Abstract
B cell lymphoma (BCL)6 and Bcl-xL are expressed in germinal center (GC) B cells and enable them to endure the proliferative and mutagenic environment of the GC. By introducing these genes into peripheral blood memory B cells and culturing these cells with factors produced by follicular helper T cells, CD40L and IL-21, we convert them to highly proliferating, cell surface BCR positive, Ig-secreting B cells with features of GC B cells including expression of activation-induced cytidine deaminase. We generated cloned lines of B cells specific for respiratory syncytial virus and used these cells as a source of antibodies that effectively neutralized this virus in vivo. This method provides a new tool to study GC B cell biology, signal transduction through antigen-specific B cell receptors, and for the rapid generation of high affinity human monoclonal antibodies.
Introduction
Stable monoclonal cell lines of immortalized human B cells that express the B cell receptor (BCR) on their cell surface while secreting antibodies would be attractive tools for studying various aspects of BCR signaling but also for the generation of human monoclonal antibodies. BCR expression on polyclonal immortalized human B cells would facilitate selection of antigen–specific cells on basis of binding of antigen to the specific BCR, while production of antibodies enables selection of B cell clones on the basis of functional activities of the secreted antibodies. While naïve and memory B cells express cell surface BCR, they do not secrete Ig. Cells that simultaneously express the BCR and secrete antibodies might be present in the light zone of the germinal center (GC). These cells may represent plasmablasts ready to be selected into the plasma cell compartment 1.
The GC consists of two areas, the dark and the light zone, which are populated by centroblasts and centrocytes, respectively 2. Antigen-activated naïve and memory B cells in the GC undergo extensive proliferation, accompanied by somatic hypermutations (SHM) and class-switch recombination (CSR) of Ig genes, processes both mediated by activation induced cytidine deaminase (AID) 2–4. Light zone GC B cells then undergo selection via BCR–antigen interaction on follicular dendritic cells 5 and receive help from follicular T helper cells, ultimately developing into memory or antibody-secreting plasma cells 6. These selected light zone GC B cells express Bcl-xL, a member of the anti apoptotic Bcl-2 protein family 7 most likely to protect them against cell death.
Mature B cells can be cultured in vitro under conditions which mimic some key aspects of the GC reaction; that is, activation of B cells with CD40 ligand (L) and the presence of cytokines like interleukin (IL)-4, IL-10 or IL-21 6,8,9. While B cells cultured with CD40L, IL-2 and IL-4 produce very little Ig, addition of IL-21 leads to differentiation to plasma cells accompanied by high Ig secretion 10,11. Although this in vitro system has proven useful to study some aspects of B cell differentiation, both naïve IgD+ B cells and switched IgD− memory B cells eventually differentiate into terminally differentiated plasma cells, which is accompanied by cell cycle arrest precluding the generation of long-term antigen-specific BCR positive cell lines.
Recent advances have provided insight into how multiple transcription factors, including B-lymphocyte-induced maturation protein 1 (BLIMP1), X-box-binding protein 1 (XBP1) and, B-cell lymphoma (BCL)6 control development of GC B cells into terminally arrested, antibody-producing plasma cells 12. The transcriptional repressor BCL6 has been shown to prevent plasma cell differentiation in GC B cells, were it facilitates expansion of B cells by downregulating p53 and prevents premature differentiation of B cells into plasma cell by negatively regulating BLIMP1 11,13,14,15,16.
Recently we reported that signal transducer and activator of transcription (STAT)5 is activated in a small subset of human GC B cells. These cells expressed relatively high levels of BCL6 and low levels of BLIMP1 17. Forced expression of constitutive active STAT5 in B cells induced expression of both BCL6 and Bcl-xL and we provided evidence that BCL6 is a direct target of STAT5 in B cells. BCL6 regulates terminal differentiation facilitates in vitro expansion of B cells since ectopic expression of BCL6 in B cells inhibited terminal differentiation in vitro 17,18. This inhibition by BCL6 facilitates in vitro expansion of activated B cells 17,19. Here we show that ectopically expressed BCL6 and Bcl-xL synergize with CD40L and IL-21 to increase the proliferative lifespan of B cells in vitro. The expanded cells have features of GC B cells, express a functional BCR while secreting antibodies, providing a novel and powerful tool to generate human BCR-positive, antibody-secreting B cell lines.
Results
Transduction of peripheral memory B cells with BCL6 and Bcl-xL
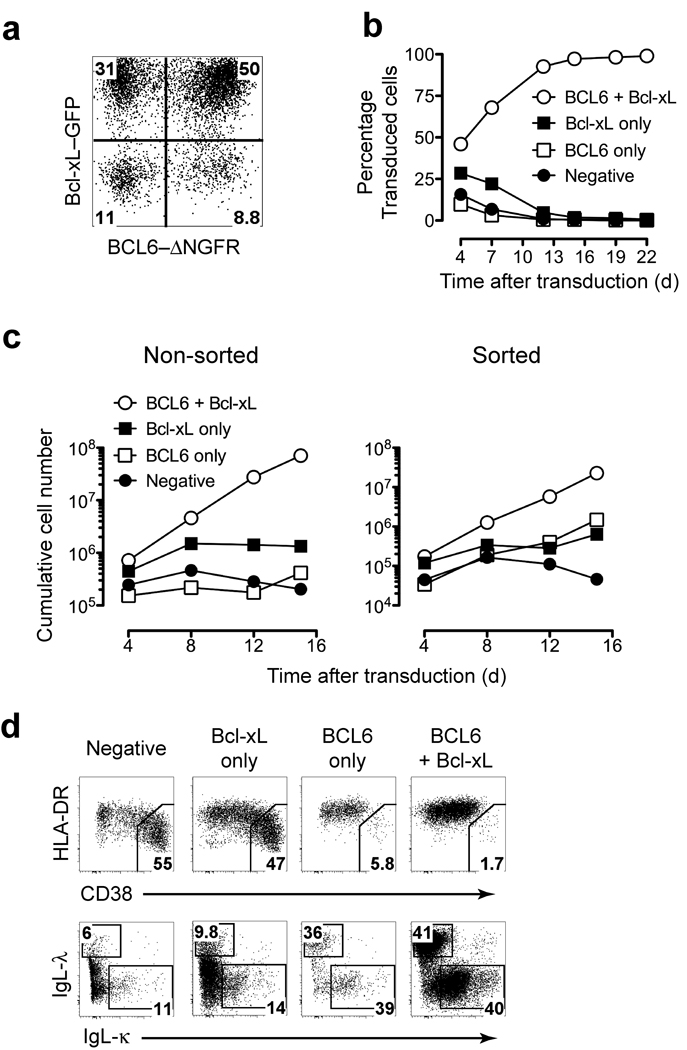
We hypothesized that BCL6 and Bcl-xL, which are expressed in GC B cells and are under control of STAT5 17 synergize to increase the proliferative and survival potential of B cells in vitro. To test this hypothesis we isolated human CD27+ memory B cells from peripheral blood and introduced BCL6 and Bcl-xL into these cells by retrovirus-mediated gene transfer. Transduced cells were cultured on irradiated CD40L expressing L cells (CD40L-L cells) in the presence of IL-21, a factor produced by follicular helper T cells in the GC that strongly induces proliferation of B cells 9. The transduced cells were visualized by their expression of the markers ΔNGFR (BCL6) and GFP (Bcl-xL) (Fig. 1a). Cells expressing both BCL6 and Bcl-xL rapidly increased in frequency to more than 95% of the culture within a two-week period (Fig. 1b). In addition, these cells exhibited a clear expansion advantage compared to cells expressing BCL6 or Bcl-xL alone (Fig. 1c, left panel). When the cell populations were sorted and cultured independently, the BCL6+Bcl-xL transduced cells showed enhanced expansion compared to BCL6 and Bcl-xL-only transduced cells (Fig. 1c, right panel). Normal human B cells rapidly differentiate to antibody-producing plasma cells when cultured on CD40L-L cells in the presence of IL-21 10 which is accompanied by a decrease in expression of surface BCR and MHC class II and an increase in expression of CD38 2. In contrast to non-transduced cells or cells expressing Bcl-xL only in the same culture, BCL6-only and BCL6+Bcl-xL cells both retained BCR expression and were HLA-DRhiCD38int (Fig. 1d) confirming that BCL6 inhibits B cell differentiation 17,18.
