Abstract
The protein tyrosine kinase (PTK) inhibitor genistein has been widely used to examine potential effects of tyrosine phosphorylation on neurotransmitter function. We report here that genistein inhibits N-methyl-D-aspartate (NMDA) receptors through a direct effect. Whole-cell NMDA-activated current was recorded in native receptors from mouse hippocampal slice culture and rat recombinant NR1aNR2A and NR1aNR2B receptors transiently expressed in HEK293 cells. Extracellular application of genistein and NMDA reversibly inhibited NMDA-activated current. The inhibition of NMDA- activated current by genistein applied externally was not affected when genistein was also preequilibrated in the intracellular solution. Daidzein, an analog of genistein that does not block PTK, also inhibited NMDA-activated current. Coapplication of lavendustin A, a specific inhibitor of PTK, had no effect on the NMDA response. Moreover, genistein-induced inhibition of NMDA-activated current displayed concentration- and voltage-dependence. Our results demonstrate that genistein has a direct inhibitory effect on NMDA receptors that is not mediated via inhibition of tyrosine kinase. Thus, other PTK inhibitors may be more suitable for studying involvement of PTKs in NMDA receptor-mediated events.
Keywords: protein tyrosine kinase, daidzein, lavendustin, patch clamp, hippocampus, HEK 293 cells
1. Introduction
Genistein (4,7,4′-trihydroxyisoflavone) is one of several known plant-derived soy isoflavones found in high abundance in most soy food products. Genistein, the most studied of the soy phytoestrogens which are structurally and functionally similar to estradiol, exerts a number of biological effects such as estrogenic (Kuiper et al., 1998), antioxidant activity (Tikkanen et al., 1998) and anticancer effects (Pan et al., 2001). Genistein is also a potent protein tyrosine kinase (PTK) inhibitor (Akiyama et al., 1987; Akiyama and Ogawara, 1991; Hanke et al., 1996) which is widely used as a pharmacological tool to assess the involvement of PTK-mediated signaling. In addition to inhibition of PTKs, genistein has been reported to directly inhibit a number of ion channels. These include voltage-gated channels selective for Ca2+ (Belevych et al., 2002; Kurejova and Lacinova, 2006; Tao et al., 2009; Yokoshiki et al., 1996), Na+ (Paillart et al., 1997), and K+ (Smirnov and Aaronson, 1995). Genistein has also been shown to inhibit ligand-gated ion channels, including both GABAA (Dunne et al., 1998; Huang et al., 1999) and glycine receptors (Huang and Dillon, 2000; Zhu et al., 2003). Moreover, genistein inhibits depolarization-induced Ca2+ influx and glutamate release from hippocampal synaptosomes, most likely through direct inhibition of voltage-gated Ca2+ channels (VDCC) and/or K+ channels (Pereira et al., 2003).
The NMDA receptor is one of three main subtypes of ionotropic glutamate receptors that mediate most fast excitatory neuronal transmission. NMDA receptors are multiprotein complexes with a central ion pore. Activation of NMDA receptors results in the opening of a non-selective cation channel, which allows flow of Na+ and Ca2+ ions into the cell. Calcium flux through NMDA receptors is thought to play a critical role in synaptic plasticity, synaptogenesis and excitotoxicity (Bliss and Collingridge, 1993; Choi, 1992; Malenka and Nicoll, 1999). A number of studies suggest that phosphorylation of the NMDA receptor is an important component in receptor function (Chen and Roche, 2007). PTKs, especially non-receptor PTKs (Src and Fyn), are important modulators of NMDA receptors (Ali and Salter, 2001). For example, NMDA currents are potentiated by increasing PTK activity and reduced by decreasing PTK activity (Wang and Salter, 1994; Wang et al., 1996). Activation of NMDA receptors is required for long-term potentiation (LTP) of excitatory synaptic transmission at hippocampal CA1 synapses. The blockade of PTK activity significantly inhibits NMDA receptor-dependent LTP (Casey et al., 2002; Huang and Hsu, 1999; O'Dell et al., 1991). Intracranial administration of PTK inhibitors impairs long-term memory formation in newborn chicks (Whitechurch et al., 1997). The involvement of PTKs in modulation of NMDA receptor-dependent processes in those studies was tested using genistein as the PTK inhibitor. The observed effect induced by genistein was interpreted as a functional consequence of inhibition of PTK activity. However, given the fact that genistein exerts a variety of complicated biological effects, especially effects on voltage- and ligand-gated ion channels that are PTK-independent, we assessed the ability of genistein to directly inhibit NMDA receptor function.
