize a wide variety of ligands including osteopontin, bone sialoprotein, fibronectin [Dalton et al, 1995] and collagen type I [Clover et al, 1992; Brighton and Albeda, 1992; Saito et al, 1994; Gronthos et al, 1997; Bennett et al, 2001]. Punctate foci of osteopontin staining along the canalicular wall was observed by McKee and Nanci (1996). Perhaps most interestingly, β1 and also β5 integrins bind directly or indirectly to proteoglycans, like versican, syndecan, and fibronectin [Woods and Couchman, 1994], some of which have been identified in osteocyte lacunae [Cowles et al, 1998; Shibata et al, 2008] and may be present within the glycocalyx-like material surrounding osteocyte cell bodies that we and others have observed [Weinger and Holtrop, 1974; Holtrop, 1975; Palumbo, 1986; You et al, 2004; Marotti et al, 1990].
CD44, a receptor for hyaluronan [Culty et al, 1990] and osteopontin [Weber et al, 1996], has previously been suggested as the putative transmembrane protein linking tethering fibers in the pericellular space to the actin cytoskeleton of osteocytes [Lacey and Underhill, 1987; You et al, 2001; Han et al, 2004]. However, integrins have been extensively studied and are commonly accepted to play a role in cellular mechanosensation by facilitating adhesion between extracellular ligands and the cytoskeleton, with its associated signal transduction molecules and membrane channels. Integrin-based adhesion occurs at focal adhesion complexes, containing clusters of integrins, termini of actin filaments and intracellular molecules like vinculin, talin and paxillin, which help to stabilize cytoskeletal interactions or facilitate downstream signalling through focal adhesion kinase (FAK) and small GTPases of the Ras family (rac, rho, cd42). Integrin-mediated signalling impinges on many intracellular pathways, including the MAP kinase pathway that has been implicated in osteoblastic response to oscillatory flow [You et al, 2001]. Furthermore, both β1 and β3 integrin classes can transduce mechanical stimuli [Hughes et al, 1993, Aarden et al, 1996; Bennett et al, 2001; Miyauchi et al, 2006], suggesting that these integrins may play a role in osteocyte mechanosensation. However, our finding that osteocytes express β1 integrins on cell bodies where they interact with a loose pericellular matrix, while expressing β3 integrins along cell processes, where they may contact bone matrix directly, indicate that osteocyte mechanotransduction may differ considerably at those sites. Indeed a recent demonstration of paxillin on osteocyte cell bodies but not cell processes [Vatsa et al, 2008] suggests that cytoplasmic elements of the mechanical signal transduction pathways in osteocytes is spatially partitioned in accordance with the organization of the actin cytoskeleton, as noted previously.
How integrin-mediated cell-matrix attachments along cell processes will influence osteocyte mechanotransduction has yet to be experimentally determined, but Wang et al [2007] recently developed a mathematical model to estimate its effects on the mechanical stimuli perceived by osteocytes. They found that β3 integrin “spot-welds” between the cell process and the rigid lacunar wall will dramatically increase focal strains (i.e., “stress concentrations”) due to load-induced fluid flow near membrane attachment sites of osteocyte processes, even at small global tissue strains. These strains are an order of magnitude larger than the hoop strains predicted in the strain amplification model of You et al [2001] and Han et al [2004], due to the tensile forces acting on the fibers that tether the processes to the canalicular wall. Similar strain amplification would not occur at cell body attachments, for several reasons. First, cell bodies interact with a loose matrix, rather than a rigid, bony substratum; second, cell bodies lack the highly organized cytoskeleton present in cell processes; and finally, cell bodies are subjected to substantially lower fluid velocity/pressure gradients than cell processes. Thus focal attachment complexes along osteocyte processes, by inducing high focal strain concentrations, can amplify small overall bone tissue strains and provide a mechanism for osteocyte excitation at low tissue loading magnitudes. This paradigm suggests that tethering fibers [Fig. 7] may function to center the dendritic processes in the canaliculi to facilitate fluid flow, but the initiation of signaling that leads to the release of either ATP or PGE2 [Reich and Frangos, 1991; Klein-Nulend et al, 1997; Genetos et al, 2007] is associated with the large local deformations associated with the high stress concentrations around integrin attachment sites. Although Nicollela et al. [2006] have proposed that large deformations can occur in the lacunar wall near the polar axis of the lacunae it is not clear how these strains can be transmitted across the much wider pericellular space surrounding the cell body since there are neither “hillock” like protuberances or tethering fibers.