Figure 1.
Overexpression of BCL6 and Bcl-xL confer a high proliferative capacity and fixed differentiation phenotype to peripheral blood CD27+IgG+ memory B cells (a) Flow cytometry identification of BCL6-transduced (ΔNGFR+), Bcl-xL-transduced (GFP+) and BCL6+Bcl-xL transduced (GFP+ΔNGFR+) CD19+ B cells in culture with CD40L-L cells and IL-21 four days post transduction. (b) Percentages of the individually transduced populations over time within a single non-sorted culture maintained with CD40L-L cells and IL-21. BCL6+Bcl-xL ○, Bcl-xL-only ■, BCL6-only □ transduced cells or non-transduced cells (●). (c) Cultured double-transduced cells were left unsorted (left panel) or the individual populations were sorted (right panel) and cultured separately. The cumulative cell number was calculated based on the original number of transduced B cells at input and illustrates the theoretical absolute cell numbers produced in culture. (d) CD19+ cells from non-sorted double transduced cultures were analyzed for CD38, HLA-DR, and surface Ig kappa (IgL-κ) or lambda (IgL-λ) light chain expression. The gates in the upper panel show the percentage terminally differentiated cells. Phenotype shown of cells 7 days post transduction. Data are representative of four separate experiments.
When BCL6 and Bcl-xL were introduced into B cells from non-human primates and mice by retrovirus-mediated gene transfer we observed rapid expansion for at least one month in the presence of IL-21 and CD40L-expressing L cells (Supplementary figure 1), similar to transduced human B cells indicating that BCL6 and Bcl-xL allow expansion of B cells of multiple species.
BCL6+Bcl-xL-transduced B cells are similar to GC B cells
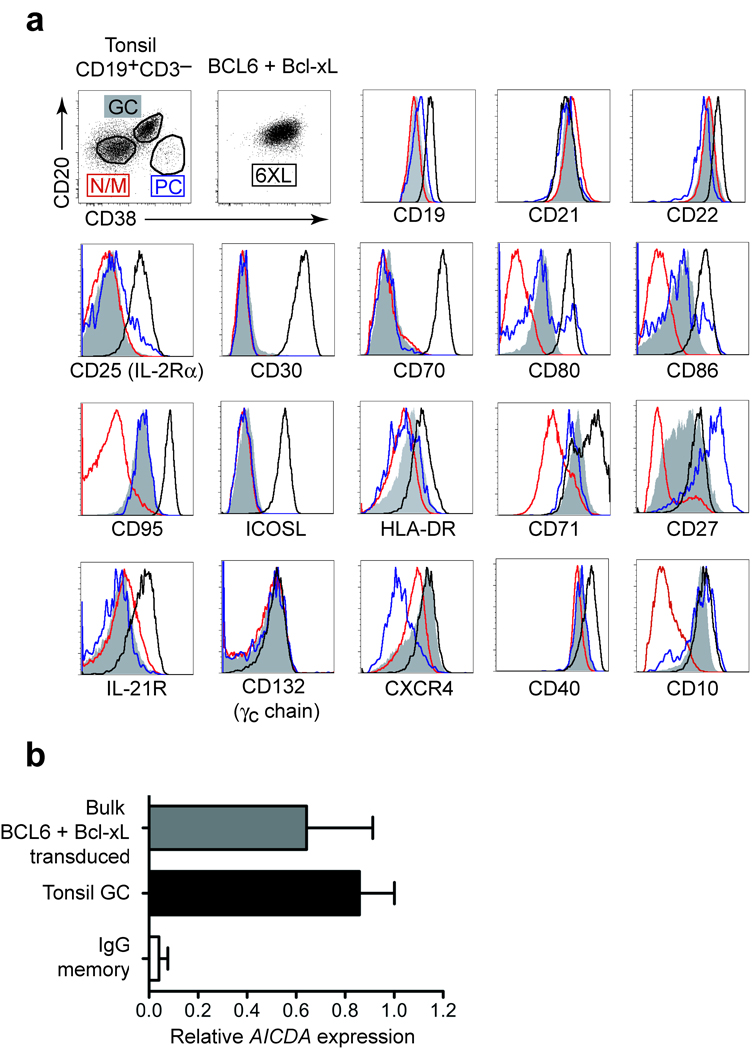
BCL6+Bcl-xL positive B cells expanded with CD40L and IL-21 express CD19, CD20, CD21 and CD22, the activation markers CD25, CD30, CD70, CD80, CD86, CD95, ICOSL, and the cytokine receptors CD132 (γc) and IL-21R. These cells express CD38 and CD20 at levels equivalent to tonsil GC cells. The expression of CD27, CXCR4, CD71, CD10 and HLA-DR on transduced cells is consistently higher compared to freshly isolated tonsil GC cells (Fig. 2a), which may be due to the activation status and increased size of the cell.
Figure 2.