2. Methods
2.1. Hippocampal neurons
Organotypic explants were derived from postnatal day (P) 4 (day of birth = P1) hippocampus, obtained from pups born of timed-pregnant C57Bl/6 mice as previously described (Singh et al., 2000). The hippocampus was first dissected out from the brain, and then sliced cross-sectionally, resulting in ∼360 μm sections. Explant slices were maintained as roller tube cultures (Gahwiler, 1981) on rat tail collagen-coated/poly-L-lysine pre-coated glass coverslips and were grown in steroid-deficient and phenol red-free maintenance medium [gelding serum (25%); Hank's BSS (22.5%); BME (50%); glucose (6 mg/ml); L-glutamine (2 mM); ascorbic acid (50 μg/ml)]. This maintenance medium was replaced 3 times a week.
2.2 Recombinant receptors
cDNAs encoding rat NR1a, NR2A and NR2B were generous gifts from Dr. David Lynch (University of Pennsylvania). Human embryonic kidney (HEK293) cell line was transiently transfected with recombinant NMDA receptor subunits using TransIT®-293 transfection reagent (Mirus, Madison, WT).
Briefly, HEK293 cells were washed and placed in fresh Dulbecco's modified eagle medium containing 10% FBS and antibiotics (penicillin 100 U/mL). NR1a along with NR2A or NR2B (0.5:0.5 μg) was added to cells growing exponentially on one poly-L-lysine coated coverslip placed in a 35-mm culture dish. Transfected cells were used for electrophysiological analysis 24- 48 h after the transfection.
2.3. Electrophysiology
Whole-cell patch recordings were made at room temperature (22-25 °C) at a holding potential of -60 mV. Patch pipettes of borosilicate glass (M1B150F, World Precision Instruments, Inc., Sarasota, FL) were pulled (Flaming/Brown, P-87/PC, Sutter Instrument Co., Novato, CA) to a tip resistance of 7-8 MΩ. The pipette solution contained (in mM): 140 CsCl, 10 EGTA, 10 HEPES, 4 Mg-ATP; 0.2 Na3-GTP, pH 7.2. The slice or transfected cells on coverslip was superfused (7-10 ml/min) with extracellular solution containing (in mM): 125 NaCl, 5.5 KCl, 5.0 CaCl2, 20 HEPES, 10 D-glucose, 10 μM glycine; pH 7.3. For brain slice recordings, 100 nM tetrodotoxin (TTX) and 5 μM bicuculline methiodide were added to the extracellular solution to suppress spontaneous activity. The cells were visualized using an upright, fixed stage microscope (Nikon Optiphot-2UD) equipped with standard Hoffman modulation contrast (HMC) optics and a video camera system (Sony model XC-75 CCD video camera module, DOT-X monitor). NMDA was prepared in extracellular solution and was applied (10 s in most cases) to cells via gravity flow using a Y-shaped tube positioned near the target cell. With this system, the 10-90% rise time of the junction potential at the open tip was 60- 120 ms. NMDA-evoked currents from the whole-cell configuration were obtained using a patch clamp amplifier (Axopatch 200A, Axon Instruments, Foster City, CA) equipped with a CV201A headstage. The currents were low-pass filtered at 5 kHz, monitored on an oscilloscope and a chart recorder (Gould TA240), and stored on a computer (pClamp 6.05, Axon Instruments) for subsequent analysis. 60-80% series resistance compensation was applied at the amplifier. To monitor the possibility that access resistance changed over time or during different experimental conditions, at the initiation of each recording we measured and stored on our digital oscilloscope the current response to a 5 mV voltage pulse. This stored trace was continually referenced throughout the recording. If a change in access resistance was observed throughout the recording period, the patch was aborted and the data were not included in the analysis.