We speculate that integrin-mediated strain amplification would be most effective if mechanosensitive membrane channels were localized at adhesion sites, and several lines of evidence indicate that integrins can regulate channel activity. Forces transmitted through integrins to channel elements, either directly or through the cytoskeleton, can activate stress-activated ion channels on the cell surface [Ingber, 2006], while many mechanosensitive ion channels lose their normal activities if the integrins are disrupted or the membrane separates from the underlying cortical cytoskeleton [Zhang et al, 2000; Howard and Bechstedt, 2004; Gui et al, 2006; Wu et al, 2001; Charras et al, 2004]. Similar results have now been observed in cultured osteocytic cells, where Miyauchi et al [2006] recently reported that the activity of volume-sensitive calcium channels was potentiated by the αvβ3 ligand osteopontin, while stretch activation of calcium signalling could be suppressed by the β3 antagonist echistatin. Finally, Charras et al [2004] found that the cell level mechanical strains needed to open half of the mechanosensitive channels on bone cells were of the same order that we expect at focal attachment sites based of the theoretical predictions of Wang et al [2007].
Conclusions
Osteocytes interact with the bone that surrounds them in a complex manner reflected in a distinctive spatial distribution of integrins. Cell bodies are immediately surrounded by a layer of pericellular matrix resembling a glycocalyx and do not contact the walls of their lacunae directly. In contrast, osteocyte processes contact walls of canaliculi, but only at discrete spots where specialized protrusions (“hillocks”) bulge from the mineralized matrix. Osteocyte cell bodies express β1 integrins while cell processes express β3 integrins, the latter in a punctate distribution similar to matrix attachment sites but involving far fewer integrins. These findings argue that osteocytes are highly specialized in terms of their interactions with extracellular matrix, and that these specializations likely have physiological consequences for mechanosensitivity.
Acknowledgments
Funding source: NIH grants AR41210 and AR48699
Grant Sponsor: National Institute of Arthritis, Musculoskeletal and Skin Diseases Grant Numbers AR41210 (MBS) and AR48699 (SW)
Supported by grants AR41210 (MBS) and AR48699 (SW) from the National Institute of Arthritis, Musculoskeletal and Skin Diseases. We thank Valerie Williams for technical assistance with the electron microscopy studies, and Damien Laudier for help in developing the immunohistochemistry protocols.
Contributor Information
LM McNamara, Email: L.McNamara@soton.ac.uk.
RJ Majeska, Email: Robert.Majeska@mssm.edu.
S Weinbaum, Email: weinbaum@ccny.cuny.edu.
Friedrich, V, Email: Victor.Friedrich@mssm.edu.
MB Schaffler, Email: Mitchell.Schaffler@mssm.edu.