CD27+ memory peripheral blood cells acquire a stable GC-like phenotype following transduction with BCL6 and Bcl-xL and subsequent culture. (a) Phenotype of BCL6+Bcl-xL transduced CD27+ memory B cells (6XL, black histogram line) compared to tonsil GC cells (GC, CD38+CD20+, shaded grey), tonsil naïve and memory cells (N/M, CD38−CD20low, red histogram), and tonsil plasma cells (PC, CD38++CD20low, blue histogram). BCL6+Bcl-xL transduced monoclonal cell lines show an identical phenotype (not shown). (b) Relative mRNA levels of AICDA (encoding AID) in CD19+IgG+CD27+ PB memory B cells and CD19+CD38+CD20+IgD− tonsillar GC B cells (value set as 1) compared to BCL6+Bcl-xL transduced bulk CD27+ memory cells using quantitative 5 RT-PCR.
BCL6+Bcl-xL transduced cells expressed AICDA (encoding the enzyme AID) at levels comparable to those expressed by freshly isolated GC B cells (Fig. 2b). As shown below, AID is functional in these cells as SHM in the Ig genes of the expanded B cells were induced. Since AID is not expressed in peripheral blood memory cells (Fig. 2b) or plasma cells 3, and transduced cells showed expression of typical GC cell surface markers, our results indicate that BCL6 and Bcl-xL expression in combination with CD40L and IL-21 signaling conferred GC-like characteristics to human CD27+ memory B cells.
Selection of B cell clones on basis of antigen binding
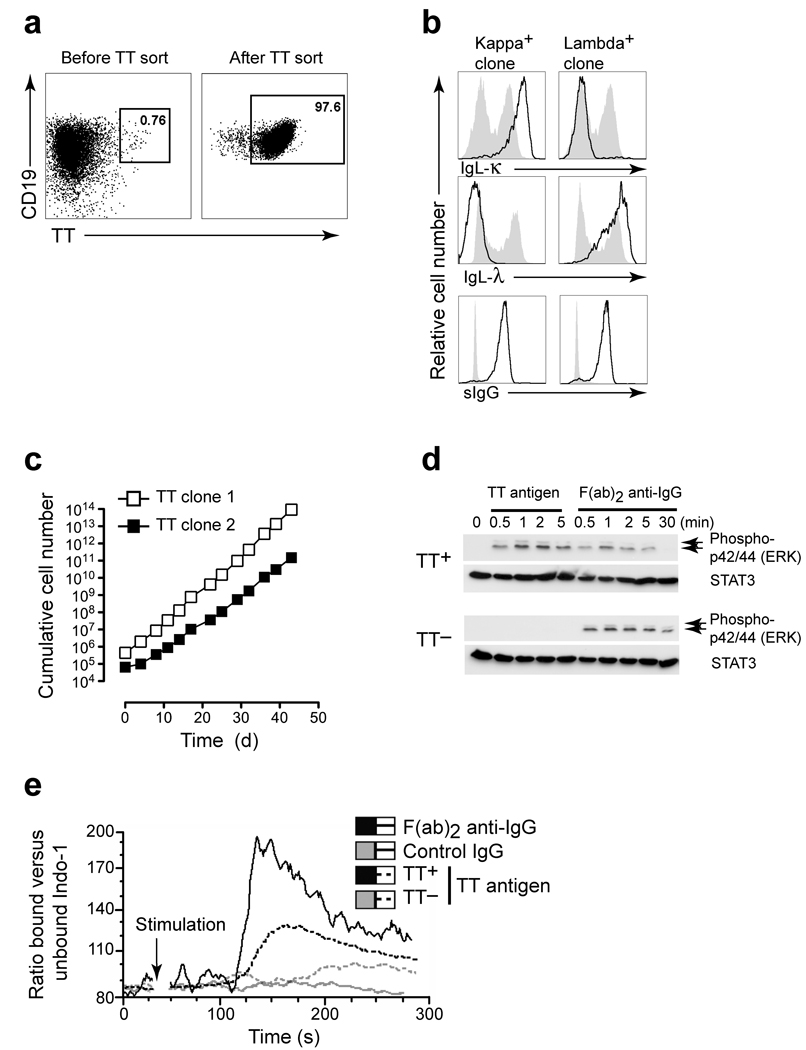
Since the transduced B cells expressed high levels of the BCR on their cell surface membrane we examined whether antigen binding to the BCR could be exploited to generate antigen-specific B cell clones. Individuals immunized with Tetanus Toxin, which is produced by Clostridium tetani, harbor memory B cells specific for this toxin. Purified peripheral CD27+ memory B cells from two healthy donors were transduced with BCL6 and Bcl-xL. In a 100% BCL6+Bcl-xL positive culture, cells were stained with phycoerythrin (PE)-labeled Tetanus toxoid (TT) (Fig. 3a). Cells that bound PE-labeled TT were FACS sorted and seeded at limiting cell numbers (0.5 and 1 cell/well). Within two weeks 39 (frequency of 68%) TT-specific B cell clones, IgG kappa or lambda light chain positive, arose and were expanded further (Fig. 3a and Fig. 3b). Cloned cells cultured with CD40L and IL-21 maintained a very short doubling time of 35 to 40 h, averaged over six weeks, (Fig. 3c) and could be maintained for extended periods of time and remained TT specific (>4 months, data not shown).
Figure 3.
Generation and characterization of Tetanus toxoid (TT)-specific monoclonal human B cell lines. (a) Cell sorting of TT-specific B cells from bulk CD19+ BCL6+Bcl-xL transduced memory B cell culture using TT-PE staining. (b) Monoclonal cell lines express surface IgG and are either IgL-λ or IgL-κ light chain positive. Shaded histograms are CD19+ tonsil B cells. (c) The cumulative cell number (as in Fig. 1c) of two representative B cell clones in time. (d) TT-specific and control TT negative cell lines were incubated with TT or F(ab)2 -human IgG antibody stimulation and phospho-ERK1/2 was determined by immunoblot analysis. STAT3 blotting was used to verify equal loading. Results are representative of two experiments (e) Calcium flux in TT specific IgG positive B cell lines. Cells were loaded with Indo-1 AM and were subsequently stimulated with TT (dashed black trace), IgG F(ab)2 antibody (solid black trace), or control IgG (solid grey trace). The Indo-1 response of a TT-negative clone stimulated with TT antigen is shown as an antigen specificity control (dashed grey trace). Shown is the ratio bound versus unbound Indo-1. Results are representative of three experiments.