2.4. Chemicals
NMDA, MK-801, bicuculline methiodide and glycine were purchased from Sigma. TTX was obtained from Tocris (Ellisville, MO) and genistein and daidzein from either Sigma or LC Laboratories (Woburn, MA). NMDA, glycine, TTX and MK-801 stocks were made in double distilled H2O. Bicuculline, genistein and daidzein were prepared in dimethyl sulfoxide (DMSO). The final concentration of DMSO was less than 0.05 % (v/v).
2.5. Data analysis
Peak currents were normalized to the initial response (100%). NMDA concentration-response profiles were fitted to the following equation: I/Imax=1(1+(EC50/[NMDA]n), where I and Imax represent the normalized NMDA-activated current at a given concentration and the maximum current induced by saturating a [NMDA], EC50 is the half-maximal effective NMDA concentration, and n is the slope factor.
All data were presented as means ± SEM. Student's t- test (paired or unpaired) or one-way ANOVA with Student–Newman–Keuls multiple comparison test was used to determine statistical significance (p< 0.05).
3. Results
3.1. Inhibition of NMDA-activated current by genistein and daidzein
The average amplitude of whole-cell current activated by 50 μM NMDA in cultured hippocampus neurons (days in culture, 8-16) was 623 ± 87 pA (n=38). The inward NMDA-activated current was completely blocked by MK-801 (10 μM), a selective NMDA receptor inhibitor (data not shown). As shown in Fig. 1, co-application of genistein with 50 μM NMDA resulted in a concentration-dependent decrease in NMDA-activated current. 50 and 100 μM genistein inhibited mean amplitude of NMDA currents to 77 ± 3.9% and 70 ± 2.7 % of the control, respectively (p<0.05, one-way ANOVA, n=4-6). Genistein mainly inhibited the current amplitude without apparently affecting steady-state current (Fig. 1A). In addition, the NMDA-current fully recovered to the control level after 1-3 min of washout (Fig. 1A). To examine whether the observed effect was due to the inhibition of PTKs, daidzein (4′,7-dihydroxyisoflavone), a genistein analogue which does not inhibit PTKs, was co-applied with NMDA to the cell. Daidzein reversibly and dose-dependently inhibited NMDA-activated current in a manner similar to that of genistein. Because of solubility limits of genistein and diadzein in the extracellular solution, we did not test them at the concentrations higher than 100 μM.
Figure 1.
Effect of coapplied genistein or daidzein on NMDA-activated currents. A, Whole-cell NMDA (50 μM)-activated current was recorded from one hippocampal neuron. Genistein (50 or 100 μM) or daidzein (50 or 100 μM) was co-applied with NMDA to the cell for 10 sec. Note that genistein- and daidzein- induced inhibition of NMDA current was reversible. B, Summary data of inhibition of NMDA response by genistein or daidzein. The current amplitude is normalized to the initial NMDA response (assigned as 100%). Each data point represents Mean ± SEM from at least 4 cells from slices at P08-P15.
3.2. Effect of intracellular genistein
The rapid effect of genistein suggested an intracellular site of action was unlikely. To test this, we examined the effect of extracellularly applied genistein or daidzein with 100 μM genistein equilibrated intracellularly through the patch pipette. If the genistein-induced inhibition of NMDA currents we had observed was via blockade of PTK activity, one would predict the inhibitory effect caused by extracellular application would be attenuated with genistein present intracellularly. Based on our previous studies, five min is sufficient to allow genistein to diffuse intracellularly (Dean et al., 1997; Huang and Dillon, 2000; Huang et al., 1999). Thus NMDA-activated currents were measured 5-10 min after rupture of the patch membrane, As shown in Fig. 2A&B, when genistein was pre-equilibrated in the intracellular solution, extracellular application of genistein or daidzein induced a reversible inhibition of NMDA currents. The mean amplitude of NMDA current was reduced to 69 ± 2.7% and 68 ± 1.6% of the control by 100 μM genistein and daidzein, respectively (n=6), comparable to that seen without intracellular genistein present (Fig. 1, P>0.05, unpaired t-test). These data firmly support an extracellular site of action of genistein and daidzein.
Figure 2.