References
- Aarden EM, Nijweide PJ, Van Der Plas A, Alblas MJ, Mackie EJ, Horton MA, Helfrich MH. Adhesive properties of isolated chick osteocytes in vitro. Bone. 1996;18:305–313. doi: 10.1016/8756-3282(96)00010-5. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- Ayasaka N, Kondo T, Goto T, Kido MA, Nagata E, Tanaka T. Differences in the transport systems between cementocytes and osteocytes in rats using microperoxidase as a tracer. Arch Oral Biol. 1992;37:363–369. doi: 10.1016/0003-9969(92)90019-5. [DOI] [PubMed] [Google Scholar]
- Bell K, Loveridge N, Power J, Garrahan N, Meggitt B, Reeve J. Regional differences in cortical porosity in the fractured femoral neck. Bone. 1999;24(1):57–64. doi: 10.1016/s8756-3282(98)00143-4. [DOI] [PubMed] [Google Scholar]
- Bennett JH, Carter DH, Alavi AL, Beresford JN, Walsh S. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch Oral Biol. 2001;46(3):229–38. doi: 10.1016/s0003-9969(00)00114-x. [DOI] [PubMed] [Google Scholar]
- Brighton CT, Albeda SM. Identification of integrin cell-substratum adhesion receptors on cultured rat bone cells. J Orthop Res. 1992;10:766–773. doi: 10.1002/jor.1100100604. [DOI] [PubMed] [Google Scholar]
- Cary LA, Han DC, Gual JL. Integrin-mediated signal transduction pathways. Histol Histopathol. 1999;14:1001–1009. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- Charras GT, Williams BA, Sims SM, Horton MA. Estimating the sensitivity of mechanosensitive ion channels to membrane strain and tension. Biophys J. 2004;87(4):2870–84. doi: 10.1529/biophysj.104.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Chatterjee A. Role of alphavbeta3 integrin receptor in the invasive potential of human cervical cancer (SiHa) cells. J Environ Pathol Toxicol Oncol. 2001;20(3):211–21. [PubMed] [Google Scholar]
- Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103:267–271. doi: 10.1242/jcs.103.1.267. [DOI] [PubMed] [Google Scholar]
- Cooper RR, Milgram JW, Robinson RA. Morphology of the osteon. An electron microscopic study. J Bone Joint Surg Am. 1966;48:1239–1271. [PubMed] [Google Scholar]
- Cowles EA, DeRome ME, Pastizzo G, Brailey LL, Gronowicz GA. Mineralization and the expression of matrix proteins during in vivo bone development. Calcif Tissue Int. 1998;62(1):74–82. doi: 10.1007/s002239900397. [DOI] [PubMed] [Google Scholar]
- Culty M, Miyake K, Kincade PW, Sikorski E, Butcher EC, Underhill C. The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol. 1990;111(6 Pt 1):2765–74. doi: 10.1083/jcb.111.6.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton BA, McFarland CD, Underwood PA, Steele JG. Role of the heparin binding domain of fibronectin in attachment and spreading of human bone-derived cells. J Cell Sci. 1995;108:2083–2092. doi: 10.1242/jcs.108.5.2083. [DOI] [PubMed] [Google Scholar]
- Devoll RE, Pinero GJ, Appelbaum ER, Dul E, Troncoso P, Butler WT, Farach-Carson MC. Improved immunohistochemical staining of osteopontin (OPN) in paraffin-embedded archival bone specimens following antigen retrieval: anti-human OPN antibody recognizes multiple molecular forms. Calcif Tissue Int. 1997;60(4):380–6. doi: 10.1007/s002239900247. [DOI] [PubMed] [Google Scholar]
- Engleman VW, Nickols GA, Ross FP, Horton MA, Griggs DW, Settle SL, Ruminski PG, Teitelbaum SL. A peptidomimetic antagonist of the αvb3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. J Clin Invest. 1997;99:2284–2292. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212(1):207–14. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- Grzesik WJ, Gehron Robey P. Bone matrix RGD glycoproteins: immunolocalisation and interaction with human primary osteblastic bone cells in vitro. J Bone Miner Res. 1994;9:487–496. doi: 10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem. 2006;281(20):14015–25. doi: 10.1074/jbc.M600433200. [DOI] [PubMed] [Google Scholar]
- Han Y, Cowin SC, Schaffler MB, Weinbaum S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc Natl Acad Sci USA. 2004;23;101(47):16689–94. doi: 10.1073/pnas.0407429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat MA. Principles and Techniques of Electron Microscopy: Biological Applications. 3. Macmillan and Co; London: CRC Press; Boca Raton, Fla: 1989. [Google Scholar]