To examine whether the BCR was functional we cultured IgG+ TT-specific and non-specific B cell lines in the absence of CD40L-L cells or IL-21 for 4 hours. The rested cells were then stimulated with TT antigen or an IgG F(ab)2 specific antibody and analyzed for phosphorylation (p) of extracellular signal–regulated kinase (ERK) 20. Within 30 seconds after addition of TT pERK was detected in the TT-specific cells but not in the TT-negative cells (Fig. 3d) indicating the presence of antigen specific functional BCR-coupled signal transduction machinery in these cells. pERK was detected in both cell lines stimulated with the IgG F(ab)2 antibody (Fig. 3d). Addition of TT or IgG F(ab)2 antibody to Indo-1-labeled B cells induced a rapid shift in the ratio bound versus unbound Indo-1 indicating that TT and IgG F(ab)2 induce Ca2+ flux (Fig. 3e). These results demonstrate that the BCR expressed on the cell surface of BCL6+Bcl-xL transduced memory B cells is signaling competent.
Selection of B cell clones secreting neutralizing antibodies
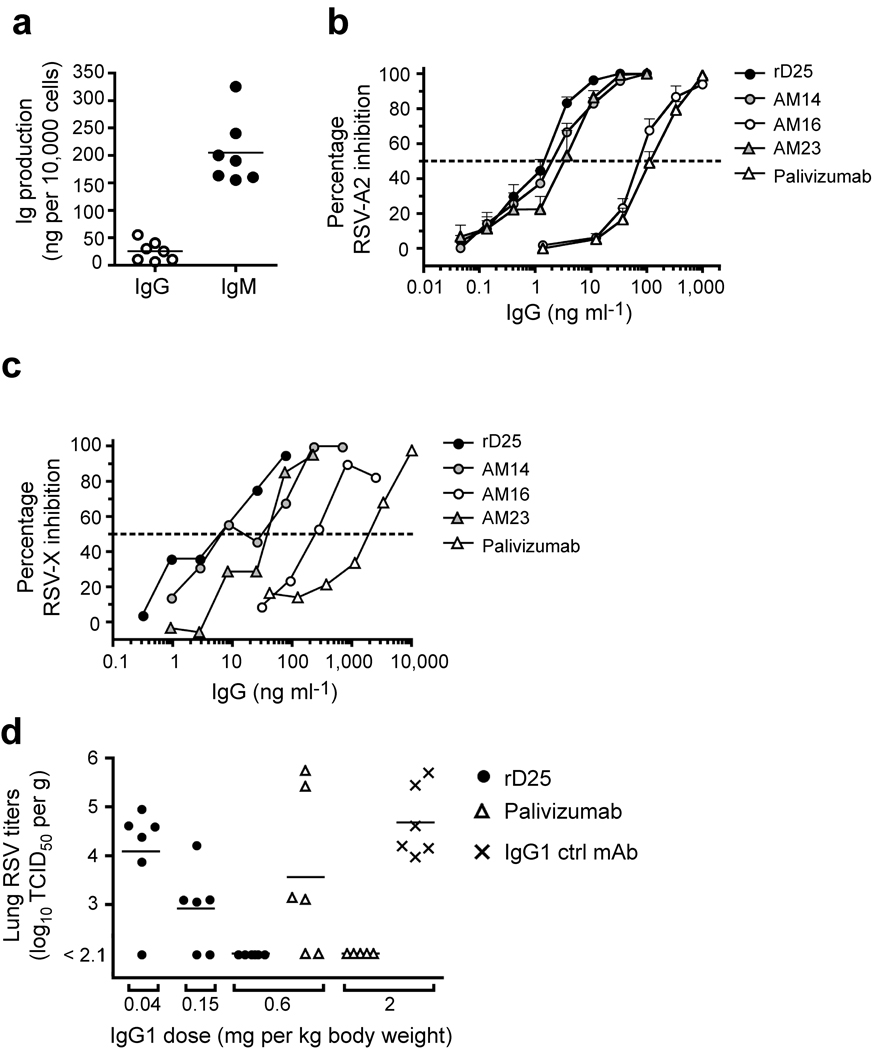
The IL-21 used to expand CD27+ B cells transduced with BCL6 and Bcl-xL is a strong inducer of differentiation into Ig-secreting cells 10. The transduced cells indeed secreted Ig (Fig. 4a) but the average production of IgG by BCL6+Bcl-xL transduced cells was lower than of plasma cells, although it was higher than that of memory B cells (Supplementary Fig. 2). Analysis of the IgG and IgM levels produced by monoclonal cell lines in a 3-day culture revealed that all clones produced Ig. The IgM-producing clones secreted five to six times more Ig than the IgG-producing clones (Fig. 4a), which has also been observed in cultured non-transduced memory cells 21. Although BCL6+Bcl-xL cells cultured with CD40L and IL-21 secrete antibody, they did not downregulate the BCR as do non-transduced in vitro–generated antibody-secreting plasma cells (Supplementary Fig. 2).
Figure 4.
Isolation of high affinity, broadly RSV neutralizing antibodies from RSV-specific B cell clones. (a) IgM or IgG production of individually cloned BCL6+Bcl-xL-immortalized cell lines, from the CD19+CD27+IgA−IgM− and CD19+CD27+IgA−IgG− memory B cell pool respectively, maintained on CD40L-L cells and IL-21 in a 3 day culture. (b) Generation of RSV neutralizing antibodies. Neutralization dose response curve against the RSV A2 virus for the newly generated RSV antibodies D25 (●),
AM14 ( ), AM16 (○), and AM23 (
), AM16 (○), and AM23 ( )
compared to palivizumab (Δ). (c) Neutralization dose response curve of the RSV antibodies D25, AM14, AM16, AM23 and palivizumab against the primary RSV isolate X. Symbols as in 4b (d) Effects of rD25 (●), palivizumab (Δ) and a control IgG1 (×) on RSV replication in cotton rat lungs. Antibodies were administered i.m. one day before intranasal challenge with RSV X (106 TCID50/animal). Lung virus titers were calculated per gram of lung tissue; control prophylaxis reached a titer of 4.7 log TCID50 g−1, while the lower limit of detection was 2.1 log10TCID50 g−1.
)
compared to palivizumab (Δ). (c) Neutralization dose response curve of the RSV antibodies D25, AM14, AM16, AM23 and palivizumab against the primary RSV isolate X. Symbols as in 4b (d) Effects of rD25 (●), palivizumab (Δ) and a control IgG1 (×) on RSV replication in cotton rat lungs. Antibodies were administered i.m. one day before intranasal challenge with RSV X (106 TCID50/animal). Lung virus titers were calculated per gram of lung tissue; control prophylaxis reached a titer of 4.7 log TCID50 g−1, while the lower limit of detection was 2.1 log10TCID50 g−1.