Effect of intracellular application of genistein on inhibitory effect produced by extracellular application genistein or daidzein. A, Whole-cell current induced by 50 μM NMDA was recorded from individual cell with 100 μM genistein included in the pipette solution. Genistein or daidzein (100 μM) was co-applied with NMDA to the cell for 10 sec. B, Summary data of inhibition of NMDA currents by genistein or daidzein. Each data point represents Mean ± SEM from at least 6 cells. Note that genistein-induced inhibition of NMDA current is reversible, and comparable to that without genistein in pipette (Fig. 1), suggesting that intracellular treatment of genistein did not prevent the inhibitory effects induced by extracellular genistein application. Data were collected from slices at P16.
3.3. Effect of lavendustin A on NMDA response
Lavendustin A, a more potent PTK inhibitor (IC50 ∼0.5 μM for pp60SRC in vitro) than genistein (IC50 18-30 μM), is structurally distinct from genistein or daidzein (Akiyama et al., 1987; Hsu et al., 1991). It has also been used as a pharmacological tool to probe PTK modulating NMDA receptor function at concentrations between 0.5-10 μM (Viviani et al., 2003; Wang and Salter, 1994). Thus we next determined whether it exerts direct inhibitory effect on the NMDA response. As shown in Fig. 3, co-application of 5 μM lavendustin A with NMDA had no effect on NMDA-activated current. Current amplitude in the presence of 1 or 5 μM lavendustin A treatment was 105 ± 5.1% (n=5) or 105 ± 3.2% (n=6) of the control response, respectively (p>0.05, paired t-test). These data further support the hypothesis that the observed genistein inhibition is due to direct action on the channels, and also demonstrate the direct inhibitory effects are not observed in all PTK inhibitors.
Figure 3.
Effect of coapplied lavendustin A on the current activated by 50 μM NMDA. A, Typical response to 50 μM NMDA recorded before, during and after coapplication of lavendustin A (Lav, 5 μM) in the same hippocampal neuron. B, Histogram summarizing the effect of lavendustin A at 5 μM on the NMDA response. Data represents 6 cells from slices at P10-P12. The current amplitude is normalized to the initial NMDA response (assigned as 100%).
3.4. Effect of genistein on recombinant NMDA receptors
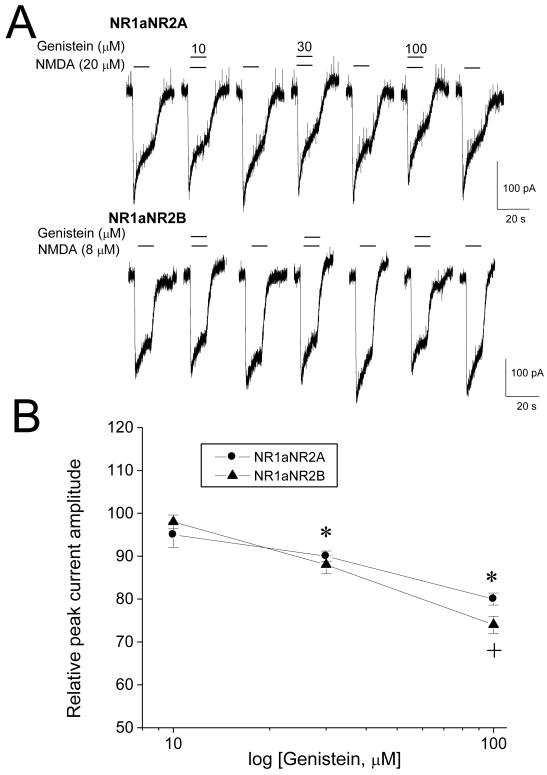
It has been shown that hippocampal neurons express NMDA receptors composing both NR1 and NR2 subunits (Sans et al., 2000). To further examine whether genistein directly blocks NMDA receptors in recombinant preparations, the effect of genistein on NMDA response was studied on the recombinant rat NMDA NR1aNR2A and NR1aNR2B transiently expressed in HEK293 cells. Fig. 4 shows the concentration-response relation for NMDA activation in NR1aNR2A and NR1aNR2B receptors. NR1aNR2A receptors exhibited relatively fast kinetics of current decay and low affinity compared to NR1aNR2B, which is consistent with previous reports (Blevins et al., 1997; Cull-Candy and Leszkiewicz, 2004). The EC50 was 44 ± 5.6 μM for NR1aNR2A (n=10), and 14 ± 1.3 μM for NR1aNR2B receptors (n=6), respectively. The effect of genistein on the NR1aNR2A and NR1aNR2B was tested with EC30 concentration of NMDA. As shown in Figure 5, coapplied genistein resulted in reversible and concentration-dependent inhibition of NMDA response (p<0.01, among different concentrations of genistein, one-way ANOVA, n=6-10). The incorporation of NR2B subunit slightly increased the sensitivity to genistein as inhibition of initial peak current in NR1aNR2B