- Hillier ML, Bell LS. Differentiating human bone from animal bone: a review of histological methods. J Forensic Sci. 2007;52(2):249–63. doi: 10.1111/j.1556-4029.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- Hirose S, Li M, Kojima T, de Freitas PH, Ubaidus S, Oda K, Saito C, Amizuka N. A histological assessment on the distribution of the osteocytic lacunar canalicular system using silver staining. J Bone Miner Metab. 2007;25(6):374–82. doi: 10.1007/s00774-007-0764-x. [DOI] [PubMed] [Google Scholar]
- Holtrop ME. The ultrastructure of bone. Ann Clin Lab Sci. 1975;5:264–271. [PubMed] [Google Scholar]
- Horton MA, Davies J. Perspectives: adhesion receptors in bone. J Bone Miner Res. 1989;4:803–806. doi: 10.1002/jbmr.5650040603. [DOI] [PubMed] [Google Scholar]
- Horton MA, Taylor ML, Arnett TR, Helfrich MH. Arg-gly-asp (RGD) peptides and the anti-vitronectin receptor antibody 23C6 inhibit dentine resorption and cell spreading by osteoclasts. Exp Cell Res. 1991;195:368–375. doi: 10.1016/0014-4827(91)90386-9. [DOI] [PubMed] [Google Scholar]
- Hotchkiss KA, Matthias LJ, Hogg PJ. Exposure of the cryptic Arg-Gly-Asp sequence in thrombospondin-1 by protein disulfide isomerase. Biochim Biophys Acta. 1998;1388(2):478–88. doi: 10.1016/s0167-4838(98)00211-8. [DOI] [PubMed] [Google Scholar]
- Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol. 2004;14(6):R224–6. doi: 10.1016/j.cub.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Huang S, Stupack D, Liu A, Cheresh D, Nemerow GR. Cell growth and matrix invasion of EBV-immortalized human B lymphocytes is regulated by expression of alpha(v) integrins. Oncogene. 2000;19(15):1915–23. doi: 10.1038/sj.onc.1203509. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993;8:527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;20;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: Putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Larsen L, Engsig MT, Lou H, Ferreras M, Lochter A, Delaisse JM, Foged NT. Matrix metalloproteinase-dependent activation of latent transforming growth factor-beta controls the conversion of osteoblasts into osteocytes by blocking osteoblast apoptosis. J Biol Chem 15. 2002;277(46):44061–7. doi: 10.1074/jbc.M207205200. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Burger EH, Semeins CM, Raisz LG, Pilbeam CC. Pulsating fluid flow stimulates prostaglandin release and inducible G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res. 1997;12:4551. doi: 10.1359/jbmr.1997.12.1.45. [DOI] [PubMed] [Google Scholar]
- Klein-Nulend J, Nijweide PJ, Burger EH. Osteocyte and bone structure. Curr Osteoporos Rep. 2003;1(1):5–10. doi: 10.1007/s11914-003-0002-y. [DOI] [PubMed] [Google Scholar]
- Knothe Tate ML, Niederer P, Knothe U. In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone. 1998;22:107–117. doi: 10.1016/s8756-3282(97)00234-2. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Underhill CB. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987;105(3):1395–404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laynon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53(Suppl 1):S102–6. S106–7. doi: 10.1007/BF01673415. [DOI] [PubMed] [Google Scholar]
- Liaw L, Lindner V, Schwartz SM, Chambers AF, Giachelli CM. Osteopontin and beta 3 integrin are coordinately expressed in regenerating endothelium in vivo and stimulate Arg-Gly-Asp-dependent endothelial migration in vitro. Circ Res. 1995;77(4):665–72. doi: 10.1161/01.res.77.4.665. [DOI] [PubMed] [Google Scholar]
- Marotti G, Cane V, Palazzini S, Palumbo C. Structure-function relationships in the osteocyte. Ital J Miner Electrolyte Metab. 1990;4:93–106. [Google Scholar]
- McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;1;33(2):141–64. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, Fujita T, Mikuni-Takagaki Y. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. J Bone Miner Metab. 2006;24(6):498–504. doi: 10.1007/s00774-006-0716-x. [DOI] [PubMed] [Google Scholar]
- Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39(9):1735–43. doi: 10.1016/j.jbiomech.2005.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Tsuji K, Nifuji A. Osteopontin: a topic from the point of bone morphology. Clin Calcium. 2003;13(4):464–6. [PubMed] [Google Scholar]
- Palumbo C. A three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryos. Cell Tissue Res. 1986;246:125–131. doi: 10.1007/BF00219008. [DOI] [PubMed] [Google Scholar]
- Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol Cell Physiol. 1991;261:C428C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- Ross FP, Alvarez JI, Chappel J, Sander D, Butler WT, Farach-Carson MC, Mintz KA, Robey PG, Teitelbaum SL, Cheresh DA. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin avb3 potentiate bone resorption. J Biol Chem. 1993;268:9901–9907. [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37(4):411–7. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- Saito T, Albeda SM, Brighton CT. Identification of integrin receptors on cultured human bone cells. J Orthop Res. 1994;12:384–394. doi: 10.1002/jor.1100120311. [DOI] [PubMed] [Google Scholar]
- Shibata S, Baba O, Oda T, Yokohama-Tamaki T, Qin C, Butler WT, Sakakura Y, Takano Y. An immunohistochemical and ultrastructural study of the pericellular matrix of uneroded hypertrophic chondrocytes in the mandibular condyle of aged c-src-deficient mice. Arch Oral Biol. 2008;53(3):220–30. doi: 10.1016/j.archoralbio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Sinha RK, Tuan RS. Regulation of human osteoblast integrin expression by orthopaedic implant materials. Bone. 1996;18:451–458. doi: 10.1016/8756-3282(96)00044-0. [DOI] [PubMed] [Google Scholar]
- Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontol. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- Vatsa A, Semeins CM, Smit TH, Klein-Nulend J. Paxillin localisation in osteocytes-Is it determined by the direction of loading? Biochem Biophys Res Commun. 2008 doi: 10.1016/j.bbrc.2007.12.174. Epub ahead of print, Jan 2008. [DOI] [PubMed] [Google Scholar]
- Wang Y, McNamara LM, Schaffler MB, Weinbaum S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc Natl Acad Sci USA. 2007;104(40):15941–6. doi: 10.1073/pnas.0707246104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Jones PH. Expression and function of the keratinocyte integrins. Dev Suppl. 1993:185–192. [PubMed] [Google Scholar]
- Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;26;271(5248):509–12. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- Weinger JM, Holtrop ME. An ultrastructural study of bone cells: the occurrence of microtubules, microfilaments and tight junctions. Calcif Tissue Res. 1974;14:15–29. doi: 10.1007/BF02060280. [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 1994;5(2):183–92. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J Biol Chem. 2001;276(32):30285–92. doi: 10.1074/jbc.M102436200. [DOI] [PubMed] [Google Scholar]
- Yamada KM. Integrin signaling. Matrix Biol. 1997;16:137–141. doi: 10.1016/s0945-053x(97)90001-9. [DOI] [PubMed] [Google Scholar]
- Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci USA. 1999;6;96(14):8156–60. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;20;276(16):13365–71. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- You L, Cowin SC, Schaffler MB, Weinbaum S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J Biomech. 2001;34(11):1375–86. doi: 10.1016/s0021-9290(01)00107-5. [DOI] [PubMed] [Google Scholar]
- You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278(2):505–13. doi: 10.1002/ar.a.20050. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao F, Popov VL, Wen JW, Hamill OP. Mechanically gated channel activity in cytoskeleton-deficient plasma membrane blebs and vesicles from Xenopus oocytes. J Physiol. 2000;15;523(Pt 1):117–30. doi: 10.1111/j.1469-7793.2000.t01-1-00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]