Given the relatively high amounts of secreted antibodies by BCL6+Bcl-xL-transduced B cells we examined whether we could select antigen-specific B cells on the basis of secretion of specific antibody. We chose the pathogenic respiratory syncytial virus (RSV) as the antigenic moiety. RSV is the most common cause of bronchiolitis and pneumonia among infants and children under 1 year of age and is a serious health problem for elderly people 22. BCL6+Bcl-xL transduced memory B cells of a healthy donor were seeded at 100 cells/well and expanded with CD40L-L cells and IL-21. After 2 weeks of culture, supernatants were harvested and screened for the presence of RSV-neutralizing antibodies in a microneutralization experiment 23. Of 384 cultures (100 cells/well), 31 prevented RSV infection of HEp2 cells. Four microcultures with the highest neutralizing activity were subcloned by limiting dilution. Four monoclonal cell lines (D25, AM14, AM16, and AM23) were selected and further characterized. We observed median half maximum inhibitory concentrations (IC50) against the RSV-A2 virus ranging from 2.1 ng ml−1 for D25 and AM14, 4.3 ng ml−1 for AM23 to 145 ng ml−1 for AM16 (Fig. 4b). By comparison, three of the four Abs exhibited significantly lower IC50 values compared to palivizumab (IC50 209 ng ml−1), a humanized, FDA-approved monoclonal antibody that is currently used as prophylaxis in infants at high risk 24. In addition, all RSV antibodies efficiently neutralized a primary RSV isolate X (RSV-X) (Fig. 4c).
D25 antibody inhibits RSV infection in cotton rats
To test the in vivo neutralizing activity of D25 in a prophylactic setting, we infected cotton rats, the standard model to test RSV infectivity and pathogenicity with the primary isolate RSV-X 25. Animals were injected i.m. with various quantities of recombinant D25 (0.6, 0.15 and 0.04 mg kg−1), palivizumab (2.0 and 0.6 mg kg−1) or control IgG1 (2.0 mg kg−1) one day before intranasal inoculation with RSV-X. Animals were sacrificed 5 days post challenge, since at this time point peak lung virus titers were observed 26. RSV-X titers in the lung were analyzed by standard TCID50 culture assay (Fig 4d). While palivizumab completely prevented in vivo viral replication at 2.0 mg kg−1, D25 showed the same efficacy already at a dose of 0.6 mg kg−1 and was partially neutralizing at a dose of 0.15 mg kg−1. These data show that also in vivo D25 is functional and potent in reducing RSV replication.
AID is active in BCL6+Bcl-xL transduced cells
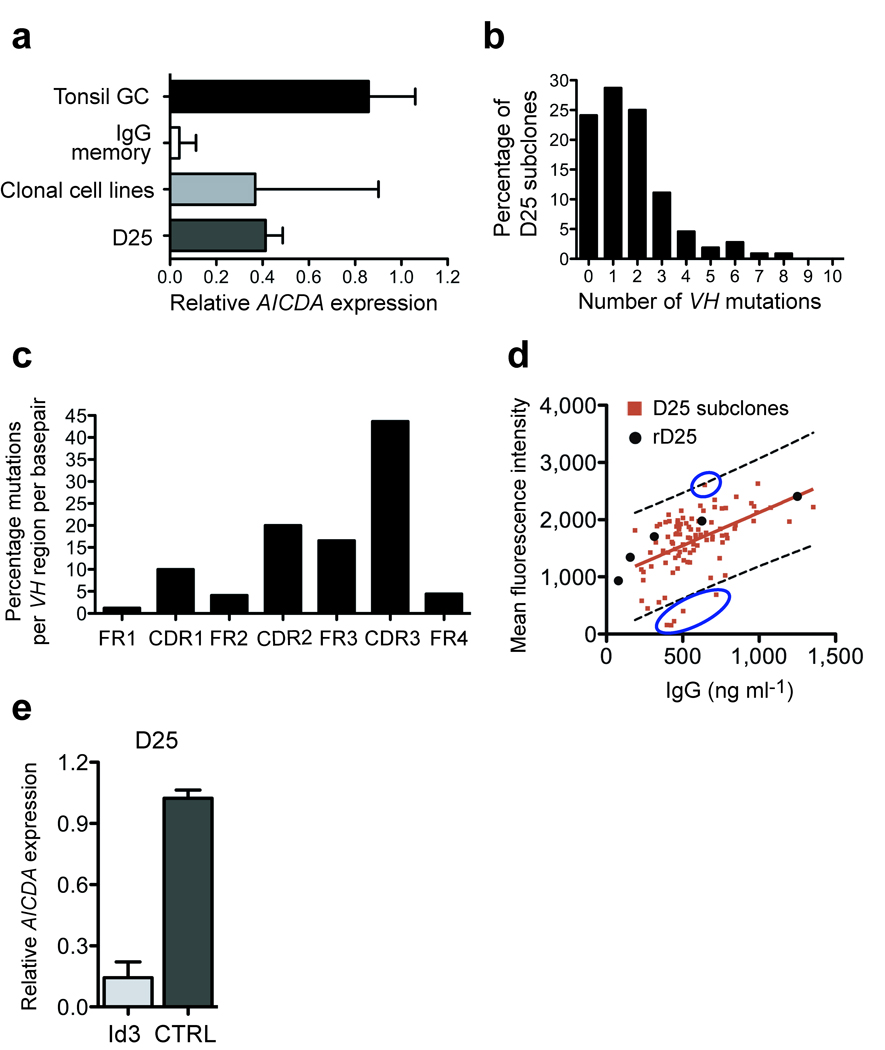
Analysis of AICDA expression in 23 monoclonal cell lines by real-time PCR revealed a variable expression in different clonal cell lines. Several clones expressed levels similar to GC tonsil B cells, whereas others were AICDA low (Fig. 5a). To determine whether AID is functional in the B cell clones, we subcloned the RSV specific monoclonal B-cell line D25 by single cell sorting and analyzed their VH genes for the presence of mutations. Sixty three percent of the wells that were seeded showed robust expansion, suggesting that expression of AID does not result in genetic instability leading to growth arrest and cell death. After 3 weeks culture supernatant of 108 subclones was harvested and the RNA from the cells isolated. Subsequently cDNA was generated and the VH region sequenced. Sequence analysis revealed a total of 184 VH mutations (107 unique mutations), resulting in an estimated mutation rate between 8.85×10−5 and 5.14×10−5 mutations per bp per cell division, which is at the lower end of the estimated AID-mediated mutation rate in vivo (10−3 to 10−5) 27. The VH genes of the individual subclones show a variable number of mutations with 65% of subclones harboring one to three mutations in their VH region (of which 23% are silent mutations) and 11% harboring more than three VH mutations. Twenty four percent of the clones had no VH mutations (Fig. 5b). The 372-basepair VH gene of D25 contains 26 AID mutational hotspots (RGYW/WRCY) that account for 30% of the total mutations. Mutations were predominantly observed in the CDR regions and FR3 (Fig 5c).