by 100 μM genistein was greater than that of NR1aNR2A (20 ±1.4% in NR1aNR2A, 26 ± 2.0% in NR1aNR2B, p<0.05, unpaired t-test). Genistein produced similar inhibition of the initial peak and stead-state current (the inhibition of amplitude at the end of current activated by EC30 NMDA was 23 ± 4.3% for NR1aNR2A, and 25 ± 3.3% for NR1aNR2B receptors, respectively, p>0.05, paired t-test, compared to their initial peak amplitude). These data suggest that genistein inhibits recombinant NMDA receptors in a manner similar to that seen in native receptors.
Figure 4.
Concentration- response relation for rat NR1aNR2A and NR1aNR2B receptors transiently expressed in HEK293 cells. A, Examples of typical response to NMDA (1-1000 μM) recorded from the cell voltage clamped at -60 mV. B, Graph plotting the relative current amplitude of NMDA-activated current as a function of NMDA concentration. Amplitude is normalized to the maximal current (assigned as 100%). Each data point is the average current from six to 10 cells. Curves shown are the best fits of the data to the logistic equation. EC50 and Hill coefficients (in parentheses) were 44 ± 5.6 μM (1.2 ± 0.13) for NR1aNR2A, and 14 ± 1.3 μM (1.2 ± 0.08) for NR1aNR2B receptors, respectively.
Figure 5.
Effect of genistein on rat NR1aNR2A and NR1aNR2B receptors transiently expressed in HEK293 cells. A, Examples of typical response to genistein in NR1aNR2A and NR1aNR2B receptors. Current activated by EC30 concentration of NMDA (20 μM for NR1aNR2A and 8 μM for NR1aNR2B) was recorded before, during and after coapplication of genistein (10-100 μM). Coapplication of genistein with NMDA resulted in a concentration-dependent and reversible decrease in current amplitude. B, Histogram summarizing the mean effect of coapplied genistein on the NMDA response. Each data point represents the mean response from six to 10 cells. * p<0.05, paired t-test, compared with the control response. + p<0.05, unpaired t-test, compared between NR1aNR2A and NR1aNR2B with 100 μM genistein.
3.5. Concentration- and voltage- dependence of genistein effect
To further reveal whether genistein effect is dependent on NMDA concentration, we tested at three different gating concentrations: 20 μM (∼EC30), 50 μM (∼EC50) and 300 μM (∼EC95+). As shown in Figure 5A&B, the ability of genistein to inhibit NMDA current decreased with increase of NMDA concentration, suggesting a competitive antagonism.
The direct inhibition induced by genistein is voltage-dependent in GABAA (Huang et al., 1999), glycine receptors (Huang and Dillon, 2000; Zhu et al., 2003) and VDCCs (Tao et al., 2009; Yokoshiki et al., 1996). We thus evaluated the possibility that transmembrane voltage gradient would alter the ability of genistein to inhibit NMDA receptors. Potential voltage-dependent of genistein block was assessed using a voltage ramp protocol in recombinant rat NR1aNR2A NMDA receptors. Transmembrane voltage was ramped from -60 to +60 mV over a 0.5 s time course in the presence of either NMDA (50 μM) alone, or NMDA plus 100 μM genistein. Genistein did not alter reversal potential, which was -20 ± 1.6 mV in the control, and -22 ± 1.7 mV in the presence of genistein (n=9, p>0.05). The inhibition of NMDA current by genistein was more pronounced at negative potentials. Genistein reduced NMDA current by 13 ± 3.1 % at -60 mV and by 5.2 ± 1.5% at +60 mV of holding potential (Figure 6C&D, n=9, p<0.05, compared to control and between -60 and +60 mV), demonstrating that blockade of NMDA receptors by genistein is voltage- dependent.
Figure 6.
Concentration- and voltage- dependence of genistein inhibitory effect on rat NR1aNR2A NMDA receptors transiently expressed in HEK293 cells. A, Representative traces recorded before, during and after coapplication of 100 μM genistein with 20, 50 and 300 μM NMDA. B, Histogram summarizing the mean effect of coapplied genistein on the response to different NMDA concentrations. Each data point represents the mean response from six to 8 cells. * p<0.05, paired t-test, compared with the control response. + p<0.05, unpaired t-test, compared between 20 and 50 μM NMDA. The data of 20 μM NMDA were replotted from Fig.5 for direct comparison. C, Representative traces recorded before, during and after coapplication of 100 μM genistein with 50 μM NMDA at transmembrane voltage ramped from -60 to +60 mV (0.24 mV/ms). D, Histogram summarizing the mean value of genistein-induced inhibition at -60 and +60 mV of membrane potential. Each data point represents the mean response from 9 cells. * p<0.05, paired t-test, compared between -60 mV and +60 mV.
4. Discussion
To our knowledge, this is the first demonstration that genistein, a widely-used PTK inhibitor, has a PTK-independent inhibitory effect on NMDA receptors. Several lines of evidence presented in our studies support this notion. First, coapplication of genistein with NMDA revealed that the genistein-induced inhibition was very rapid in onset, and was reversible upon removal of the drug. Such rapid (in ms scale) and reversible inhibition can not be readily explained by an intracellular PTK-mediated event. Second, when cells were intracellularly pre-equilibrated with genistein, extracellular application of genistein produced a similar degree of inhibition. In addition, daidzein, a structural analog of genistein devoid of PTK inhibitory effects, produced an inhibitory action on NMDA currents comparable to that observed with genistein. The similar genistein-induced blockade was also duplicated in recombinant NMDA receptors transiently expressed in HEK293 cells. Finally, lavendustin A, a potent PTK inhibitor that is structurally unrelated to genistein, produced little effect while it was extracellularly coapplied with NMDA. Taken together, these data demonstrate that genistein directly blocks NMDA receptors via a PTK-independent, extracellular site of action.
Whereas an extracellular site of action for genistein is apparent, a more specific site of action cannot be identified at this time. In the case of ligand-gated GABAA and glycine receptors, a site of action in the ion channel itself has been postulated (Huang and Dillon, 2000). Moreover, in both of these receptors, the inhibitory effects of genistein were shown to be voltage- dependent and non-competitive fashion. Use-dependent blockade of genistein is also consistent with the hypothesis that genistein appears to be open- channel blocker in these two channels (Huang and Dillon, 2000; Huang et al., 1999; Zhu et al., 2003). However, genistein seems to inhibit NMDA receptors in a competitive manner without apparent use-dependent effects. Whether this is truly “competition” at the agonist recognition site is not clear. For example, the convulsant pentylenetetrazole (PTZ) inhibits GABAA receptors in what appears to be a competitive manner (Huang et al., 2001), but in fact does not inhibit agonist binding at the GABA binding site. Allosteric competitive inhibition has been reported for other ligand-receptor interactions (Bertrand et al., 1992; Lynch et al., 1995).
The inhibition of NMDA receptors by genistein also displays a voltage-dependent component, being much more pronounced at negative membrane potential. GABAA and glycine receptors belong to a structurally distinct class of ligand-gated ion channels compared to the NMDA receptors; the channel domains of the two classes share no significant homology. Thus, lack of a conserved site of action would not be unexpected. A clearer understanding of the mechanism and site of action of genistein inhibition of NMDA receptors will require further investigation.