Figure 5.
Expression and activity of AID in BCL6+Bcl-xL transduced cells. (a) mRNA levels of AICDA (encoding AID) in CD19+CD38+CD20+IgD− tonsillar GC B cells and CD19+IgG+CD27+ PB memory B cells compared to 23 BCL6+Bcl-xL transduced monoclonal cell lines and monoclonal cell line D25 using quantitative RT-PCR. (b) Percentage of subclones with indicated number of VH mutations, as percentage of total number of subclones sequenced. (c) Location of VH mutations, percentage of mutations per VH region per basepair. (d) Binding of D25-subclone Ig to RSV infected HEp2 cells. Red squares are individual D25 subclones, black circles rD25. Blue circles indicate clones with deviating binding activity. (e) mRNA levels of AICDA in the monoclonal cell line D25 transduced with Control-YFP or Id3-YFP using quantitative RT-PCR.
While the supernatants of the majority of D25 subclones bound to RSV-infected HEp2 cells similar to recombinant D25, those of some clones bound either less or better than D25 (Fig. 5d). The differences in binding activities were associated with mutations in the VH regions.
For some applications it would be desirable to inhibit AID to prevent accumulation of mutations in the Ig genes of BCL6+Bcl-xL transduced B cell clones. To achieve this we took advantage of the fact that AID is regulated by the basic Helix Loop Helix transcription factor E47 28. We overexpressed the Helix loop Helix factor inhibitor of DNA binding (Id)3 which is known to form transcriptionally inactive complexes with E47, thereby inhibiting AID expression in D25 28. As shown in Figure 5e, overexpression of Id3 strongly reduced AICDA levels in the D25 cell line without influencing proliferation of the D25 cells (data not shown). Thus modulation of Id3 levels provides a method to prevent AID induced mutations in BCL6+Bcl-xL transduced B cells.
Discussion
Here we describe an efficient and rapid method for the generation of antigen-specific, BCR positive B cell lines. By retroviral introduction of the GC-expressed genes BCL6 and Bcl-xL, we converted peripheral blood memory cells into GC-like B cells that can be maintained for prolonged periods in culture. Both BCL6 and Bcl-xL are involved in the GC response: BCL6 is required for GC formation 13,14, while Bcl-xL is an important anti-apoptotic factor in the GC 7. The BCL6+Bcl-xL transduced cells express markers for human GC B cells including AID, CD38, CXCR4 and ICOSL, the later has been shown to be important for GC formation 29.
It is evident from our data that the double-transduced cells resemble GC B cells with respect to surface markers and AICDA expression, but whether they can be classified as centroblasts or centrocytes is less clear. We suggest that they are centrocyte-like in nature, perhaps representing a small subset of centrocytes, since the transduced B cells express a functional BCR, which has long been held as a characteristic of light zone centrocytes 1. This BCR participates in the selection by follicular dendritic cells which occurs in the light zone 5. Moreover, expansion of these B cells is depended upon CD40 signaling and the dark zone is mostly devoid of CD154+ (CD40L) T cells, while these activated T cells are present in the light zone. In addition, nuclear translocation of NF-kB, which is induced by CD40 signaling, has only been observed in B cells in the light zone 30.
The expression of AICDA in the cell lines raised the question whether AID is functional. Although AID was expressed in all clones that were derived from BCL6+Bcl-xL transduced CD27+ peripheral blood memory cells, the level was variable. Moreover we did not observe isotype switching in these cells over time, indicating that AICDA expression by itself is insufficient to drive this process at least in the time frame and culture conditions we applied. In contrast we did observe the occurrence of somatic hypermutations at relative low frequency in subclones of one RSV-specific B cell clone. Screening of the mAb containing culture supernatant of these subclones on RSV infected cells demonstrated variations in binding capacity. This result suggested that the additional VH gene mutations of some subclones resulted in changes in the antigen-binding affinity. These data raise the possibility that ongoing AID-mediated mutational activity in B cell clones can be used to select higher (or lower) affinity clones obviating the need for deliberate molecular engineering of the relevant Ig genes. It is conceivable that in some cases mutations in the Ig genes of established cloned B cell lines need to be avoided. We show that following introduction of Id3 into B cells that already overexpress BCL6 and Bcl-xL AICDA levels are strongly reduced consistent with findings published by Sayeg et al. 28. Thus overexpression of Id3 should prevent ongoing AID-mediated mutational activity, if a frozen VH/VL repertoire is required. Despite the expression of AICDA in BCL6 and Bcl-xL-transduced IgG, IgM and IgA memory B cells we did not observe CSR in these cells (data not shown). This might be related to the different requirements for AID-mediated SHM and CSR 31,32.
We previously showed that IL-21 was able to elicit Ig secretion from polyclonal BCL6-expressing B cells 18. In combination with the stable BCR expression, which allows for selection of antigen specific B cells by binding of fluorescent-labeled antigen, Ig secretion allows for functional screening of secreted Ig. Both methods provide a tool for rapid isolation of potential therapeutic antibodies. Indeed we were able to generate unique RSV neutralizing antibodies within 3 months by direct functional screens following immortalization of B cells obtained from an RSV-exposed individual.
Recently another method of generating monoclonal antibodies from human B cells was described based on Epstein-Barr virus (EBV) transformation of B cells activated with Toll like receptor-9 antagonists 33. Both the method described by Traggiai et al. and ours utilize human B cells of exposed individuals to select monoclonal antibodies against a variety of microbes without the need of recent vaccination of the donors. However, in contrast to B cells transformed by EBV 34 all B cell lines obtained with BCL6+Bcl-xL genetic programming stably express high levels of a signaling-competent, membrane-bound BCR. This high level of cell surface BCR facilitates isolation of B cells on the basis of antigen binding which greatly increases the frequency of antigen specific cells and abrogates the need for extensive subcloning. Whereas the generation of antigen specific clones using the EBV method takes 3 months 33, the genetic programming approach reduces this to 1 month. The fact that rare B cells can be easily cloned compares our method favorably with a recently described single cell cloning approach used to isolate influenza-specific antibodies 35. In this study, Ig DNA was isolated 7 days after influenza vaccination from a population of CD27highCD38high B cells which contained a relatively high frequency of only several Influenza virus-specific B cells. The feasibility of this technique depends on a relative high frequency of antigen-specific B cells, that can be obtained by vaccination or very recent exposure to a microbe 35,36. As discussed above expression of AID in the genetically modified clones may be utilized to select antibodies with higher affinity providing an advantage compared to the EBV transformation and single cell PCR methods as platforms to generate human monoclonal antibodies.