It should be pointed out that our experimental conditions were designed solely to uncover a potential direct effect of genistein on NMDA receptors, not to assess PTK-dependent modulation of NMDA receptors. Indeed, PTKs play an important role in modulation of NMDA receptor function as demonstrated previously (see (Ali and Salter, 2001) for review). Rather, the importance of the present work is the demonstration that genistein, commonly used as a pharmacological tool to assess the role of PTKs, is also able to directly inhibit NMDA receptors at concentrations higher than 30 μM. Genistein inhibits PTKs with an IC50 of 18-30 μM in vitro, depending on the PTKs inhibited (Akiyama et al., 1987; Akiyama and Ogawara, 1991). Importantly, the concentrations of genistein used as a pharmacological tool to examine PTK involvement in NMDA receptor-associated processes were 50 μM (Collett and Collingridge, 2004), 100 μM (Collett and Collingridge, 2004; Huang and Hsu, 1999; O'Dell et al., 1991; Wang and Salter, 1994; Wang et al., 1996), and in some studies has been used at concentrations up to 250 μM (Casey et al., 2002) or even 500 μM (Whitechurch et al., 1997). Our data demonstrate conclusively that at such concentrations, genistein produces marked direct suppression of NMDA receptors.
Thus, genistein has now been shown to directly inhibit several ion channels, in the range of concentration used to inhibit PTK activity. This fact, coupled with the fact that genistein also directly inhibits other enzymes such as DNA topoisomerase I and II, (Okura et al., 1988; Peterson, 1995), S6 kinase (Yamashita et al., 1990) and cAMP phosphodiesterase (Nichols and Morimoto, 1999), suggests great caution should be taken when genistein is used to probe regulation of target proteins by PTKs. With regard specifically to NMDA receptors, lavendustin A would seem to be a preferable PTK inhibitor, as it has no detectable direct action on NMDA receptors.
Acknowledgments
We thank Dr. David R Lynch for providing the recombinant NMDA receptor subunit cDNAs. We also thank Ms. Cathy L Bell-Horner for preparing HEK293 cell lines and amplifying cDNAs.
Grant sponsors: the American Heart Association TX Affiliate grants 0565054Y, 0655028Y and National Institute of Aging AG022550
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Belevych AE, Warrier S, Harvey RD. Genistein inhibits cardiac L-type Ca(2+) channel activity by a tyrosine kinase-independent mechanism. Mol Pharmacol. 2002;62:554–565. doi: 10.1124/mol.62.3.554. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Devillers-Thiery A, Revah F, Galzi JL, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux JP. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci U S A. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins T, Mirshahi T, Chandler LJ, Woodward JJ. Effects of acute and chronic ethanol exposure on heteromeric N-methyl-D-aspartate receptors expressed in HEK 293 cells. J Neurochem. 1997;69:2345–2354. doi: 10.1046/j.1471-4159.1997.69062345.x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Casey M, Maguire C, Kelly A, Gooney MA, Lynch MA. Analysis of the presynaptic signaling mechanisms underlying the inhibition of LTP in rat dentate gyrus by the tyrosine kinase inhibitor, genistein. Hippocampus. 2002;12:377–385. doi: 10.1002/hipo.10036. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Collett VJ, Collingridge GL. Interactions between NMDA receptors and mGlu5 receptors expressed in HEK293 cells. Br J Pharmacol. 2004;142:991–1001. doi: 10.1038/sj.bjp.0705861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Dean JB, Huang RQ, Erlichman JS, Southard TL, Hellard DT. Cell-cell coupling occurs in dorsal medullary neurons after minimizing anatomical-coupling artifacts. Neuroscience. 1997;80:21–40. doi: 10.1016/s0306-4522(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Dunne EL, Moss SJ, Smart TG. Inhibition of GABAA receptor function by tyrosine kinase inhibitors and their inactive analogues. Mol Cell Neurosci. 1998;12:300–310. doi: 10.1006/mcne.1998.0717. [DOI] [PubMed] [Google Scholar]