Another advantage of the method described here is that it can be used to obtain stable lines of B cells from species other than humans. We demonstrate that BCL6+Bcl-xL-transduced B cells of mice and non-human primates proliferate for at least one month in the presence of CD40L and IL-21. We also generated a series of cloned lines of mouse B cells (results not shown). It is therefore feasible to assume that our method will allow for expansion of antigen-specific B cells of multiple species including rabbits and goats. Such lines cannot be obtained by using EBV, which is only capable of transforming B cells of a variety of monkey species such as Chimpanzee, Marmoset, Squirrel, Owl and Cebus monkeys 37.
The monoclonal cell lines that can be generated with this technology are very useful not only for generation of monoclonal antibodies, but also allow for detailed mechanistic studies of BCR-mediated signal transduction induced by specific antigens. They may be used to study signaling of BCR variants that differ in affinity for antigen. Furthermore, these cell lines can be useful tools to study the effects of a variety of external signals like antigen or cytokines on the function of AID and the induction of SHM.
Methods
B cell isolation
We obtained B cells from peripheral blood (Sanquin) by Ficoll separation and CD22 MACS microbeads (Miltenyi Biotech). Subsequently we sorted these cells for CD19+CD3−CD27+IgM−IgA− (IgG memory cells) or CD19+CD3−CD27+IgG−IgA− (IgM memory cells) on a FACSAria (Becton Dickinson). We obtained Tonsil B cells from routine tonsillectomies performed at the Department of Otolaryngology at the Academic Medical Center, Amsterdam, The Netherlands. The use of these tissues was approved by the medical ethical committees of the institution and was contingent on informed consent.
Cell culture
We maintained B cells (2×105 cells ml−1) in IMDM (Gibco) culture medium containing 8% FBS (HyClone), penicillin/streptomycin (Roche) supplemented with recombinant mouse IL-21 (25 ng ml−1, R&D systems) and co-cultured them on γ-irradiated (50Gy) mouse L cell fibroblasts stably expressing CD40L (CD40L-L cells, 105 cells ml−1). We used rhIL-4 (R&D) at 50 ng ml−1, and rhIL-2 at 100 U ml−1. We tested cells routinely by PCR for the presence of mycoplasma and EBV (data not shown).
Retroviral transduction
The BCL6 and Id3 retroviral constructs were described previously 19,38. We cloned human Bcl-xL cDNA into the LZRS retroviral vector. We performed transfections and production of Amphotropic and Ecotropic virus by Phoenix packaging cells as described before 17,19 and co-transduced memory B cells by retroviruses after activation on CD40L-L cells in the presence of rmIL-21 for 36 h as before 18, but then we centrifuged cells and virus at room temperature for 60 min at 360 × g (1800 RPM).
Flow cytometry
We analyzed stained cells on an LSRII (BD) and processed flow cytometry data with FlowJo software (Tree Star). The antibodies are described in the online supplementary methods section.
RT-PCR
We carried out quantitative RT-PCR with a BioRad iCycler and used the 2−(ΔΔCT) method to calculate relative mRNA expression levels normalized to ACTIN. Primers for AICDA (encoding AID) are described 39.
ELISA
We coated plates with either anti-human IgG or IgM Fc-fragment (Jackson ImmunoResearch Laboratories) at 5 µg ml−1 in PBS for 1 h at 37 °C or o/n at 4 °C and washed them in ELISA wash buffer (PBS, 0.5% Tween-20). 4% milk in PBS was used as blocking agent, before we added serial dilutions of cell culture supernatants and HRP-conjugated detection Abs (dilutions 1:2500 for HRP-conjugated IgG antibody (Jackson), dilution 1:5000 for HRP-conjugated IgM antibody (Jackson)). We used TMB substrate solution (Biosource) for development of the ELISAs.
Immunoblotting
We lysed cells in Triton Lysis Buffer supplemented with HALT protease inhibitor (Roche) and 1 µM Na2VO4. We separated equal amounts of protein by 10% SDS-PAGE, transferred it to nitrocellulose, and probed using phospho-ERK1/2 antibody (Thr202/Tyr204) (Cell Signaling Technologies) and STAT3 antibody (Santa Cruz). We used enhanced chemiluminescence (Pierce Biotechnology) and x-ray film (Hyperfilm, GE Healthcare Life Sciences) for detection.
Ca2+ flux
We cultured transduced B cells for two days without CD40L-L cells in the presence of IL-21, subsequently we loaded the cells with 2 µM Indo-1 AM (Sigma-Aldrich). Fluorescence ratios of Indo-1 emission at 405/485 nm were measured by flow cytometry on a FACSVantage SE (BD).
RSV culture and neutralization assay
We seeded 104 HEp2 cells in flat bottom 96 well plates (Costar). The next day we pre-incubated 100 TCID50 of RSV A2 or X virus mixed with B cell culture supernatant for 30 min at 37 °C before adding it in triplicate to HEp2 cells for 1 h at 37 °C. After 2 days we fixed the cells with 80% acetone and stained them with polyclonal RSV-HRP antibody (Biodesign) and 3-amino-9-ethylcarbazole for detection and visualization of RSV plaques by light microscopy (plaques were counted).
Cloning of D25
We isolated total RNA with the RNeasy® mini kit (Qiagen), generated cDNA, performed PCR and cloned the heavy and light chain variable regions into the pCR2.1 TA cloning vector (Invitrogen). To rule out reverse transcriptase or DNA polymerase induced mutations, we performed several independent cloning experiments. To produce recombinant D25 mAb we cloned D25 heavy and light variable regions in frame with human IgG1 and Kappa constant regions into a pcDNA3.1 (Invitrogen) based vector and transiently transfected 293T cells. We purified recombinant D25 from the culture supernatant with Protein A.
Cotton rat experiments
7–9 week old Cotton rats (Sigmodon hispidus, Charles River) were anesthetized with isoflurane, bled and given 0.1 ml of purified mAb per 100 gram body weight (bw) by intramuscular (i.m.) injection at doses of 2.0 or 0.6 for palivizumab, 0.6, 0.15 or 0.04 for D25 and 2.0 mg kg−1 bw for the control antibody. Twenty-four hours later, animals were anesthetized and challenged by intranasal instillation of 106 TCID50 RSV-X (100 µl). Five days later animals were sacrificed and their lungs were harvested. Lung virus titers were determined and the lowest limit of detection was 2.1 log10TCID50 g−1. The Animal Experiment Committee of the Netherlands Vaccine Institute approved all procedures involving cotton rats.
Supplementary Material
Acknowledgements
We thank B. Hooijbrink for his excellent help with FACS sorting and maintenance of the flow cytometry facility, J. Boes for her excellent work on virus titrations at NVI, R. Molenkamp and D. Pajkrt of the Dept. of Clinical Virology of the AMC for helpful discussions. Human Bcl-xL cDNA was kindly provided by S. Korsmeyer. Howard Hughes Medical Institute, Harvard Medical School, Boston, MA, USA. S.A.D. is supported by NIH-NIAID grant F32AI063846, M.v.L is supported by grant OZF-02-004 of the Wilhelmina Research Fund.
Abbreviations
- BCR
B cell receptor
- RSV
respiratory syncytial virus
- GC
germinal center
- CSR
class-switch recombination
- Ig
immunoglobulin
- IL
interleukin
- SHM
somatic hypermutation
- TT
tetanus toxoid
- EBV
Epstein-Barr virus
- AID
activation induced cytidine deaminase
- BLIMP1
B-lymphocyte-induced maturation protein 1
- XBP1
X-box-binding protein 1
- BCL6
B-cell lymphoma 6
- STAT5
signal transducer and activator of transcription 5
- ERK
extracellular signal–regulated kinase
- Id3
inhibitor of DNA binding 3
Footnotes
Financial interests
SAD, FAS, HS and TB are listed on patent applications describing this technology.
Author contributions
MJK, SAD, and TB performed experiments, analyzed data and wrote the paper. HS and TB organized research. EY, AQB and CVG performed experiments. MVL, GMvB, HM, AR and WMJMB contributed valuable reagents and developed assays. MNW performed cotton rat experiments, FAS analyzed data and provided valuable suggestions.
References
- 1.Close PM, Pringle JH, Ruprai AK, West KP, Lauder I. Zonal distribution of immunoglobulin-synthesizing cells within the germinal centre: an in situ hybridization and immunohistochemical study. J Pathol. 1990;162:209–216. doi: 10.1002/path.1711620306. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–126. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem. 1999;274:18470–18476. doi: 10.1074/jbc.274.26.18470. [DOI] [PubMed] [Google Scholar]
- 4.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 5.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- 6.Kim CH, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuscano JM, et al. Bcl-x rather than Bcl-2 mediates CD40-dependent centrocyte survival in the germinal center. Blood. 1996;88:1359–1364. [PubMed] [Google Scholar]
- 8.Banchereau J, de Paoli P, Valle A, Garcia E, Rousset F. Long-term human B cell lines dependent on interleukin-4 and antibody to CD40. Science. 1991;251:70–72. doi: 10.1126/science.1702555. [DOI] [PubMed] [Google Scholar]
- 9.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 11.Kuo TC, et al. Repression of BCL-6 is required for the formation of human memory B cells in vitro. J Exp Med. 2007;204:819–830. doi: 10.1084/jem.20062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro-Shelef M, et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 13.Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 1997;276:589–592. doi: 10.1126/science.276.5312.589. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, et al. Disruption of the Bcl6 gene results in an impaired germinal center formation. J Exp Med. 1997;186:439–448. doi: 10.1084/jem.186.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 17.Scheeren FA, et al. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol. 2005;6:303–313. doi: 10.1038/ni1172. [DOI] [PubMed] [Google Scholar]
- 18.Diehl SA, et al. STAT3-Mediated Up-Regulation of BLIMP1 Is Coordinated with BCL6 Down-Regulation to Control Human Plasma Cell Differentiation. J Immunol. 2008;180:4805–4815. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shvarts A, et al. A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev. 2002;16:681–686. doi: 10.1101/gad.929302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 21.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 22.Hall CB, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson S, et al. A direct comparison of the activities of two humanized respiratory syncytial virus monoclonal antibodies: MEDI-493 and RSHZl9. J Infect Dis. 1999;180:35–40. doi: 10.1086/314846. [DOI] [PubMed] [Google Scholar]
- 24.Sidwell RW, Barnard DL. Respiratory syncytial virus infections: recent prospects for control. Antiviral Res. 2006;71:379–390. doi: 10.1016/j.antiviral.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Domachowske JB, Bonville CA, Rosenberg HF. Animal models for studying respiratory syncytial virus infection and its long term effects on lung function. Pediatr Infect Dis J. 2004;23:S228–S234. doi: 10.1097/01.inf.0000144672.81955.a4. [DOI] [PubMed] [Google Scholar]
- 26.Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Invest. 1999;79:1385–1392. [PubMed] [Google Scholar]
- 27.Peled JU, et al. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 28.Sayegh CE, Quong MW, Agata Y, Murre C. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- 29.Dong C, Temann UA, Flavell RA. Cutting edge: critical role of inducible costimulator in germinal center reactions. J Immunol. 2001;166:3659–3662. doi: 10.4049/jimmunol.166.6.3659. [DOI] [PubMed] [Google Scholar]
- 30.Basso K, et al. Tracking CD40 signaling during germinal center development. Blood. 2004;104:4088–4096. doi: 10.1182/blood-2003-12-4291. [DOI] [PubMed] [Google Scholar]
- 31.Shinkura R, et al. Separate domains of AID are required for somatic hypermutation and class-switch recombination. Nat Immunol. 2004;5:707–712. doi: 10.1038/ni1086. [DOI] [PubMed] [Google Scholar]
- 32.McBride KM, et al. Regulation of class switch recombination and somatic mutation by AID phosphorylation. J Exp Med. 2008;205:2585–2594. doi: 10.1084/jem.20081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traggiai E, et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dykstra ML, Longnecker R, Pierce SK. Epstein-Barr virus coopts lipid rafts to block the signaling and antigen transport functions of the BCR. Immunity. 2001;14:57–67. doi: 10.1016/s1074-7613(01)00089-9. [DOI] [PubMed] [Google Scholar]
- 35.Wrammert J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwick MB, Gach JS, Burton DR. A welcome burst of human antibodies. Nat Biotechnol. 2008;26:886–887. doi: 10.1038/nbt0808-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deinhardt F, Falk LA, Wolfe LG. Transformation of nonhuman primate lymphocytes by Epstein-Barr virus. Cancer Res. 1974;34:1241–1244. [PubMed] [Google Scholar]
- 38.Jaleco AC, et al. Genetic modification of human B-cell development: B-cell development is inhibited by the dominant negative helix loop helix factor Id3. Blood. 1999;94:2637–2646. [PubMed] [Google Scholar]
- 39.Smit LA, et al. Expression of activation-induced cytidine deaminase is confined to B-cell non-Hodgkin's lymphomas of germinal-center phenotype. Cancer Res. 2003;63:3894–3898. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.