- Gahwiler B. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Persons PE, Spada AP, Bednar RA, Levitzki A, Zilberstein A. Kinetic analysis of the inhibition of the epidermal growth factor receptor tyrosine kinase by Lavendustin-A and its analogue. J Biol Chem. 1991;266:21105–21112. [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. J Physiol. 1999;520(Pt 3):783–796. doi: 10.1111/j.1469-7793.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–995. [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Direct inhibition of glycine receptors by genistein, a tyrosine kinase inhibitor. Neuropharmacology. 2000;39:2195–2204. doi: 10.1016/s0028-3908(00)00046-0. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Fang MJ, Dillon GH. The tyrosine kinase inhibitor genistein directly inhibits GABAA receptors. Brain Res Mol Brain Res. 1999;67:177–183. doi: 10.1016/s0169-328x(99)00061-3. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kurejova M, Lacinova L. Effect of protein tyrosine kinase inhibitors on the current through the Ca(V)3.1 channel. Arch Biochem Biophys. 2006;446:20–27. doi: 10.1016/j.abb.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Rajendra S, Barry PH, Schofield PR. Mutations affecting the glycine receptor agonist transduction mechanism convert the competitive antagonist, picrotoxin, into an allosteric potentiator. J Biol Chem. 1995;270:13799–13806. doi: 10.1074/jbc.270.23.13799. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Nichols MR, Morimoto BH. Tyrosine kinase-independent inhibition of cyclic-AMP phosphodiesterase by genistein and tyrphostin 51. Arch Biochem Biophys. 1999;366:224–230. doi: 10.1006/abbi.1999.1200. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Kandel ER, Grant SG. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988;157:183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- Paillart C, Carlier E, Guedin D, Dargent B, Couraud F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J Pharmacol Exp Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- Pan W, Ikeda K, Takebe M, Yamori Y. Genistein, daidzein and glycitein inhibit growth and DNA synthesis of aortic smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Nutr. 2001;131:1154–1158. doi: 10.1093/jn/131.4.1154. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Carvalho AP, Duarte CB. Genistein inhibits Ca2+ influx and glutamate release from hippocampal synaptosomes: putative non-specific effects. Neurochem Int. 2003;42:179–188. doi: 10.1016/s0197-0186(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Peterson G. Evaluation of the biochemical targets of genistein in tumor cells. J Nutr. 1995;125:784S–789S. doi: 10.1093/jn/125.suppl_3.784S. [DOI] [PubMed] [Google Scholar]
- Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr, Guan X, Frail DE, Toran-Allerand CD. Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-alpha knock-out mice. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ Res. 1995;76:310–316. doi: 10.1161/01.res.76.2.310. [DOI] [PubMed] [Google Scholar]
- Tao J, Zhang Y, Li S, Sun W, Soong TW. Tyrosine kinase-independent inhibition by genistein on spermatogenic T-type calcium channels attenuates mouse sperm motility and acrosome reaction. Cell Calcium. 2009;45:133–143. doi: 10.1016/j.ceca.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Tikkanen MJ, Wahala K, Ojala S, Vihma V, Adlercreutz H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc Natl Acad Sci U S A. 1998;95:3106–3110. doi: 10.1073/pnas.95.6.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Wang YT, Yu XM, Salter MW. Ca(2+)-independent reduction of N-methyl-D-aspartate channel activity by protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1996;93:1721–1725. doi: 10.1073/pnas.93.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitechurch RA, Ng KT, Sedman GL. Tyrosine kinase inhibitors impair long-term memory formation in day-old chicks. Brain Res Cogn Brain Res. 1997;6:115–120. doi: 10.1016/s0926-6410(97)00022-0. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Kawada S, Nakano H. Induction of mammalian topoisomerase II dependent DNA cleavage by nonintercalative flavonoids, genistein and orobol. Biochem Pharmacol. 1990;39:737–744. doi: 10.1016/0006-2952(90)90153-c. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Sumii K, Sperelakis N. Inhibition of L-type calcium current in rat ventricular cells by the tyrosine kinase inhibitor, genistein and its inactive analog, daidzein. J Mol Cell Cardiol. 1996;28:807–814. doi: 10.1006/jmcc.1996.0075. [DOI] [PubMed] [Google Scholar]
- Zhu L, Jiang ZL, Krnjevic K, Wang FS, Ye JH. Genistein directly blocks glycine receptors of rat neurons freshly isolated from the ventral tegmental area. Neuropharmacology. 2003;45:270–280. doi: 10.1016/s0028-3908(03)00151-5. [DOI] [PubMed] [Google Scholar]